Abstract
Matrix metalloproteinases (MMPs) are secreted endopeptidases that play an essential role in remodeling the extracellular matrix (ECM). MMPs are primarily active during development, when the majority of ECM remodeling events occurs. In adults, elevated MMP activity has been observed in many pathological conditions such as cancer and osteoarthritis. The proteolytic activity of MMPs is controlled by their natural inhibitors - the tissue inhibitor of metalloproteinases (TIMPs). In addition to blocking MMP-mediated proteolysis, TIMPs have a number of MMP-independent functions including binding to cell surface proteins thereby stimulating signaling cascades. TIMP-2, the most studied member of the family, can both inhibit and activate MMPs directly, as well as inhibit MMP activity indirectly by upregulating expression of RECK, a membrane anchored MMP regulator. While TIMP-2 has been shown to play important roles in breast cancer, we describe how the MMP-independent effects of TIMP-2 can modulate the invasiveness of MCF-7, T47D and MDA-MB-231 breast cancer cells. Using an ALA + TIMP-2 mutant which is devoid of MMP inhibition, but still capable of initiating specific cell signaling cascades, we show that TIMP-2 can differentially affect MMP activity and cellular invasiveness in both an MMP dependent and independent manner. More specifically, MMP activity and invasiveness is increased with the addition of exogenous TIMP-2 in poorly invasive cell lines whereas it is decreased in highly invasive cells lines (MDA-MB-231). Conversely, the addition of ALA + TIMP-2 resulted in decreased invasiveness regardless of cell line.
Keywords: TIMP, MMP proteolysis, MCF-7, T47D, MDA-MB-231, Breast cancer
Introduction
The extracellular matrix (ECM) is a dynamic network of interacting macromolecules whose components mediate cell behavior by creating influential cellular environments. The turnover and remodeling of the ECM is an integral part of normal and pathological processes such as development, cell growth, differentiation, and metastasis (Lambert et al. 2004). ECM remodeling is predominantly accomplished by a family of proteins known as matrix metalloproteinases (MMPs) (Ra and Parks 2007). MMPs consist of 24 proteins that collectively cleave all components of the ECM. Although the role of MMPs was traditionally thought to be restricted to catabolism of structural ECM components, there is a growing consensus that the role of MMPs is much broader. (Mott and Werb 2004; Walsh and Damjanovski 2011) Some established functions for MMPs now include the release of sequestered growth factors, cleavage of growth factor receptors from the cell surface, shedding of cell adhesion molecules, and the activation of other MMPs (Gill et al. 2010).
To avoid excessive proteolysis and tissue damage, the activity of MMPs is carefully regulated in a number of different ways. One regulatory mechanism is the proteolytic inhibition of MMPs by tissue inhibitors of metalloproteinases (TIMPs) (Zucker et al. 1998). The human TIMP family consists of four members, designated TIMP-1,-2, -3, and -4, that can bind to and directly inhibit the catalytic activity of MMPs (Lambert et al. 2003). Although TIMPs were originally characterized as proteolytic inhibitors of MMPs, it has been shown that TIMPs are multifaceted proteins capable of MMP- independent functions. TIMP-2 is of particular interest because it is the most extensively studied TIMP both in disease and development (Stetler-Stevenson 2008; Walsh et al. 2007). There are two known MMP dependent functions of TIMP-2. The first is binding and inhibiting the proteolytic activity of MMPs, while paradoxically the second is the activation of proMMP-2 (Seo et al. 2003). All MMPs are translated as inactive zymogens and as such, require activation through cleavage of a pro-domain (Ra and Parks 2007). TIMP-2 aids in the activation of proMMP-2 by binding and association with two MMP-14 proteases at the cell surface that cleave the pro-domain of MMP-2 and release it into the ECM in its active form (Yana and Seiki 2002).
TIMP inhibition of MMPs occurs through the TIMP N-terminal domain. TIMP functions that are independent of MMP binding are poorly understood although there is evidence they involve the C-terminal of the TIMP molecule. The C-terminal domain of TIMP-2 has been shown to bind to cell-surface α3β1 integrin (Seo et al. 2003). Binding to this integrin induces a signaling cascade that results in the regulation of growth, and upregulation of RECK (‘reversion-inducing-cysteine-rich protein with kazal motifs’) (Oh et al. 2004). The RECK gene codes for a membrane bound MMP regulator that can inhibit MMP activity in three ways: direct inhibition of the proteolytic activity of MMP-2, -9, and -14; inhibition of proMMP-2 activation by preventing proMMP-2/TIMP-2/MMP-14 complex formation; and by inhibiting the secretion of MMP-9 (Rhee and Coussens 2002). It has been shown in numerous studies that RECK expression is downregulated in various human cancers and that high RECK expression is correlated with a positive prognosis and increased patient survival (Noda & Takahashi, 2007). This also correlates with the finding that RECK mRNA is expressed ubiquitously in normal human tissue, while it is undetectable in a number of tumour-derived cell lines (Rhee & Coussens, 2002). Interestingly, these cell lines showed reduced tumour angiogenesis, invasion and metastasis when stably transfected with RECK.
The balance between MMPs and TIMPs in cancer progression has recently received considerable attention as these two protein families are implicated as important regulators of several key steps in carcinogenesis (Stetler-Stevenson and Seo 2005). By virtue of their activity, MMPs are most closely associated with the invasive and metastatic aspect of cancer progression, as increased MMP activity allows cancer cells to invade through the ECM and metastasize into other tissues (Zhang et al. 2006). MMPs are overexpressed in almost all human cancers and high MMP expression usually correlates with a poor prognosis (Rhee and Coussens 2002). Accordingly, an attempt was made to create synthetic MMP inhibitors as a cancer therapy. However, clinical trials of these synthetic MMP inhibitors did not produce significant results for reasons not yet understood (Stetler-Stevenson 2008). The failure of synthetic MMP inhibitors as a cancer therapy, along with the many roles of TIMP-2, highlights that our understanding of TIMP and MMP biology is incomplete and more complicated than initially thought (Dove 2002). The intricacy of MMP/TIMP interactions, complicated by the possible role of molecules such as RECK, has made understanding the regulation of matrix remodeling difficult as the relative roles and expression levels of these and other molecules likely varies between cell types.
The purpose of this study is to further understand the dynamic relationship between TIMP-2 and MMPs by treating breast cancer cells with conditioned media containing high levels of exogenous TIMP-2 or ALA + TIMP-2. ALA + TIMP-2 is a TIMP-2 mutant containing an additional amino terminal alanine, which renders it unable to inhibit MMPs (Wingfield et al. 1999). As such, while ALA + TIMP-2 cannot inhibit MMPs directly, or activate proMMPs, it is still capable of binding to cell surface receptors, such as α3β1 integrin, and inducing integrin signaling (Seo et al. 2003). Here we show that treatment of breast cancer cells lines with ALA + TIMP-2 resulted in decreased invasiveness concomitant with decreased MMP activity regardless of cell line. Interestingly, wild-type TIMP-2 treatment resulted in opposing effects depending on the aggressiveness of the cell line. Noninvasive cells revealed increased MMP activity and increased invasiveness after treatment, while invasive cells revealed the opposite. Accordingly we propose the TIMP-2 activation/inhibition activity may be dependent on endogenous levels of MMPs.
Materials and methods
Kaplan-Meier analysis
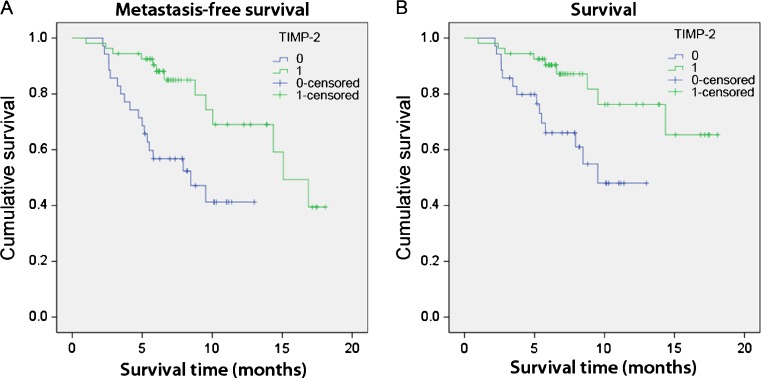
An available DNA microarray database (van 't Veer et al. 2002) from the analysis of tumors of 117 young patients whose prognosis and outcome were correlated to specific genes signatures, was used to examine the relationship between TIMP-2 expression and survival in breast cancer patients. Patients whose TIMP-2 expression levels were within one standard deviation of the mean were removed. The remaining data points were designated as either “0” for low expressors, or “1” for high expressors. Invariant analysis of survival was estimated by the Kaplan-Meier method (using SPPS software) and compared using the log rank test. Analysis was performed to examine the relationship between low “0”, and high “1”, TIMP-2 expression and metastasis-free survival (Fig. 1a), as well as overall patient survival (Fig. 1b). Log rank p < 0.05 was considered statistically significant.
Fig. 1.
High tissue of inhibitor of metalloproteinases-2 (TIMP-2) expression is correlated with prolonged and metastasis-free survival in breast cancer patients. Kaplan-Meier survival plot demonstrated that TIMP-2 expression stratified subjects in two groups with significantly different a metastasis-free survival or b cumulative survival after diagnosis. P-values correspond to the log-rank test comparing survival curves. Subjects with high TIMP-2 expression are in blue, and those with the low TIMP-2 expression are in green. Values of p < 0.05 are considered significant
Generation of the TIMP-2 and ALA + TIMP-2 expression constructs
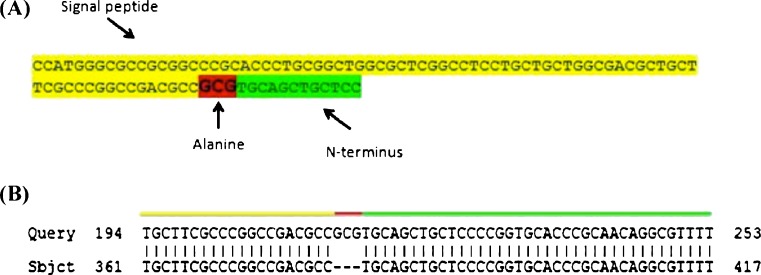
A DNA plasmid vector containing the full-length cDNA sequence of human TIMP-2 (Origene) was used to generate the TIMP-2 and ALA + TIMP-2 constructs. To PCR amplify the TIMP-2 cDNA, primers 20 base pairs (bp) long were used that were complementary to the 5’ and 3’ ends of the TIMP-2 coding region. To PCR amplify the ALA + TIMP-2 sequence from the TIMP-2 cDNA, the 3’ primer used was the same, but the 5’ primer was much longer in order to add an alanine codon after (3’ of ) the signal sequence as TIMP-2 is a secreted protein (Fig 2a). Following PCR, amplicons were ligated in pcDNA™ 3.3 TOPO mammalian constitutive expression vectors as per kit instructions. The correct DNA sequence was verified by the DNA Sequencing Facility at the Robarts Research Institute (The University of Western Ontario).
Fig. 2.
Schematic representation of ALA + TIMP-2 construct. a The 93 base pair primer designed to generate the ALA + TIMP-2 construct by PCR amplification. The signal peptide sequence of TIMP-2 is highlighted in yellow, the partial N-terminus of the protein in green, and the added alanine codon in red. b Sequence data of the ALA + TIMP-2 construct analyzed by NCBI BLAST. ALA + TIMP-2 (Query) shows 100% amino acid identity with TIMP-2 (Sbjct) except for the alanine codon, GCG, at the N-terminus (red). The signal peptide (which is removed after secretion) is highlight in yellow and amino-terminus of the wildtype mature protein is in green
MCF-7 cell transfection and conditioned media collection
MCF-7 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) media (Invitrogen) with 10% Fetal bovine serum (Invitrogen) and 1% Penicillin (100 U/mL) Streptomycin (100 μg/mL). Cells were passaged by disassociating them with TrypLETM Express (Invitrogen) before they reached 80% confluency. Cells were transfected with TIMP-2, ALA + TIMP-2 or GFP (Control) plasmids using LipofectamineTM 2000 (Invitrogen). The cells were incubated with the lipofectamine/DNA mixture in Opti-Mem serum free media (Invitrogen) for 6 h and then switched to full growth media for 24 h to recover. After recovery, and several washes with PBS, transfected MCF-7 cells were switched to Human Mammary Epithelial Cell (HuMEC) basal serum-free media (Invitrogen) for 24 h before conditioned media was collected. The collected media was assayed for levels of TIMP-2 and ALA + TIMP-2 using a human TIMP-2 enzyme-linked immunosorbent assay (ELISA) kit (R&D systems) as per kit instructions. The levels of TIMP-2 and ALA + TIMP-2 were quantified by measuring absorbance at 450 nm using a Molecular Devices M2E microplate reader.
Matrigel invasion assay following treatment with conditioned media
The breast cancer cell lines MCF-7, T47D and MDA-MB-231 were obtained from the American Type Culture Collection (ATCC) and were used in accordance with UWO biosafety protocols. These cell lines were chosen because of their variable expression of TIMPs, MMPs and invasiveness (Figueira et al. 2009). Fifty thousand untransfected cells were added per transwell invasion chamber coated with 1 mg/ml Matrigel (reconstituted basement membrane; BD Biosciences). Cells were treated with conditioned media from TIMP-2, ALA + TIMP-2, or GFP (Control) transfectants, and allowed to invade for 24 h. Transwell inserts were subsequently fixed in methanol, stained with 1% crystal violet and invaded cells counted. Three experimental replicates were performed and invading cell numbers averaged.
Metalloproteinase activity assay following treatment with conditioned media
Untransfected breast cancer cells grown for 24 h were treated with conditioned media collected from TIMP-2, ALA + TIMP-2, or GFP transfected MCF-7 cells. After treatment for 24 h the media was removed from each cell type for proteolytic analysis. The media collected after treatments was used to assay MMP activity by using a Mca-K-P-L-G-L-Dpa-A-R-NH2 Fluorogenic Peptide Substrate (R&D systems). Following BCA quantification to ensure equal protein levels, MMP activity was quantified by measuring fluorescence with an excitation at 320 nm and emission at 405 nm using a Molecular Devices M2E microplate reader.
Gelatin zymography
After treatment for 24 h the culture media was removed from each cell type and analyzed by gelatin zymography as described previously (Toth and Fridman 2001). Briefly, following BCA quantification to ensure equal protein levels, aliquots of media were loaded without reduction on a 0.1% gelatin-10% acrylamide gels. After electrophoresis, gels were washed with 2.5% Triton X-100 to remove SDS and re-nature the MMP proteins in the gels. The gels were then incubated in developing buffer for 24 h at 37°C to induce gelatin lysis by the re-natured proteases. Areas of proteolytic activity were visualized as clear bands after staining the gels with Coomassie blue. Gels were visualized and photographed using Bio-Rad Quantity One 4.4.0 software.
Viability assay
The viability of cells after 24 h of TIMP-2 or ALA + TIMP-2 conditioned media treatment was assayed using a DHL™ Cell Viability and Proliferation Assay Kit (Anaspec) as per manufacturer’s instructions. Cell viability was quantified by measuring fluorescence with an excitation at 590 nm and emission at 530 nm using a Molecular Devices M2E microplate reader.
Statistical analysis
Statistical analyses of multiple comparisons for parametric data were performed using a one-way ANOVA followed by a Tukey–Kramer Comparisons Post-Hoc test. Data were expressed as mean ± S.E.M. for replicate values. Data comparisons for all experiments were considered statistically significant at p < 0.05.
Results
High levels of TIMP-2 expression are correlated with prolonged and metastasis-free survival in breast cancer patients
Using data available from a database generated by van 't Veer et al. (2002) we wanted to establish the relationship between levels of TIMP-2 expression, metastasis and patient survival. Patients that expressed high levels of TIMP-2 had a significantly higher rate of metastasis-free survival (Fig. 1a). Fifty percent of low TIMP-2 expressors survived metastasis-free at 8 months compared to 15 months for the high TIMP-2 expressors. Similarly, patients that expressed high levels of TIMP-2 survived significantly longer than patients that expressed low levels of TIMP-2 (Fig. 1b). Low TIMP-2 expressors had a cumulative survival rate of about 50% after 10 months, while high TIMP-2 expressors had a cumulative survival rate of about 80% after 10 months.
Generation of the TIMP-2 and ALA + TIMP-2 expression constructs
Sequencing of the TIMP-2 and ALA + TIMP-2 constructs showed 100% identity with human TIMP-2 and the additional amino-terminal alanine codon in the ALA + TIMP-2 mutant was in the correct position (Fig. 2).
Transfection of TIMP-2 and ALA + TIMP-2 expression constructs resulted in high levels of secreted protein
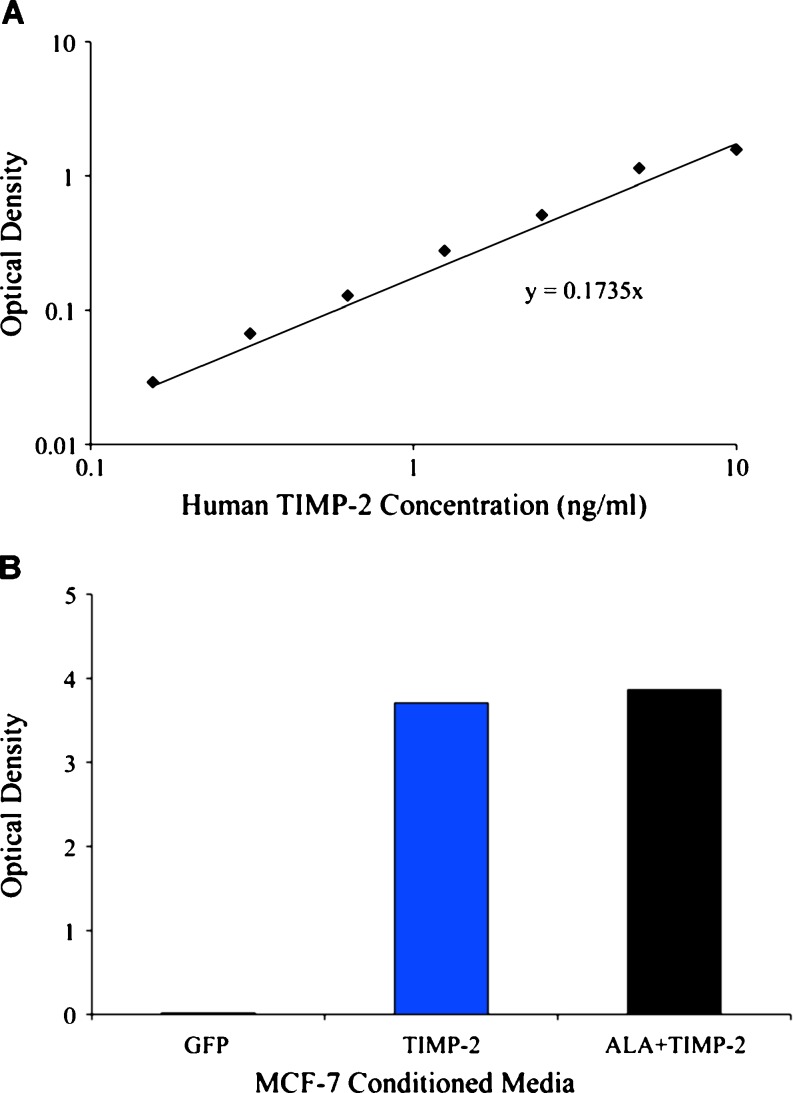
To confirm that transfection of the expression constructs resulted in the production of elevated levels of secreted proteins, media from transfected cells was analyzed by ELISA for TIMP-2 protein. MCF-7 cells transfected with the TIMP-2 or ALA + TIMP-2 constructs secreted high levels of respective proteins. GFP transfected cells showed significantly lower (endogenous) expression of TIMP-2 as compared to TIMP-2 or ALA + TIMP-2 transfected cells. Based on known standards, the concentration of TIMP-2 and ALA + TIMP-2 in the respective conditioned media was determined to be 22 ng/ml while the concentration of TIMP-2 in the media from GFP transfected MCF-7 cells was determined to be 0.1 ng/ml (Fig. 3).
Fig. 3.
TIMP-2 concentrations in conditioned medium isolated from MCF-7 cells transfected with TIMP-2, ALA + TIMP-2 or GFP. a Known concentrations of recombinant TIMP-2 were assayed using a TIMP-2 ELISA kit and the optical density of each was plotted against thcphe concentration of TIMP-2. b Levels of TIMP-2 in the conditioned media isolated from MCF-7 cells transfected with GFP, TIMP-2 or ALA + TIMP-2 constructs. TIMP-2 levels were determined to be 0.1, 22 and 22 ng/ml in the media from GFP, TIMP-2, or ALA + TIMP-2 transfected cells respectively (as determined by comparing the optical density to the standard curve)
TIMP-2 conditioned media increased the invasiveness of MCF-7 cells
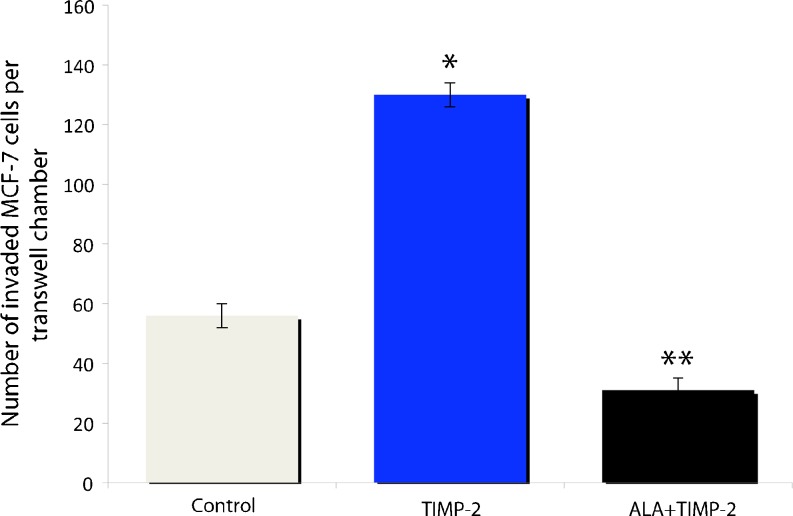
Untrasfected MCF-7 cells were treated with TIMP-2 or ALA + TIMP-2 conditioned media to investigate effects on invasiveness. MCF-7 cells treated with TIMP-2 conditioned media showed an over 100% increase in invasiveness as compared to control cells treated with conditioned media from GFP (control) transfectants (Fig. 4). Conversely, MCF-7 cells treated with ALA + TIMP-2 conditioned media showed a 50% decrease in invasiveness compared to control (Fig. 4). MCF-7 cells treated with TIMP-2 or ALA + TIMP-2 conditioned media did not show any changes in viability after 24 h of treatment as compared to control cells treated with conditioned media from GFP transfectants (data not shown).
Fig. 4.
Invasiveness of MCF-7 cells treated with elevated levels of TIMP-2 or ALA + TIMP-2. TIMP-2 more than doubled the number of invading cells, while ALA + TIMP-2 treatment decreased the number of invading cells by ~50% compared to the GFP control. Invasion data is shown as the average number of invaded cells per transwell chamber. All data are mean ± s.e.m based on 3 replicates. * represents a significant difference between the treatment and the control, while ** represents significant differences between treatments (p < 0.05)
T47D cells treated with TIMP-2 or ALA + TIMP-2 conditioned media displayed changes in invasiveness consistent with MCF-7 cells
Given that MCF-7 cells treated with TIMP-2 conditioned media paradoxically resulted in increased invasiveness, we decided to determine the effects of TIMP-2 treatment on a similar cell line. T47D cells are an estrogen receptor-positive, low MMP expressing, and poorly invasive cell line similar to MCF-7 cells. T47D cells treated with 22 ng/ml TIMP-2 conditioned media did not significantly increase their invasiveness. However, T47D cells treated with ALA + TIMP-2 conditioned media resulted in a 66% decrease in invasiveness (Fig. 5a-top).
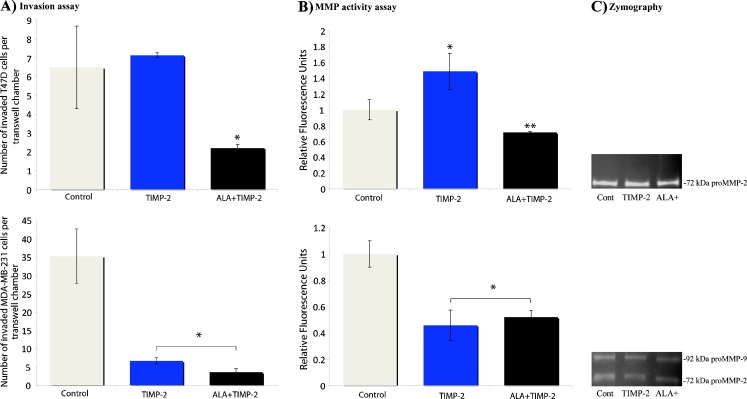
Fig. 5.
Invasiveness and metalloproteinase activity of T47D and MDA-MB-231 cells treated with TIMP-2 or ALA + TIMP-2 conditioned media. a-top) T47D cells treated with 22 ng/ml TIMP-2 conditioned media did not significantly increase invasiveness whereas T47D cells treated with 22 ng/ml ALA + TIMP-2 resulted in a 66% decrease in invasiveness. a-bottom) MDA-MB-231 cells treated with 22 ng/ml TIMP-2 or ALA + TIMP-2 conditioned media resulted in a greater than 80% reductions in invasiveness compared to control. b-top) T47D conditioned media displayed a 45% increase in metalloproteinase activity with the addition of TIMP-2 and a 35% decrease in metalloproteinase activity with the addition of ALA + TIMP-2. b-bottom) Decreases in MDA-MB-231 invasiveness with treatment with TIMP-2 or ALA + TIMP-2 were concomitant with greater than 40% and 50% decreases in metalloproteinase activity respectively. c Conditioned media from control, TIMP-2 or ALA + TIMP-2 treated cells were assayed for MMP activity using zymography. c-top) T47D conditioned media displayed consistent levels of pro-MMP-2 regardless of treatment. c-bottom) MDA-MB-231 conditioned media displayed consistent levels of both pro-MMP-2 and pro-MMP-9, regardless of treatment. a and b–all data are mean ± s.e.m based on 3 replicates. * represents a significant difference between the treatment and the control, while ** represents significant differences between treatments (p < 0.05). C is a representative zymography with several repeats showing consistent levels between treatments
T47D cells displayed changes in protease activity when treated with TIMP-2 or ALA + TIMP-2 conditioned media consistent with invasiveness
To determine if the changes seen in invasiveness correlated with changes in MMP activity, a broad spectrum MMP activity assay was used with conditioned media isolated from T47D cells treated with TIMP-2 or ALA + TIMP2 for 24 h. Interestingly, T47D conditioned media displayed a 45% increase in metalloproteinase activity with the addition of TIMP-2, but a 35% decrease in metalloproteinase activity with the addition of ALA + TIMP-2 (Fig. 5b-top).
MDA-MB-231 breast cancer cells decreased in invasiveness and protease activity when treated with TIMP-2 conditioned media
Contrasting MCF-7 and T47D cells, MDA-MB-231 cells are estrogen receptor-negative, highly invasive and secrete elevated levels of MMPs. When treated with 22 ng/ml TIMP-2 conditioned media, MDA-MB-231 cells displayed a greater than 80% reduction in invasiveness compared to control (Fig. 5a-bottom). This reduction in invasiveness is concomitant with a 50% reduction in MMP activity (Fig. 5b-bottom). Treatment of MDA-MB-231 with ALA + TIMP-2 conditioned media also resulted in a >80% reduction in invasiveness that is also accompanied by a >50% reduction in MMP activity (Fig. 5).
proMMP-2 and proMMP-9 levels in T47D and MDA-MB-231 were not altered after treatment with either TIMP-2 or ALA + TIMP-2 conditioned media
Treatment with TIMP-2 or ALA + TIMP-2 conditioned media did not significantly change the activation profiles of pro-MMP-2 in T47D (Fig. 5c-top), nor pro-MMP-2 and pro-MMP-9 in MDA-MB-231 cells (Fig. 5c-bottom). Pro-MMP-9 was detectable at very low levels, while levels of pro-MMP-2 and -9 were comparable in MDA-MB-231cells under all treatment conditions.
Discussion
Given the known benefits associated with elevated TIMP levels in a number of cancers (Alakus et al. 2008; Stetler-Stevenson and Seo 2005; Svagzdys et al. 2011), it is disappointing that TIMP utilization as a therapeutic agent has not been overly successful (Stetler-Stevenson 2008). Indeed, our examination of TIMP-2 expression in breast cancer using Kaplan-Meier analysis demonstrated that elevated TIMP-2 levels are associated with higher overall and metastasis-free survival. While elevated TIMP-2 levels may be beneficial, the overall action of TIMP-2 is dependent on its ability to both inhibit MMPs with its N-terminal domain, as well as interact with the cell surface with its C-terminal domain. As such, its specific actions will be dependent on both the makeup of the ECM environment as well as the cell surface. Other research has shown that elevated TIMPs are most beneficial when MMP levels are also high (Alakus et al. 2008; Svagzdys et al. 2011).
In this study, low and high TIMP/MMP expressing cancer cells were treated with TIMP-2 or ALA + TIMP-2 conditioned media to further our understanding of the molecular mechanisms underlying TIMP-2 biology. Wild-type TIMP-2 is both a direct inhibitor of MMP activity, and an activator or pro-MMP-2 (with MMP-14), as well as a potential ligand for cell surface receptors, such as α3β1 integrin, that induce the upregulation of MMP suppressive proteins such as RECK. By combining direct MMP inhibitory potential with cell signaling activities TIMP-2 utilizes two complementary mechanisms that provide for the negative regulation of MMP activity, and concomitant decrease in cellular invasive potential. However, TIMP-2 can also participate in the activation of MMPs, thereby potentiating MMP activity. Previous studies have shown that TIMP-2 inhibits tumour growth and invasion both in vivo and in vitro. However, these effects were obtained with TIMP-2 levels ranging from 2.5 to 10 μg/ml which are 25 to 100-fold higher than found in normal tissues or biological fluids (10-100 ng/ml) (Larsen et al. 2005). In this study, we treat breast cancer cells with TIMP-2/ALA + TIMP-2 conditioned media containing 22 ng/ml of the respective proteins, a level well within and in the low end of physiological concentrations.
Surprisingly, when we assayed for the invasiveness of MCF-7 cells treated with TIMP-2 conditioned media, we saw a significant increase in the invasive nature of these cells. Accordingly, we decided to look at additional cell lines to see if this increased invasiveness in the presence of an MMP inhibitor was a peculiarity of MCF-7 cells. We examined cells that are both similar to, and different from, MCF-7 cells with respect to characteristics crucial to this study. T47D cells, like MCF-7 cells are regarded as less tumorigenic when compared to MDA-MB-231 cells. Of importance to us is that both MCF-7 and T47D cells show low levels of expression of TIMP-2, MMP2 and many other MMPs (Balduyck et al. 2000; Figueira et al. 2009; Jones et al. 2003). Further, MCF-7 and T47D share similar invasive capabilities, and their invasive capabilities mirror each other even when cells are treated with extracellular reagents, such as the protein tenascin-C (Hancox et al. 2009). These shared attributes of low MMP expression levels and low invasiveness are not shared by MDA-MB-231 cells which express high levels of TIMPs and MMPs, and whose invasive characteristics are also distinct (Balduyck et al. 2000; Hancox et al. 2009; Jones et al. 2003).
Here we demonstrate that T47D cells, which are similar to MCF-7 cells, also increased their invasiveness, though not significantly, when treated with TIMP-2 conditioned media. When we isolated media from these treated T47D cells to assay for MMP activity, we saw that it was significantly increased in MMP activity. As MCF-7 and T47D cells both have low endogenous levels of MMPs, we hypothesize that high levels of exogenous TIMP-2 works to activate pro-MMPs when they are expressed at low levels by these cell lines. Conversely, when we treated MDA-MB-231 cells (which express high levels of active MMPs) with TIMP-2, this resulted in a significant decrease in both MMP activity and invasiveness. Here addition of exogenous TIMP-2 inhibited the active MMPs that are already present at high levels. Gelatin zymography was then used to examine the activation of specific MMPs. However, it has been previously reported that gelatin zymography is not sensitive enough to detect subtle changes in proMMP-2 activation, especially in cell lines that endogenously express low levels of TIMPs and MMPs (Ratnikov et al. 2002). Indeed studies have published that proMMP-2 and -9 activity is not detectable in MCF7 cell media using zymography (Ehrenfeld et al. 2011; Lauber and Gooderham 2011), while others demonstrate the contrary (Abdallah et al. 2007; Bartsch et al. 2003). Similarly, zymography reports using conditioned media from T47D cells have conflicting results. Some report the absence of pro-MMP-2 and -9 activity (Janowska-Wieczorek et al. 2006) while others report the presence (Abdallah et al. 2007). However, despite differences in the absolute levels of pro-MMP-2 or -9 in MCF-7, T47D or MDA-MB-231 media, these reports agree on the relative differences between these cells lines, with MDA-MB-231 showing the highest levels of activity, and MCF-7 the lowest amongst these 3 cell lines (Ehrenfeld et al. 2011; Janowska-Wieczorek et al. 2006; Jones et al. 2003). Here we demonstrate a TIMP-2 dependent increase in global MMP activity that is not detected through zymography. Given that other MMPs in addition to MMPs 2 and 9 could be activated, and with limitations and inconsistencies of zymography in detecting subtle changes, other approaches need to be utilized to understand how TIMP-2 is facilitating changes in cell behavior.
To further investigate the nature of this phenomenon, we used an ALA + TIMP-2 mutant that cannot inhibit MMP activity (Wingfield et al. 1999). We found that treatment with ALA + TIMP-2 conditioned media suppressed MMP activity and invasiveness in both T47D and MDA-MB-231 cell lines. The reduction in MMP activity seen with ALA + TIMP-2 is consistent with functions attributed to α3β1 integrin signaling that would still be possible through the C-terminal domain of the ALA + TIMP-2 mutant, as the upregulation of RECK mRNA occurs through TIMP-2 mediated α3β1 integrin signaling (Oh et al. 2004). However, further investigation of the cell signaling cascades and RECK regulation by cells is crucial to complete our understanding of TIMP biology.
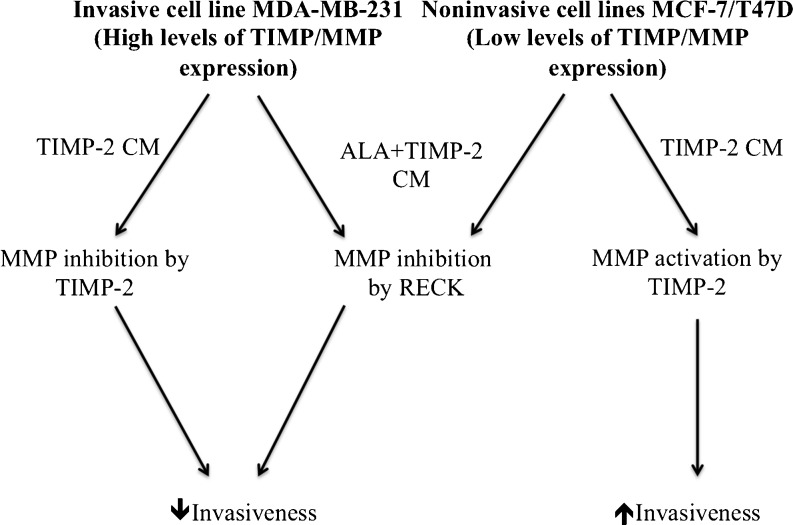
Our assays using wild-type TIMP-2 and ALA + TIMP-2 constructs allowed us to infer the relative importance of the MMP binding and cell signaling activities. Results indicated that the fully functional inhibitor preferentially acts through direct MMP interaction, whether in an inhibitory or activating context. Thus in an environments of low MMP activity, such as provided by MCF7 and T47D cells, TIMP-2 predominantly activated pro-MMPs. In contrast, in an environment of high MMP activity, such as provided by MDA-MB-231 cells, TIMP-2 predominantly inhibits MMP activity. When devoid of MMP binding potential (as is the ALA + TIMP-2 construct), TIMP-2 exclusively suppresses MMP activity and invasiveness through cell signaling events, that include RECK, that are independent of the cellular environment. These interactions are summarized in Fig. 6.
Fig. 6.
Schematic model of TIMP-2’s biphasic effects on cellular behavior. Regardless of cell type, the addition ALA + TIMP-2 conditioned media results in global MMP inhibition. This is likely achieved by the upregulation of MMP inhibiting proteins such as RECK via established MMP independent signaling pathways such as α3β1 integrins (middle path). Decreases in MMP activity is concomitant with decreased cellular invasiveness. In non-invasive cell types that produce relatively low levels of active MMPs, the addition of TIMP-2 facilitates MMP activation and provides a mechanism for these cells to increases their cellular invasiveness. Conversely, highly invasive MDA-MB-231 cells, which already produce high levels of active MMPs, TIMP-2 inhibits MMP activity resulting in decreased invasiveness. CM; conditioned media
Taken together, the relationship between the proteolytic activity of a cell and its invasive character need to be considered in a broader context. While proteolytic activity and invasiveness are very strongly correlated, with highly invasive cells often secreting many active MMPs, the regulation of this activity is complex. Using the ALA + TIMP-2 mutant we demonstrated that different cells have specific abilities to utilize full-length TIMP-2. While the addition of TIMP-2 can result in the inhibition of metalloproteinase activity, this may be dependent on cell type, endogenous MMP expression levels and likely other factors. Our data suggests that TIMP-2 MMP-independent roles may be to exclusively down-regulate invasiveness. Such exclusive down regulation of invasiveness could be potentially exploited in future therapeutics, though more experimentation is needed to further elucidate this complex phenomenon.
Glossary
- ATCC
American tissue culture collection
- CM
Conditioned media
- DMEM
Dulbecco's modified eagle medium
- ECM
extracellular matrix
- ELISA
enzyme-linked immunosorbent assay
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GFP
green fluorescent protein
- HuMEC
Human Mammary Epithelial Cell media
- MMP
matrix metalloproteinase
- PCR
polymerase chain reaction
- PBS
phosphate buffered saline
- RECK
reversion-inducing cysteine rich protein
- TIMP
tissue inhibitor of metalloproteinase
- UWO
University of Western Ontario
Contributor Information
Logan A. Walsh, Phone: +1-519-6612111, FAX: +1-519-6613935, Email: walshl@mskcc.org
Mario A. Cepeda, Phone: +1-519-6612111, FAX: +1-519-6613935, Email: mcepeda@uwo.ca
Sashko Damjanovski, Phone: +1-519-6612111, FAX: +1-519-6613935, Email: sdamjano@uwo.ca.
References
- Abdallah MA, Abdullah HI, Kang S, Taylor DD, Nakajima ST, Gercel-Taylor C. Effects of the components of hormone therapy on matrix metalloproteinases in breast-cancer cells: an in vitro study. Fertil Steril. 2007;87:978–981. doi: 10.1016/j.fertnstert.2006.08.091. [DOI] [PubMed] [Google Scholar]
- Alakus H, Grass G, Hennecken JK, Bollschweiler E, Schulte C, Drebber U, Baldus SE, Metzger R, Hölscher AH, Mönig SP. Clinicopathological significance of MMP-2 and its specific inhibitor TIMP-2 in gastric cancer. Histol Histopathol. 2008;23:917–923. doi: 10.14670/HH-23.917. [DOI] [PubMed] [Google Scholar]
- Balduyck M, Zerimech F, Gouyer V, Lemaire R, Hemon B, Grard G, Thiebaut C, Lemaire V, Dacquembronne E, Duhem T, Lebrun A, Dejonghe MJ, Huet G. Specific expression of matrix metalloproteinases 1, 3, 9 and 13 associated with invasiveness of breast cancer cells in vitro. Clin Exp Metastasis. 2000;18:171–178. doi: 10.1023/A:1006762425323. [DOI] [PubMed] [Google Scholar]
- Bartsch JE, Staren ED, Appert HE. Matrix metalloproteinase expression in breast cancer. J Surg Res. 2003;110:383–392. doi: 10.1016/S0022-4804(03)00007-6. [DOI] [PubMed] [Google Scholar]
- Dove A. MMP inhibitors: glimmers of hope amidst clinical failures. Nat Med. 2002;8:95. doi: 10.1038/nm0202-95. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld P, Conejeros I, Pavicic MF, Matus CE, Gonzalez CB, Quest AF, Bhoola KD, Poblete MT, Burgos RA, Figueroa CD. Activation of kinin B1 receptor increases the release of metalloproteases-2 and -9 from both estrogen-sensitive and -insensitive breast cancer cells. Cancer Lett. 2011;301:106–118. doi: 10.1016/j.canlet.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Figueira RC, Gomes LR, Neto JS, Silva FC, Silva ID, Sogayar MC. Correlation between MMPs and their inhibitors in breast cancer tumor tissue specimens and in cell lines with different metastatic potential. BMC Cancer. 2009;9:20. doi: 10.1186/1471-2407-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SE, Kassim SY, Birkland TP, Parks WC. Mouse models of MMP and TIMP function. Methods Mol Biol. 2010;622:31–52. doi: 10.1007/978-1-60327-299-5_2. [DOI] [PubMed] [Google Scholar]
- Hancox RA, Allen MD, Holliday DL, Edwards DR, Pennington CJ, Guttery DS, Shaw JA, Walker RA, Pringle JH, Jones JL. Tumour-associated tenascin-C isoforms promote breast cancer cell invasion and growth by matrix metalloproteinase-dependent and independent mechanisms. Breast Cancer Res. 2009;11:R24. doi: 10.1186/bcr2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowska-Wieczorek A, Marquez-Curtis LA, Wysoczynski M, Ratajczak MZ. Enhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cells. Transfusion. 2006;46:1199–1209. doi: 10.1111/j.1537-2995.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Jones JL, Shaw JA, Pringle JH, Walker RA. Primary breast myoepithelial cells exert an invasion-suppressor effect on breast cancer cells via paracrine down-regulation of MMP expression in fibroblasts and tumour cells. J Pathol. 2003;201:562–572. doi: 10.1002/path.1483. [DOI] [PubMed] [Google Scholar]
- Lambert V, Wielockx B, Munaut C, Galopin C, Jost M, Itoh T, Werb Z, Baker A, Libert C, Krell HW, Foidart JM, Noël A, Rakic JM. MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. FASEB J. 2003;17:2290–2302. doi: 10.1096/fj.03-0113fje. [DOI] [PubMed] [Google Scholar]
- Lambert E, Dasse E, Haye B, Petitfrere E. TIMPs as multifacial proteins. Crit Rev Oncol Hematol. 2004;49:187–198. doi: 10.1016/j.critrevonc.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Larsen MB, Stephens RW, Brunner N, Nielsen HJ, Engelholm LH, Christensen IJ, Stetler-Stevenson WG, Hoyer-Hansen G. Quantification of tissue inhibitor of metalloproteinases 2 in plasma from healthy donors and cancer patients. Scand J Immunol. 2005;61:449–460. doi: 10.1111/j.1365-3083.2005.01585.x. [DOI] [PubMed] [Google Scholar]
- Lauber SN, Gooderham NJ. The cooked meat-derived mammary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine promotes invasive behaviour of breast cancer cells. Toxicology. 2011;279:139–145. doi: 10.1016/j.tox.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Takahashi C. Recklessness as a hallmark of aggressive cancer. Cancer Sci. 2007;98:1659–1665. doi: 10.1111/j.1349-7006.2007.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Seo DW, Diaz T, Wei B, Ward Y, Ray JM, Morioka Y, Shi S, Kitayama H, Takahashi C, Noda M, Stetler-Stevenson WG. Tissue inhibitors of metalloproteinase 2 inhibits endothelial cell migration through increased expression of RECK. Cancer Res. 2004;64:9062–9069. doi: 10.1158/0008-5472.CAN-04-1981. [DOI] [PubMed] [Google Scholar]
- Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–956. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnikov BI, Deryugina EI, Strongin AY. Gelatin zymography and substrate cleavage assays of matrix metalloproteinase-2 in breast carcinoma cells overexpressing membrane type-1 matrix metalloproteinase. Lab Invest. 2002;82(11):1583–90. doi: 10.1097/01.lab.0000038555.67772.db. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Coussens LM. RECKing MMP function: implications for cancer development. Trends Cell Biol. 2002;12:209–211. doi: 10.1016/S0962-8924(02)02280-8. [DOI] [PubMed] [Google Scholar]
- Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/S0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev. 2008;27:57–66. doi: 10.1007/s10555-007-9105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Seo DW. TIMP-2: an endogenous inhibitor of angiogenesis. Trends Mol Med. 2005;11:97–103. doi: 10.1016/j.molmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Svagzdys S, Lesauskaite V, Pangonyte D, Saladzinskas Z, Tamelis A, Pavalkis D. Matrix metalloproteinase-9 is a prognostic marker to predict survival of patients who underwent surgery due to rectal carcinoma. Tohoku J Exp Med. 2011;223:67–73. doi: 10.1620/tjem.223.67. [DOI] [PubMed] [Google Scholar]
- Toth M, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9 by gelatin zymography. Methods Mol Med. 2001;57:163–174. doi: 10.1385/1-59259-136-1:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van't Veer LJ, Dai H, Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- Walsh LA, Carere DA, Cooper CA, Damjanovski S. Membrane type-1 matrix metalloproteinases and tissue inhibitor of metalloproteinases-2 RNA levels mimic each other during Xenopus laevis metamorphosis. PLoS One. 2007;2:e1000. doi: 10.1371/journal.pone.0001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh LA, Damjanovski S. IGF-1 increases invasive potential of MCF 7 breast cancer cells and induces activation of latent TGF-β1 resulting in epithelial to mesenchymal transition. Cell Commun Signal. 2011;9:10. doi: 10.1186/1478-811X-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield PT, Sax JK, Stahl SJ, Kaufman J, Palmer I, Chung V, Corcoran ML, Kleiner DE, Stetler-Stevenson WG. Biophysical and functional characterization of full-length, recombinant human tissue inhibitor of metalloproteinases-2 (TIMP-2) produced in Escherichia coli, Comparison of wild type and amino-terminal alanine appended variant with implications for the mechanism of TIMP functions. J Biol Chem. 1999;274:21362–21368. doi: 10.1074/jbc.274.30.21362. [DOI] [PubMed] [Google Scholar]
- Yana I, Seiki M. MT-MMPs play pivotal roles in cancer dissemination. Clin Exp Metastasis. 2002;19:209–215. doi: 10.1023/A:1015527220537. [DOI] [PubMed] [Google Scholar]
- Zhang A, Meng L, Wang Q, Xi L, Chen G, Wang S, Zhou J, Lu Y, Ma D. Enhanced in vitro invasiveness of ovarian cancer cells through up-regulation of VEGF and induction of MMP-2. Oncol Rep. 2006;15:831–836. [PubMed] [Google Scholar]
- Zucker S, Drews M, Conner C, Foda HD, DeClerck YA, Langley KE, Bahou WF, Docherty AJ, Cao J. Tissue inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic domain of the cell surface receptor, membrane type 1-matrix metalloproteinase 1 (MT1-MMP) J Biol Chem. 1998;273:1216–1222. doi: 10.1074/jbc.273.2.1216. [DOI] [PubMed] [Google Scholar]