Abstract
Antioxidant enzymes, such as glutathione peroxidase (GPx), catalase (CAT), and peroxiredoxin (Prx), are essential components in cells to eliminate excessive reactive oxygen species such as hydrogen peroxide (H2O2). GPx, CAT, and Prx genes have been reported in penaeid shrimp, and they showed different expression profiles at transcription or protein level when shrimps were challenged by microbes. In order to learn the relationship among the above three genes in their function, GPx, CAT, and Prx transcripts were analyzed, and the variation of GPx and CAT enzyme activity was detected when shrimp was injected with H2O2 or one antioxidant enzyme gene was silenced in shrimp by double-strand RNA injection. The results indicated that there existed some relationships among three antioxidant enzyme genes, CAT, GPx, and Prx in shrimp at transcriptional level. The transcription of CAT and GPx could be directly induced by H2O2 injection, while the transcription of Prx cannot be induced by H2O2. Decreased transcription level of CAT or GPx could lead to increased transcription of the other two genes, which suggested that there existed some compensation among these three antioxidant enzyme genes. These data can help us to understand the roles of antioxidant enzymes in crustacean.
Keywords: Antioxidant enzymes, RNA interference (RNAi), Transcription expression, Enzyme activity, Penaeid shrimp
Introduction
Invertebrates do not possess an adaptive immune system, which is based on highly specific antibodies and antigen receptors. They must rely on efficient innate immune defense to protect themselves against invaders (Iwanaga and Lee 2005). Great economical requirement of penaeid shrimp gives rise to researches on their innate immunity, but the aquaculture of shrimp is still limited by the occurrence of infectious diseases.
Phagocytosis is an important immune defense reaction when the organism is attacked by bacteria or viruses (Lee and Soderhall 2002; Smith et al. 2003). During phagocytosis in mammals, the host’s NADPH oxidase gets activated, which in turn enhances the glycolytic reactions. They increase the consumption of oxygen and induce the production of a mass of reactive oxygen species (ROS) such as superoxide anion (O·−2), hydrogen peroxide (H2O2), and hydroxyl radical (OH·) (Bogdan et al. 2000; Roch 1999). Although ROS play an important role in host defense against microbial infection, their over expression and the residual ROS can cause cellular damage (Yu 1994). Most cells have acquired the relevant protective mechanisms to maintain the lowest possible level of ROS inside the cell. The protective mechanisms include both non-enzyme (ascorbic acid, β-carotene, glutathione, and α-tocopherol) and enzyme antioxidant systems, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and peroxiredoxin (Prx) (Aruoma 1998; Holmblad and Soderhall 1999; Schwarz 1996). SOD can scavenge the superoxide anions and detoxify them by converting to hydrogen peroxide and oxygen. Hydrogen peroxide is then transformed to water and oxygen by CAT, GPx, or Prx (Nordberg and Arnér 2001).
CAT is an important antioxidant enzyme and exists virtually in all oxygen-respiring organisms (Klotz et al. 1997). The central role of CAT is eliminating excessive hydroperoxide and maintaining cellular redox balance. An extracellular immune-regulated catalase mediates a key host defense system that is necessary during host–microbe interaction in the gastrointestinal tract, highlighting the importance of these enzymes in the innate immune system of invertebrates (Ha et al. 2005a,b; Ryu et al. 2006). GPx plays an important role in detoxifying lipids and hydroperoxides, which protects biomembranes and other cellular components from oxidative damage by catalyzing the reduction of a variety of hydroperoxides, using glutathione as the reducing substrate during phagocytosis and/or physiological metabolism (Arthur 2000; Liu et al. 2004; Mills 1957; Speier et al. 1985). Prx, also named thioredoxin peroxidase (TPx), as an important peroxidase, was the latterly identified antioxidant protein, which can eliminate hydroperoxide with thioredoxin as an immediate hydrogen donor (Chae et al. 1994; Lim et al. 1993). Prx can protect the organisms against various oxidative stresses and regulate the intracellular signal transduction (Kang et al. 2005; Rhee et al. 2005). Expression of Prx gene can be induced when the organisms faced with oxidative stress caused by virus infection, temperature, or H2O2 stimulation (Kim et al. 2005; Lee et al. 2005). Due to the important roles of antioxidant enzymes, researchers are paying more attention to the antioxidant systems of shrimp in recent years, and made some progress already.
In penaeid shrimp, the complementary DNA (cDNA) sequence of CAT gene was identified in white shrimp, Litopenaeus vannamei (Tavaressanchez 2004) and Chinese shrimp, Fenneropenaeus chinensis (Zhang et al. 2008). The transcription level of CAT gene in hemocytes and hepatopancreas was upregulated when shrimp was infected by white spot syndrome virus (WSSV), which suggested that CAT might be involved in the shrimp immune response (Zhang et al. 2008). The full-length cDNA of GPx was cloned and characterized in L. vannamei (Liu et al. 2007), Penaeus monodon (Liu et al. 2010), and F. chinensis (Ren et al. 2009), and its transcript level and protein activity were significantly upregulated when shrimp were subjected to pathogen invasion, which suggested that GPx might play important role in shrimp immunity. Similarly, Prx genes have been isolated from several penaeid shrimp species including F. chinensis (Zhang et al. 2007) and Marsupeneaus japonicus (Bacano Maningas et al. 2008), and their expression at transcription level was changed when shrimp was challenged by bacteria or virus.
Although three antioxidant enzymes (CAT, GPx, and Prx), which might function in transforming H2O2 to harmless water and oxygen, were found in penaeid shrimp, no any study was reported on the relationship between these three genes. Shrimp has an open circulating system in which hemocytes distribute in most of the tissues. Besides hemocytes, hepatopancreas, and lymphoid organ, gills are also involved the immune function of shrimp. Since most of the organs are located in the cephalothorax of shrimp; therefore, the cephalothoraxes were usually used to represent the whole shrimp. In the present study, the relative expression of Prx, CAT, and GPx in the cephalothorax of Chinsese shrimp F. chinensis was studied at transcription and translation level after one of them was silenced by double-strand RNA (dsRNA) injection. The data can help us to understand the relationship of these genes in antioxidant reactions in shrimp.
Materials and methods
Shrimp source
Healthy juvenile shrimp with body weight of 6.80 ± 2.29 g used in this study were reared in our lab from the nauplius stage. These shrimp were siblings from the same parents to ensure that they had the same genetic background. These shrimp would be used for H2O2 injection and dsRNA injection experiments.
Preparation of total RNA and cDNA synthesis by reverse transcription
Total RNA was extracted from cephalothorax of juvenile shrimp mentioned above with Unizol Reagent (Biostar Genechip Inc., Shanghai, China) as described in the manufacturer’s protocol. RNA quality was assessed by electrophoresis on 1% agarose gel. The cDNA was synthesized from the total RNA using Moloney murine leukemia virus reverse transcriptase (Promega, USA) according to the manufacturer’s instruction.
Construction of recombinant plasmids for dsRNA synthesis
In order to synthesize double-strand RNA, three plasmids, including pGEM-T-FcGPx, pGEM-T-FcCAT, and pGEM-T-FcPrx, were constructed based on the pGEM-T easy vector (Takara, Japan). The cDNA sequences used in dsRNA synthesis of FcGPx, FcCAT, and FcPrx are shown in Fig. 1. For the construction of plasmid pGEM-T-FcGPx, cDNA fragment of GPx with length of 646 bp was amplified from the cDNA synthesized above with forward primer GPx-F1 (5′-TCCTCTCCCCCCAATCTT-3′) and reverse primer GPx-R1 (5′-CGGTCTTCTTTGAATAACTT-3′). PCR program for amplification of GPx cDNA was 94°C for 4 min; followed by 35 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min; then extended at 72°C for 10 min. The cDNA fragment of GPx was ligated into the pGEM-T easy vector, and then the restriction enzymes, ApaI or SalI, were used to make the plasmid be linearized separately. For plasmid pGEM-T-FcCAT, cDNA fragment of CAT with length of 996 bp was amplified with forward primer CAT-OF1 (5′-ACACTTCGACCGTGAGCGCAT-3′) and reverse primer CAT-OR1 (5′-ACACACATGGGGCCATCCCT-3′). PCR program for amplification of FcCAT cDNA was 94°C for 4 min; followed by 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min; then extended at 72°C for 10 min. The cDNA fragment of CAT was ligated into the pGEM-T easy vector, and the restriction enzyme, NcoI or SalI, was used to make the plasmid be linearized separately. For plasmid pGEM-T-FcPrx, the cDNA fragment of Prx with length of 835 bp, was amplified with forward primer Prx-TF (5′- ATGAGCAACACTGTCCCA-3′) and reverse primer Prx-TR (5′- CTGATACTGTGAAATAACGC-3′). The PCR program for amplification of Prx cDNA was the same as that in FcCAT cDNA amplification. The amplified cDNA fragment of Prx was ligated into the pGEM-T easy vector, and the restriction enzymes NcoI or SalI were used to make plasmid be linearized separately.
Fig. 1.
The nucleotide sequences of cDNA fragment for GPx, CAT, and Prx double strand RNA synthesis. a The cDNA sequence for DsGPx synthesis. b The cDNA sequence for DsCAT synthesis. c The cDNA sequence for DsPrx synthesis
Preparation of dsRNA for FcGPx, FcCAT, and FcPrx
Procedures for the preparation of dsRNA, detection of silencing efficiency were the same as described previously (Li et al. 2009). Briefly, two single-stranded RNAs were transcribed in vitro from the linearized plasmid constructs (pGEM-T-FcGPx, pGEM-T-FcCAT, or pGEM-T-FcPrx) using T7 and SP6 RNA polymerases (Takara, Japan), and the DNA template was degraded by addition of DNase I (Promega, USA) at a ratio of 1 U/μg of template. The single-strand RNA transcripts obtained from T7 and SP6 RNA polymerase were then mixed and annealed by incubation at 95°C for 5 min, and then put at room temperature overnight to let them form dsRNA of FcGPx, FcCAT, and FcPrx (DsGPx, DsCAT, and DsPrx). The formation of DsGPx, DsCAT, or DsPrx was monitored by determining the size shift in agarose gel electrophoresis, and the concentration of dsRNA was measured spectrophotometrically after purification by phenol chloroform. The silencing efficiency was detected as described previously (Li et al. 2009).
H2O2 injection into shrimp
Different concentrations, including 0.1% and 0.01% of H2O2 diluted by phosphate-buffered saline (PBS), were used for juvenile shrimp injection. In the control group, each shrimp was injected with 20 μL PBS. In the experimental group, each shrimp was injected with 20 μL 0.01% or 0.1% H2O2. Cephalothoraxs of four shrimps in each group were dissected out and preserved in liquid nitrogen at 0 and 2 h post-injection (hpi). The sampled cephalothoraxs of shrimp were used for further activity assay and RNA isolation to analyze the transcriptional expression profiles.
DsGPx, DsCAT, or DsPrx injection into shrimp
Twenty micrograms of DsGPx, 30 μg DsCAT or DsPrx in volume of 20 μL PBS were injected into each juvenile shrimp separately in the RNA interference (RNAi) experiments (three experimental groups). At the same time, shrimp injected with 20 μL PBS were used as a control group. Before injection, cephalothoraxes of four shrimp were collected to be preserved in liquid nitrogen. Then cephalothoraxs of four shrimps in each RNAi group and control group were dissected out at 48 hpi and preserved in liquid nitrogen. These samples will be used for RNA isolation or enzyme activity analysis for FcGPx, FcCAT, or FcPrx.
H2O2 injection into shrimp after FcGPx was silenced for 48 h
Each shrimp injected with 20 μg DsGPx in the volume of 20 μL PBS were taken as RNAi groups and the shrimp injected with the same volume of PBS as the control group. Four shrimp in each group were taken out at 0 h and 48 hpi, and their cephalothoraxes were preserved in liquid nitrogen. After 48 h of post-DsGPx or PBS injection, the shrimp from each group were injected with 0.1%H2O2 or PBS, respectively. Then, their cephalothoraxes in each group were dissected out at 2 hpi and preserved in liquid nitrogen, respectively. These samples will be used for RNA isolation to analyze the transcriptional expression profiles or activity measurement.
Quantification of gene transcript by real-time PCR
Based on the cDNA sequences of GPx (Ren et al. 2009), CAT (EU102287.1), and Prx (DQ205423) in GenBank, primers used for real-time PCR analysis were designed. The primer sequences and the expected size of the amplified fragments with the annealing temperature are shown in Table 1. The synthesized cDNAs from RNA isolated from samples preserved above were used as template for real-time PCR analysis. 18S rRNA was quantified as a stably expressed reference gene. The amplification conditions were as follows: 1 cycle of 94°C for 2 min; 40 cycles of 94°C for 15 s, annealing for 20 s, and 72°C for 20 s. In order to confirm whether only one specific PCR product was amplified, a melt cycle, in which PCR product was denatured from 68°C to 100°C, was added to each thermal profile to produce the melting curves. Expression of other target genes was normalized to 18S rRNA, which served as an internal control for calibrating the quantity of RNA isolated from each sample. Experiments were performed in quadruplicate, and at least three shrimps were analyzed for each sample. The relative expression of each gene was calculated using the comparative Ct method with the formula 2−ΔΔCt (where ΔCt = ΔCtsample − ΔCtreference). Data obtained from real-time PCR analysis were subjected to one-way analysis of variance using SPSS v 16.0, and P < 0.05 were considered statistically significant.
Table 1.
Primer sequence and corresponding annealing temperature of genes
| Gene | Primer name | Direction | Primer sequence(5′–3′) | Expected size (bp) | annealing temperature (°C) |
|---|---|---|---|---|---|
| GPx | GPx-RTF1 | Forward | TGCAACCAGTTCGGGCACCA | 173 | 69 |
| GPx-RTR1 | Reverse | AGTGGCAGTCGCTCCTTCAGGT | |||
| CAT | CAT-RT-F | Forward | ACTCCCATTGCTGTTCGT | 114 | 58.5 |
| CAT-RT-R | Reverse | ATCCCAATTTCCTTCTTCTG | |||
| Prx | Prx-RT-F | Forward | GCTTGCTCTACAGACTCCC | 295 | 58.5 |
| Prx-RT-R | Reverse | ATACTTCACCGTGCTCATC | |||
| 18S rRNA | 18S2F | Forward | TATACGCTAGTGGAGCTGGAA | 147 | 55 |
| 18S2R | Reverse | GGGGAGGTAGTGACGAAAAAT |
The activity assay of GPx and CAT
GPx and CAT activity assays were performed respectively using the GPx and CAT activity assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instruction provided by the producer. U was expressed as the activity unit of the enzyme. One unit of GPx activity means the amount of enzyme in 1 mg tissue proteins that catalyze the reaction to make the concentration of reduced glutathione to decrease for 1 μmol/L in a minute without regard to non-enzymatic effects. One unit of CAT activity means the amount of enzyme in 1 mg tissue proteins that can reduce 1 μmol H2O2 in a second.
Results
Expression of FcGPx, FcCAT, and FcPrx in shrimp after H2O2 injection
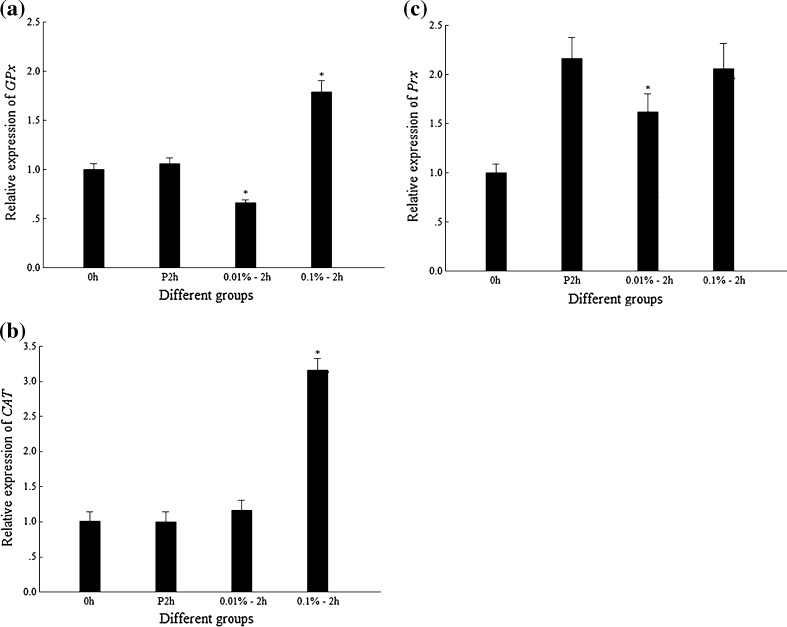
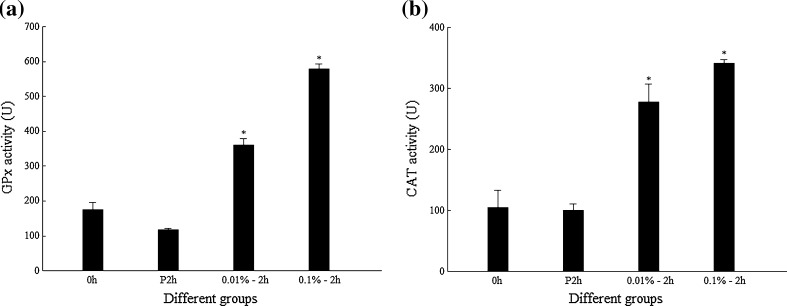
The transcription level of the genes encoding different enzymes in juvenile shrimp after injection of different concentration of H2O2 is shown in Fig. 2. The data showed that injection of 0.1% H2O2 could significantly induce the upregulation of FcGPx and FcCAT on the transcription level at 2 hpi compared to those with 0.01% H2O2 or PBS injection (Fig. 2a, b), while the transcription level of FcPrx showed no apparent upregulation when shrimp was injected with 0.1% H2O2 (Fig. 2c). The activity assay of FcGPx and FcCAT showed that the activities of both FcGPx and FcCAT were upregulated when shrimp were injected by 0.01% or 0.1% H2O2 compared with their PBS controls (Fig. 3).
Fig. 2.
Transcriptional expression profiles of GPx, CAT, and Prx in shrimp after H2O2 injections. 0h blank group without any injection, P2h 2 hpi of PBS, 0.01%–2 h 2 hpi of 0.01% H2O2; 0.1%–2 h 2 hpi of 0.1% H2O2. Asterisk means that the difference between 0.01%–2 h or 0.1%–2 h and P2h group is statistically significant. a Expression profile of GPx. b Expression profile of CAT. c Expression profile of Prx
Fig. 3.
Enzyme activities of GPx and CAT in shrimp after H2O2 injections. Asterisk means that the difference between 0.01%–2 h or 0.1%–2 h and P2h group is statistically significant. a GPx activity. b CAT activity
Detection on the expression of FcCAT, FcGPx, and FcPrx in DsCAT, DsGPx, or DsPrx injected shrimp
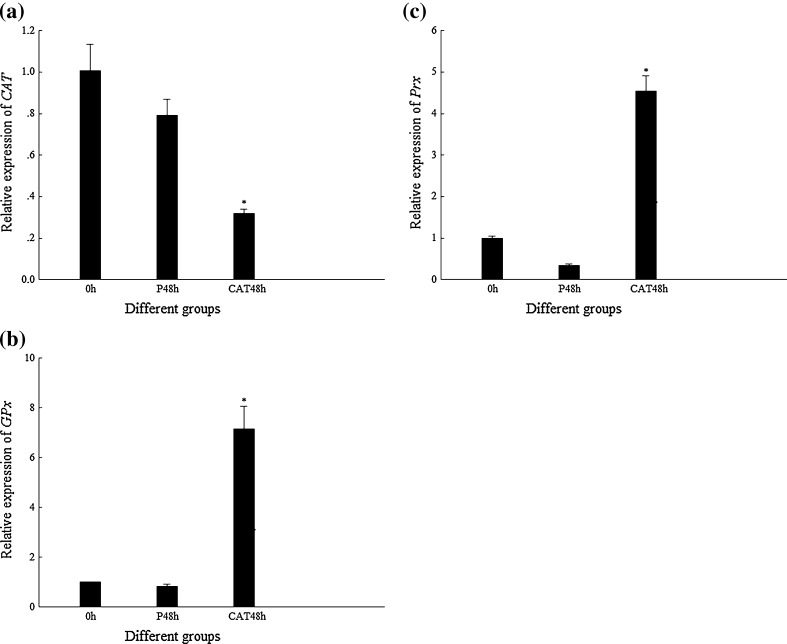
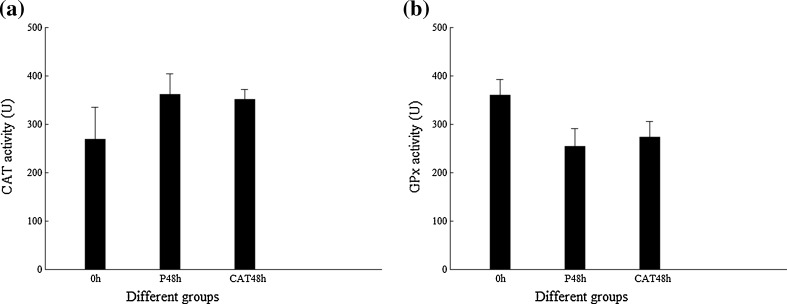
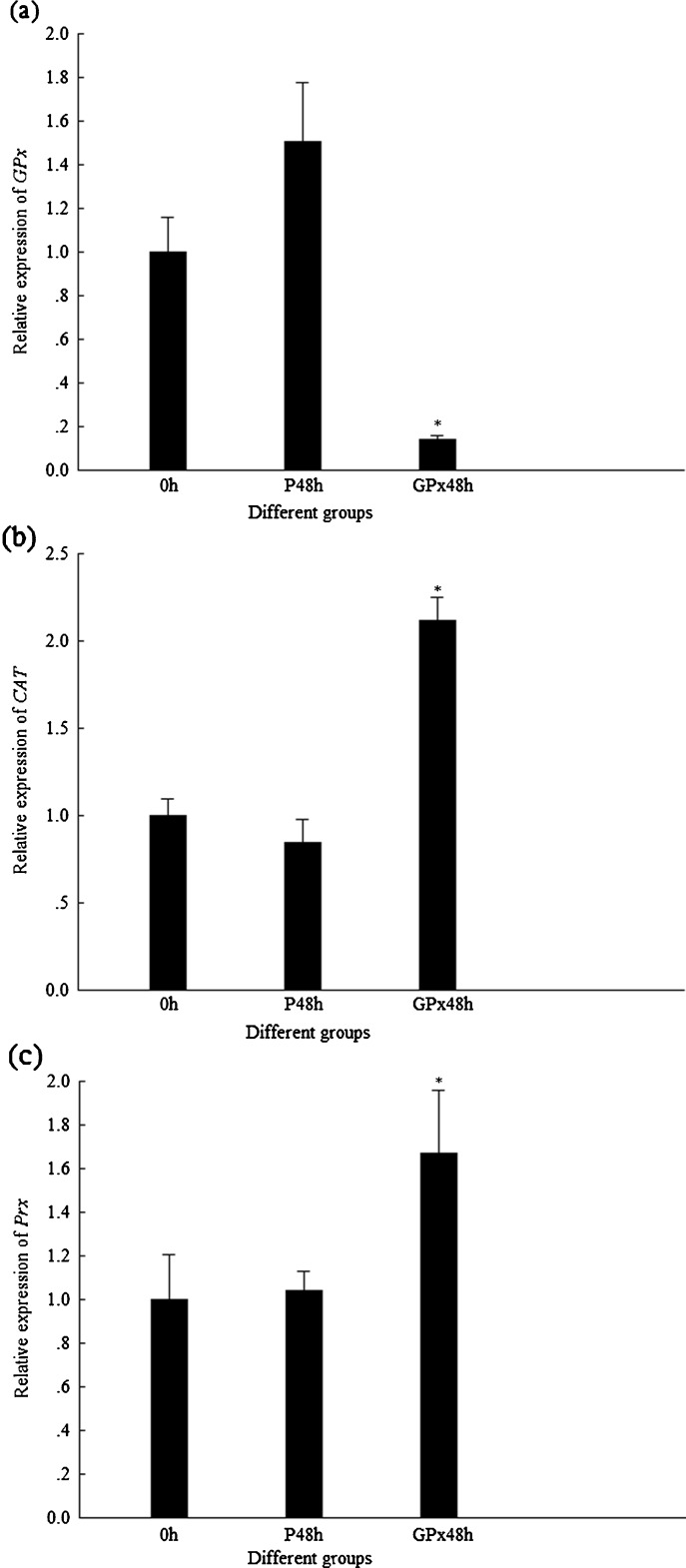
The transcription level of genes encoding different antioxidant enzymes, FcCAT, FcGPx, and FcPrx in shrimp, is shown in Fig. 4 when FcCAT were silenced by DsCAT injection for 48 h. The transcription level of FcCAT in DsCAT-silenced shrimp was much lower than that of its control. In this situation, the transcription levels of FcGPx and FcPrx were much higher than those of their controls. However, the enzyme activities of FcGPx and FcCAT in DsCAT-silenced shrimp did not show any difference from their PBS controls (Fig. 5).
Fig. 4.
Transcriptional expression profiles of CAT, GPx, and Prx in shrimp when CAT were silenced by DsCAT. P48h 48 hpi of PBS, CAT48h 48 hpi of DsCAT. Asterisk means that the difference between CAT48h and P48h group is statistically significant. a Expression profile of CAT. b Expression profile of GPx. c Expression profile of Prx
Fig. 5.
Enzyme activities of CAT and GPx in shrimp when CAT were silenced by DsCAT. a CAT activity. b GPx activity
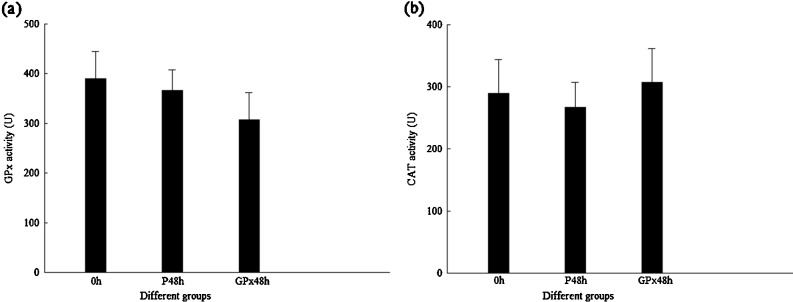
In DsGPx-silenced shrimp, the transcription level of FcGPx was much lower than that in their control group, while the transcription levels of FcCAT and FcPrx were significantly higher than those in their control groups (Fig. 6). Similar with the results of DsCAT-silenced shrimp, the enzyme activities of FcGPx and FcCAT in DsGPx-silenced shrimp did not show any difference from their PBS controls (Fig. 7).
Fig. 6.
Transcriptional expression profiles of GPx, CAT, and Prx in shrimp when GPx were silenced by DsGPx. P48h 48 hpi of PBS, GPx48h 48 hpi of DsGPx. Asterisk means that the difference between GPx48h and P48h group is statistically significant. a Expression profile of GPx. b Expression profile of CAT. c Expression profile of Prx
Fig. 7.
Enzyme activity of GPx and CAT in shrimp when GPx were silenced by DsGPx. a GPx activity. b CAT activity
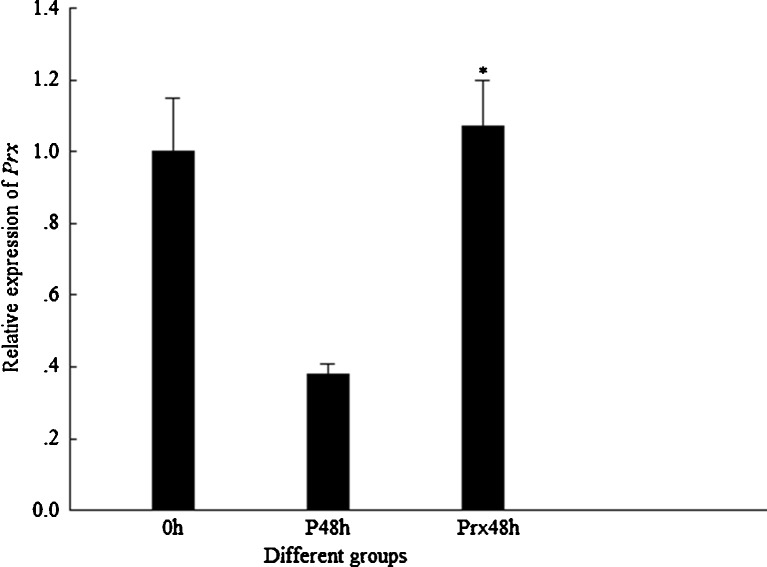
In DsPrx-injected shrimp, the transcription level of FcPrx did not show any suppression compared with their control group; in reverse, it showed upregulation (Fig. 8). Therefore, the injection of DsPrx in shrimp could not silence the expression of FcPrx. In this case, the expression of FcGPx and FcCAT was not detected further.
Fig. 8.
Transcriptional expression profile of Prx in shrimp after injected by DsPrx. P48h 48 hpi of PBS, Prx48h 48 hpi of DsPrx. Asterisk means that the difference between Prx48h and P48h group is statistically significant
Expression analysis on FcGPx, FcCAT, and FcPrx when DsGPx-silenced shrimp were injected with H2O2
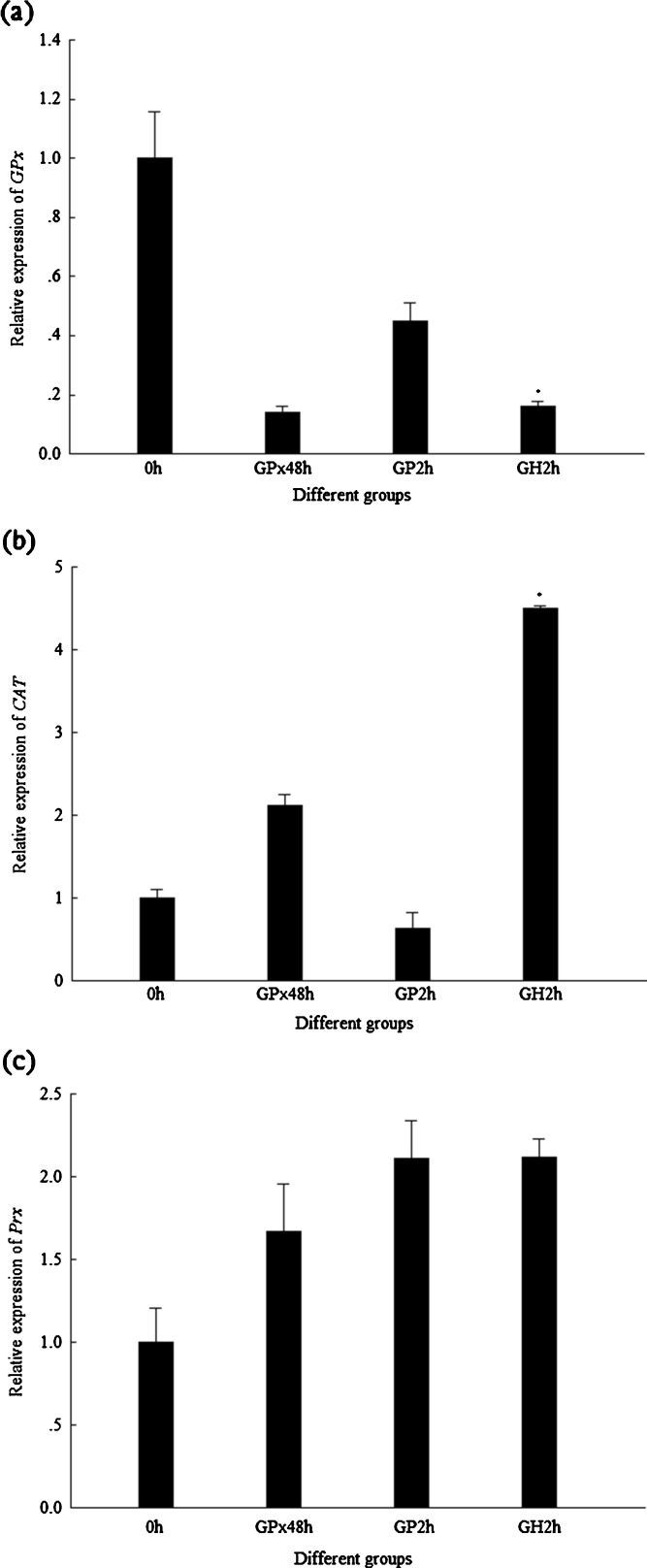
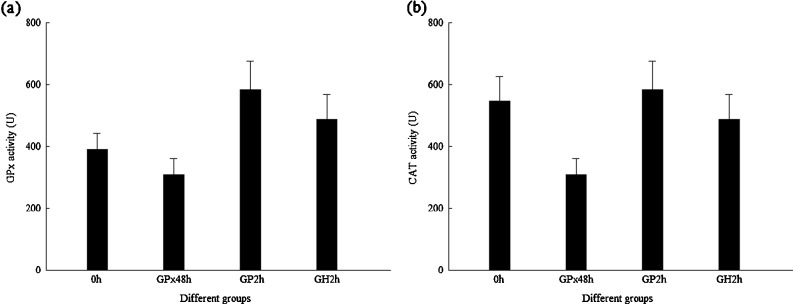
In order to know whether there existed some compensation among the expression of these three genes, the transcriptions of FcCAT and FcPrx were analyzed when DsGPx-silenced shrimp were injected with H2O2. The transcription levels of FcGPx, FcCAT, and FcPrx in DsGPx-silenced shrimp injected with 0.1% H2O2 for 2 h are shown in Fig. 9. When shrimps were injected with PBS for 2 h, no any upregulation at transcription level for FcGPx, FcCAT, and FcPrx was detected; however, when shrimps were injected with H2O2, although the transcription level of FcGPx and FcPrx was not upregulated, the transcription level of FcCAT was greatly upregulated. However, there was no big difference for the enzyme activity of FcGPx and FcCAT (Fig. 10).
Fig. 9.
Transcriptional expression profiles of GPx, CAT, and Prx in DsGPx-silenced shrimp injected with 0.1% H2O2. GPx48h 48 hpi of DsGPx, GP2h 2 hpi of PBS after DsGPx silenced, GH2h 2 hpi of H2O2 after DsGPx silenced. Asterisk means that the difference between GH2h and GP2h is statistically significant. a Expression profile of GPx. b Expression profile of CAT. c Expression profile of Prx
Fig. 10.
Enzyme activities of GPx and CAT in DsGPx-silenced shrimp injected with 0.1% H2O2. a GPx activity. b CAT activity
Discussion
In high animals, the produced superoxide radicals after cell stress will be converted to hydrogen peroxide (H2O2) and oxygen (O2) by SOD, and the residual H2O2 will be catalyzed into harmless water (H2O) and oxygen (O2) by CAT, GPx, and Prx (Aruoma 1998; Holmblad and Soderhall 1999; Schwarz 1996). The role of the antioxidant defense system in invertebrates could be of even greater importance than that in vertebrates, since phagocytosis—a highly ROS and reactive oxygen intermediate (ROI) producer process—is a major line of defense against invading microorganisms due to their lack of antibodies and acquired immunity (Johansson et al. 1999). The respiratory burst increased significantly in giant freshwater prawn, Macrobrachium rosenbergii, to eliminate the invading pathogen; therefore, the up-regulation of antioxidant enzyme activity and messenger RNA transcription were upregulated in order to convert the excess ROS caused by pathogen infection (Yeh et al. 2009). In black tiger shrimp P. monodon, respiratory bursts of Photobacterium damsela and WSSV-injected shrimp significantly increased (Liu et al. 2010). Therefore, the antioxidant enzymes might play key roles in the innate immunity of shrimp.
In the present study, when shrimp were injected by two doses of H2O2 (0.01% and 0.1%), a low dose (0.01%) did not induce upregulation of GPx and CAT at transcription level, but a high dose (0.1%) led to significant upregulation of GPx and CAT. In contrast to the variation at transcription level, the activity of GPx and CAT increased after both doses of H2O2 injection. These data suggested that both GPx and CAT were important for shrimp to eliminate H2O2. However, two doses of H2O2 injection did not induce any upregulation of Prx at the transcription levels; this might be related to the existence of abundant Prx protein in the cell. It was reported that Prxs are fairly abundant proteins and present in an approximate range from 1 to 10 μg/mg of soluble protein (Chae et al. 1999). On the other hand, it might be possible that the dose of 0.1% H2O2 is not enough to induce the variation of Prx at transcription or protein level. When CAT was silenced by DsRNA injection, the transcription of GPx and Prx was significantly upregulated. When GPx was silenced by DsRNA injection, the transcription level of CAT and Prx was apparently upregulated. Therefore, we inferred that the expression of CAT, GPx, and Prx could compensate each other to sustain the homeostasis of the cell in shrimp. When CAT or GPx was silenced for 48 h, we did not detect any variations of their activity. The reason might be that the decreased activity of CAT or GPx would be shown later than 48 h. DsRNA injection for 48 h could only cause the depressing at the transcription level of CAT or GPx, but their activity variation might be at a later time. In the present study, we did not succeed in silencing the expression of Prx by DsRNA injection in shrimp, this might also be caused by high amount of Prx that existed in the cell. In order to confirm the roles of CAT, GPx, and Prx in eliminating H2O2 in shrimp, we injected H2O2 in DsGPx-silenced shrimp. In GPx-silenced shrimp, the transcription level of GPx at 2 h post-0.1% H2O2 injection was not upregulated, which was different from the data that the transcription level of GPx was elevated in normal shrimp at 2 h post-0.1% H2O2 injection. This might indicate that the injected double-strand RNA of GPx was still effective in shrimp to silence GPx. Therefore, even if more transcripts of GPx were synthesized, they would be degraded due to the existence of double-strand RNA of GPx. Although injection of H2O2 could not cause the upregulation of Prx at transcription level, silencing of GPx or CAT can lead to upregulation of Prx at transcription level, which suggest that potential relationships among CAT, GPx, and Prx might exist in penaeid shrimp just like the situation in higher animals. When we detect the enzyme activities of FcGPx and FcCAT in DsGPx or DsCAT-silenced shrimp for 48 h, we did not detect any variation on GPx or CAT activity. We guess that the expression at translation level might compensate each other in later time because the regulation at translation level is always later than that at transcription level.
In summary, close relationships among three antioxidant enzymes, CAT, GPx, and Prx, existed in shrimp. The transcription of CAT and GPx could be directly induced by H2O2 injection; however, the transcription of Prx cannot be induced by H2O2, but decreased expression of CAT and GPx at transcription level could lead to increased transcription of Prx. Above data suggested that there exist some compensation among these three antioxidant enzyme genes.
Acknowledgments
This work was financially supported by the General Program of National Natural Science Foundation of China (31072203, 31001134), Major State Basic Research Development Program of China (973 program; 2012CB114403), China Agriculture Research system (CARS-47), and Special Fund for Agro-scientific Research in the Public Interest (201103034).
References
- Arthur JR. The glutathione peroxidases. Cell Mol Life Sci. 2000;57(13):1825–1835. doi: 10.1007/PL00000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma OI. Free radicals, oxidative stress, and antioxidants in human health and disease. J Am Oil Chem Soc. 1998;75(2):199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacano Maningas MB, Koyama T, Kondo H, Hirono I, Aoki T. A peroxiredoxin from kuruma shrimp, Marsupenaeus japonicus, inhibited by peptidoglycan. Dev Comp Immunol. 2008;32(3):198–203. doi: 10.1016/j.dci.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Bogdan C, Rollinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12(1):64–76. doi: 10.1016/S0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- Chae HZ, Robison K, Poole LB, Church G, Storz G, Rhee SG. Cloning and sequencing of thiol-specific antioxidant from mammalian brain—alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc Natl Acad Sci U S A. 1994;91(15):7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae HZ, Kim HJ, Kang SW, Rhee SG. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res Clin Pract. 1999;45(2–3):101–112. doi: 10.1016/S0168-8227(99)00037-6. [DOI] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310(5749):847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- Ha EM, Oh CT, Ryu JH, Bae YS, Kang SW, Jang IH, Brey PT, Lee WJ. An antioxidant system required for host protection against gut infection in Drosophila. Dev Cell. 2005;8(1):125–132. doi: 10.1016/j.devcel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Holmblad T, Soderhall K. Cell adhesion molecules and antioxidative enzymes in a crustacean, possible role in immunity. Aquaculture. 1999;172(1–2):111–123. doi: 10.1016/S0044-8486(98)00446-3. [DOI] [Google Scholar]
- Iwanaga S, Lee BL. Recent advances in the innate immunity of invertebrate animals. J Biochem Mol Biol. 2005;38(2):128–150. doi: 10.5483/BMBRep.2005.38.2.128. [DOI] [PubMed] [Google Scholar]
- Johansson MW, Holmblad T, Thornqvist PO, Cammarata M, Parrinello N, Soderhall K. A cell-surface superoxide dismutase is a binding protein for peroxinectin, a cell-adhesive peroxidase in crayfish. J Cell Sci. 1999;112(6):917–925. doi: 10.1242/jcs.112.6.917. [DOI] [PubMed] [Google Scholar]
- Kang SW, Rhee SG, Chang T-S, Jeong W, Choi MH. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends Mol Med. 2005;11(12):571–578. doi: 10.1016/j.molmed.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Lee KS, Hwang JS, Ahn MY, Li JH, Sohn HD, Jin BR. Molecular cloning and characterization of a peroxiredoxin gene from the mole cricket, Gryllotalpa orientalis. Comp Biochem Phys B. 2005;140(4):579–587. doi: 10.1016/j.cbpc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Klotz MG, Klassen GR, Loewen PC. Phylogenetic relationships among prokaryotic and eukaryotic catalases. Mol Biol Evol. 1997;14(9):951–958. doi: 10.1093/oxfordjournals.molbev.a025838. [DOI] [PubMed] [Google Scholar]
- Lee SY, Soderhall K. Early events in crustacean innate immunity. Fish Shellfish Immunol. 2002;12(5):421–437. doi: 10.1006/fsim.2002.0420. [DOI] [PubMed] [Google Scholar]
- Lee KS, Kim SR, Park NS, Kim I, Kang PD, Sohn BH, Choi KH, Kang SW, Je YH, Lee SM, Sohn HD, Jin BR. Characterization of a silkworm thioredoxin peroxidase that is induced by external temperature stimulus and viral infection. Insect Biochem Mol Biol. 2005;35(1):73–84. doi: 10.1016/j.ibmb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Li F, Yan H, Wang D, Priya TAJ, Li S, Wang B, Zhang J, Xiang J. Identification of a novel relish homolog in Chinese shrimp Fenneropenaeus chinensis and its function in regulating the transcription of antimicrobial peptides. Dev Comp Immunol. 2009;33(10):1093–1101. doi: 10.1016/j.dci.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Lim YS, Cha MK, Kim HK, Uhm TB, Park JW, Kim K, Kim IH. Removals of hydrogen-peroxide and hydroxyl radical by thiol-specific antioxidant protein as a possible role in vivo. Biochem Biophys Res Commun. 1993;192(1):273–280. doi: 10.1006/bbrc.1993.1410. [DOI] [PubMed] [Google Scholar]
- Liu JQ, Zhang K, Ren XJ, Luo GM, Shen JC. Bioimprinted protein exhibits glutathione peroxidase activity. Anal Chim Acta. 2004;504(1):185–189. doi: 10.1016/S0003-2670(03)00763-3. [DOI] [Google Scholar]
- Liu CH, Tseng MC, Cheng W. Identification and cloning of the antioxidant enzyme, glutathione peroxidase, of white shrimp, Litopenaeus vannamei, and its expression following Vibrio alginolyticus infection. Fish Shellfish Immunol. 2007;23(1):34–45. doi: 10.1016/j.fsi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Liu KF, Yeh MS, Kou GH, Cheng WT, Lo CF. Identification and cloning of a selenium-dependent glutathione peroxidase from tiger shrimp, Penaeus monodon, and its transcription following pathogen infection and related to the molt stages. Dev Comp Immunol. 2010;34(9):935–944. doi: 10.1016/j.dci.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Mills GC. Hemoglobin catabolism.1. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem. 1957;229(1):189–197. [PubMed] [Google Scholar]
- Nordberg J, Arnér ESJ. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31(11):1287–1312. doi: 10.1016/S0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- Ren Q, Sun R-R, Zhao X-F, Wang J-X (2009) A selenium-dependent glutathione peroxidase (Se-GPx) and two glutathione S-transferases (GSTs) from Chinese shrimp (Fenneropenaeus chinensis). Comp Biochem Physiol C Toxicol Pharmacol 149 (4):613–623. doi:10.1016/j.cbpc.2009.01.007 [DOI] [PubMed]
- Rhee SG, Kang SW, Jeong W, Chang TS, Yang KS, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17(2):183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Roch P. Defense mechanisms and disease prevention in farmed marine invertebrates. Aquaculture. 1999;172(1–2):125–145. doi: 10.1016/S0044-8486(98)00439-6. [DOI] [Google Scholar]
- Ryu JH, Ha EM, Oh CT, Seol JH, Brey P T, Jin I, Lee DG, Kim J, Lee D, Lee WJ. An essential complementary role of NF-kappa B pathway to microbicidal oxidants in Drosophila gut immunity. EMBO J. 2006;25(15):3693–3701. doi: 10.1038/sj.emboj.7601233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz KB. Oxidative stress during viral infection: a review. Free Radic Biol Med. 1996;21(5):641–649. doi: 10.1016/0891-5849(96)00131-1. [DOI] [PubMed] [Google Scholar]
- Smith VJ, Brown JH, Hauton C. Immunostimulation in crustaceans: does it really protect against infection? Fish Shellfish Immunol. 2003;15(1):71–90. doi: 10.1016/S1050-4648(02)00140-7. [DOI] [PubMed] [Google Scholar]
- Speier C, Baker SS, Newburger PE. Relationships between invitro selenium supply, glutathione-peroxidase activity, and phagocytic function in the Hl-60 human myeloid cell-line. J Biol Chem. 1985;260(15):8951–8955. [PubMed] [Google Scholar]
- Tavaressanchez O. Catalase from the white shrimp Penaeus (Litopenaeus) vannamei: molecular cloning and protein detection*1. Comp Biochem Physiol B Biochem Mol Biol. 2004;138(4):331–337. doi: 10.1016/j.cbpc.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Yeh SP, Liu KF, Chiu ST, Jian SJ, Cheng W, Liu CH. Identification and cloning of a selenium dependent glutathione peroxidase from giant freshwater prawn, Macrobrachium rosenbergii. Fish Shellfish Immunol. 2009;27(2):181–191. doi: 10.1016/j.fsi.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994;74(1):139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- Zhang QL, Li FH, Zhang JQ, Wang B, Gao HW, Huang BX, Jiang H, Xiang JH. Molecular cloning, expression of a peroxiredoxin gene in Chinese shrimp Fenneropenaeus chinensis and the antioxidant activity of its recombinant protein. Mol Immunol. 2007;44(14):3501–3509. doi: 10.1016/j.molimm.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li F, Zhang X, Dong B, Zhang J, Xie Y, Xiang J. cDNA cloning, characterization and expression analysis of the antioxidant enzyme gene, catalase, of Chinese shrimp Fenneropenaeus chinensis. Fish Shellfish Immunol. 2008;24(5):584–591. doi: 10.1016/j.fsi.2008.01.008. [DOI] [PubMed] [Google Scholar]