Abstract
Background & Aims
Vitamin D influences innate immunity, which is believed to be involved in the pathogenesis of Crohn's disease (CD) and ulcerative colitis (UC). However, data examining vitamin D status in relation to risk of CD and UC are lacking.
Methods
We conducted a prospective cohort study of 72,719 women (age, 40–73 y) enrolled in the Nurses' Health Study. In 1986, women completed an assessment of diet and lifestyle, from which a 25-hydroxy vitamin D [25(OH)D] prediction score was developed and validated against directly measured levels of plasma 25(OH)D. Through 2008, we confirmed reported diagnoses of incident CD or UC through medical record review. We used Cox proportional hazards modeling to examine the hazard ratio (HR) for incident CD or UC after adjusting for potential confounders.
Results
During 1,492,811 person-years of follow-up evaluation, we documented 122 incident cases of CD and 123 cases of UC. The median predicted 25(OH)D level was 22.3 ng/mL in the lowest and 32.2 ng/mL in the highest quartiles. Compared with the lowest quartile, the multivariate-adjusted HR associated with the highest quartile of vitamin D was 0.54 (95% confidence interval [CI], 0.30–.99) for CD (Ptrend = .02) and 0.65 (95% CI, 0.34–1.25) for UC (Ptrend = .17). Compared with women with a predicted 25(OH)D level less than 20 ng/mL, the multivariate-adjusted HR was 0.38 (95% CI, 0.15–0.97) for CD and 0.57 (95% CI, 0.19–1.70) for UC for women with a predicted 25(OH)D level greater than 30 ng/mL. There was a significant inverse association between dietary and supplemental vitamin D and UC, and a nonsignificant reduction in CD risk.
Conclusions
Higher predicted plasma levels of 25(OH)D significantly reduce the risk for incident CD and nonsignificantly reduce the risk for UC in women.
Keywords: Ultraviolet Exposure, Nutrition, Inflammatory Bowel Disease, IBD
Ulcerative colitis (UC) and Crohn's disease (CD), known collectively as inflammatory bowel diseases (IBDs), are chronic, immunologically mediated disorders with an increasing incidence worldwide.1, 2 A key pathogenic mechanism in the development of these diseases is an inappropriate immune response to intestinal microbial flora in a genetically susceptible host.2, 3 Despite the identification of nearly 100 genetic loci associated with CD or UC affecting innate or adaptive immune responses and intestinal barrier function,4,5 the risk of IBD attributable to these known genetic factors is estimated to be less than 25%.2 Thus, other, as yet undefined, genetic factors and environmental influences appear to play an important role.
Several lines of evidence support vitamin D as a particularly promising environmental factor that substantially may influence the risk of developing IBD. First, ecologic studies have suggested that lower levels of vitamin D associated with reduced solar ultraviolet-B radiation exposure could account for a north-south gradient, with increased incidence of IBD among populations at higher latitudes.6 Second, studies have linked single-nucleotide polymorphisms in the vitamin D receptor (VDR) to increased susceptibility to CD and UC.7–10 Third, deficiency of 1,25(OH)2D3 and VDR knockout in mouse models increases the severity of dextran sodium sulfate–induced colitis and administration of 1,25(OH)2D3 suppresses the expression of several tumor necrosis factor-α–related genes.11,12 Administration of cholecalciferol also suppresses peripheral blood mononuclear cell responsiveness to relevant multiple sclerosis disease antigens.13 However, despite this compelling data, more direct evidence supporting a role for vitamin D in modulating risk of incident CD and UC in human beings is lacking. Specifically, there are no prior studies examining estimates of prediagnostic plasma 25(OH)D, widely considered the best integrated measure of vitamin D status,14,15 in relation to risk of incident CD or UC.
We therefore sought to examine the association between vitamin D status among women enrolled in a large prospective cohort, the Nurses' Health Study (NHS), for which detailed information about dietary and lifestyle predictors of 25(OH)D status have been collected and validated in relation to plasma vitamin D levels. This cohort offered us a unique opportunity to examine 25(OH)D and dietary intake of vitamin D several years before diagnosis of CD or UC, thus minimizing any biases associated with the nutritional deficiencies associated with preclinical symptoms.
Materials and Methods
Study Population
The NHS is a prospective cohort that began in 1976 when 121,700 US female registered nurses, ages 30 to 55 years, completed a mailed health questionnaire. With a follow-up response rate of more than 90%, questionnaires have been mailed every 2 years to update health information. In 1980, comprehensive dietary and supplement information was obtained through a validated semiquantitative food frequency questionnaire from which intake of vitamin D could be derived.16,17 In 1986 the semiquantitative food frequency questionnaire was expanded, and a validated physical activity assessment also was administered. For this analysis, we included the 72,719 women who returned the 1986 questionnaire with data on dietary intake and physical activity and did not have a prior history of CD, UC, or cancer (except nonmelanoma skin cancer).
Ascertainment of CD and UC
Since 1976, on each biennial questionnaire, participants in the NHS I have reported newly diagnosed diseases, including UC and CD. When a diagnosis was reported, we sent a supplemental questionnaire to obtain more detailed information and request permission to review all pertinent medical records. All records were reviewed by 2 independent gastroenterologists and cases of UC and CD with dates of diagnosis were confirmed according to strict diagnostic criteria. Patients were considered to have UC based on a typical clinical presentation of 4 weeks or more and one of either endoscopic, surgical, or radiologic changes consistent with chronic UC. A diagnosis of CD was made based on typical clinical history for 4 weeks or more along with at least one typical finding on endoscopy, radiologic evaluation showing small-bowel involvement, or surgical findings consistent with CD in combination with pathology suggesting transmural inflammation or granuloma. Disagreements were resolved through consensus. Such disagreements occurred in fewer than 1% of the cases. Through the 2008 questionnaire cycle, 46% of the 1937 women with initial self-report of CD or UC confirmed their diagnosis on a detailed supplementary questionnaire. We were able to obtain medical records from 82% of the women who confirmed diagnosis on the follow-up questionnaire and allowed access to medical records, among whom 78% of cases were confirmed as having CD or UC based on the aforementioned criteria (Figure 1). Nonresponders were similar to responders in age (mean age, 53.5 vs 52.6 y, respectively), body mass index (25.3 vs 25.4 kg/m2, respectively), physical activity (13.3 vs 13.2 met-h/wk, respectively), white race (93.8% vs 94.5%, respectively), never smoking (40.9% vs 36.6%, respectively), predicted vitamin D score (27.4 vs 27.3 ng/mL, respectively), and dietary (196.7 vs 196.3 IU/day, respectively) or supplemental vitamin D intake (143.7 vs 138.3 IU/day, respectively) (P > .20 for all comparisons).
Figure 1.
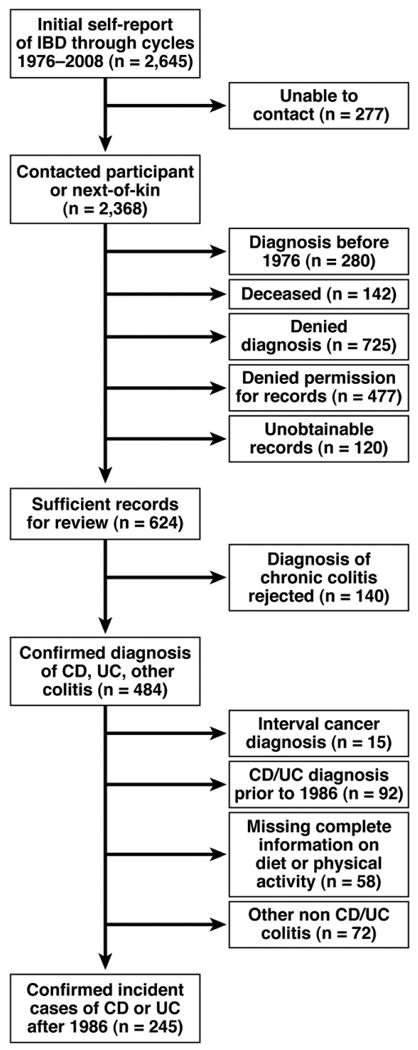
Flow of potential cases of incident Crohn's disease or ulcerative colitis.
Assessment of Plasma Vitamin D Status
We assessed predicted plasma 25(OH)D status using a previously published and validated regression model.18 Briefly, this linear regression model to predict the plasma 25(OH)D level was developed in a parallel cohort of 911 men enrolled in the Health Professionals Follow-up Study, who had directly measured plasma 25(OH)D levels. By using lifestyle predictors, which included dietary and supplemental vitamin D intake, exposure to sunlight, race, body mass index, and regional ultraviolet-B radiation intensity, a linear regression model with differential weights assigned to the distribution of the individual predictor variables was developed. Each coefficient was adjusted for season of blood draw.18 The regression model subsequently was validated in an independent sample of 542 men from the Health Professionals Follow-up Study and applied to women in the NHS.19,20 By using this regression model, predicted plasma 25(OH)D status has been correlated with other disease end points in the NHS that subsequently were validated using direct measurements of plasma 25(OH)D.21–24
Statistical Analysis
We classified predicted plasma 25(OH)D status and total vitamin D intake (from diet and supplements) in 1986 according to the quartile distribution of the cohort. We also used predefined categories of vitamin D intake according to the Institute of Medicine recommendations regarding dietary reference intakes of vitamin D.25 In our primary analysis, participants accrued follow-up time beginning on the date of the return of the baseline questionnaire and ending on the date of diagnosis of CD or UC, date of death, or at the end of the follow-up period on June 30, 2008, whichever came first. We calculated the hazard ratio (HR) by dividing the incidence rate in each exposure category by the incidence rate in the reference category. We used Cox proportional hazards modeling stratified by age and time period, to determine the HRs and 95% confidence intervals (CI) while controlling for potential confounding variables. The covariates included in our multivariate models were determined a priori based on their known association with CD or UC. Smoking is a well-recognized risk factor for CD and UC.26,27 Oral contraceptive use, and possibly postmenopausal hormone use, has been recognized as a risk factor for IBD in recent meta-analyses.28 Consistent with prior studies of vitamin D, we also adjusted for body mass index and physical activity in our final model. We modeled smoking status, oral contraceptive use, and hormone use as time varying covariates up to the questionnaire cycle immediately before IBD diagnosis. We modeled vitamin D status, body mass index, and physical activity according to information collected at baseline in 1986, consistent with previous studies of other end points.18,20 In secondary analyses, we analyzed the individual contributions of dietary vitamin D (from food) and supplemental vitamin D intake on risk. We assessed for linear trend by including the median value of each category of vitamin D in our multivariate models. We also performed a 2-year lagged analysis by excluding cases diagnosed within 2 years of assessment of 25(OH)D status. We evaluated the proportional hazards assumption by likelihood ratio tests comparing models with and without time-dependent variable-by-variable interaction terms. All models satisfied the proportionality of hazards assumption. We used SAS software (version 9.1) (SAS Institute Inc, Cary, NC) for all analyses. All P values are 2 sided.
Results
Among the 72,719 women included, we documented 122 cases of CD and 123 cases of UC during 1,492,811 person-years of follow-up evaluation. At baseline, the median age of participants was 53 years (range, 40–73 y). The median predicted 25(OH)D level was 27.6 ng/mL (range, 7.3–38.6 ng/mL). Table 1 presents the characteristics of the study cohort in 1986 according to the quartile distribution of predicted plasma 25(OH)D level. As expected, women in the lowest quartile of predicted 25(OH)D level had a significantly higher body mass index, were less physically active, were more likely to reside in the Northern or Midwestern United States, and had lower levels of both dietary and supplemental vitamin D intake.
Table 1.
Baseline Characteristics of the Study Population According to Predicted Plasma Vitamin D Levels
| Quartile 1 (n = 18,183) | Quartile 2 (n = 18,034) | Quartile 3 (n = 18,262) | Quartile 4 (n = 18,240) | Total (n = 72,719) | |
|---|---|---|---|---|---|
| Median age, y (SD) | 53.0 (7.0) | 53.0 (7.1) | 53.0 (7.2) | 53.0 (7.2) | 53.0 (7.2) |
| Median body mass index, kg/m2 (SD) | 29.2 (5.2) | 25.0 (4.4) | 23.2 (3.5) | 22.1 (2.6) | 24.2 (4.8) |
| Activity, met-h/wk (SD) | 5.5 (9.4) | 10.3 (16.2) | 16.5 (20.9) | 24.0 (26.9) | 7.7 (20.6) |
| Race, % | |||||
| White | 91.1 | 95.6 | 95.5 | 95.6 | 94.5 |
| Non-white | 8.9 | 4.4 | 4.5 | 4.4 | 5.5 |
| Region of residence, % | |||||
| Midwest | 19.6 | 19.3 | 18.0 | 20.5 | 19.4 |
| North | 62.9 | 60.4 | 56.2 | 51.3 | 57.7 |
| South | 7.4 | 9.3 | 11.6 | 13.6 | 10.5 |
| West | 10.1 | 11.0 | 14.2 | 14.7 | 12.5 |
| Smoking status, % | |||||
| Never | 44.8 | 43.7 | 43.7 | 44.8 | 44.3 |
| Past | 33.3 | 33.9 | 34.6 | 36.9 | 34.7 |
| Current | 21.9 | 22.5 | 21.7 | 18.3 | 21.1 |
| Oral contraceptive use, % | |||||
| Never | 53.9 | 52.1 | 50.2 | 50.0 | 51.6 |
| Past | 40.4 | 41.8 | 43.4 | 43.6 | 42.3 |
| Current | 5.7 | 6.1 | 6.4 | 6.4 | 6.1 |
| Premenopausal, % | 33.2 | 33.8 | 34.1 | 31.3 | 33.1 |
| Post-menopausal hormone use, %a | |||||
| Never | 59.2 | 53.7 | 48.6 | 43.0 | 51.0 |
| Past | 22.3 | 22.5 | 22.5 | 22.6 | 22.5 |
| Current | 18.5 | 23.8 | 28.9 | 34.4 | 26.5 |
| Vitamin D supplemental intake, IU/day (SD)b | 31.6 (108.2) | 84.2 (176.6) | 140.9 (213.8) | 302.4 (239.2) | 140 (216.3) |
| Vitamin D dietary intake, IU/day (SD)c | 155.5 (85.4) | 181.2 (95.8) | 200.0 (101.7) | 256.4 (124.7) | 178.1 (109.4) |
| Median predicted vitamin D level, ng/mL [range (SD)] | 22.9 (7.3–24.9) | 26.3 (24.9–27.6) | 28.7 (27.6–30.0) | 31.8 (30.0–38.6) | 27.6 (7.3–38.6) |
Percentages among post-menopausal women only.
Includes vitamin D intake from multivitamins and vitamin D supplements.
Includes energy-adjusted vitamin D intake from food.
The median age at diagnosis was 64.0 years (range, 48–80 y) for CD and 63.5 years (range, 44–85 y) for UC, respectively. The median interval between assessment of predicted plasma 25(OH)D level and disease diagnosis was 12 years for UC and 10 years for CD. For each 1-ng/mL increase in plasma 25(OH)D level, we observed a 6% relative reduction in risk of CD (multivariate HR, 0.94; 95% CI, 0.89–0.99; P = .03) and a nonsignificant 4% reduction in risk of UC (multivariate HR, 0.96; 95% CI, 0.91–1.02). Compared with women in the lowest quartile of predicted plasma 25(OH)D level, women in the highest 2 quartiles had a significantly lower risk of CD with multivariate HRs of 0.50 (95% CI, 0.28–0.90) and 0.55 (95% CI 0.30–1.00), respectively (Ptrend = .02) (Table 2). For UC, we observed similar associations, although the comparison of the highest quartile with the lowest quartile of predicted plasma 25(OH)D level did not reach statistical significance (HR, 0.68; 95% CI, 0.5–1.31).
Table 2. Risk of CD and UC According to Quartiles of Predicted Plasma 25(OH)D Level.
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Ptrend | |
|---|---|---|---|---|---|
| Person-years of follow-up evaluation | 380,369 | 384,505 | 377,108 | 378,763 | |
| Crohn's disease | |||||
| Number of cases | 37 | 37 | 22 | 26 | |
| Age-adjusted HR (95% CI) | 1.0 | 0.99 (0.63–1.56) | 0.60 (0.35–1.01) | 0.70 (0.42–1.15) | .059 |
| Multivariate HR (95% CI)a | 1.0 | 0.89 (0.55–1.45) | 0.50 (0.28–0.90) | 0.55 (0.30–1.00) | .018 |
| Ulcerative colitis | |||||
| Number of cases | 35 | 36 | 29 | 23 | |
| Age-adjusted HR (95% CI) | 1.0 | 1.02 (0.64–1.62) | 0.84 (0.51–1.37) | 0.66 (0.39–1.12) | .096 |
| Multivariate HR (95% CI)a | 1.0 | 0.98 (0.59–1.63) | 0.84 (0.47–1.48) | 0.68 (0.35–1.31) | .17 |
Adjusted for age, smoking (ever or never), oral contraceptive use (current, past, or never), postmenopausal hormone therapy use (premenopausal, current, past, or never), physical activity (quintiles), and body mass (kg/m2) index (≤21, 21–22.9, 23–24.9, 25–28.9, ≥29).
We also examined the association of 25(OH)D and risk of CD and UC according to predefined categories of vitamin D sufficiency (plasma 25(OH)D ≥ 30 ng/mL), insufficiency (plasma 25(OH)D = 20–30 ng/mL), and deficiency (plasma 25(OH)D < 20 ng/mL). Compared with women who were predicted to be vitamin D deficient, the multivariate HR of CD was 0.38 (95% CI, 0.15–0.97) for women predicted to be vitamin D sufficient (Ptrend = .048). The corresponding multivariate HR of UC was 0.57 95% CI (0.19–1.70) for women predicted to be vitamin D sufficient (Ptrend = .20).
For vitamin D intake from diet and supplements, each 100-IU/day increase in total vitamin D intake resulted in a 10% reduction in UC risk (multivariate HR, 0.90; 95% CI, 0.83–0.98; P = .02) and a 7% reduction in CD risk (multivariate HR, 0.93; 95% CI, 0.86–1.01; P = .09). Table 3 presents the association between total vitamin D intake from diet and supplements according to the quartile distribution of the cohort with incident CD or UC. There was a statistically significant linear trend showing an inverse association between vitamin D intake and incidence of UC (Ptrend = .04); this relationship was weaker for CD (Ptrend = .22). Compared with vitamin D intake less than 150 IU/day, intake higher than the reference dietary intake of 600 IU/day was associated with a reduction in risk of incident UC (multivariate HR, 0.63; 95% CI, 0.32– 1.22; Ptrend = .026) and a nonsignificant reduction for CD (multivariate HR, 0.60; 95% CI, 0.31–1.17; Ptrend = 0.18). Intake of vitamin D greater than 800 IU/day (the recommended intake for age > 70 y) resulted in correspondingly greater reductions in risk of incident UC (multivariate HR, 0.48; 95% CI, 0.15–1.58; Ptrend = .04) and CD (multivariate HR, 0.15; 95% CI, 0.02–1.12; Ptrend = .099).
Table 3. Risk of CD and UC According to Quartiles of Dietary and Supplemental Vitamin D Intake.
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Ptrend | |
|---|---|---|---|---|---|
| Person-years of follow-up evaluation | 380,151 | 380,256 | 380,299 | 380,039 | |
| Crohn's disease | |||||
| Number of cases | 34 | 33 | 28 | 27 | |
| Age-adjusted HR (95% CI) | 1.0 | 0.95 (0.59–1.54) | 0.80 (0.48–1.32) | 0.76 (0.46–1.26) | .24 |
| Multivariate HR (95% CI)a | 1.0 | 0.96 (0.60–1.56) | 0.80 (0.49–1.32) | 0.76 (0.46–1.27) | .22 |
| Ulcerative colitis | |||||
| Number of cases | 34 | 37 | 31 | 21 | |
| Age-adjusted HR (95% CI) | 1.0 | 1.08 (0.68–1.72) | 0.91 (0.56–1.48) | 0.61 (0.35–1.05) | .04 |
| Multivariate HR (95% CI)a | 1.0 | 1.10 (0.69–1.76) | 0.93 (0.57–1.52) | 0.64 (0.37–1.10) | .04 |
Adjusted for age, smoking (ever or never), oral contraceptive use (current, past, or never), postmenopausal hormone therapy use (premenopausal, current, past, or never), physical activity (quintiles), and body mass (kg/m2) index (≤21, 21–22.9, 23–24.9, 25–28.9, ≥29).
We considered the possibility of reverse causation, with symptoms associated with active disease influencing predicted 25(OH)D levels before a formal diagnosis of CD or UC. However, excluding cases of CD or UC diagnosed within the first 2 years after the assessment of predicted 25(OH)D did not materially alter our risk estimate for CD (HR, 0.53; 95% CI, 0.29–0.95) or UC (HR, 0.60; 95% CI, 0.30–1.17), comparing the highest quartile of 25(OH)D with the lowest. We also considered the possibility that the associations we observed with vitamin D status may be owing to a healthy user effect in which women who take vitamin D supplements may be more likely to observe other healthy behaviors associated with lower risk of CD and UC. Thus, we repeated the analysis adjusting for intake of other commonly used supplements that also would be reasonably expected to correlate with greater health-seeking behaviors. The reduction in CD incidence associated with a 1-ng/mL increase in predicted plasma vitamin D was not materially altered after adjusting for regular use of vitamin B complex supplements (multivariate HR, 0.94; 95% CI, 0.89–0.99) or vitamin E supplements (multivariate HR, 0.95; 95% CI, 0.89–1.00). Similarly, we did not observe any significant changes in our risk estimates for UC in models that included these covariates.
We examined effect modification according to smoking status or oral contraceptive use. The reduction in CD risk with a 1-ng/mL increase in predicted plasma vitamin D level was similar among never-smokers (multivariate HR, 0.95; 95% CI, 0.88–1.01) compared with ever-smokers (HR, 0.95; 95% CI, 0.87–1.03) (Pinteraction = .61). Similar associations for CD were observed in strata of women who never used oral contraceptives (multivariate HR, 0.95; 95% CI, 0.89–1.01) or ever used oral contraceptives (multivariate HR, 0.93; 95% CI, 0.85–1.02) (Pinteraction = .84). We also did not observe any effect modification by smoking or oral contraceptive use for risk of UC according to the predicted plasma vitamin D levels (Pinteraction > .20).
Discussion
Although deficiency of vitamin D commonly has been described in patients with newly diagnosed IBD,29 it is unclear if this is a consequence of the disease or a contributor to its pathogenesis. In this prospective cohort study, we show that predicted levels of prediagnosis plasma 25(OH)D are associated with a significant reduction in risk of incident CD and nonsignificant reduction in risk of UC over a follow-up period of 22 years. Taken together, our results suggest that vitamin D may be an important mediator in the pathogenesis of CD and possibly UC.
Our results are supported by data highlighting the role of vitamin D in the innate immune system and inflammatory response.6, 12, 30 VDRs are located on peripheral blood T-lymphocytes and antigen presenting cells. Through these receptors, 1,25(OH)2D3, the active form of vitamin D, suppresses the proliferation of T-helper 1 (Th1) cells and inhibits the secretion of interleukin-2 and interferon-γ.6,13 1,25(OH)2D3 also induces proliferation of regulatory T cells that down-regulate the inflammatory response. In addition to its effect on the T-cell population, 1,25(OH)2D3 inhibits differentiation of peripheral blood monocytes into dendritic cells and blocks dendritic cell-mediated activation of Th1 cells.6 Deficiency of 1,25(OH)2D3, and VDR knockout increases the severity of dextran sodium sulfate–induced colitis in mice.11,12,31–33
Although there are no comparable human data examining vitamin D status with the incidence of CD or UC, our findings are supported by other studies that have linked vitamin D deficiency with several chronic diseases that may be immunologically mediated, including multiple sclerosis and type 1 diabetes.34 There also are ecologic studies that have shown a higher incidence of IBD at higher latitudes where populations typically are exposed to less ultraviolet radiation, the greatest environmental determinant of plasma 25(OH)D.35,36
Our results suggesting a potentially stronger association of 25(OH)D with CD than UC also are biologically plausible. First, although CD and UC share several common features, there are clear differences in their pathogenesis. Among the 99 susceptibility loci that have been identified to date as associated with either CD or UC, less than half appear to be common to both diseases.37 Immunologically, CD appears to be Th1-mediated with an increase of Th1-related cytokines including tumor necrosis factor α and interleukin-12. In contrast, UC is a Th2-mediated process associated with an increase of interleukin-4 and interleukin-13.37 Second, vitamin D may influence risk of CD through its actions on VDR as a ligand-activated transcription factor. Ramagopalan et al38 performed chromatin immunoprecipitation sequencing of VDR in relation to several known autoimmune susceptibility loci. They found that administration of calcitriol resulted in 3.5-fold enrichment of VDR binding over basal levels at sites adjacent to several known loci associated with susceptibility to CD but not UC. Third, administration of 1,25(OH)D3 results in the Nucleotide-binding oligomerization domain-containing protein 2 (NOD2)/nuclear factor-κB–mediated expression of defensin β 2 (DFB2/HDB2), an effect that is abrogated by the loss of function mutation in the NOD2 locus associated with CD, but not UC.39 Nonetheless, our data do suggest a possible weaker association between plasma vitamin D status and UC with an inverse association for dietary and supplemental vitamin D intake, suggesting that vitamin D status may play a significant role in UC pathogenesis as well.
There was a significant trend toward reduction in UC risk with increasing vitamin D intake, although the hazard ratios for each of the individual quartiles did not reach statistical significance. A weaker association was observed for CD. One reason for this lack of a statistically significant reduction in our study is the relatively narrow range of intake of vitamin D across the cohort with only a relatively small proportion of women with intake significantly greater than the reference dietary intake.25 Intervention trials of vitamin D supplementation in Crohn's disease40 and multiple sclerosis13 that showed a benefit to vitamin D supplementation typically used a higher dose than the median intake in our highest quartile. Indeed, we observed the greatest reduction in disease risk for vitamin D intake greater than 800 IU/day, although the small numbers of cases in this stratum precluded precise comparisons. These results also are consistent with findings from the randomized Women's Health Initiative trial in which a relatively low dose of vitamin D supplementation (400 IU/day) did not reduce the risk of colorectal cancer, despite an inverse association observed among trial participants who did achieve a 25(OH)D level greater than 35 ng/mL,41 as we also have shown in the NHS cohort.21
Our study had several strengths including the prospective study design, large number of participants, information on predictors of vitamin D status validated in relation to measured plasma 25(OH)D level, detailed data regarding potential confounders, and long-term follow-up evaluation over 22 years. The prospective design of our study avoids the potential recall and selection biases of retrospective, case-control studies that collect data on diet and lifestyle after diagnosis of CD or UC. Our assessment of 25(OH)D a median of 10–12 years before diagnosis also minimizes the potential for reverse causation in which preclinical symptoms or disease activity before a formal diagnosis of CD or UC is established can directly influence 25(OH)D levels. Last, we confirmed all cases of CD and UC through medical record review, a significant advantage over studies that rely on self-report or discharge codes, which may not accurately reflect true diagnoses.
There were several limitations of our study that merit acknowledgement. First, our cohort was composed entirely of women, most of whom are Caucasian. However, CD and UC incidence rates observed were similar to rates from other well-established epidemiologic cohorts. It is unlikely that biological relations among women in our cohorts will differ from the US population in general because age or gender differences have not been described consistently with other well-established environmental risk factors. Second, we used predicted 25(OH)D status based only on information derived from our baseline questionnaire, consistent with prior studies of vitamin D in the NHS that subsequently have been validated with direct measurements of plasma 25(OH)D.19,42,43 However, a single assessment of 25(OH)D appears to be representative of long-term levels.44 Both published studies in other cohorts and a prior analysis in NHS has shown high intraclass correlation coefficients of 0.72 for measures of plasma 25(OH)D taken 3 years apart and 0.52 for measures taken 10 years apart.44 These intraclass correlation coefficients are consistent with those of plasma cholesterol (0.65), which is widely considered a reasonable measure of long-term cardiovascular risk. In addition, using a baseline assessment of vitamin D status may be associated more plausibly with risk given the latency between specific environmental exposure and disease onset required before the initiating events in IBD pathogenesis lead to clinically apparent disease. Our approach also minimized the likelihood of reverse causation in which symptoms of subclinical disease may lead to modification of diet or physical activity before a formal diagnosis. Third, in lieu of directly measuring plasma 25(OH)D levels in each of our participants, we estimated plasma 25(OH)D levels by using a regression model that has been validated in the NHS and has yielded disease associations consistent with those identified from direct plasma measurements. Fourth, our dietary vitamin D information was obtained through use of a semiquantitative food frequency questionnaire, which may be subject to reporting bias. However, we anticipate the occurrence of such bias to be infrequent, of a low magnitude, and nondifferential given the high level of education and medical knowledge of our study participants. In addition, our dietary instruments have been validated previously and have showed strong correlation with a 1-year food diary and biochemical measurements of nutrient status.45–48 Nonetheless, all such dietary instruments are limited by the accuracy of participant reporting. Fifth, the small number of cases in each quartile precluded our being able to conduct meaningful analyses to explore effect modification or interaction with known disease risk factors. It also may lead to overfitting of multivariate models and limit our power to examine weaker associations. Sixth, it is possible that the regular use of vitamin D supplements may be associated with other health-seeking behaviors. However, vitamin D supplement intake is only a minor determinant of vitamin D status. More than 50% of plasma 25(OH)D level is attributable to conversion in the skin.14 In addition, compared with the lowest level of vitamin D supplement intake, intake of more than 400 IU/day of vitamin D supplements increased plasma 25(OH)D levels by less than 1 ng/mL.19 Thus, our overall associations with vitamin D status are unlikely to be attributable to differing propensity to use dietary supplements. Nonetheless, as with all observational studies, we cannot exclude the potential influence of unmeasured confounders on our risk estimates. Finally, the median age of diagnosis in our cohort was older than many patients with IBD. However, the influence of other well-described risk factors, such as smoking, with risk of CD and UC in the NHS appear consistent with other population-based cohorts, suggesting that our findings may be generalizable to broader populations. Nonetheless, studies to examine vitamin D status in younger cohorts are essential.
Our data have several important implications. First, previous studies support a role for vitamin D in influencing disease activity.40,49 Our results strengthen the rationale for considering vitamin D supplementation both for treatment of active CD or prevention of disease flares. Despite the recognized prevalence of vitamin D deficiency in CD patients, assessment of plasma 25(OH)D level is not yet part of routine care. Thus, studies of vitamin D supplementation, particularly among vitamin D–deficient patients, to modify disease activity in patients with established CD, are warranted. Second, our data suggest a possible role for routine screening for vitamin D deficiency or vitamin D supplementation among individuals at high risk for development of CD (eg, those identified as high risk according to genetic predisposition). Last, our results suggest that vitamin D possibly may play a role in the pathogenesis of IBD. Such insights may lead to the development of novel lines of therapy as well as interventions that may modulate the risk of incident disease.
In conclusion, we showed that prediagnosis predicted plasma 25(OH)D is associated significantly with a lower risk of incident CD with a weaker, nonsignificant association with UC. However, the small number of cases within each quartile in our study precluded firm establishment of the plausible range of effect sizes for this association. Future studies are needed to confirm these findings. Further studies on the mechanism of this association and interaction with known disease-specific and other genetic loci also are necessary. In addition, larger trials of vitamin D supplementation in at-risk individuals as well as those with established disease may be warranted.
Supplementary Material
Acknowledgments
The authors thank Gideon Aweh for computer programming expertise and acknowledge the dedication of the Nurses Health Study participants and members of Channing Laboratory.
Funding: This work was supported by a Research Scholars Award of the American Gastroenterological Association (A.N.A.), the IBD Working Group (H.K.), the Broad Medical Research Program of the Broad Foundation (A.T.C), and the National Institutes of Health (R01 CA137178, P01 CA87969, P30 DK043351, and K08 DK064256).
The research presented in this manuscript is original. The contents of this article are solely the responsibility of the authors. The American Gastroenterological Association and the Broad Medical Research Foundation had no role in the collection, management, analysis, or interpretation of the data and had no role in the preparation, review, or approval of the manuscript.
Abbreviations used in this paper
- CD
Crohn's disease
- CI
confidence interval
- HR
hazard ratio
- IBD
inflammatory bowel disease
- NHS
Nurses' Health Study
- Th1
T-helper 1
- UC
ulcerative colitis
- VDR
vitamin D receptor
Footnotes
Supplementary Material: Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2011.11.040.
Conflicts of interest: These authors disclose the following: Joshua Korzenik has been a consultant for Procter and Gamble, Shire Pharmaceuticals, CytokinePharma, and receives research support from Procter and Gamble and Warner Chilcott; James Richter is a consultant for Policy Analysis, Inc; and Andrew Chan has served as a consultant for Bayer HealthCare and Millennium Pharmaceuticals. The remaining authors disclose no conflicts.
References
- 1.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 4.Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franke A, McGovern DP, Barrett JC, et al. Genome-wide metaanalysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim WC, Hanauer SB, Li YC. Mechanisms of disease: vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:308–315. doi: 10.1038/ncpgasthep0215. [DOI] [PubMed] [Google Scholar]
- 7.Martin K, Radlmayr M, Borchers R, et al. Candidate genes colocalized to linkage regions in inflammatory bowel disease. Digestion. 2002;66:121–126. doi: 10.1159/000065592. [DOI] [PubMed] [Google Scholar]
- 8.Naderi N, Farnood A, Habibi M, et al. Association of vitamin D receptor gene polymorphisms in Iranian patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2008;23:1816–1822. doi: 10.1111/j.1440-1746.2008.05525.x. [DOI] [PubMed] [Google Scholar]
- 9.Simmons JD, Mullighan C, Welsh KI, et al. Vitamin D receptor gene polymorphism: association with Crohn's disease susceptibility. Gut. 2000;47:211–214. doi: 10.1136/gut.47.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dresner-Pollak R, Ackerman Z, Eliakim R, et al. The BsmI vitamin D receptor gene polymorphism is associated with ulcerative colitis in Jewish Ashkenazi patients. Genet Test. 2004;8:417–420. doi: 10.1089/gte.2004.8.417. [DOI] [PubMed] [Google Scholar]
- 11.Cantorna MT, Munsick C, Bemiss C, et al. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 12.Cantorna MT, Zhu Y, Froicu M, et al. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–1720S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 13.Kimball S, Vieth R, Dosch HM, et al. Cholecalciferol plus calcium suppresses abnormal PBMC reactivity in patients with multiple sclerosis. J Clin Endocrinol Metab. 2011;96:2826–2834. doi: 10.1210/jc.2011-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willett WC, Reynolds RD, Cottrell-Hoehner S, et al. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc. 1987;87:43–47. [PubMed] [Google Scholar]
- 17.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127:188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 18.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 19.Ng K, Wolpin BM, Meyerhardt JA, et al. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer. Br J Cancer. 2009;101:916–923. doi: 10.1038/sj.bjc.6605262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao Y, Ng K, Wolpin BM, et al. Predicted vitamin D status and pancreatic cancer risk in two prospective cohort studies. Br J Cancer. 2010;102:1422–1427. doi: 10.1038/sj.bjc.6605658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feskanich D, Ma J, Fuchs CS, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Bio-markers Prev. 2004;13:1502–1508. [PubMed] [Google Scholar]
- 22.Freedman DM, Looker AC, Chang SC, et al. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99:1594–1602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- 23.Holick MF. Calcium plus vitamin D and the risk of colorectal cancer. N Engl J Med. 2006;354:2287–2288. doi: 10.1056/NEJMc060753. [DOI] [PubMed] [Google Scholar]
- 24.Wu K, Feskanich D, Fuchs CS, et al. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. 2007;99:1120–1129. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 25.Ross AC, Taylor CL, Yaktine AL, et al., editors. Institute of Medicine. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium Food and Nutrition Board. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 26.Cosnes J. Tobacco and IBD: relevance in the understanding of disease mechanisms and clinical practice. Best Pract Res Clin Gastroenterol. 2004;18:481–496. doi: 10.1016/j.bpg.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Cosnes J. What is the link between the use of tobacco and IBD? Inflamm Bowel Dis. 2008;14(Suppl 2):S14–S15. doi: 10.1002/ibd.20555. [DOI] [PubMed] [Google Scholar]
- 28.Cornish JA, Tan E, Simillis C, et al. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 2008;103:2394–2400. doi: 10.1111/j.1572-0241.2008.02064.x. [DOI] [PubMed] [Google Scholar]
- 29.Leslie WD, Miller N, Rogala L, et al. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Am J Gastroenterol. 2008;103:1451–1459. doi: 10.1111/j.1572-0241.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 30.Cantorna MT, Mahon BD. D-hormone and the immune system. J Rheumatol Suppl. 2005;76:11–20. [PubMed] [Google Scholar]
- 31.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Froicu M, Weaver V, Wynn TA, et al. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 33.Froicu M, Zhu Y, Cantorna MT. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology. 2006;117:310–318. doi: 10.1111/j.1365-2567.2005.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant WB. Epidemiology of disease risks in relation to vitamin D insufficiency. Prog Biophys Mol Biol. 2006;92:65–79. doi: 10.1016/j.pbiomolbio.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Lakatos PL. Environmental factors affecting inflammatory bowel disease: have we made progress? Dig Dis. 2009;27:215–225. doi: 10.1159/000228553. [DOI] [PubMed] [Google Scholar]
- 36.Peyrin-Biroulet L, Oussalah A, Bigard MA. Crohn's disease: the hot hypothesis. Med Hypotheses. 2009;73:94–96. doi: 10.1016/j.mehy.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Waterman M, Xu W, Stempak JM, et al. Distinct and overlapping genetic loci in Crohn's disease and ulcerative colitis: correlations with pathogenesis. Inflamm Bowel Dis. 2011;17:1936–1942. doi: 10.1002/ibd.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramagopalan SV, Heger A, Berlanga AJ, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352–1360. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang TT, Dabbas B, Laperriere D, et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285:2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jorgensen SP, Agnholt J, Glerup H, et al. Clinical trial: vitamin D3 treatment in Crohn's disease—a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–383. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 41.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 42.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 43.Ng K, Meyerhardt JA, Wu K, et al. Circulating 25-hydroxyvitamin D levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26:2984–2991. doi: 10.1200/JCO.2007.15.1027. [DOI] [PubMed] [Google Scholar]
- 44.Kotsopoulos J, Tworoger SS, Campos H, et al. Reproducibility of plasma and urine biomarkers among premenopausal and post-menopausal women from the Nurses' Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19:938–946. doi: 10.1158/1055-9965.EPI-09-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willett WC, Stampfer MJ, Underwood BA, et al. Validation of a dietary questionnaire with plasma carotenoid and alpha-tocopherol levels. Am J Clin Nutr. 1983;38:631–639. doi: 10.1093/ajcn/38.4.631. [DOI] [PubMed] [Google Scholar]
- 46.Ascherio A, Stampfer MJ, Colditz GA, et al. Correlations of vitamin A and E intakes with the plasma concentrations of carotenoids and tocopherols among American men and women. J Nutr. 1992;122:1792–1801. doi: 10.1093/jn/122.9.1792. [DOI] [PubMed] [Google Scholar]
- 47.Romieu I, Stampfer MJ, Stryker WS, et al. Food predictors of plasma beta-carotene and alpha-tocopherol: validation of a food frequency questionnaire. Am J Epidemiol. 1990;131:864–876. doi: 10.1093/oxfordjournals.aje.a115577. [DOI] [PubMed] [Google Scholar]
- 48.Hunter DJ, Rimm EB, Sacks FM, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol. 1992;135:418–427. doi: 10.1093/oxfordjournals.aje.a116302. [DOI] [PubMed] [Google Scholar]
- 49.Ulitsky A, Ananthakrishnan AN, Naik A, et al. Vitamin D deficiency in inflammatory bowel disease patients: association with disease activity and quality of life. J Parenter Enteral Nutr. 2011;35:308–316. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


