Abstract
In Drosophila imaginal epithelia, cells mutant for the endocytic neoplastic tumor suppressor gene vps25 stimulate nearby untransformed cells to express Drosophila Inhibitor-of-Apoptosis-Protein-1 (DIAP-1), conferring resistance to apoptosis non-cell autonomously. Here, we show that the non-cell autonomous induction of DIAP-1 is mediated by Yorkie, the conserved downstream effector of Hippo signaling. The non-cell autonomous induction of Yorkie is due to Notch signaling from vps25 mutant cells. Moreover, activated Notch in normal cells is sufficient to induce non-cell autonomous Yorkie activity in wing imaginal discs. Our data identify a novel mechanism by which Notch promotes cell survival non-cell autonomously and by which neoplastic tumor cells generate a supportive microenvironment for tumor growth.
Introduction
Imbalances in the cell-cell communication that coordinates cell proliferation, cell differentiation, and cell death can trigger cancer development. Most epithelial cancers arise from single cells that have acquired multiple oncogenic lesions while initially being surrounded by normal cells [1]–[3]. Cell-cell communication between oncogenic cells and surrounding normal cells can create a context that promotes tumor growth and progression.
In Drosophila, the genes avalanche, Rab5, vacuolar protein sorting 25 (vps25) and tumor susceptibility gene 101 (tsg101, also known as vps23 and erupted) are classified as endocytic neoplastic tumor suppressor genes (nTSGs) because homozygous mutant larvae develop multilayered and invasive tumors with neoplastic characteristics [4]–[9]. Tsg101 and Vps25 are components of the Endosomal Sorting Complex Required for Transport-I (ESCRT-I) and ESCRT-II complexes, respectively, and are necessary to regulate endocytic trafficking of ubiquitylated proteins into internal cellular compartments [10]–[12]. Mutations of tsg101 or vps25 cause an endosomal sorting defect resulting in cell-autonomous activation of Notch, Jak/Stat and JNK signaling, loss of apicobasal polarity, and inability to enter a cellular differentiation program [5]–[8]. Nevertheless, when mutant cells of these nTSGs are surrounded by wild-type cells, they undergo JNK-mediated cell death [6], [13]–[15], and only if cell death is blocked, they unleash their tumor-promoting capacity [6], [7].
Unexpectedly, although mutant cells of these nTSGs are highly apoptotic, they are able to non-cell autonomously promote overgrowth of adjacent wild-type tissue before they die. This overgrowth appears to result, at least in part, from altered trafficking of the Notch receptor [5]–[8]. Notch is trapped in abnormal early endsosomes, leading to increased Notch activity as assessed by transcriptional reporters of Notch signaling. Ectopic Notch activation induces increased expression of the secreted cytokine Unpaired (Upd) which stimulates tissue growth in surrounding wild-type cells through activation of the Jak/STAT pathway [5], [7], [8].
In addition to non-cell autonomous overgrowth, our previous studies have shown that vps25 oncogenic cells can promote non-cell autonomous resistance to apoptotic signals in neighboring cells [6]. This is mediated via non-cell autonomous accumulation of DIAP-1, a potent inhibitor of apoptotic caspases [6], [14]. However, the non-cell autonomous accumulation of DIAP-1 in vps25 mosaics is not mediated via Upd [6] and has remained unknown. The Hippo/Warts/Yorkie (Hpo/Wts/Yki) pathway is known to control diap1 expression (reviewed in [16]–[18]). The core components Hpo and Wts negatively regulate the Yki transcription factor through phosphorylation by Wts [16]–[18]. Once Hpo and Wts are inactive, Yki is dephosphorylated and induces target genes such as diap1 and expanded (ex). Therefore, we considered the Hpo/Wts/Yki pathway as candidate for non-cell autonomous diap1 expression in vps25 mosaics.
Here, we show that activation of Notch, but not of JNK or JAK/STAT, in vps25 mutant cells can induce non-cell autonomous protection from apoptosis by inducing expression of diap1. This increase in DIAP-1 is mediated at the transcriptional level by Yki activity. Additionally, Notch signaling is both necessary and sufficient to induce non-cell autonomous activation of Yki. Therefore, this study identifies a novel mechanism by which Notch signaling can affect growth and cell survival non-cell autonomously in tissues mosaic for mutations in endocytic nTSGs and in general.
Results and Discussion
Imaginal Cells Mutant for vps25 Induce Non-cell Autonomous Yki Activity
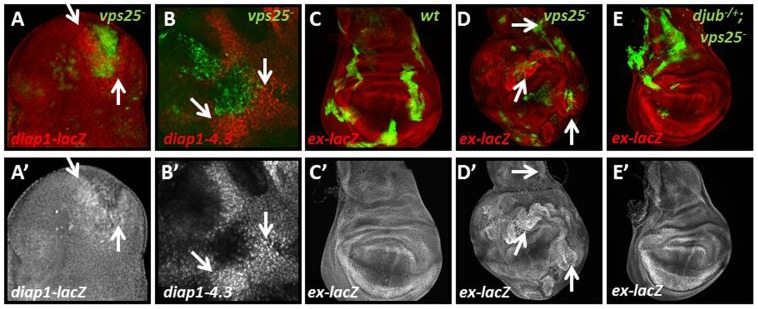
In Drosophila imaginal epithelia, clones of cells mutant for the endocytic nTSG vps25 can induce neighboring wild-type cells to express DIAP-1 protein [6]. To identify the mechanism by which vps25 mutant cells regulate DIAP-1 levels in surrounding normal tissue, we first addressed if the accumulation of DIAP-1 is a transcriptional response. Using a diap1-lacZ reporter, an enhancer-trap insertion that monitors diap1 transcription [19], we found increased β-Gal labeling surrounding vps25 mutant cells suggesting a transcriptional response (Figure 1A). Interestingly, the non-cell autonomous expression of diap1-lacZ is position-dependent and was not observed around every clone. Specifically, in the eye disc this non-cell autonomous effect was more pronounced by vps25 clones located anterior to the morphogenetic furrow (MF) as compared to posterior to the MF. In the wing disc, vps25 clones located in the hinge and notum did trigger non-cell autonomous up-regulation of diap1-lacZ, while clones in the center of the wing pouch did not (see also below).
Figure 1. vps25 mutant cells can induce non-cell autonomous Yorkie activity.
Shown are mosaic imaginal discs. Control and vps25 mutant clones are marked in green. diap1-lacZ and ex-lacZ are detected by β-Gal labeling (red or grayscale). Arrows point to representative examples in the panels. (A,A’) vps25 mutant cells (green) induce non-cell autonomous diap1-lacZ expression (red and gray) in imaginal discs. (B,B’) vps25 mutant cells (green) induce non-cell autonomous Diap1-4.3GFP (red and grayscale). Please note that GFP is presented in red to match the color code in the other panels. vps25 mutant clones were identified by ubiquitylation-specific FK1/2 labeling which is known to be increased in vps25 mutant clones [14] (60×magnification). (C,C’) ex-lacZ expression (red and grayscale) in wild-type (wt) control mosaic wing discs. (D,D’) vps25 mutant cells (green) induce non-cell autonomous ex-lacZ in 71% of wing discs analyzed (n = 28) (red and grayscale). (E,E’) The non-cell autonomous induction of ex-lacZ (red and grayscale) by vps25 mutant clones (green) can be suppressed by removing one copy of djub (only 29% of wing discs still showed non-cell autonomous ex-lacZ (n = 28)). Genotypes: (A) yw hs-FLP; FRT42D Tub-Gal80/FRT42D vps25N55 y+; Tub-Gal4, UAS-CD8-GFP/thj5c8 (B) yw hs-FLP; FRT42D piMyc/FRT42D vps25N55 y+; Diap1-4.3GFP/+ (C) yw hs-FLP; FRT42D Tub-Gal80/ex697 FRT42D y+; Tub-Gal4, UAS-CD8-GFP/+ (D) yw hs-FLP; FRT42D Tub-Gal80/ex697 FRT42D vps25N55 y+; Tub-Gal4, UAS-CD8-GFP/+ (E) yw hs-FLP/djubΔII; FRT42D Tub-Gal80/ex697 FRT42D vps25N55 y+; Tub-Gal4, UAS-CD8-GFP/+.
The non-cell autonomous up-regulation of diap1-lacZ in vps25 mosaics suggests a transcriptional response. The Hpo/Wts/Yki pathway is known to transcriptionally regulate diap1 [20]–[24]. Therefore, we tested for an involvement of the Hpo/Wts/Yki pathway for the non-cell autonomous up-regulation of diap1 by vps25 mutant cells by assaying the expression of a diap1-4.3GFP reporter transgene which contains a minimal enhancer responding to Yki [25]. Consistently, we found that diap1-4.3GFP expression was increased non-cell autonomously surrounding clones of vps25 mutant cells, shown in Figure 1B for the notum of a wing disc, providing evidence that non-cell autonomous induction of diap1 expression is mediated via Yki activation.
To assay specifically for Yki activity in vps25 mosaic discs, we examined the expression of ex-lacZ, a convenient reporter of Yki activity [26], [27]. Compared to controls (Figure 1C), we found that ex-lacZ was increased non-cell autonomously surrounding patches of vps25 mutant cells in imaginal discs (Figure 1D). Again, a position-dependence of vps25 clones was noted, restricting the non-cell autonomous up-regulation of ex-lacZ to clones in the hinge, some lateral areas of the pouch and notum region of the wing disc, similar to diap1-lacZ. It has recently been reported that RNAi knockdown of tsg101 and vps25 can lead to autonomous Yki activity via JNK signaling [28]. Consistently, we observed autonomous and non-cell autonomous induction of ex-lacZ when vps25 was knocked down using RNAi (Figure S1), though autonomous induction of ex-lacZ was rarely seen in null vps25 clones, likely reflecting a difference between hypomorphic and null conditions. Importantly, regardless of the strength of the vps25 mutation, a non-cell autonomous induction of ex-lacZ was consistently observed.
To confirm that the non-cell autonomous increase of ex-lacZ in vps25 mosaics is indeed due to Yki activity, we genetically removed one gene dose of a positive regulator of Yki activity, Drosophila Ajuba Lim protein (djub), thereby reducing Yki activity [29]. Consistently, heterozygosity of djub dominantly suppressed the non-cell autonomous increase of ex-lacZ seen in vps25 mosaics (Figure 1E). Taken together, these data suggest that Yki activity is non-cell autonomously increased in wild-type cells adjacent to vps25 mutant cells, and this increased activity triggers diap1 transcription promoting non-cell autonomous resistance to apoptosis.
Blocking Notch Signaling in vps25 Mutant Cells Suppresses Non-cell Autonomous Induction of Yorkie Activity
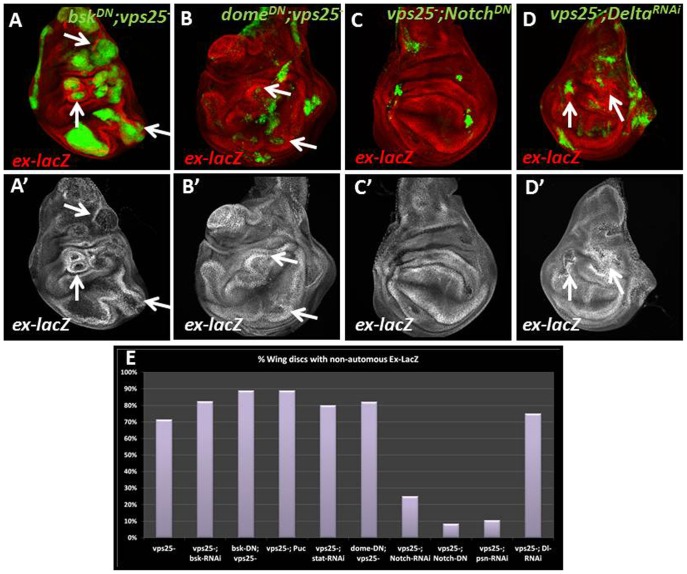
The signaling events that occur between oncogenic and normal cells in an epithelium are largely unknown. In order to determine which signaling pathways could mediate the non-cell autonomous activation of Yki signaling in vps25 mosaics, we inhibited pathways known to be activated within vps25 mutant cells in wing and eye imaginal discs and assayed for effects on non-cell autonomous induction of the ex-lacZ reporter. JNK signaling is active in vps25 mutant cells (Figure S2A) and mediates autonomous apoptosis of vps25 mutant cells [6], [15]. However, autonomous inhibition of the JNK pathway using RNAi to the Drosophila JNK ortholog basket (bsk), expression of a dominant negative form of Bsk, or overexpression of an inhibitor of the JNK pathway, puckered (puc), did not block non-cell autonomous ex-lacZ in vps25 mosaics (Figure 2A and E). In contrast, due to inhibition of JNK-induced apoptosis under these conditions, vps25 mutant clones are larger and the non-cell autonomous induction of ex-lacZ is even more clearly visible. These observations suggest that JNK activation in vps25 clones does not play a role for non-cell autonomous activation of Yki signaling.
Figure 2. Autonomous inhibition of Notch signaling suppresses non-cell autonomous ex-lacZ in vps25 mosaics.
Shown are MARCM-induced vps25 mosaic wing discs expressing the transgenes indicated. vps25 mutant cells are marked in green. ex-lacZ is detected by β-Gal labeling (red or grayscale). Arrows point to representative examples. (A,A’,B,B’) Autonomous expression of dominant negative bsk (bskDN; A,A’) (89% of wing discs showed non-cell autonomous ex-lacZ (n = 9)) and domeless (domeDN; B,B’) (82% of wing discs showed non-cell autonomous ex-lacZ (n = 28)) does not suppress the non-cell autonomous induction of ex-lacZ in vps25 mosaic discs. (C,C’) Autonomous expression of dominant negative Notch (NotchDN) suppresses the non-cell autonomous induction of ex-lacZ in vps25 mosaic discs (8% of wing discs showed non-cell autonomous ex-lacZ (n = 12)). (D,D’) Autonomous RNAi-induced knockdown of Delta (DeltaRNAi) does not suppress the non-cell autonomous induction of ex-lacZ in vps25 mosaic discs (75% of wing discs showed non-cell autonomous ex-lacZ (n = 12)). (E) Summary of the effects on non-cell autonomous ex-lacZ when JNK, Jak/STAT or Notch activity are autonomously inhibited in vps25 mosaic wing discs. At least 10 discs were assayed/genotype. Genotypes: (A,B) yw hs-FLP/UAS-bskDN or UAS-domeDN; FRT42D Tub-Gal80/ex697 FRT42D vps25N55 y+; Tub-Gal4, UAS-CD8-GFP/+ (C) yw hs-FLP; FRT42D Tub-Gal80/ex697 FRT42D vps25N55 y+; Tub-Gal4, UAS-CD8-GFP/UAS- NotchDN (D) yw hs-FLP; FRT42D Tub-Gal80/ex697 FRT42D vps25N55 y+; Tub-Gal4, UAS-CD8-GFP/UAS-DeltaRNAi.
Jak/STAT signaling is thought to mediate non-cell autonomous overgrowth surrounding vps25 mutant cells [6]–[8]. However, we also detect strong autonomous labeling in vps25 clones using the phosphoSTAT antibody that detects phosphorylated and thus activated STAT92 protein (Figure S2B). Therefore, we tested for an autonomous involvement of Jak/STAT signaling for the non-cell autonomous control of ex-lacZ in vps25 mosaics. However, autonomous reduction of Jak/STAT signaling either by RNAi to stat92E, which encodes the transcription factor in the Jak/STAT pathway, or expression of a dominant-negative form of Domeless (domeDN), the receptor of the pathway, did not inhibit the non-cell autonomous activation of Yki signaling in vps25 mosaics (Figure 2B and E).
Finally, Notch signaling is known to be ectopically activated in vps25 mutant cells [6]–[8] as verified by strong up-regulation of the Notch signaling reporter Gbe-Su(H)-lacZ (Figure S2C). In contrast to Jak/STAT and JNK signaling, autonomous inhibition of Notch signaling in vps25 clones by expression of a dominant negative form of Notch in vps25 clones lead to suppression of non-cell autonomous expression of ex-lacZ (Figure 2C). Similarly, Notch RNAi caused a dramatic decrease in the number of clones that displayed non-cell autonomous induction of ex-lacZ in vps25 mosaics (summarized in Figure 2E). This was also true when RNAi to presenilin, a positive regulator of Notch signaling, was expressed in vps25 mutant cells (Figure 2E). Interestingly, the activation of Notch and induction of ex-lacZ in vps25 mosaics appear to be ligand independent, as RNAi to the Notch ligand Delta had no effect on the penetrance of non-cell autonomous ex-lacZ in vps25 mosaic wing discs (Figure 2D and E) although knock-down was effective because Delta protein is lost in Delta-RNAi clones (data not shown). This ligand-independent control of Notch activation in vps25 mutants is consistent with previous findings obtained in S2 cultured cells [7]. Taken together, these data suggest that ligand-independent Notch signaling from within vps25 neoplastic cells induces Yki activity in adjacent, wild-type cells.
Notch is Sufficient to Induce Non-cell Autonomous Yorkie Activity
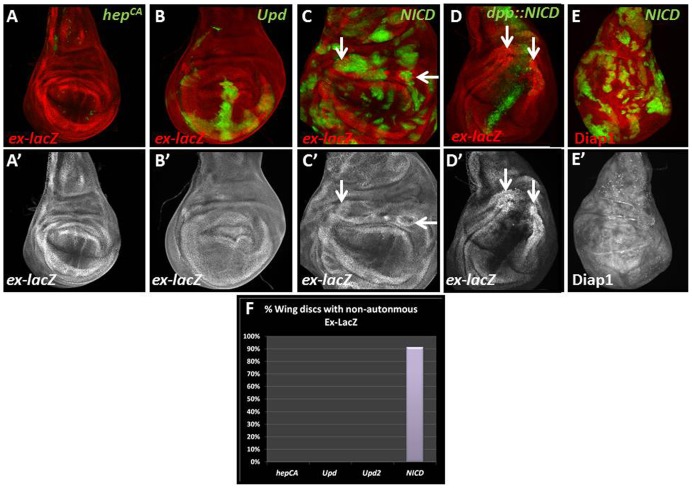
Next, we sought to determine if autonomous hyperactivation of JNK, Jak/STAT or Notch signaling in otherwise wild-type cells is sufficient to induce non-cell autonomous expression of ex-lacZ. First, mosaic overexpression of a constitutively active form of hemipterous (hepCA), the Drosophila JNKK ortholog, yielded very small clones due to JNK-induced apoptosis. However, the few clones that did survive showed no apparent effect on ex-lacZ (Figure 3A and F). Second, we expressed the ligands Upd and Upd2 to ectopically activate Jak/STAT signaling and saw no effect on ex-lacZ (Figure 3B and F). These results suggest that the JNK and Jak/STAT signaling pathways are not sufficient to induce non-cell autonomous Yki activity.
Figure 3. Activation of Notch signaling is sufficient to induce Yorkie activity.
Shown are MARCM-induced mosaic wing discs (A,B,C, E) expressing the indicated transgenes in otherwise wild-type clones marked in green. (D) expresses NICD under dpp-Gal4. ex-lacZ is detected by β-Gal labeling (red or grayscale). Arrows point to representative examples. (A,A’,B,B’) Overexpression of hepCA (0% of wing discs showed non-cell autonomous ex-lacZ (n = 13)) and upd (0% of wing discs showed non-cell autonomous ex-lacZ (n = 11)) does not lead to non-cell autonomous induction of ex-lacZ. (C,C’) Overexpression of NICD leads to non-cell autonomous induction of ex-lacZ (91% of wing discs showed non-cell autonomous ex-lacZ (n = 23)). (D,D’) Expression of NICD using dpp-Gal4 induces strong non-cell autonomous upregulation of ex-lacZ in the hinge and notum, but not the wing pouch. (E,E’) Overexpression of NICD leads to non-cell autonomous accumulation of Diap1 protein. (F) Summary of the effects on non-cell autonomous ex-lacZ when JNK, Jak/STAT or Notch activity are autonomously induced in wild-type mosaic wing discs. At least 10 discs were assayed/genotype. Genotypes: (A,B) yw hs-FLP; FRT42D Tub-Gal80/ex697FRT42D y+; Tub-Gal4, UAS-CD8-GFP/UAS- hepCA or UAS-upd (C) yw hs-FLP; FRT42D Tub-Gal80/ex697FRT42D y+; Tub-Gal4, UAS-CD8-GFP UAS-NICD (D) ex-lacZ; dpp-Gal4 UAS-GFP/UAS-NICD (E) yw hs-FLP; FRT42D Tub-Gal80/ex697FRT42D y+; Tub-Gal4, UAS-CD8-GFP/UAS-NICD.
Finally, we tested if activation of Notch is sufficient to induce non-cell autonomous expression of ex-lacZ and thus activity of Yki. Indeed, expression of the activated form of Notch, the intracellular domain (NICD), is sufficient to induce non-cell autonomous expression of ex-lacZ in wing imaginal discs (Figure 3C and F). Similar to diap1-lacZ and ex-lacZ expression in vps25 mosaics, we noted a position-dependence of NICD-expressing clones for ex-lacZ expression. To further characterize the position-dependence, we expressed NICD using dpp-Gal4 along the anterioposterior axis of the wing disc. Consistently, strong non-cell autonomous ex-lacZ expression is observed in the hinge region (see arrows in Figure 3D and D’). In contrast, expression of NICD in the center of the wing pouch does not cause non-cell autonomous ex-lacZ expression. Finally, NICD expression was also able to induce non-cell autonomous accumulation of DIAP-1 (Figure 3E), suggesting that Notch can control cell survival non-cell autonomously.
In summary, our study identifies a novel role of Notch signaling for non-cell autonomous control of apoptosis via induction of Yki activity in neighboring cells. It has previously been shown that Notch signaling controls cell proliferation both autonomously and non-cell autonomously in the developing eye [30]. The non-cell autonomous component of proliferation control was attributed to Notch-dependent activation of Jak/Stat signaling [31] although that recently came into question [32]. Nevertheless, Jak/Stat activation is not sufficient to mediate the effect of Notch on non-cell autonomous control of apoptosis [6] (Figure 3B,E). Here, we identify the Hpo/Wts/Yki pathway as a target of Notch signaling for the non-cell autonomous control of apoptosis both in eye and wing imaginal discs. Because the Hpo/Wts/Yki pathway also controls proliferation, it is likely that Notch promotes non-cell autonomous proliferation through both Jak/Stat and Hpo/Wts/Yki activities.
It is also interesting to note that this non-autonomous control of the Hpo/Wts/Yki pathway by Notch occurs in a position-dependent manner. For example, vps25 mutant clones or NICD-expressing clones located in the hinge and notum of wing discs triggered non-cell autonomous up-regulation of ex-lacZ, while clones in the wing pouch did not (Figure 1D, 3D). Additionally, vps25 mutant clones located anterior to the morphogenetic furrow triggered non-cell autonomous up-regulation of ex-lacZ, while clones in the posterior of the eye disc did not. The reason for this position-dependence is unknown. However, the regions which do not induce Hpo/Wts/Yki signaling non-autonomously correspond to the zone of non-proliferating (ZNP) cells in the wing disc and post-mitotic, differentiating cells in the eye disc [33]. Therefore, one potential reason for the position-dependence may be that the post-mitotic nature of the cells in the ZNP of the wing pouch and in the posterior of the eye disc render them inert to growth-promoting signals that trigger the Hpo/Wts/Yki pathway. However, while this is one possibility, there may also be additional mechanisms that influence the response to growth-promoting signals.
How Notch exerts this non-autonomous effect is an important and interesting question. Based on its function as a transcriptional regulator, it is possible that increased Notch signaling in vps25 mutant cells could lead to transcription of a secreted or transmembrane protein that communicates to surrounding tissue and induces Yki activity. Expression of proteins known to non-cell autonomously activate Yki signaling such as Fat, Dachsous (Ds) and Four-Jointed as well as ds-lacZ (reviewed in [16]–[18]), however, are not altered in vps25 mosaic discs (data not shown). Identification of this non-cell autonomous signaling mechanism may also be critical for understanding tumorigenesis, as mutations in the Notch pathway, the Hippo pathway and in ESCRT components have been implicated in many different types of human cancer (reviewed in [17], [34]–[37]). In conclusion, this study provides a mechanism by which neoplastic cells influence the behavior of neighboring wild-type cells, which may be critical for generating a supportive microenvironment for tumor growth by preventing cell death and promoting the proliferation of wild-type cells.
Materials and Methods
Fly Stocks
The following mutants and transgenic lines were used: vps25N55 [6]; djubΔII [29]; diap1-lacZ = thj5c8 [19]; diap1-4.3GFP [25]; ex-lacZ = ex697 [26]; UAS-bskDN [38]; UAS-domeDN [39]; UAS-NICD and UAS-NotchDN [40]; UAS-NotchRNAi (VDRC); UAS-DeltaRNAi (VDRC); UAS-psnRNAi (VDRC); UAS-vps25RNAi (VDRC); UAS-hepCA [41]; UAS-upd [42]; UAS-upd2 [43]; puc-lacZ [44]; dpp-Gal4 and ptc-Gal4 (Bloomington); Su(H)-Gbe-lacZ [45].
Mosaics
Mosaics were generated using the MARCM (mosaic analysis using a repressible cell marker) technique which allows expression of transgenes such as UAS-GFP in mutant clones [46]. Heat shocks were administered for 1 hour at 37°C at 48 and 72 hours after egg laying to induce clones.
Immunohistochemistry
Imaginal discs were dissected from 3rd instar larvae and stained using standard protocols. The following antibodies were used: mouse α-βGal (1∶500; DSHB), guinea pig α-DIAP1 (1∶1000; kind gift of Pascal Meier), and rabbit pStat (1∶100; Cell Signaling Technology). Cy3-conjugated anti-guinea pig and anti-mouse (Jackson ImmunoResearch) were used as secondary antibodies. Images were obtained using an OlympusFV500 confocal microscope and processed using Adobe Photoshop CS4.
Supporting Information
vps25 RNAi induces autonomous and non-cell autonomous induction of ex - lacZ (related to Figure 1 ). vps25 was knocked down by RNAi along the anteroposterior boundary using ptc-Gal4 (grey in A”). (A) is the merged image of GFP (ptc-Gal4) and red (ex-lacZ). Yellow stippled lines in (A’) indicates the ptc-Gal4 expression domain based on (A”). Both autonomous and non-cell autonomous (white arrow) expression of ex-lacZ is detectable. However, in the center of the wing pouch area (green arrow) neither autonomous nor non-cell autonomous ex-lacZ is induced, suggesting position-dependence of the location of vps25 inhibition on ex-lacZ induction. Genotype: ex-lacZ ptc-Gal4 UAS-GFP; UAS-vps25RNAi
(TIF)
JNK, Jak/STAT and Notch signaling are activated in vps25 mutant cells (related to Figure 2 ). Shown are MARCM-induced vps25 mosaic wing discs with the indicated gene reporters. vps25 mutant cells are marked in green. puc-lacZ (A) and Su(H)-Gbe-lacZ (C) are detected by β-Gal labeling (red or grayscale). Arrows point to representative examples. (A,A’) puc-lacZ is increased in vps25 mutant clones. (B,B’) Phosphorylated Stat (pStat) protein (red and grayscale) is increased in vps25 mutant clones. (C,C’) Su(H)-Gbe-lacZ is increased in vps25 mutant clones. Genotypes: (A) yw hs-FLP; FRT42D Tub-Gal80/FRT42D vps25N55 y+; Tub-Gal4, UAS-CD8-GFP/puc-lacZ (B) yw hs-FLP; FRT42D Tub-Gal80/FRT42D vps25N55 y+; Tub-Gal4, UAS-CD8-GFP/+ (C) yw hs-FLP; FRT42D Tub-Gal80/FRT42D vps25N55 y+; Tub-Gal4, UAS-CD8-GFP/Su(H)-Gbe-lacZ
(TIF)
Acknowledgments
We thank Spyros Artavanis-Tsakonas, Sarah Bray, Erika Bach, Hugo Bellen, Dirk Bohmann, Michael Galko, Jin Jiang, Greg Longmore, Pascal Meier, the Bloomington Stock Center and the Developmental Studies Hybridoma Bank (DSHB) for fly lines and antibodies. We also thank Zhihong Chen, Christian Antonio and Jacob Hernandez for excellent technical support.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the National Institutes of Health (GM068016 to AB and GM67997 to GH) and the Cancer Prevention and Research Institute of Texas (CPRIT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Fialkow PJ. Clonal origin of human tumors. Biochim Biophys Acta. 1976;458:283–321. doi: 10.1016/0304-419x(76)90003-2. [DOI] [PubMed] [Google Scholar]
- 3.Nowell P, Jensen J, Gardner F, Murphy S, Chaganti RS, et al. Chromosome studies in “preleukemia”. III. Myelofibrosis. Cancer. 1976;38:1873–1881. doi: 10.1002/1097-0142(197611)38:5<1873::aid-cncr2820380502>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1232–1239. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- 5.Moberg KH, Schelble S, Burdick SK, Hariharan IK. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell. 2005;9:699–710. doi: 10.1016/j.devcel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Herz HM, Chen Z, Scherr H, Lackey M, Bolduc C, et al. vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development. 2006;133:1871–1880. doi: 10.1242/dev.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson BJ, Mathieu J, Sung HH, Loeser E, Rorth P, et al. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell. 2005;9:711–720. doi: 10.1016/j.devcel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell. 2005;9:687–698. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Menut L, Vaccari T, Dionne H, Hill J, Wu G, et al. A mosaic genetic screen for Drosophila neoplastic tumor suppressor genes based on defective pupation. Genetics. 2007;177:1667–1677. doi: 10.1534/genetics.107.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nature Reviews Molecular Cell Biology. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 11.Saksena S, Sun J, Chu T, Emr SD. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci. 2007;32:561–573. doi: 10.1016/j.tibs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Hariharan IK, Bilder D. Regulation of imaginal disc growth by tumor-suppressor genes in Drosophila. Annu Rev Genet. 2006;40:335–361. doi: 10.1146/annurev.genet.39.073003.100738. [DOI] [PubMed] [Google Scholar]
- 13.Herz HM, Bergmann A. Genetic analysis of ESCRT function in Drosophila: a tumour model for human Tsg101. Biochem Soc Trans. 2009;37:204–207. doi: 10.1042/BST0370204. [DOI] [PubMed] [Google Scholar]
- 14.Herz HM, Woodfield SE, Chen Z, Bolduc C, Bergmann A. Common and distinct genetic properties of ESCRT-II components in Drosophila. PLoS One. 2009;4:e4165. doi: 10.1371/journal.pone.0004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igaki T, Pastor-Pareja JC, Aonuma H, Miura M, Xu T. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev Cell. 2009;16:458–465. doi: 10.1016/j.devcel.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh H, Irvine KD. Yorkie: the final destination of Hippo signaling. Trends Cell Biol. 2010;20:410–417. doi: 10.1016/j.tcb.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryoo HD, Bergmann A, Gonen H, Ciechanover A, Steller H. Regulation of Drosophila IAP1 degradation and apoptosis by reaper and ubcD1. Nature Cell Biology. 2002;4:432–438. doi: 10.1038/ncb795. [DOI] [PubMed] [Google Scholar]
- 20.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 21.Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 22.Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 23.Kango-Singh M, Nolo R, Tao C, Verstreken P, Hiesinger PR, et al. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development. 2002;129:5719–5730. doi: 10.1242/dev.00168. [DOI] [PubMed] [Google Scholar]
- 24.Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17:2514–2519. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Ren F, Zhang Q, Chen Y, Wang B, et al. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boedigheimer M, Laughon A. Expanded: a gene involved in the control of cell proliferation in imaginal discs. Development. 1993;118:1291–1301. doi: 10.1242/dev.118.4.1291. [DOI] [PubMed] [Google Scholar]
- 27.Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 28.Robinson BS, Moberg KH. Drosophila endocytic neoplastic tumor suppressor genes regulate Sav/Wts/Hpo signaling and the c-Jun N-terminal kinase pathway. Cell Cycle. 2011;10 doi: 10.4161/cc.10.23.18243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das Thakur M, Feng Y, Jagannathan R, Seppa MJ, Skeath JB, et al. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr Biol. 2010;20:657–662. doi: 10.1016/j.cub.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reynolds-Kenneally J, Mlodzik M. Notch signaling controls proliferation through cell-autonomous and non-autonomous mechanisms in the Drosophila eye. Dev Biol. 2005;285:38–48. doi: 10.1016/j.ydbio.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 31.Chao JL, Tsai YC, Chiu SJ, Sun YH. Localized Notch signal acts through eyg and upd to promote global growth in Drosophila eye. Development. 2004;131:3839–3847. doi: 10.1242/dev.01258. [DOI] [PubMed] [Google Scholar]
- 32.Gutierrez-Avino FJ, Ferres-Marco D, Dominguez M. The position and function of the Notch-mediated eye growth organizer: the roles of JAK/STAT and four-jointed. EMBO Rep. 2009;10:1051–1058. doi: 10.1038/embor.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brochta DA, Bryant PJ. A zone of non-proliferating cells at a lineage restriction boundary in Drosophila. Nature. 1985;313:138–141. doi: 10.1038/313138a0. [DOI] [PubMed] [Google Scholar]
- 34.Saksena S, Emr SD. ESCRTs and human disease. Biochem Soc Trans. 2009;37:167–172. doi: 10.1042/BST0370167. [DOI] [PubMed] [Google Scholar]
- 35.Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: it’s NOTCH what you think. J Exp Med. 2011;208:1931–1935. doi: 10.1084/jem.20111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka N, Kyuuma M, Sugamura K. Endosomal sorting complex required for transport proteins in cancer pathogenesis, vesicular transport, and non-endosomal functions. Cancer Sci. 2008;99:1293–1303. doi: 10.1111/j.1349-7006.2008.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy M, Pear WS, Aster JC. The multifaceted role of Notch in cancer. Curr Opin Genet Dev. 2007;17:52–59. doi: 10.1016/j.gde.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Adachi-Yamada T, Fujimura-Kamada K, Nishida Y, Matsumoto K. Distortion of proximodistal information causes JNK-dependent apoptosis in Drosophila wing. Nature. 1999;400:166–169. doi: 10.1038/22112. [DOI] [PubMed] [Google Scholar]
- 39.Brown S, Hu N, Hombria JC. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr Biol. 2001;11:1700–1705. doi: 10.1016/s0960-9822(01)00524-3. [DOI] [PubMed] [Google Scholar]
- 40.Go MJ, Eastman DS, Artavanis-Tsakonas S. Cell proliferation control by Notch signaling in Drosophila development. Development. 1998;125:2031–2040. doi: 10.1242/dev.125.11.2031. [DOI] [PubMed] [Google Scholar]
- 41.Lee SB, Park J, Jung JU, Chung J. Nef induces apoptosis by activating JNK signaling pathway and inhibits NF-kappaB-dependent immune responses in Drosophila. J Cell Sci. 2005;118:1851–1859. doi: 10.1242/jcs.02312. [DOI] [PubMed] [Google Scholar]
- 42.Harrison DA, McCoon PE, Binari R, Gilman M, Perrimon N. Drosophila unpaired encodes a secreted protein that activates the JAK signaling pathway. Genes Dev. 1998;12:3252–3263. doi: 10.1101/gad.12.20.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hombria JC, Brown S, Hader S, Zeidler MP. Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev Biol. 2005;288:420–433. doi: 10.1016/j.ydbio.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 44.Ring JM, Martinez Arias A. Dev Suppl; 1993. puckered, a gene involved in position-specific cell differentiation in the dorsal epidermis of the Drosophila larva. pp. 251–259. [PubMed] [Google Scholar]
- 45.Furriols M, Bray S. A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr Biol. 2001;11:60–64. doi: 10.1016/s0960-9822(00)00044-0. [DOI] [PubMed] [Google Scholar]
- 46.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends in Neurosciences. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
vps25 RNAi induces autonomous and non-cell autonomous induction of ex - lacZ (related to Figure 1 ). vps25 was knocked down by RNAi along the anteroposterior boundary using ptc-Gal4 (grey in A”). (A) is the merged image of GFP (ptc-Gal4) and red (ex-lacZ). Yellow stippled lines in (A’) indicates the ptc-Gal4 expression domain based on (A”). Both autonomous and non-cell autonomous (white arrow) expression of ex-lacZ is detectable. However, in the center of the wing pouch area (green arrow) neither autonomous nor non-cell autonomous ex-lacZ is induced, suggesting position-dependence of the location of vps25 inhibition on ex-lacZ induction. Genotype: ex-lacZ ptc-Gal4 UAS-GFP; UAS-vps25RNAi
(TIF)
JNK, Jak/STAT and Notch signaling are activated in vps25 mutant cells (related to Figure 2 ). Shown are MARCM-induced vps25 mosaic wing discs with the indicated gene reporters. vps25 mutant cells are marked in green. puc-lacZ (A) and Su(H)-Gbe-lacZ (C) are detected by β-Gal labeling (red or grayscale). Arrows point to representative examples. (A,A’) puc-lacZ is increased in vps25 mutant clones. (B,B’) Phosphorylated Stat (pStat) protein (red and grayscale) is increased in vps25 mutant clones. (C,C’) Su(H)-Gbe-lacZ is increased in vps25 mutant clones. Genotypes: (A) yw hs-FLP; FRT42D Tub-Gal80/FRT42D vps25N55 y+; Tub-Gal4, UAS-CD8-GFP/puc-lacZ (B) yw hs-FLP; FRT42D Tub-Gal80/FRT42D vps25N55 y+; Tub-Gal4, UAS-CD8-GFP/+ (C) yw hs-FLP; FRT42D Tub-Gal80/FRT42D vps25N55 y+; Tub-Gal4, UAS-CD8-GFP/Su(H)-Gbe-lacZ
(TIF)