Abstract
We recently show that CCN3 is a counter-regulatory molecule for the pro-fibrotic protein CCN2, and a potentially novel fibrosis therapy. The goal of this study was to assess the role of CCN3 in fibroproliferative/fibrotic responses in human dermal fibroblasts exposed to Omniscan, one of the gadolinium-based contrast agents associated with development of nephrogenic systemic fibrosis (NSF) a rare but life-threatening disease thought to be complication of NMR diagnostics in renal impaired patients. Human dermal fibroblasts were exposed to Omniscan; or to platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β) as controls. Proliferation was assessed along with matrix metalloproteinase-1, tissue inhibitor of metalloproteinases-1 and type 1 procollagen in the absence and presence of CCN3. In parallel, CCN3 production was assessed in control and Omniscan-treated cells. The results showed that PDGF stimulated fibroblast proliferation, production of Timp-1 and MMP-1 whereas exogenous CCN3 inhibited, in a dose response manner, cell proliferation (approx. 50 % max.) and production of MMP-1 (approx 35 % max.) but had little effect on TIMP-1. TGF-β stimulated type 1 procollagen production but not proliferation, Timp-1 or MMP-1 compared to non-TGF-ß treated control cells, and CCN3 treatment blocked (approx. 80 % max.) this up-regulation. Interestingly, untreated, control fibroblasts produced high constitutive levels of CCN3 and concentrations of Omniscan that induced fibroproliferative/fibrogenic changes in dermal fibroblasts correspondingly suppressed CCN3 production. The use of PDGF and TGF-β as positive controls, and the study of differential responses, including that to Omniscan itself, provide the first evidence for a role of fibroblast-derived CCN3 as an endogenous regulator of pro-fibrotic changes, elucidating possible mechanism(s). In conclusion, these data support our hypothesis of a role for fibroblast-derived CCN3 as an endogenous regulator of pro-fibrotic changes in these cells, and suggest that CCN3 may be an important regulatory molecule in NSF and a target for treatment in this and other fibrotic diseases.
Keywords: Nephroblastoma overexpressed gene (NOV) [CCN3], Gadolinium-based contrast agent (GBCA), Matrix metalloproteinase-1 (MMP-1), Platelet-derived growth factor (PDGF), Tissue inhibitor of metalloprotienases-1 (TIMP-1), Nephrogenic systemic fibrosis (NSF)
Introduction
Nephrogenic systemic fibrosis (NSF) is a clinical syndrome linked, in individuals with renal failure, to gadolinium-based contrast agent (GBCA) exposure during magnetic resonance imaging (MRI) procedures (Cowper et al. 2000; Swartz et al. 2003; Grobner 2006; Marckmann et al. 2006; Mendoza et al. 2006; Khurana et al. 2007; Yerram et al. 2007; Deo et al. 2007; Rydahl et al. 2008; Zou et al. 2011). The disease has been likened to scleroderma, based on histopathology, which includes the presence of dense, thickened collagen bundles in lesional skin. However, most pathologists describe the lesions as more fibroplastic than that typically observed in scleroderma (Cowper et al. 2000; McNeill and Barr 2002; Swartz et al. 2003; Neudecker et al. 2005), based on the presence of numerous, “plump” fibroblast-like cells. Some studies have reported only a minor increase in the number of proliferating interstitial cells, while others have reported a marked fibroplasia, resembling the cellular phase of acute wound healing (Mendoza et al. 2006).
The mechanistic events that link GBCA exposure to NSF are not fully understood. However, past studies have shown that fibroblasts undergo a proliferative response to gadolinium-containing compounds (Edward et al. 2008; Varani et al. 2009; Bhagavathula et al. 2009, 2010; DaSilva et al. 2010; Edward et al. 2010; Perone et al. 2010; Wiesinger et al. 2010; MacNeil et al. 2011). Our own studies demonstrated that exposure of human skin in organ culture or human dermal fibroblasts in monolayer culture to the clinically-used GBCAs (as well as to gadolinium chloride) increased the elaboration of both matrix metalloproteinase-1 (MMP-1) and tissue inhibitor of metalloproteinases-1 (TIMP-1) (Bhagavathula et al. 2009; Varani et al. 2009; Perone et al. 2010). Based on these observations, it was concluded that resident fibroblasts are, themselves, a potential target of GBCA stimulation. Subsequent studies demonstrated that in the presence of gadolinium, signaling pathways connected to platelet-derived growth factor (PDGF), including phosphatidylinositol 3-kinase (PI3) kinase and mitogen-activated protein (MAP) kinase signaling pathways were activated (Bhagavathula et al. 2009, 2010) and likely important.
The present study continues our effort to elucidate the events that underlie dermal fibroblast responses to gadolinium. Here the focus is on nephroblastoma overexpressed gene (NOV), renamed CCN3, as an endogenous regulator of fibroblast function. CCN3 plays a critical role in embryogenesis, but its role in adult physiology and disease pathology is less clear (Chevalier et al. 1998). A multi-functional secreted matricellular protein; it has been reported to be important in angiogenesis, cell migration, and in the regulation of cancer cell growth and metastases (McCallum et al. 2006). Recent studies have shown that CCN3 is able to suppress CCN2, a molecule critical in the initiation and progression of renal fibrosis, and, likely, other forms of fibrosis as well (Riser et al. 2009). In the model, renal cell exposure to TGF-β up-regulates CCN2, driving increased collagen deposition, while simultaneously downregulating CCN3. Concomitant exposure of the cells to CCN3 markedly down-regulates collagen production and deposition, indicating that CCN3 is a co-regulatory molecule for CCN2 (Riser et al. 2010). Others have shown using the anti-Thy-1 model of proliferative glomerulonephritis, that PDGF appears to be a central player, driving mesangial cell proliferation and concomitantly down-regulating CCN3, whereas exogenous CCN3 blocks PDGF-induced mesangial cell growth in culture (van Roeyen et al. 2008). Given this background, we posited that CCN3 might play a role as a negative regulator of fibroblast responses to gadolinium-containing compounds. Indeed, here we demonstrate that exogenous CCN3 is able to suppress fibroblast responses to Omniscan, one of the clinically used GBCAs. Furthermore, our studies demonstrate that concentrations of Omniscan that induce these fibroblast responses concomitantly down-regulate CCN3 production. Taken together, these data are consistent with a role for fibroblast-derived CCN3 as an endogenous regulator of pro-fibrotic changes in these cells.
Materials and methods
Reagents
CCN3 from two sources was utilized here. One was a recombinant CCN3 produced in our laboratory from the human embryonic kidney cell line (HEK-293) transfected with the complete human CCN3 gene, and producing an approximate 55 kDa, full-length CCN3 product. A second source of CCN3 was obtained from Pepro Tech Corporation (Rocky Hill, NJ). The commercial peptide was produced in a prokaryote system from the full human gene, but was approximately 36 kDa, apparently the result of the absence of glycosylation as observed in the mammalian cell product. PDGF and TGF-β1 were obtained from R&D Systems (Minneapolis, MN). Omniscan (GE Healthcare) was obtained from Investigational Drug Services at the University of Michigan Hospitals. Omniscan is a sterile aqueous solution of gadodiamide at 287 mg per ml (0.5 M with respect to gadodiamide) along with 12 mg per ml of sodium caldiamide (same chelator but without gadolinium), suitable for injection. Antibodies against human MMP-1 were from Chemicon (San Diego, CA).
Human dermal fibroblasts
Human foreskin tissue was obtained from neonatal circumcisions performed at the University of Michigan Hospitals. De-identified tissue samples were obtained under an exemption from IRB oversight. Fibroblasts were grown using Dulbecco's Modified Minimal Essential Medium supplemented with nonessential amino acids and 10 % fetal bovine serum (DMEM-FBS) as culture medium. Maintenance was at 37°C in an atmosphere of 95 % air and 5 % CO2. Cells were used at passage 2–3. The isolation and handling of dermal fibroblasts has been described previously (Varani et al. 1994).
Proliferation assay
Fibroblasts were added to wells of a 24 well dish at 2 × 104 cells per well in DMEM-FBS, and allowed to attach. The wells were then washed two times with 1 mL of keratinocyte Growth Medium (KGM) (Lonza, Walkersville, MD) supplemented with extracellular calcium to a final concentration of 1.5 mM. KGM is a low-calcium (0.15 mM) modification of MCDB-153 medium containing a mixture of growth factors including EGF, insulin, and bovine pituitary extract. Our past studies have demonstrated that when KGM is supplemented with extracellular calcium to a final concentration of 1.5 mM, it provides a potent fibroblast growth medium (Varani et al. 1994). After washing, duplicate wells were counted to provide accurate “zero-time” values. One mL of calcium-supplemented KGM was added to each remaining well. Omniscan was added at the desired amount with or without additional reagents as indicated in the Results Section. Incubation was for 3 days at 37°C in an atmosphere of 95 % air and 5 % CO2. At the end of the incubation period, culture fluids were harvested for assessment of MMP-1, TIMP-1 and type I procollagen as described below. Cells were harvested with trypsin-EDTA and counted. In some experiments, PDGF (1–25 ng/mL) or TGF-β (1–10 ng/mL) was used in place of Omniscan.
MMP-1 production by Western blotting
Western blotting with a rabbit polyclonal anti-MMP-1 antibody was used to assess MMP-1 levels (Bhagavathula et al. 2009; Varani et al. 2009). Briefly, samples consisting of 3-day culture fluids were separated in SDS-PAGE under denaturing and reducing conditions and transferred to nitrocellulose membranes. After blocking with a 5 % nonfat milk solution in Tris-buffered saline with 0.1 % Tween (TTBS) at 4°C overnight, membranes were incubated for 1 h at room temperature with the primary antibody, diluted 1:1000 in 5 % nonfat milk/TTBS. Thereafter, the membranes were washed with TTBS and bound antibody detected using the Phototope-HRP Western blot detection kit (Cell Signaling Technologies, Inc; Beverely, MS). Images were scanned, digitized and quantified. Prior to loading the gels, protein levels in each sample were determined using the BCA protein determination kit (Pierce Biotechnology; Rockford, IL) and equal amounts of protein were loaded onto each lane. In some experiments, the nitrocellulose filters were exposed to the Ponceau S reversible staining solution (Pierce Biotechnology) to visualize the transferred proteins and to confirm that comparable amounts of total protein were transferred.
MMP-1 production by enzyme-linked immunosorbant assay
Culture fluids were also assayed for MMP-1 by enzyme-linked immunosorbent assay (ELISA) using a commercially-available assay kit (R&D Systems).
MMP-2 production by gelatin zymography
We assessed MMP-2 by gelatin zymography as described previously (Varani et al. 1994, 2009). MMP-2 is commonly used as a control for secreted proteins in general, and MMP-1, specifically (Fisher et al. 1996).
TIMP-1 production by ELISA
Culture fluids were assayed for TIMP-1 by enzyme-linked immunosorbent assay (ELISA) using a commercially-available assay kit (R&D Systems).
Type I procollagen by ELISA
Culture fluids were assayed for type I procollagen by ELISA (Takara, Inc.; Madison, WS). Type I procollagen contains the N- and C-terminal peptide sequences that are present at synthesis and, therefore, provides a measure of newly synthesized collagen precursor.
Quantitative real-time RT-PCR for CCN3
Quantitative real-time RT PCR was performed to assess transcript levels for CCN3. Preparation of the RNA samples and transcript assessment were carried out as previously described (Bhagavathula et al. 2009), using reagents and TaqMan Gene Expression primers and probes from Applied Biosystems, Foster City, CA.
CCN3 and CCN2 by ELISA
CCN2 and CCN3 was assessed using an ELISA developed in our laboratory (Riser et al. 2009). Briefly, the plate was first coated with MAB1640 as a CCN3-specific trapping antibody (R&D Systems). After blocking, plates were incubated with sample or standard then washed before the addition of AF 1640 (R&D Systems) as a primary anti-CCN3 antibody. After further washing, horseradish peroxidase-conjugated secondary antibody (bovine anti-goat (cat# 805-035-180); Jackson ImmunoResearch, West Grove, PA) was added, followed by more washing and the addition of horseradish peroxidase substrate (Enhanced K-Blue TMB substrate, 308175; Neogen Corp., Lexington, KY). The color intensity was allowed to develop before being read at 650 nm using a microplate reader (Thermo Max;Molecular Devices Corp., Sunnydale, CA). MAB1640 is a purified monoclonal antibody produced in response to mouse myeloma cell line NS0-derived recombinant human CCN3/NOV (Thr32 - Met357) as the immunogen. MAB 1640 is reported as specific for human CCN3 in ELISA and Western blots and does not reportedly cross react with mouse CCN3, rhCCN2/CTGF, or rhWISP-1. Epitope mapping has not been done. AF1640 is an affinity purified polyclonal goat IgG, produced in response to the same rhCCN3 described above. It is reported to have approximately 7 % cross reactivity to mCCN3 in a sandwich ELISA using AF1640 and BAF1640.
Statistical analysis
Data were analyzed using one-way analysis of variance (ANOVA) followed by the Bonferroni post-test for selected pairs (GraphPad Prism version 4.00 for Windows, GraphPad Software). Data were considered significant at p < 0.05. Asterisks have been added to the appropriate bars in each figure to denote values that are significantly different from the respective control values.
Results
Effects of exogenous CCN3 on human dermal fibroblast responses to PDGF and TGF-β
In the first set of studies we assessed the effects of exogenous CCN3 on fibroblast responses in the presence of PDGF and TGF-β. As seen in Fig. 1 (upper left panel), PDGF stimulation was associated with markedly increased fibroblast proliferation. Concomitant treatment with CCN3 substantially blocked the PDGF-driven proliferation response and in a dose-dependent manner. At the highest concentration (100 μM), there was a greater than 50 % reduction in growth. TGF-β exposure, as expected, had no effect on proliferation, and CCN3 did not alter baseline proliferation. Accompanying PDGF-induced growth was increased production of TIMP-1 and MMP-1 (Fig. 1, lower left and upper right, respectively). CCN3 treatment down-regulated the increase in MMP-1 in a dose dependent manner, but had little or no effect on TIMP-1. In contrast, TGF-β had no stimulatory effect on MMP-1 and TIMP-1, and baseline levels were not affected by exposure to CCN3. The same culture fluids from PDGF-treated and TGF-β –treated fibroblasts were also assessed for type I procollagen. Consistent with our previous reports, PDGF did not stimulate type I procollagen production at any concentration examined either in the presence or absence of CCN3 (Fig. 1, lower right panel). Also, consistent with our previous findings TGF-β exposure stimulated increased type I procollagen production. However, CCN3 was able to suppress this production, and in a dose dependent manner. At 100 μM CCN3, inhibition was virtually complete. The data shown in Fig. 1 were obtained using the commercially-available (approximate 36 kDa) rhCCN3 produced in prokaryotic cells. Virtually identical results were obtained with the rhCCN3 produced (approximate 55 kDa) in our laboratory in HEK-293 cells (not shown).
Fig. 1.
Effects of CCN3 on human dermal fibroblast proliferation and production of MMP-1, TIMP-1 and type I procollagen in response to PDGF or TGF-β. Proliferation was assessed by cell counting; MMP-1, TIMP-1 and Type I procollagen by ELISA. Values shown are means and standard errors based on fibroblast isolates from 5 different normal donors for proliferation and MMP-1 assays, and 3 donors for TIMP-1 and type I procollagen assays. Statistical significance of the data was assessed by ANOVA, followed by paired-group comparisons. *indicates statistically significant increase compared to controls at p < 0.05 level. **indicates statistically significant decrease compared to positive control at p < 0.05 level
Effects of exogenous CCN3 on human dermal fibroblast response to Omniscan stimulation
In a recent study (Varani et al. 2009), human dermal fibroblasts from both adult skin and neonatal foreskin were examined for response to Omniscan, one of the commonly-used GBCAs. Cells from both sources demonstrated a proliferative response over a wide range of Omniscan concentrations (0.5 μM to 2.5 mM), with a peak response at 50–250 μM. Enhanced proliferation was observed with cells incubated in either of two highly-enriched growth media as well as in basal medium. In conjunction with the proliferative response, there were increased amounts of MMP-1 and TIMP-1 released into the culture fluid (Bhagavathula et al. 2009; Varani et al. 2009). The increase in secreted protein was associated with increased mRNA levels (Bhagavathula et al. 2009). As part of the present study, exogenously added CCN3 was examined for its ability to alter gadolinium-induced cell growth. Consistent with our past findings, cell growth was increased in fibroblasts exposed to Omniscan. The response was near totally blocked by CCN3 (Fig. 2, upper panel). Also consistent with previous results, MMP-1 was increased in response to Omniscan and this was also markedly suppressed in the presence of exogenous CCN3, as demonstrated by Western blotting (Fig. 2, middle panel) and ELISA (Fig. 2, lower panel). In contrast, MMP-2 was not significantly up-regulated by Omniscan, nor down-regulated in the presence of CCN3 (not shown). We did not assess the effects of CCN3 on either TIMP-1 or type I procollagen since CCN3 had little effect on TIMP-1 induced by PDGF (Fig. 1 above) and type I procollagen decreased in response to Omniscan (as shown previously, Fisher et al. 1996).
Fig. 2.
Effects of CCN3 on human dermal fibroblast proliferation and production of MMP-1, in response to Omniscan. Upper panel: Proliferation: Values shown are means and standard errors based on separate experiments with fibroblast isolates from five different individuals. Middle panel. MMP-1 assessed by Western blotting. Insert: Example Western blot: lane 1 = control; lane 2 = Omniscan; lane 3 = Omniscan and CCN3. Lower panel. MMP-1 assessed by ELISA. Values shown are means and standard errors based separate experiments with fibroblast isolates from three different individuals. For all analysis, statistical significance of the data was assessed by ANOVA, followed by paired-group comparisons. *indicates statistically significant increase compared to control at p < 0.05 level. **indicates statistically significant decrease compared to positive control at p < 0.05 level
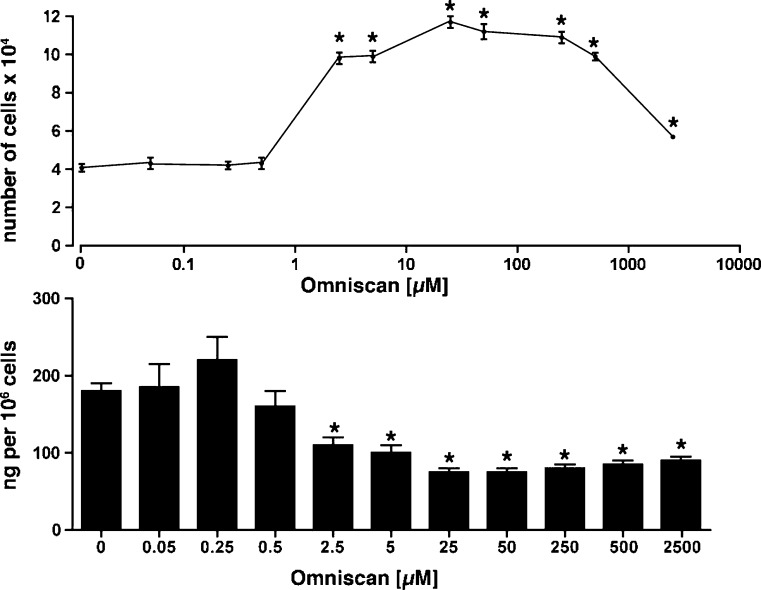
Effects of Omniscan on human dermal fibroblast production of CCN3
The above findings demonstrate that exogenously added CCN3 is able to down-regulate fibroblast responses to Omniscan. Last, we sought to determine a role for endogenous CCN3 in the same fibroblast responses. Accordingly, human dermal fibroblasts were exposed to Omniscan over a wide range of concentrations. Proliferation was assessed in parallel with CCN3 production (Fig. 3). The gadolinium-containing compound induced a significant increase in cell number at concentrations between 2.5 and 500 μM (Fig. 3, upper panel). At optimal concentrations, the rate of fibroblast doubling was increased greater than 2.5-fold. It can also be seen from Fig. 3 (lower panel) that dermal fibroblasts produce and secrete substatinal amounts of CCN3. Most importantly, the growth-inducing concentrations of Omniscan were associated with a sharp decrease in CCN3. Specifically, as increasing concentrations of Omniscan produced increased cell proliferation, there was a corresponding decrease in the amount of CCN3 secreted. In support of these findings, RT-PCR demonstrated a time- and concentration-dependent decrease in CCN3 mRNA expression in response to Omniscan (Table 1).
Fig. 3.
Effects of Omniscan on CCN3 production by dermal fibroblasts. Upper panel. Proliferation: Values shown are means and standard errors based on separate experiments with fibroblast isolates from five different individuals. Lower panel. Values for CCN3 were determined by ELISA and are mean and standard errors based on the same 5 separate donors. For both panels, statistical significance of the data was assessed by ANOVA, followed by paired-group comparisons. *indicates statistically significant change compared to untreated control at p < 0.05 level
Table 1.
CCN3 transcript in Omniscan-treated fibroblasts
| CCN3 mRNA (fold-change) | |
|---|---|
| Omniscan concentration (μM) | |
| 0 | 0.97 ± 0.06 |
| 5 | 0.87 ± 0.18 |
| 25 | 0.56 ± 0.03* |
| 50 | 0.55 ± 0.05* |
| 250 | 0.72 ± 0.22 |
| 500 | 0.83 ± 0.10 |
| Time (hours) | |
| 8 | 1.34 ± 0.22 |
| 16 | 1.23 ± 0.13 |
| 24 | 1.02 ± 0.13 |
| 48 | 0.69 ± 0.06* |
Concentration study: Fibroblasts were incubated for 3 days in varying concentrations of Omniscan. At the end of the incubation period, RNA was prepared and processed for RT-PCR as described in the Materials and Methods Section. The results are presented as fold-change relative to the control and based on three independent assessments for each transcript. *indicates statistically significant decrease compared to control at p < 0.05 level
Time study: Fibroblasts were incubated for varying periods of time with 50 μM Omniscan. At the end of the incubation period, RNA was prepared and processed for RT-PCR as described in the Materials and Methods Section. The results are presented as fold-change relative to the control and based on three independent assessments for each transcript. *indicates statistically significant decrease compared to control at p < 0.05 level
In both studies, MMP-1 was used as a positive control. mRNA increases ranged from 2.3-fold to 8.7 fold
In parallel, CCN2 was examined in Omniscan-treated fibroblasts. The skin fibroblasts produced measurable levels of CCN2 under control, untreated conditions. Changes similar to those seen with CCN3 were also observed with CCN2. That is, there was a sharp decline in CCN2 levels in the culture fluid at concentrations of Omniscan that induced proliferation (Fig. 4). A corresponding decline in CCN2 mRNA expression also occurred (not shown).
Fig. 4.
Effects of Omniscan on CCN2 production by dermal fibroblasts. Values for CCN2 were determined by ELISA and are mean and standard deviations based on 5 separate donors
Discussion
Our recent studies demonstrated that exposure of human dermal fibroblasts to Omniscan (as well as to any of several other GBCAs or to gadolinium chloride) resulted in increased fibroblast proliferation, along with increased production of MMP-1 and TIMP-1 (Varani et al. 2009). The level of TIMP-1 was sufficient such that virtually all of the MMP-1 was complexed with the inhibitor (Perone et al. 2010). Based on these findings, we hypothesized that the fibrotic changes seen in the skin of individuals with NSF might reflect a contrast agent-induced increase in fibroblast numbers in the lesional skin, and an effect on the enzyme/inhibitor system that regulates collagen turnover as opposed to an effect on collagen synthesis, per se. Subsequent studies demonstrated that gadolinium-containing compounds activated signaling pathways tied to the PDGF receptor in responsive cells (Bhagavathula et al. 2009, 2010). Specifically, both PI3 kinase and MAP kinase signaling were stimulated in response to Omniscan. Of interest, MAP kinase signaling was associated with increased proliferation, as well as with increased production of both MMP-1 and TIMP-1, while PI3 kinase signaling promoted proliferation and MMP-1 elaboration, but appeared to suppress TIMP-1 (Bhagavathula et al. 2009). In the present study, which is part of our continued effort to understand patho-physiologic events in NSF, we show that CCN3 is a potent inhibitor of PDGF-induced responses (proliferation and MMP-1 production) in the fibroblast population. The same responses to Omniscan, one of the gadolinium-containing MRI contrast agents, are inhibited in parallel. Perhaps most interesting, the fibroblasts produced high constitutive levels of CCN3 and the concentrations of Omniscan that induced fibroblast growth and increased MMP-1 production concomitantly suppressed this CCN3 production. The decrease in CCN3 protein was observed when culture fluids from Omniscan-treated cells were examined at 72 h. The changes in CCN3 expression as measured by RT-PCR were consistent with the observed protein changes in terms of concentration- and time-dependence, indicating that the effects of Omniscan were likely occurring at both the transcriptional and translational levels. Although the studies conducted here utilized Omniscan as a source of gadolinium, our previous studies have shown than other GBCAs as well as gadolinium chloride stimulated the same responses in human dermal fibroblasts as shown here with Omniscan. It is reasonable to suggest, then that CCN3 would interfere with responses induced by other GBCAs as well as by Omniscan. Taken together, our findings support the notion that CCN3 may be part of the mechanism regulating pro-fibrotic changes in NSF, and suggest that the gadolinium-containing compounds may act, in part at least, by down-regulating endogenous production of this protein in collagen-producing cells. Additional research will be needed to determine if gadolinium-mediated suppression of CCN3 can be modulated as a strategy for interfering with events that contribute to fibrosis in NSF.
Past studies have convincingly demonstrated that gadolinium compounds do not directly increase synthesis of type I procollagen by human dermal fibroblasts – at least under the conditions examined (Varani et al. 2009). In fact, there is a decline in procollagen synthesis seen in both organ-cultured skin isolated dermal fibroblasts. Although the underlying mechanism for reduced procollagen production has not been delineated, reduced type I procollagen synthesis is consistent with our observation of a reduction in CCN2. Unlike the finding we reported in renal cells where a decrease in CCN3 is driven by TGF-β, thus allowing for increased CCN2 expression and activity with a downstream elevation of collagen type 1 synthesis, here in the absence of increased TGF-β, we observe a simultaneous decrease in both CCN3 and CCN2 levels that is accompanied by a lack of increase in collagen synthesis and an increase in the major enzyme/enzyme inhibitor system regulating collagen turnover. Altered collagen turnover could, ultimately, be conducive to an increase in collagen accumulation even without increased synthesis. Additional studies will need to be done to address this issue.
Although type I procollagen does not appear to be directly up-regulated by Omnican, past studies by other investigators have demonstrated a persistent increase in collagen production by fibroblasts isolated from NSF lesional skin as compared to control fibroblasts (Piera-Velazquez et al. 2010). Increased collagen production occurring in NSF lesional skin fibroblasts could reflect an indirect response to GBCA stimulation rather than a direct effect. Specifically, studies in animals (Steger-Hartmann et al. 2009) as well as studies using human blood-derived mononuclear cells in culture (Wermuth et al. 2009; Del Galdo et al. 2010) have demonstrated that GBCA exposure up-regulates cytokines such as IL-13, that, in turn, induce TGF-β production in collagen-synthesizing cells. Regardless of whether TGF-β is up-regulated directly or indirectly, down-regulation of CCN3 in collagen-producing cells would be expected to have the same permissive effect on new collagen synthesis. Thus, one could envision a model whereby exposure to the gadolinium-containing contrast agent could induce fibroproliferative/fibrogenic events in human dermal fibroblasts through multiple mechanisms driven by both PDGF and TGF-β. Down-regulation of endogenous CCN3 in the target cells would be permissive to all of these events.
The findings reported here may have relevance beyond NSF. Roles for TGF-β and PDGF have been described in systemic sclerosis (Del Galdo et al. 2010; Beyer et al. 2010) as well as in fibrotic changes occurring in lung, liver and kidney (Del Galdo et al. 2010). Along these lines, van Roeyen et al. (van Roeyen et al. 2008) reported that PDGF is able to stimulate cultured mesangial cell proliferation and simultaneously suppresses CCN3 expression. Further, in a mesangial proliferative model of glomerulonephritis, they also showed that CCN3 was decreased at the same time that PDGF was increased, whereas in late stage as proliferation subsided CCN3 increased toward baseline levels. This study did not investigate the effects of CCN3 on fibrosis. More recently it was shown in models of liver fibrosis that CCN3 is up-regulated, the location within the liver dependent on the insult (Borkham-Kamphorst et al. 2012). In the same study, siRNA induced suppression of CCN3 in a variety of liver cells in vitro and simultaneously enhanced the expression pro-fibrotic marker genes including collagen type I. The work presented in the present study supports our original data and hypothesis of an inhibitory role for CCN3 in the initiation and progression of fibrosis in the kidney and in other organs (Riser et al. 2009, 2010). Finally, the same pathways that lead to fibrotic changes in various disease processes also function during the normal wound-healing response. It will be of interest to determine if CCN3 can act as a “brake” to limit scar formation at the time of wound closure.
Acknowledgments
This study was supported by a grant from Baxter Healthcare Corporation (BLR), and grants from the National Institutes of Health, Bethesda, MD (CA140760; JV), and from the Association for International Cancer Research, St. Andrews, Fife, Scotland (11–0577 JV).
References
- Beyer C, Schett G, Distler O, Distler JHW. Animal models of systemic scherosis: a review. Arthritis Rheum. 2010;62:2831–2844. doi: 10.1002/art.27647. [DOI] [PubMed] [Google Scholar]
- Bhagavathula N, DaSilva M, Aslam MN, Dame MK, Warner RL, Xu Y, Fisher JG, Swartz R, Varani J. Regulation of collagen turnover in human skin fibroblasts exposed to a gadolinium-based contrast agent. Invest Radiol. 2009;44(8):433–439. doi: 10.1097/RLI.0b013e3181a4d7e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagavathula N, Dame MK, DaSilva M, Jenkins W, Aslam MN, Perone P, Varani J. Fibroblast response to gadolinium: role for platelet-derived growth factor receptor. Invest Radiol. 2010;45(12):769–777. doi: 10.1097/RLI.0b013e3181e943d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkham-Kamphorst E, Roeyen CR, Leur E, Floege J, Weiskirchen R. CCN3/NOV small interfering RNA enhances fibrogenic gene expression in primary hepatic stellate cells and cirrhotic fat storing cell line CFSC. J Cell Commun Signal. 2012;6(1):11–25. doi: 10.1007/s12079-011-0141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier G, Yeger H, Martinerie C, Laurent M, Alami J, Schofield PN, Perbal B. novH: differential expression in developing kidney and Wilm's tumors. Am J Pathol. 1998;152(6):1563–1575. [PMC free article] [PubMed] [Google Scholar]
- Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000–1001. doi: 10.1016/S0140-6736(00)02694-5. [DOI] [PubMed] [Google Scholar]
- DaSilva M, O'Brien Deming M, Fligiel SE, Dame M, Johnson KJ, Swartz R, Varani J. Responses of human skin in organ culture and human skin fibroblasts to a gadolinium-based MRI contrast agent: comparison of skin from patients with end-stage renal disease and skin from healthy subjects. Invest Radio. 2010;45(11):733–739. doi: 10.1097/RLI.0b013e3181e9436b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdo F, Wermuth PJ, Addya S, Addya S, Fortina P, Jimenez SA. NFkappaB activation and stimulation of chemokine production in normal human macrophages by the gadolinium-based magnetic resonance contrast agent Omniscan: possible role in the pathogenesis of nephrogenic systemic fibrosis. Ann Rheum Dis. 2010;69(11):2024–2033. doi: 10.1136/ard.2010.134858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol. 2007;2:264–267. doi: 10.2215/CJN.03921106. [DOI] [PubMed] [Google Scholar]
- Edward M, Quinn JA, Mukherjee S, Jensen M-BV, Jardine AG, Mark PB, Burden AD. Gadodiamide contrast agent 'activates' fibroblasts: a possible cause of nephrogenic systemic fibrosis. J Pathol. 2008;214(5):584–593. doi: 10.1002/path.2311. [DOI] [PubMed] [Google Scholar]
- Edward M, Quinn JA, Burden AD, Newton BB, Jardine AG. Effect of different classes of gadolinium-based contrast agents on control and nephrogenic systemic fibrosis-derived fibroblast proliferation. Radiology. 2010;256(3):735–743. doi: 10.1148/radiol.10091131. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Wang ZQ, Datta SC, Talwar HS, Kang S, Varani J, Voorhees JJ. Up-regulation of collagen- and elastin-degrading matrix metalloproteinases by doses of ultraviolet B light (UVB) too low to cause sunburn. Nature. 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Grobner T. Gadolinium - a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transpl. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- Khurana A, Runge VM, Narayanan M, Green JF, Nickel AE. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (Omniscan) Invest Radiol. 2007;42:139–145. doi: 10.1097/01.rli.0000253505.88945.d5. [DOI] [PubMed] [Google Scholar]
- MacNeil S, Bains S, Johnson C, Idee J-M, Factor C, Jestin G, Fretellier N, Morcos SK. Gadolinium contrast agent associated stimulation of human fibroblast collagen production. Invest Radiol. 2011;46:711–717. doi: 10.1097/RLI.0b013e31822b1f38. [DOI] [PubMed] [Google Scholar]
- Marckmann P, Skov L, Rossen K, DuPont A, Damholt MB, Heaf JG, Thomsen HS. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359–2362. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- McCallum L, Price S, Planque N, Perbal B, Pierce A, Whitton AD, Irvine AE. A novel mechanism for BCR-ABL action: stimulated secretion of CCN3 is involved in growth and differentiation regulation. Blood. 2006;108(5):1716–1723. doi: 10.1182/blood-2006-04-016113. [DOI] [PubMed] [Google Scholar]
- McNeill AM, Barr RJ. Scleromyxedema-like fibromucinosis in a patient undergoing hemodialysis. Int J Derm. 2002;41:364–367. doi: 10.1111/j.1365-4632.2002.1488_3.x. [DOI] [PubMed] [Google Scholar]
- Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jiminez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheu. 2006;35:238–249. doi: 10.1016/j.semarthrit.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudecker BA, Stern R, Mark LA, Steinberg S. Scleromyxedema-like lesions of patients in renal failure contain hyaluronan: a possible pathophysiological mechanism. J Cutan Pathol. 2005;32:612–615. doi: 10.1111/j.0303-6987.2005.00415.x. [DOI] [PubMed] [Google Scholar]
- Perone P, Weber S, DaSilva M, Varani J. Collagenase activity is suppressed by elevated tissue inhibitor of metalloproteinase-1 (TIMP-1) in human skin exposed to a gadolinium-based MRI contrast agent. Invest Radiol. 2010;45:42–48. doi: 10.1097/RLI.0b013e3181bf95eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piera-Velazquez S, Louneva N, Fertala J, Wermuth PJ, Del Galdo F, Jimenez SA (2010) Persistent activation of dermal fibroblasts from patients with gadolinium-associated nephrogenic systemic fibrosis. Ann Rheum Dis. doi:10.1136/ard127761 [DOI] [PMC free article] [PubMed]
- Riser BL, Najmabadi F, Perbal B, Peterson DR, Rambow JA, Riser ML, Sukowski E, Yeger H, Riser SC. CCN3 (Nov) is a negative regulator of CCN2 (CTGF) and a novel endogenous inhibitor of the fibrotic pathway in an in vitro model of renal disease. Am J Pathology. 2009;174:1725–1734. doi: 10.2353/ajpath.2009.080241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riser BL, Najmabadi F, Perbal B, Rambow JA, Riser ML, Sukowski E, Yeger H, Riser SC, Peterson DR. CCN3/CCN2 regulation and the fibrosis of diabetic renal disease. J Cell Commun Signal. 2010;1:39–50. doi: 10.1007/s12079-010-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydahl C, Thomsen H, Marckmann R. High prevalence of nephrogenic systemic fibrosis in chronic renal failure patients exposed to Gadodiamide, a gadolinium-containing magnetic resonance contrast agent. Invest Radiol. 2008;43:141–144. doi: 10.1097/RLI.0b013e31815a3407. [DOI] [PubMed] [Google Scholar]
- Steger-Hartmann T, Raschke M, Riefke B, Pietsch H, Sieber MA, Walter J. The involvement of pro-inflammatory cytokines in nephrogenic systemic fibrosis - a mechanistic hypothesis based on preclinical results from a rat model treated with gadodiamide. Exp Toxicol Pathol. 2009;61(6):537–552. doi: 10.1016/j.etp.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Swartz RD, Crofford LJ, Phan SH, Ike RW, Su LD. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563–572. doi: 10.1016/S0002-9343(03)00085-8. [DOI] [PubMed] [Google Scholar]
- Roeyen CRC, Eitner F, Scholl T, Boor P, Kunter U, Planque N, Grone H-J, Bleau AM, Perbal B, Ostendorf T, Floege J. CCN3 is a novel endogenous PDGF-regulated inhibitor of glomerular cell proliferation. Kidney Int. 2008;73:86–94. doi: 10.1038/sj.ki.5002584. [DOI] [PubMed] [Google Scholar]
- Varani J, Perone P, Griffiths CEM, Kang S, Fisher GJ, Vorrhees All-trans retinoic acid (RA) stimulates events in organ-cultured human skin that underlie repair. Adult skin from sun-protected and sun-exposed sites responds in an identical manner to RA while neonatal foreskin responds differently. J Clin Invest. 1994;94:1747–1756. doi: 10.1172/JCI117522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, DaSilva M, Warner RL, O’Brien Deming M, Barron AG, Johsnon KJ, Swartz R. Effects of gadolinium-based magnetic resonance imaging contrast agents on human skin in organ culture and human skin fibroblasts. Invest Radiol. 2009;44(2):74–81. doi: 10.1097/RLI.0b013e31818f76b5. [DOI] [PubMed] [Google Scholar]
- Wermuth PJ, Galdo F, Jimenez SA. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009;60(5):1508–1518. doi: 10.1002/art.24471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesinger B, Kehlbach R, Bebin J, Hemsen J, Bartleon R, Schmehl J, Dietz K, Clausen CD, Wiskirchen J. Effects of MRI contrast agents on human embryonic lung fibroblasts. Invest Radiol. 2010;45(9):513–519. doi: 10.1097/RLI.0b013e3181eb2fe7. [DOI] [PubMed] [Google Scholar]
- Yerram P, Saab G, Karuparthi PR, Hayden MR, Khanna R. Nephrogenic systemic fibrosis: a mysterious disease in patients with renal failure-role of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate. Clin J Am Soc Nephrol. 2007;2:258–263. doi: 10.2215/CJN.03250906. [DOI] [PubMed] [Google Scholar]
- Zou Z, Zhang HL, Roditi GH, Leiner T, Kucharczyk W, Prince MR. Nephrogenic systemic fibrosis: review of 370 biopsy-confirmed cases. J Am Coll Cardiol Img. 2011;4:1206–1216. doi: 10.1016/j.jcmg.2011.08.013. [DOI] [PubMed] [Google Scholar]