Abstract
The gain of plasticity by a subset of cancer cells is a unique but common sequence of cancer progression from epithelial phenotype to mesenchymal phenotype (EMT) that is followed by migration, invasion and metastasis to a distant organ, and drug resistance. Despite multiple studies, it is still unclear how cancer cells regulate plasticity. Recent studies from our laboratory and others’ proposed that CCN5/WISP-2, which is found intracellularly (in the nucleus and cytoplasm) and extracellularly, plays a negative regulator of plasticity. It prevents the EMT process in breast cancer cells as well as pancreatic cancer cells. Multiple genetic insults, including the gain of p53 mutations that accumulate over the time, may perturb CCN5 expression in non-invasive breast cancer cells, which ultimately helps cells to gain invasive phenotypes. Moreover, emerging evidence indicates that several oncogenic lesions such as miR-10b upregulation and activation of TGF-β-signaling can accumulate during CCN5 crisis in breast cancer cells. Collectively, these studies indicate that loss of CCN5 activity may promote breast cancer progression; application of CCN5 protein may represent a novel therapeutic intervention in breast cancer and possibly pancreatic cancer.
Keywords: Breast cancer, Cell signaling, Invasion and apoptosis
Human breast cancer: an overview
Breast cancer, a genetically heterogeneous disease (Lichy et al. 2000; Polyak 2011), is the most commonly identified malignant disease in Western women after nonmelanocytic skin cancer. It attacks one in eight women (~12%), impacting nearly every family worldwide. It is estimated that more than 1 in 4 cancers are breast cancer. Approximately 30% of these women will develop the invasive form of the disease, which is ultimately incurable. Therefore, it is a major health issue for women. Incidence of breast cancer generally increases with age. Women approaching or at menopause have an increased risk of breast cancer. In addition to age, several other factors (i.e., personal and environmental) are associated with the development of this disease. Fortunately, the breast cancer related death rate has been reduced recently due, in part, to early diagnosis and decreased intake of carcinogenic hormones or other unknown factors. However, our current therapeutic options for advanced stages of breast cancer are still fairly limited and ineffective. Thus, there is a need to better understand the molecular basis of the genesis of breast cancer and its progression in order to design targeted, molecularly based therapies.
Like other cancers, the genesis of ductal carcinoma in the breast is the result of unprogrammed genetic and epigenetic changes, which lead to mal-regulation of cellular growth. Nevertheless, a foremost clinical turning point in the progression of this disease is the formation of an “invasive front” (invasion and metastatic spread to the distant organs) through the changes in the adhesive and migratory capabilities of non-invasive breast cancer cells (Christofori 2006). The invasive front is a complex, multistep process in which primary tumor cells invade adjacent tissue, enter the systemic circulation (intravasation), translocate through circulation, extravasate into a secondary site, re-colonize/homing and expand for metastatic growth of cancer cells (Christofori 2006). In recent years, multiple studies have revealed the participation of several protein-encoding genes and non-coding genes such as miRNA in the formation of an invasive front (Christofori 2006). However, the specific molecular changes needed to execute the initial step of the noninvasive to invasive phenotypic switch remain an enigma. Loss of cellular adhesion and increased cell motility are the fundamental aspects of the invasive front (Gupta and Massague 2006). Therefore, identification and characterization of molecules that control adhesion and cell motility are critical to our understanding of cancer dissemination.
Most human tumors including human breast tumors are epithelial in origin. However, the noninvasive to invasive phenotypic switch is often associated with the loss of epithelial phenotype and acquisition of a fibroblast or mesenchymal-like phenotype [epithelial-mesenchymal transitions (EMT)] (Kang and Massague 2004; Radisky 2005; Liang 2011). Although initially some researchers believed that there is no convincing evidence for conversion of epithelial cells into mesenchymal cell lineages in vivo and therefore did not consider EMT important for neoplasia (Tarin et al. 2005), several studies from different laboratories established that EMT is an important and coordinated series of events through the dysfunction of certain genes including, but not limited to E-cadherin, where epithelial cells lose many of their epithelial features including polarity, cell-cell interactions and remodeling of cytoskeleton and acquired mesenchymal properties (Thiery 2003; Kang and Massague 2004; Shah and Gallick 2007). EMT was initially attributed as an important feature of embryogenesis (Thiery and Sleeman 2006). However, recently, several studies have manifested that EMT is a vital step in the progression of many cancers including ductal adenocarcinoma in the breast (Kang and Massague 2004) (Radisky et al. 2005; Trimboli et al. 2008). The involvement of several proteins with different pathobiological functions (i.e., Snail, Twist, p53, RANKL, CXCR4 and ligands) and their molecular cross talks have been reported to trigger EMT followed by invasive changes (Muller et al. 2001; Kang and Massague 2004; Thiery and Sleeman 2006; Jones et al. 2006; Aylon and Oren 2007; Oren and Bartek 2007; Weisz et al. 2007). Nevertheless, the definite mechanism underlying EMT during carcinogenic development of breast cancer remains unclear.
The p53 transcriptional factor is a tumor suppressor protein and a vital component for the regulation of normal cellular physiology (Friend 1994; Levine 1997; Rozan and El-Deiry 2007; Kastan and Berkovich 2007). Functions of normal p53 protein are frequently mislaid in various human cancers including breast tumors primarily through the mutations or deletions of this gene (Hollstein et al. 1991; Kastan et al. 1991; Vogelstein et al. 2000). Accumulating evidence indicates that the mutational changes, which prolonged the half-life of this protein, not only perturb the normal tumor suppressor function of this gene, but also simultaneously exhibit a dominant negative effect and gain new tumor-promoting functions through the inactivation of DNA damage response genes or transactivation of target genes associated with the cell proliferation, apoptosis, tumorigenesis and tissue invasiveness including EMT (Cadwell and Zambetti 2001; Willis et al. 2004; Bossi et al. 2006; Song et al. 2007; Kastan 2007). However, the possible mechanism of p53 mutant mediated induction of invasive front remains elusive.
Structural, biological and pathobiological properties of CCN5/WISP-2
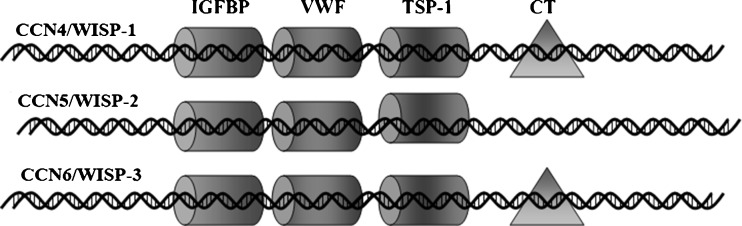
CCN5 [which is also known as WISP-2 (Wnt-1-induced signaling protein-2)] is a ~29 kDa secreted protein identified as a member of CCN [Cyr61(cysteine rich 61), CTGF (connective tissue growth factor), and Nov (nephroblastoma over-expressed gene)] family of matricellular proteins (Jun and Lau 2011) which plays a critical role in growth factors’ mediated cell proliferation (Banerjee et al. 2005; Dhar et al. 2007b). This family shares conserved multimodular domains with diverse biological functions (Pennica et al. 1998; Brigstock 2003; Holbourn et al. 2008; Jun and Lau 2011), which include angiogenesis, stem cell differentiation and carcinogenesis (Pennica et al. 1998; Brigstock 2003; Schutze et al. 2005; Holbourn et al. 2008; Jun and Lau 2011). Although CCN5 protein is mainly localized in cytoplasm (Saxena et al. 2001), it is also sometimes localized transiently in the nucleus of different breast cancer cells for diverse functional needs (Russo and Castellot 2010; Wiesman et al. 2010; Sabbah et al. 2011). The nucleotide and protein sequence of CCN5 exhibits a 30–40% sequence homology within other family members, largely CCN4 (WISP-1) and CCN6 (WISP-3) that contain an IGF-binding protein type (IGFBP) domain, a Von Willebrand type C (VWC) domain, a Thrombospondin-1 (TSP-1) domain and C-terminal Cysteine-knot (CT) domain. The modular architectures of CCN5 are identical with other family members except in their C-terminal Cysteine-knot (CT) domain, which is absent in the CCN5 gene (Pennica et al. 1998) (see Fig. 1). Although the functional roles of these four modules in these genes are unclear, it can be speculated that each individual domain may play critical biological functions under a specific microenvironment and niche, such as TSP-1 motif is necessary for the regulation of endothelial cell proliferation (Karagiannis and Popel 2007). CCN5 has both growth promoting and growth arresting competence, depending on cell types and the microenvironment of the cells. For example, overproduction of CCN5 by EGF or IGF-1 is required for the mitogenic action in estrogen-receptor-positive, non-invasive breast tumor cells (Banerjee et al. 2005; Sengupta et al. 2006; Dhar et al. 2007b), while it acts as a growth arrest specific (gas) gene in vascular smooth muscle cells and prostate cancer cells (Lake and Castellot 2003). In addition, it is most likely that CCN5 plays a preventive role in the progression of pancreatic cancer as it participates in morphological alterations from mesenchymal to epithelial transition (MET) of pancreatic adenocarcinoma cells (Dhar et al. 2007a) and breast cancer cells (Banerjee et al. 2008).
Fig. 1.
Modular structures of WISP-subfamily of CCN family growth factors. There are three genes under WISP (Wnt-1-induced signaling protein) sub-family. These include WISP-1/CCN4, WISP-2/CCN5 and WISP-3/CCN6. The modular domains of WISP-subfamily are analogous except in their C-terminal domains, which is absent in the CCN5/WISP-2 gene
The roles of CCN5/WISP-2 in breast carcinogenesis
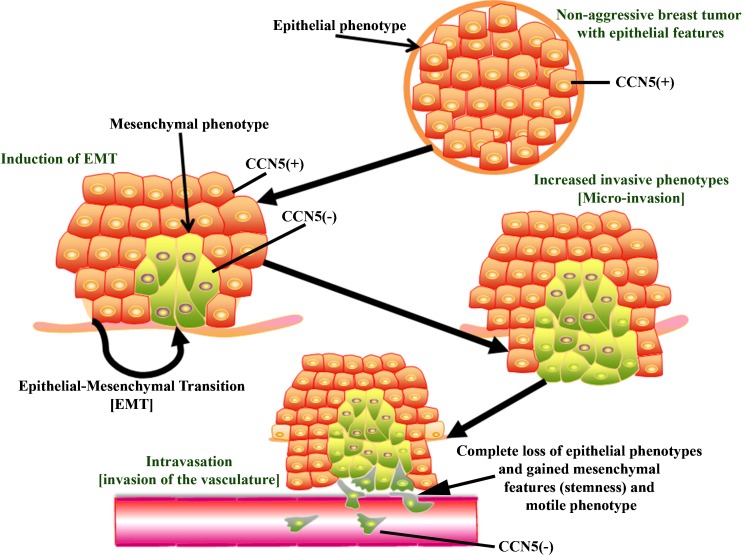
CCN5 has been implicated as having an important role in carcinogenesis with particular relevance to human breast disease (Saxena et al. 2001; Banerjee et al. 2003; Banerjee et al. 2005). Our studies have shown for the first time that CCN5 is differentially expressed in breast tumor cell lines and human breast samples (Saxena et al. 2001). Its expression is mainly detected in non-invasive breast cancer cell lines and noninvasive lesions (i.e., ADH and DCIS) compared with adjacent invasive lesions where expression levels are gradually decreased to an undetected level as the disease progresses from well-differentiated to poorly differentiated stages (Fig. 2) (Banerjee et al. 2003; Banerjee et al. 2008). Collectively, these studies suggest a preventive role of CCN5 in the progression of human breast cancer. Our in vitro results were further supported by other studies (Davies et al. 2007; Fritah et al. 2008; Sabbah et al. 2011). However, the results of the immunohistochemical analysis in human tissue samples of Davies et al. (Davies et al. 2007) contradicted its own in vitro data as well as our in vivo results. It does not corroborate our claim as it proposed opposite reports (Davies et al. 2007). This is because their studies were not designed to evaluate the CCN5 expression profile in different non-invasive and invasive breast tumor samples. Accordingly, our studies are not in agreement with the work of Davies et al. 2007 (Davies et al. 2007).
Fig. 2.
Pathobiological implication of CCN5/WISP-2. CCN5/WISP-2 is differentially expressed in different cellular environments of normal breast and breast cancer samples. CCN5/WISP-2 expression in undetected or minimally detected in normal breast epithelial and its expression is predominantly in the nucleus. CCN5/WISP-2 over expressed in non-invasive breast cancer cells and its expression gradually disappears as the disease progressed from non-invasive to invasive cancers with or without ER-α positivity. Highly aggressive cells show no expression of CCN5/WISP-2
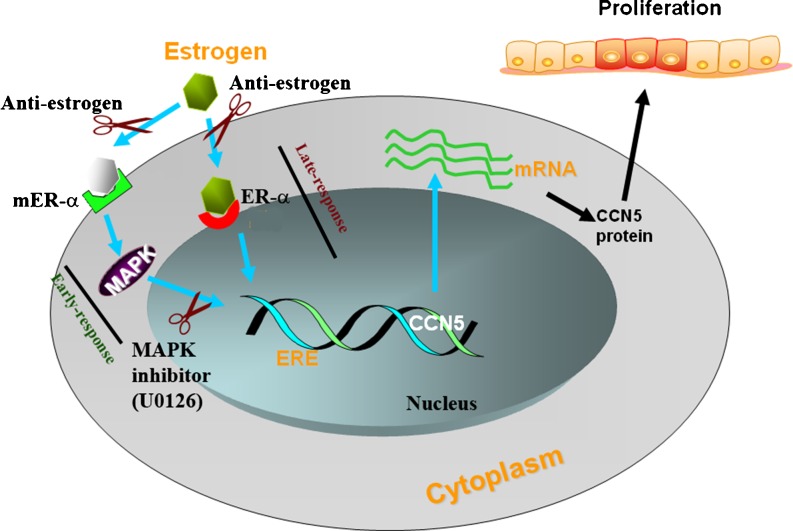
CCN5 expression can be induced by various stimulants (i.e., estrogen, progesterone and growth factors such as IGF-1, EGF, PMA and serum) and it seems to be essential to one element of the multi-component, hormones and growth factor-induced mitogenic response in ER-positive, non-invasive breast tumor cells (Inadera et al. 2000; Zoubine et al. 2001; Inadera et al. 2002; Banerjee et al. 2003; Banerjee et al. 2005; Ray et al. 2005; Fritah et al. 2006; Sengupta et al. 2006; Dhar et al. 2007b) (Figs. 3 and 4). We found that CCN5/WISP-2 is a 17β-estradiol (E2; natural estrogen) -induced early as well as late response gene in ER-α positive MCF-7 cells, and the expression was continuously increased to reach a maximum level at 24 h. The estrogen effect can be blocked by a pure antiestrogen (ICI 182,780) (Banerjee et al. 2003). Non-transformed human mammary epithelial cells (HMEC) in which CCN5/WISP-2 expression was undetected, responded to 17β-estradiol by up regulating the CCN5 gene after stable transfection of human estrogen receptor-α (hER-α). This response provides further supporting evidence that active ER-α is critical for the upregulation of CCN5 in HMEC. Overexpression of CCN5 mRNA by estrogen may be accomplished by both transcriptional activation and stabilization (Banerjee et al. 2003).
Fig. 3.
Regulation of CCN5/WISP-2 by estrogen. Estrogen up regulates CCN5/WISP-2 expression through both genomic and none genomic signaling pathways. Constitutively expressed CCN5 in breast cancer epithelial cells shows no effect on proliferation. In contrast, estrogen-induced upregulation of CCN5/WISP-2 is associated with the estrogen-induced proliferation of ER-positive breast cancer cells. mER-α: membrane estrogen receptor-α; ER-α: nuclear ER-α; ERE: estrogen-response element
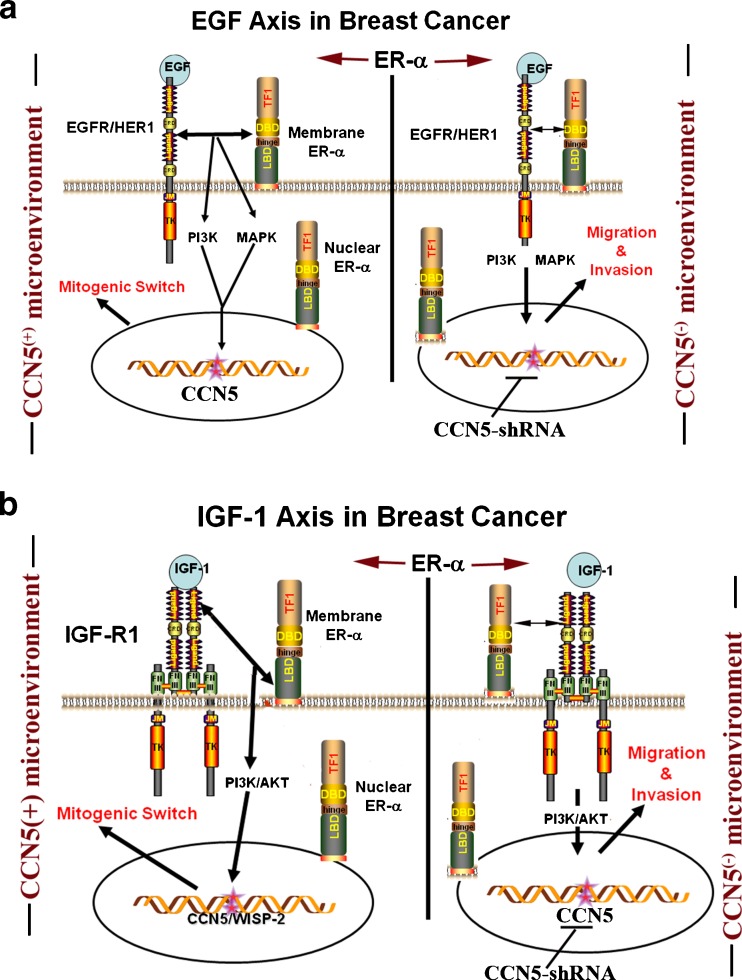
Fig. 4.
CCN5/WISP-2 plays critical roles in EGF and IGF-1-induced cell proliferation and migration and invasion. EGF and IGF-1 growth factors regulate breast cancer cell proliferation or migration and invasion under two different micro-environments. In ER-α − CCN5 positive microenvironment, EGF and IGF-1 induce cell proliferation, while in CCN5 negative microenvironment they help breast cancer cells migrate to and invade the distant places
Estrogen has been considered a potent stimulator of proliferation, differentiation, maturation, angiogenesis and other crucial functions of target tissues and organs (Sengupta et al. 2004; Teede 2007; Watson et al. 2010). These effects of estrogen are usually elicited through classical intracellular estrogen receptors (ERs; nuclear receptor). Despite this fact, multiple studies found that estrogen can rapidly influence cellular physiology in many cell types via the activation of a diverse array of intracellular non-nuclear/non-genomic signaling pathways (Wehling 1997; Watson et al. 1999; Kousteni et al. 2001; Manolagas and Kousteni 2001; Levin 2002; Pedram et al. 2002; Sengupta et al. 2004). For example, rapid induction of intracellular Ca2 + (Bulayeva et al. 2005) and G-protein signaling (Wyckoff et al. 2001), activation of extracellular regulated kinases (ERK1/2) (Ballare et al. 2003; Watson et al. 2005), PI3K, and MAPK-ERK (Simoncini et al. 2000; Pedram et al. 2002; Levin 2003), or inhibition of TSP-1 expression (Sengupta et al. 2004) can be achieved by estrogen treatment. Although, mechanistic details of these non-genomic actions are poorly understood, some of the rapid effect can be mediated through the binding of E2 with membrane-ERs (mER) (Manolagas and Kousteni 2001; Levin 2002; Sengupta et al. 2004). Our studies suggest that the rapid transcriptional activation CCN5/WISP-2 by estrogen in breast cancer cells could be mediated through non-genomic pathways by activating mER-α-MAPK signaling, while delayed transcriptional activation is mediated through a direct transcriptional activation effect (genomic effect) via a classical intracellular estrogen receptors (Banerjee et al. 2003) that interact with a specific response element (ERE) on promoter or coregulators of the general transcription machinery (Beato et al. 1989). These concepts are developed from our studies which indicated that rapid action of estrogen on CCN5/WISP-2 expression (Banerjee et al. 2003) can be blocked by MAPK inhibitor (U0126) (Dhar K et.al. unpublished data) (Fig. 3). Interestingly, the non-genomic pathway is also involved in IGF and EGF-induced upregulation of CCN5/WISP-2 in ER positive breast cancer cells (Banerjee et al. 2005; Dhar et al. 2007b) (Fig. 4).
Although mitogen-induced up-regulation of CCN5/WISP-2 participates in cell proliferation events of ER positive breast cancer cells, the basal level of CCN5 exhibits no mitogenic response in these cells (Banerjee et al. 2005; Sengupta et al. 2006; Dhar et al. 2007b), but rather, it protect cells from gaining invasive phenotypes. For example, silencing of CCN5 in MCF-7 non-invasive carcinoma cells by CCN5-specific shRNA significantly enhances motility and EMT, and it modulates the expression of some genes associated with invasive phenotypes of cancer cells (Banerjee et al. 2008; Dhar et al. 2008; Fritah et al. 2008). In CCN5 knockout ER-α positive breast cancer cells, IGF-1 and EGF lost their mitogenic effect (Banerjee et al. 2005; Dhar et al. 2007b), but possibly gained aggressive phenotypes (i.e., migration and invasion) (Banerjee et al., Manuscript in preparation) (Fig. 4). In contrast, induced expression of CCN5 or supplementation of CCN5 protein in CCN5 (-) invasive cancer cells reduces the rate of proliferation, migration and invasion (Fritah et al. 2008; Banerjee et al. 2008). Collectively, the contrasting pathobiological roles (i.e., their participation in steroids and growth factors-induced proliferation and their protection of cells from EMT, migration and invasion) of CCN5 under different microenvironments suggest that CCN5 may be a two-faced cancer gene, and the major role of CCN5, at least under culture conditions, is to protect the cells from gaining invasive phenotypes. However, to confirm this hypothesis thorough investigations are warranted.
Although CCN5/WISP-2 has been recognized as an estrogen-response gene by multiple studies, regulation of ER-α by CCN5/WISP-2 has also been appreciated by recent in vitro study (Fritah et al. 2008) and a CCN5-conditional tri-transgenic mouse model (Majumder et al. 2011). The in vitro studies showed that the knocking down of CCN5 expression in breast cancer cells markedly reduced the expression of ER-α expression. The CCN5-conditional tri-transgenic mouse model demonstrated that the activation of CCN5 in breast ductal epithelial cells by administration of doxycycline (a derivative of tetracycline) significantly induces ER-α expression and activity along with no morphological changes. The functional relevance of CCN5 mediated activation of ER-α in ductal epithelial cells in the breast is uncertain and therefore, further investigations are needed.
CCN5 is a negative regulator of micro-RNA 10b
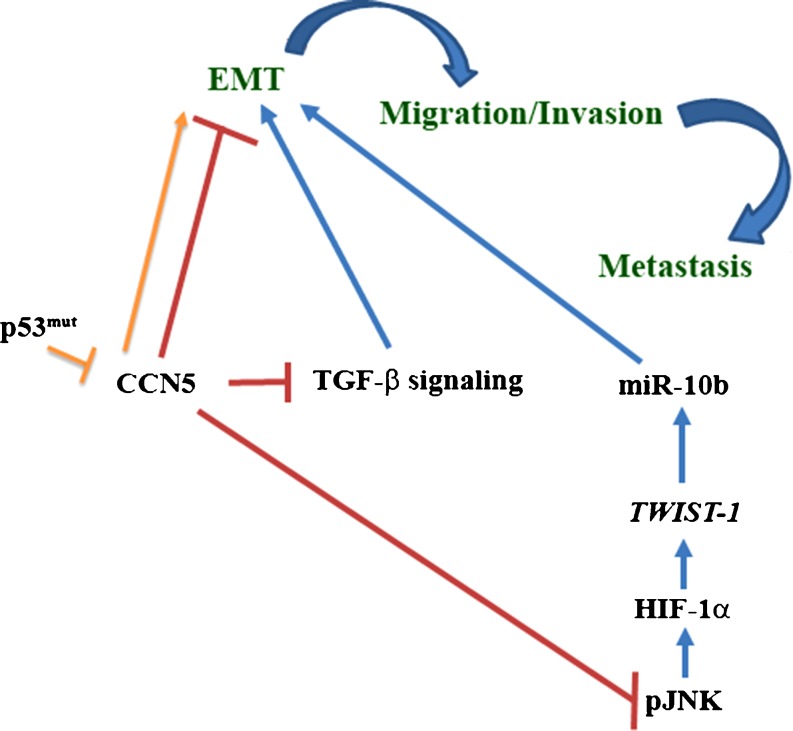
Advances in high throughput transcriptome studies have produced an explosion of new information about the mammalian genome and indicate many different complex families of RNAs are transcribed from the mammalian genome. Many small non-coding RNAs (micro-RNAs), counterpart of transcriptome, are among the greatest surprises. They have been identified with both biological functions (Lu et al. 2005), as well as pathobiological functions including the regulation of tumorigenesis in various organs through modulation of oncogenic or tumor suppressor pathways (Esquela-Kerscher and Slack 2006; Calin and Croce 2006a, b; Blenkiron et al. 2007; Ma and Weinberg 2008; Ventura and Jacks 2009; Xiang and Wu 2010; Yu et al. 2010; Farazi et al. 2011; Song et al. 2011). In 2007, Weingberg’s laboratory identified micro RNA (miRNA) miR-10b, which plays a critical role in micro-invasion and metastasis of breast cancer cells (Ma et al. 2007), and therefore they anticipated a therapeutic importance of miR-10b in inhibition of metastatic growth of breast cancer (Ma et al. 2010). They also found that miR-10b is induced by a transcription factor, TWIST, a pro-metastatic gene (yang et al. 2004), through direct binding to the putative promoter of miR-10b. TWIST-induced miR-10b inhibits translation of homeobox D10 (HOXD10), resulting in the increased expression of the pro-metastatic gene RHOC (Ma et al. 2007; Steeg 2007). Recently, we found that under CCN5/WISP-2 deficient (knockout) microenvironment the stability and activity of HIF-1α protein markedly increased in breast cancer cells, which ultimately leads to the increase in TWIST-1 mediated miR-10b expression parallel with migration and invasion of these cells (Haque et al. 2011). This effect can be repealed by restoring CCN5/WISP-2 in deficient cells through genetic manipulations (i.e., enforced ectopic expression of CCN5/WISP-2) (Haque et al. 2011). HIF-1α stabilization in CCN5-deficient cells is enhanced by c-Jun N-terminal kinase (JNK) (Haque et al. 2011). However, the activation is presumably mediated through the phosphorylation of this protein by the p42/p44 MAPK signaling pathway as MAPK is activated in CCN5/WISP-2 knockout cells (Haque et al. 2011) (Haque et.al. unpublished data) and is essential for promoting HIF-1α phosphorylation in quiescent cells (Richard et al. 1999). The present finding does not concur with the previous view (Gort et al. 2008) that TWIST-1 is a direct target of HIF-2α but not HIF-1α. The rational of this inconsistency is uncertain. However, one could anticipate that the regulation of TWIST-1 by HIFs could be specific to cell types and microenvironments, a possibility that requires further investigation. Significantly, these studies suggest, on the basis of our findings, that CCN5/WISP-2-induced inhibition of miR-10b expression in breast cancer cells is critical for the anti-invasive function of this gene (Dhar et al. 2008; Banerjee et al. 2008; Fritah et al. 2008), and is mediated through the inhibition of JNK-HIF-1α-TWIST1 signaling cascades (Fig. 5).
Fig. 5.
EMT is regulated by CCN5/WISP-2 through the modulation of multiple signaling pathways in breast cancer cells. CCN5/WISP-2 is a negative regulator of EMT. It blocks miR-10b expression and TGF-β-signaling in breast cancer cells, which are prime regulators of EMT in breast cancer cells. The mutant p53 proteins, which are also EMT inducers, activate the EMT event in breast cancer cells through the down regulation of CCN5/WISP-2
Transforming growth factor β (TGF − β)-signaling is blocked by CCN5/WISP-2
The gain of invasive phenotypes of breast cancer cells is dependent on the gain of the plasticity of epithelial tumor cells, which help in migration and invasion of these cells. The gain of this plasticity is achieved through epithelial to mesenchymal transition (EMT) (Kalluri and Weinberg 2009). Multiple signaling pathways have been shown to promote EMT in various cancers (Kalluri 2009). Despite having both tumor growth promoting and inhibiting roles of TGF-β-signaling in breast carcinogenesis (Singh et al. 2010; Novitskiy et al. 2011), many studies found TGF-β-signaling can induce EMT under specific microenvironments (Sato et al. 2010; Takahashi et al. 2010). TGF-β-signaling-induced EMT is Smad 3/4 dependent, which eliminates the p53 tumor suppressor function by activating the E3 ubiquitin ligase (Araki et al. 2010) which degrades this protein. Recent studies of Sabbah et al. (2011) found that CCN5 is an upstream negative regulator of TGF-β-signaling (Sabbah et al. 2011), and they anticipate that an anti-invasive role of CCN5 may be mediated through TGF-β-signaling (Fig. 5). The studies showed that RNAi-based loss of CCN5/WISP-2 in non-invasive ER positive MCF-7 breast cancer cells increased key components of TGF-β signaling (i.e., TGF-β RII, and a target of the Smad signaling pathway PAI1) which ultimately induces EMT. This study claimed that CCN5 could possibly act as a transcriptional repressor, and this could be achieved through association with histone deacetylase 1.
Gain of carcinogenic function of p53 tumor suppressor gene suppresses CCN5/WISP-2 expression in breast cancer cells
Mutations (principally, but not exclusively, missense) in the p53 tumor suppressor gene are the most common genetic insult in breast cancer and these mutations are intimately associated with the aggressiveness of this disease and poor survival rates (Gasco et al. 2002). Our studies found that over expression of the p53 mutant protein is inversely correlated with CCN5 expression in pancreatic cancer samples (Dhar et al. 2007a) and breast cancer samples (Dhar et al. 2008). We also found that ectopic expression of p53 mutants suppresses CCN5 expression in breast cancer cell lines for the induction of invasive phenotypes, including the induction of morphologic changes from the epithelial-to-mesenchymal type along with the alterations of hallmark proteins of these cell types and an augmentation of the migration of these cells (Banerjee et al. 2008). Interestingly, the studies also found that mutant p53-mediated-silencing of CCN5 can be overruled by estrogen treatment, which transcriptionally activated CCN5 expression in these cells. Moreover, p53-mutant-induced invasive phenotypes can be mimicked by blocking the CCN5 expression through RNAi. Thus these studies postulated that p53 mutant proteins may exert their malignant behavior through the inactivation of CCN5 (Fig. 5). However, it is uncertain how the mutant p53 silences CCN5 expression in non-invasive breast cancer cells to potentiate invasive phenotypes.
Summary and future goal
An increasing number of publications report that in advanced cancer cells an inactivation of CCN5/WISP-2 promotes EMT and hence contributes to the progression of breast and pancreatic cancers. These studies would predict that reactivation of CCN5, either alone or in combination with current therapeutic regimens, could provide a unique, alternative strategy for treating breast and pancreatic cancer.
Acknowledgements
We thank members of CRU for reading the manuscript and their helpful discussions. This work is supported by VA Merit Award funds to SB and SKB
Contributor Information
Sushanta K. Banerjee, Email: sbanerjee2@kumc.edu, Email: publication@vamccancerresearchunit.org
Snigdha Banerjee, Phone: +1-816-8614700, FAX: +1-816-9223320, Email: sbanerjee@kumc.edu.
References
- Araki S, Eitel JA, Batuello CN, Bijangi-Vishehsaraei K, Xie XJ, Danielpour D, Pollok KE, Boothman DA, Mayo LD. TGF-beta1-induced expression of human Mdm2 correlates with late-stage metastatic breast cancer. J. Clin. Invest. 2010;120:290–302. doi: 10.1172/JCI39194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y, Oren M. Living with p53, dying of p53. Cell. 2007;130:597–600. doi: 10.1016/j.cell.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Ballare C, Uhrig M, Bechtold T, Sancho E, Domenico M, Migliaccio A, Auricchio F, Beato M. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol. Cell Biol. 2003;23:1994–2008. doi: 10.1128/MCB.23.6.1994-2008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Saxena N, Sengupta K, Tawfik O, Mayo MS, Banerjee SK. WISP-2 Gene in Human Breast Cancer: estrogen and progesterone inducible expression and regulation of tumor cell proliferation. Neoplasia. 2003;5:63–73. doi: 10.1016/s1476-5586(03)80018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Sengupta K, Saxena NK, Dhar K, Banerjee SK. Epidermal growth factor induces WISP-2/CCN5 expression in estrogen receptor-{alpha}-positive breast tumor cells through multiple molecular cross-talks. Mol. Cancer Res. 2005;3:151–162. doi: 10.1158/1541-7786.MCR-04-0130. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Dhar G, Haque I, Kambhampati S, Mehta S, Sengupta K, Tawfik O, Phillips TA, Banerjee SK. CCN5/WISP-2 expression in breast adenocarcinoma is associated with less frequent progression of the disease and suppresses the invasive phenotypes of tumor cells. Cancer Res. 2008;68:7606–7612. doi: 10.1158/0008-5472.CAN-08-1461. [DOI] [PubMed] [Google Scholar]
- Beato M, Chalepakis G, Schauer M, Slater EP. DNA regulatory elements for steroid hormones. J. Steroid Biochem. 1989;32:737–747. doi: 10.1016/0022-4731(89)90521-9. [DOI] [PubMed] [Google Scholar]
- Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE, Green AR, Ellis IO, Tavare S, Caldas C, Miska EA. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossi G, Lapi E, Strano S, Rinaldo C, Blandino G, Sacchi A. Mutant p53 gain of function: reduction of tumor malignancy of human cancer cell lines through abrogation of mutant p53 expression. Oncogene. 2006;25:304–309. doi: 10.1038/sj.onc.1209026. [DOI] [PubMed] [Google Scholar]
- Brigstock DR. The CCN family: a new stimulus package. J. Endocrinol. 2003;178:169–175. doi: 10.1677/joe.0.1780169. [DOI] [PubMed] [Google Scholar]
- Bulayeva NN, Wozniak AL, Lash LL, Watson CS. Mechanisms of membrane estrogen receptor-alpha-mediated rapid stimulation of Ca2+ levels and prolactin release in a pituitary cell line. Am. J. Physiol Endocrinol. Metab. 2005;288:E388–E397. doi: 10.1152/ajpendo.00349.2004. [DOI] [PubMed] [Google Scholar]
- Cadwell C, Zambetti GP. The effects of wild-type p53 tumor suppressor activity and mutant p53 gain-of-function on cell growth. Gene. 2001;277:15–30. doi: 10.1016/S0378-1119(01)00696-5. [DOI] [PubMed] [Google Scholar]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- Calin GA, Croce CM. MicroRNAs and chromosomal abnormalities in cancer cells. Oncogene. 2006;25:6202–6210. doi: 10.1038/sj.onc.1209910. [DOI] [PubMed] [Google Scholar]
- Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- Davies SR, Watkins G, Mansel RE, Jiang WG. Differential expression and prognostic implications of the CCN family members WISP-1, WISP-2, and WISP-3 in human breast cancer. Ann. Surg. Oncol. 2007;14:1909–1918. doi: 10.1245/s10434-007-9376-x. [DOI] [PubMed] [Google Scholar]
- Dhar G, Banerjee S, Dhar K, Tawfik O, Mayo MS, Vanveldhuizen PJ, Banerjee SK. Gain of oncogenic function of p53 mutants induces invasive phenotypes in human breast cancer cells by silencing CCN5/WISP-2. Cancer Res. 2008;68:4580–4587. doi: 10.1158/0008-5472.CAN-08-0316. [DOI] [PubMed] [Google Scholar]
- Dhar G, Mehta S, Banerjee S, Gardner A, McCarty BM, Mathur SC, Campbell DR, Kambhampati S, Banerjee SK. Loss of WISP-2/CCN5 signaling in human pancreatic cancer: a potential mechanism for epithelial-mesenchymal-transition. Cancer Lett. 2007;254:63–70. doi: 10.1016/j.canlet.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Dhar K, Banerjee S, Dhar G, Sengupta K, Banerjee SK. Insulin-like growth factor-1 (IGF-1) induces WISP-2/CCN5 via multiple molecular cross-talks and is essential for mitogenic switch by IGF-1 axis in estrogen receptor-positive breast tumor cells. Cancer Res. 2007;67:1520–1526. doi: 10.1158/0008-5472.CAN-06-3753. [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J. Pathol. 2011;223:102–115. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend S. p53: a glimpse at the puppet behind the shadow play. Science. 1994;265:334–335. doi: 10.1126/science.8023155. [DOI] [PubMed] [Google Scholar]
- Fritah A, Redeuilh G, Sabbah M. Molecular cloning and characterization of the human WISP-2/CCN5 gene promoter reveal its upregulation by oestrogens. J. Endocrinol. 2006;191:613–624. doi: 10.1677/joe.1.07009. [DOI] [PubMed] [Google Scholar]
- Fritah A, Saucier C, De WO, Bracke M, Bieche I, Lidereau R, Gespach C, Drouot S, Redeuilh G, Sabbah M. Role of WISP-2/CCN5 in the maintenance of a differentiated and noninvasive phenotype in human breast cancer cells. Mol. Cell Biol. 2008;28:1114–1123. doi: 10.1128/MCB.01335-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasco M, Shami S, Crook T. The p53 pathway in breast cancer. Breast Cancer Res. 2002;4:70–76. doi: 10.1186/bcr426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gort EH, Van HG, Verlaan I, Groot AJ, Plasterk RH, Shvarts A, Suijkerbuijk KP, Van LT, Wall E, Raman V, Diest PJ, Tijsterman M, Vooijs M. The TWIST1 oncogene is a direct target of hypoxia-inducible factor-2alpha. Oncogene. 2008;27:1501–1510. doi: 10.1038/sj.onc.1210795. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Haque I, Banerjee S, Mehta S, De A, Majumder M, Mayo MS, Kambhampati S, Campbell DR, Banerjee SK. Cysteine-rich 61-connective tissue growth factor-nephroblastoma-overexpressed 5 (CCN5)/Wnt-1-induced signaling protein-2 (WISP-2) regulates MicroRNA-10b via hypoxia-inducible factor-1alpha-TWIST signaling networks in human breast cancer cells. J. Biol. Chem. 2011;286:43475–43485. doi: 10.1074/jbc.M111.284158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem. Sci. 2008;33:461–473. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Inadera H, Dong HY, Matsushima K. WISP-2 is a secreted protein and can be a marker of estrogen exposure in MCF-7 cells. Biochem. Biophys. Res. Commun. 2002;294:602–608. doi: 10.1016/S0006-291X(02)00530-2. [DOI] [PubMed] [Google Scholar]
- Inadera H, Hashimoto S, Dong HY, Suzuki T, Nagai S, Yamashita T, Toyoda N, Matsushima K. WISP-2 as a novel estrogen-responsive gene in human breast cancer cells. Biochem. Biophys. Res. Commun. 2000;275:108–114. doi: 10.1006/bbrc.2000.3276. [DOI] [PubMed] [Google Scholar]
- Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, Komnenovic V, Kong YY, Schreiber M, Dixon SJ, Sims SM, Khokha R, Wada T, Penninger JM. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat. Rev. Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J. Clin. Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Karagiannis ED, Popel AS (2007) Peptides derived from type I thrombospondin repeat-containing proteins of the CCN family inhibit proliferation and migration of endothelial cells. Int J Biochem Cell Biol [DOI] [PMC free article] [PubMed]
- Kastan MB. Wild-type p53: tumors can’t stand it. Cell. 2007;128:837–840. doi: 10.1016/j.cell.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Berkovich E. p53: a two-faced cancer gene. Nat. Cell Biol. 2007;9:489–491. doi: 10.1038/ncb0507-489. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- Lake AC, Castellot JJ., Jr CCN5 modulates the antiproliferative effect of heparin and regulates cell motility in vascular smooth muscle cells. Cell Commun. Signal. 2003;1:5. doi: 10.1186/1478-811X-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67:471–475. doi: 10.1016/S0039-128X(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol. Endocrinol. 2003;17:309–317. doi: 10.1210/me.2002-0368. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/S0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Liang X. EMT: new signals from the invasive front. Oral Oncol. 2011;47:686–687. doi: 10.1016/j.oraloncology.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Lichy JH, Dalbegue F, Zavar M, Washington C, Tsai MM, Sheng ZM, Taubenberger JK. Genetic heterogeneity in ductal carcinoma of the breast. Lab Invest. 2000;80:291–301. doi: 10.1038/labinvest.3780034. [DOI] [PubMed] [Google Scholar]
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- Ma L, Weinberg RA. MicroRNAs in malignant progression. Cell Cycle. 2008;7:570–572. doi: 10.4161/cc.7.5.5547. [DOI] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW, Weinberg RA. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat. Biotechnol. 2010;28:341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder M, Banerjee S, Mehta S, De A, Dhar K, Tawfik O, Larson MA, Banerjee SK (2011) Estrogen receptor-alpha is activated in Breast ductal epithelial cells by CCN5 in CCN5-conditional Tri Transgenic mice. 102[102nd Annual American Association of Cancer Research Meeting]. 2011. Philadelphia, PA 19106, AACR. 4-4-0011. Ref Type: Conference Proceeding
- Manolagas SC, Kousteni S. Perspective: nonreproductive sites of action of reproductive hormones. Endocrinology. 2001;142:2200–2204. doi: 10.1210/en.142.6.2200. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Novitskiy SV, Pickup MW, Gorska AE, Owens P, Chytil A, Aakre M, Wu H, Shyr Y, Moses HL. TGF-beta receptor II loss promotes mammary carcinoma progression by Th17-dependent mechanisms. Cancer Discovery. 2011;1:431–441. doi: 10.1158/2159-8290.CD-11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M, Bartek J. The sunny side of p53. Cell. 2007;128:826–828. doi: 10.1016/j.cell.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Aitkenhead M, Hughes CC, Levin ER. Integration of the non-genomic and genomic actions of estrogen. Membrane-initiated signaling by steroid to transcription and cell biology. J. Biol. Chem. 2002;277:50768–50775. doi: 10.1074/jbc.M210106200. [DOI] [PubMed] [Google Scholar]
- Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M, Watanabe C, Cohen RL, Melhem MF, Finley GG, Quirke P, Goddard AD, Hillan KJ, Gurney AL, Botstein D, Levine AJ. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14717–14722. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K. Heterogeneity in breast cancer. J. Clin. Invest. 2011;121:3786–3788. doi: 10.1172/JCI60534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC. Epithelial-mesenchymal transition. J. Cell Sci. 2005;118:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray G, Banerjee S, Saxena NK, Campbell DR, Van VP, Banerjee SK. Stimulation of MCF-7 tumor progression in athymic nude mice by 17beta-estradiol induces WISP-2/CCN5 expression in xenografts: a novel signaling molecule in hormonal carcinogenesis. Oncol. Rep. 2005;13:445–448. [PubMed] [Google Scholar]
- Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 1999;274:32631–32637. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- Rozan LM, El-Deiry WS. p53 downstream target genes and tumor suppression: a classical view in evolution. Cell Death. Differ. 2007;14:3–9. doi: 10.1038/sj.cdd.4402058. [DOI] [PubMed] [Google Scholar]
- Russo JW, Castellot JJ. CCN5: biology and pathophysiology. J. Cell Commun. Signal. 2010;4:119–130. doi: 10.1007/s12079-010-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah M, Prunier C, Ferrand N, Megalophonos V, Lambein K, De WO, Nazaret N, Lachuer J, Dumont S, Redeuilh G. CCN5, a novel transcriptional repressor of the transforming growth factor beta signaling pathway. Mol. Cell Biol. 2011;31:1459–1469. doi: 10.1128/MCB.01316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Harada K, Itatsu K, Ikeda H, Kakuda Y, Shimomura S, Shan RX, Yoneda N, Sasaki M, Nakanuma Y. Epithelial-mesenchymal transition induced by transforming growth factor-{beta}1/Snail activation aggravates invasive growth of cholangiocarcinoma. Am. J. Pathol. 2010;177:141–152. doi: 10.2353/ajpath.2010.090747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena N, Banerjee S, Sengupta K, Zoubine MN, Banerjee SK. Differential expression of WISP-1 and WISP-2 genes in normal and transformed human breast cell lines. Mol. Cell Biochem. 2001;228:99–104. doi: 10.1023/A:1013338912642. [DOI] [PubMed] [Google Scholar]
- Schutze N, Noth U, Schneidereit J, Hendrich C, Jakob F. Differential expression of CCN-family members in primary human bone marrow-derived mesenchymal stem cells during osteogenic, chondrogenic and adipogenic differentiation. Cell Commun. Signal. 2005;3:5. doi: 10.1186/1478-811X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta K, Banerjee S, Saxena NK, Banerjee SK. Thombospondin-1 disrupts estrogen-induced endothelial cell proliferation and migration and its expression is suppressed by estradiol. Mol. Cancer Res. 2004;2:150–158. [PubMed] [Google Scholar]
- Sengupta K, Banerjee S, Dhar K, Saxena N, Mehta S, Campbell DR, Banerjee SK. WISP-2/CCN5 is involved as a novel signaling intermediate in phorbol ester-protein kinase Cα-mediated breast tumor cell proliferation. Biochemistry. 2006;45:10698–10709. doi: 10.1021/bi060888p. [DOI] [PubMed] [Google Scholar]
- Shah AN, Gallick GE. Src, chemoresistance and epithelial to mesenchymal transition: are they related? Anticancer Drugs. 2007;18:371–375. doi: 10.1097/CAD.0b013e32801265d7. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G, Singh SK, Konig A, Reutlinger K, Nye MD, Adhikary T, Eilers M, Gress TM, Fernandez-Zapico ME, Ellenrieder V. Sequential activation of NFAT and c-Myc transcription factors mediates the TGF-beta switch from a suppressor to a promoter of cancer cell proliferation. J. Biol. Chem. 2010;285:27241–27250. doi: 10.1074/jbc.M110.100438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Hollstein M, Xu Y. p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nat. Cell Biol. 2007;9:573–580. doi: 10.1038/ncb1571. [DOI] [PubMed] [Google Scholar]
- Song YX, Yue ZY, Wang ZN, Xu YY, Luo Y, Xu HM, Zhang X, Jiang L, Xing CZ, Zhang Y. MicroRNA-148b is frequently down-regulated in gastric cancer and acts as a tumor suppressor by inhibiting cell proliferation. Mol. Cancer. 2011;10:1. doi: 10.1186/1476-4598-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg PS. Cancer: micromanagement of metastasis. Nature. 2007;449:671–673. doi: 10.1038/449671a. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Nagano O, Ishimoto T, Yae T, Suzuki Y, Shinoda T, Nakamura S, Niwa S, Ikeda S, Koga H, Tanihara H, Saya H. Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J. Biol. Chem. 2010;285:4060–4073. doi: 10.1074/jbc.M109.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. [DOI] [PubMed] [Google Scholar]
- Teede HJ. Sex hormones and the cardiovascular system: effects on arterial function in women. Clin. Exp. Pharmacol. Physiol. 2007;34:672–676. doi: 10.1111/j.1440-1681.2007.04658.x. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Cell adhesion in cancer. Comptes Rendus Physique. 2003;4:289–304. doi: 10.1016/S1631-0705(03)00031-8. [DOI] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Trimboli AJ, Fukino K, De BA, Wei G, Shen L, Tanner SM, Creasap N, Rosol TJ, Robinson ML, Eng C, Ostrowski MC, Leone G. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- Ventura A, Jacks T. MicroRNAs and cancer: short RNAs go a long way. Cell. 2009;136:586–591. doi: 10.1016/j.cell.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Watson CS, Norfleet AM, Pappas TC, Gametchu B. Rapid actions of estrogens in GH3/B6 pituitary tumor cells via a plasma membrane version of estrogen receptor-alpha. Steroids. 1999;64:5–13. doi: 10.1016/S0039-128X(98)00107-X. [DOI] [PubMed] [Google Scholar]
- Watson CS, Bulayeva NN, Wozniak AL, Finnerty CC. Signaling from the membrane via membrane estrogen receptor-alpha: estrogens, xenoestrogens, and phytoestrogens. Steroids. 2005;70:364–371. doi: 10.1016/j.steroids.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Watson CS, Jeng YJ, Kochukov MY. Nongenomic signaling pathways of estrogen toxicity. Toxicol. Sci. 2010;115:1–11. doi: 10.1093/toxsci/kfp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling M. Specific, nongenomic actions of steroid hormones. Annu. Rev. Physiol. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- Weisz L, Damalas A, Liontos M, Karakaidos P, Fontemaggi G, Maor-Aloni R, Kalis M, Levrero M, Strano S, Gorgoulis VG, Rotter V, Blandino G, Oren M. Mutant p53 enhances nuclear factor kappaB activation by tumor necrosis factor alpha in cancer cells. Cancer Res. 2007;67:2396–2401. doi: 10.1158/0008-5472.CAN-06-2425. [DOI] [PubMed] [Google Scholar]
- Wiesman KC, Wei L, Baughman C, Russo J, Gray MR, Castellot JJ. CCN5, a secreted protein, localizes to the nucleus. J. Cell Commun. Signal. 2010;4:91–98. doi: 10.1007/s12079-010-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis A, Jung EJ, Wakefield T, Chen X. Mutant p53 exerts a dominant negative effect by preventing wild-type p53 from binding to the promoter of its target genes. Oncogene. 2004;23:2330–2338. doi: 10.1038/sj.onc.1207396. [DOI] [PubMed] [Google Scholar]
- Wyckoff MH, Chambliss KL, Mineo C, Yuhanna IS, Mendelsohn ME, Mumby SM, Shaul PW. Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Galpha(i) J. Biol. Chem. 2001;276:27071–27076. doi: 10.1074/jbc.M100312200. [DOI] [PubMed] [Google Scholar]
- Xiang J, Wu J. Feud or friend? The role of the miR-17-92 cluster in tumorigenesis. Curr. Genomics. 2010;11:129–135. doi: 10.2174/138920210790886853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yu Z, Baserga R, Chen L, Wang C, Lisanti MP, Pestell RG. microRNA, cell cycle, and human breast cancer. Am. J. Pathol. 2010;176:1058–1064. doi: 10.2353/ajpath.2010.090664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoubine MN, Banerjee S, Saxena NK, Campbell DR, Banerjee SK. WISP-2: a serum-inducible gene differentially expressed in human normal breast epithelial cells and in MCF-7 breast tumor cells. Biochem. Biophys. Res Commun. 2001;282:421–425. doi: 10.1006/bbrc.2001.4584. [DOI] [PubMed] [Google Scholar]