Abstract
Much evidence supports that oxidative stress plays an important role in the pathogenesis of neurodegenerative diseases, such as Alzheimer’s disease. Herein, we studied the compensatory/adaptive mechanisms involved in 3-thiomethyl-5,6-(diphenyl)-1,2,4-triazine and 3-thiomethyl-5,6-(dichlorophenyl)-1,2,4-triazine neuroprotection. We found that these compounds could counteract H2O2-induced rupture of neurite outgrowth in differentiated PC12 cells. In addition, we found that pretreatment of cells with triazine derivatives could modulate the expression of heat shock proteins Hsp70, Hsp90, and Hsp32 in H2O2-treated PC12 cells. These compounds could also increase nuclear level of stress sensing transcription factor, NF-E2 related factor 2, which contributes to redox homeostasis and cell survival following stress. As a result, the elevated levels of glutamylcysteine synthetase, glutathione peroxidase-1, and glutathione, as well as superoxide dismutase and catalase, increased cellular antioxidant capacity. Studying the relation between structure and activity of these compounds will pave the way for exploiting preventive and/or therapeutic strategies for the management of oxidative stress-mediated disorders.
Keywords: Alzheimer’s disease, HSPs, Nrf2, Oxidative stress, PC12 cells, Triazine derivatives
Introduction
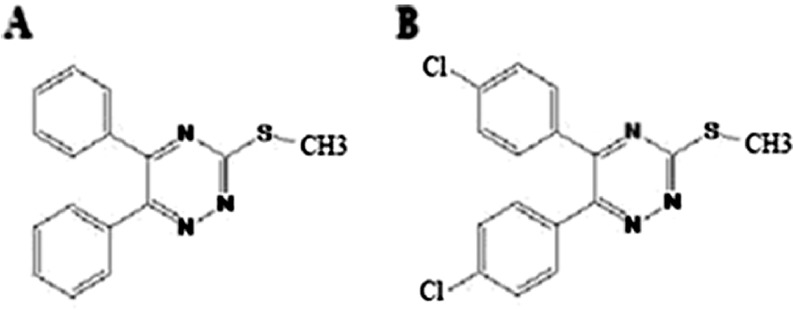
There is increasing evidence of the ubiquitous role of oxidative stress in neurodegenerative diseases, such as AD (Zhu et al. 2007). Oxidative stress occurs when there is a surplus of free radicals, a decrease in antioxidant levels or both. Moreover, numerous studies have provided evidence that β-amyloid aggregates are involved in reactive oxygen species (ROS) generation (Butterfield et al. 1999). The imbalance between production and removal of ROS results in bio-molecular damage which can generate secondary products that can be as damaging as the initial ROS (Circu and Aw 2010). Moreover, H2O2 at lower concentrations activates signaling pathways that regulate a variety of cellular responses including sensing of oxidative stress (Taupin 2010). Accordingly, many therapeutic strategies have been adopted to prevent oxidative stress-induced cell death. Triazine compounds possess a wide range of biological activities including anti-inflammatory (Mullick et al. 2009), radical scavenging (Iwashita et al. 2003), β-sheet breakage (Kim et al. 2006), and antifungal activity (Pandey et al. 2009). In our previous study, we found that different triazine derivatives have protective effects in neuron-like PC12 cells. We have shown that triazine derivatives protect PC12 cells from apoptosis induced by H2O2 through decrease of nuclear factor-kappa B which plays an important role in inflammatory and antioxidant responses (Irannejad et al. 2010). The present experiment was designed to investigate the mechanism of two protective triazines, 3-thiomethyl-5,6-(diphenyl)-1,2,4-triazine (TMDPT) and 3-thiomethyl-5,6-(dichlorophenyl)-1,2,4-triazine (TMDCPT) (Fig. 1), in cellular responses essential for sensing environmental changes and adapting to them.
Fig. 1.
Chemical structure of TMDPT (a) and TMDCPT (b)
Heat shock proteins (HSPs) were first discovered in 1962 (Ritossa 1962) as a set of highly conserved proteins whose expressions are induced by different kinds of stresses such as heat shock, ischemia damage, infections, oxidants, and heavy metals (Kalmar and Greensmith 2009; Söti and Csermely 2007). They act as molecular chaperones that prevent protein denaturation and facilitate protein folding (Welch 1991). In addition, HSPs may protect cells by mechanisms unrelated to their chaperone function (Yenari 2002). Increasing evidence shows HSPs have critical roles in protecting the cell against a wide range of physiological stresses through inhibition of apoptosis (Yenari et al. 2005).
NF-E2 related factor 2 (Nrf2) is a central transcription factor involved in transcriptional activation of phase II detoxifying enzymes via the antioxidant response element (ARE) (Jaiswal 2004). Increasing evidence indicates that the Nrf2 signaling pathway plays an important role in the adaptive responses induced by ROS and lipid peroxidation products (Osburn and Kensler 2008). Oxidative stress results in the activation of the Nrf2 pathway through the release of Nrf2 from its cytoplasmic inhibitor, Kelch-like ECH-associated protein 1 (Keap1), and translocation of Nrf2 to the nucleus, where it activates transcription of ARE-driven genes, such as Hsp32 and glutamylcysteine synthetase (γ-GCS) (Kobayashi and Yamamoto 2006). Activated Nrf2 has a short half-life and degrades rapidly through the ubiquitin–proteasome system, so that Nrf2 stabilization by pharmacological modulation might be a helpful strategy in preventing oxidative stress-related neuronal cell injury (Ansari et al. 2011; Eftekharzadeh et al. 2010; Li et al. 2005). Interestingly, Zhao et al. have reported that Nrf2 promotes neuronal cell differentiation. Primary neurons isolated from Nrf2-null mice showed a slower progress in differentiation, compared to those from wild-type mice (Zhao et al. 2009).
The signaling mechanism involved in neuronal differentiation processes has not been well studied and is probably a complex process, requiring the interplay of many signaling events. Recently, several studies have focused on the effect of different neurotoxins on the impairment of neurite outgrowth (Frankel et al. 2009; Rand et al. 2009). In the current study, we examined the effect of triazine derivatives on the protection of neurite outgrowth, which is a hallmark of neuronal function, against the toxicity of H2O2. We focused on the effect of TMDPT and TMDCPT on HSPs levels and on Nrf2 nuclear translocation. We also provided some insights into the relation between the structural changes of this core system and the effectiveness of TMDPT and TMDCPT.
Materials and methods
Materials
Antibodies directed against Hsp90, Hsp70, and β-actin were obtained from Cell Signaling Technology (Beverly, MA, USA). γ-GCS, glutathione peroxidase-1 (GPx1), and Hsp32 antibodies were from Abcam (Cambridge, UK). The polyclonal antibodies against Nrf2 (C-20) and lamin B2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). FITC-goat anti-rabbit IgG was purchased from Invitrogen (Carlsbad, CA, USA). TMDPT and TMDCPT were synthesized as described previously (Irannejad et al. 2010). All the other reagents, unless otherwise stated, were from Sigma Aldrich (St. Louis, MO, USA).
Cell culture, differentiation, and treatment conditions
Rat pheochromocytoma (PC12) cells obtained from Pasteur Institute (Tehran, Iran) were grown in Dulbecco’s modified Eagle’s medium, supplemented with 10% horse serum, 5% fetal bovine serum, and 1% antibiotic mixture comprising penicillin–streptomycin in a humidified atmosphere at 37°C with 5% CO2. The growth medium was changed three times a week. Cells were differentiated by treating with nerve growth factor (NGF) (50 ng/ml) every other day for 6 days. Differentiated PC12 cells, plated in 75 cm2 culture flasks, were incubated with TMDPT and TMDCPT (10 μM) for 3 h prior to our experiments. The cells were then treated with H2O2 (150 μM) for 24 h.
Morphological analysis of differentiated PC12 cells
For morphological analysis, two random images were acquired from each well. A minimum of 50 cells per treatment were quantified. Criteria for selection were that the cell body and processes were completely within the field of view and that the cell body was distinct from neighboring cell bodies. Cells fitting these criteria were analyzed and their cell body area, average neurite length, average neurite width, number of primary neurites, and bipolar morphology were quantified. Data analysis was done by using the Cell^A program. Cell body area was defined as the area of the cell exclusive of neurite processes. Neurite length was calculated by summing up the lengths of the primary process and all associated branches. To establish the average neurite width, the area was divided by the length of the neurite. Primary neurites were defined as clear protrusions from the cell body greater than 10 μM length. Cells were considered “bipolar” if they displayed a cell body with one process at either end. To evaluate neurite networks, images were analyzed using the cell counter plugin to score all branching nodes at each image. Nodes were defined as sites at which individual neurites branched or separate neurites contacted each other. All measurements expressed as proportions used the number of cells displaying the characteristic as a sub-population of the total number of cells that met selection criteria described above.
Western blot analysis
For western blot analysis, cells were lysed in buffer containing complete protease inhibitor cocktail. The total proteins were electrophoresed in 12% SDS-PAGE gels, transferred to polyvinylidene fluoride membranes, and probed with specific antibodies. Immunoreactive polypeptides were detected by chemiluminescence using enhanced ECL reagents (Amersham Bioscience, USA) and subsequent autoradiography. Quantification of the results was performed by densitometric scan of films. Data analysis was done by Image.J, measuring integrated density of bands after background subtraction. Protein concentrations were determined according to Bradford’s method (Bradford 1976). A standard plot was generated using bovine serum albumin. Nuclear and cytoplasmic proteins were isolated as described by Kutuk and Basaga (2003).
Immunofluorescent staining of Nrf2
Localization of Nrf2 in the cells was assessed by immunofluorescent staining. PC12 cells seeded in eight-well chamber slides were treated with triazine derivatives (10 μM) for 3 h, followed by adding H2O2 for 24 h. Fixed PC12 cells were permeabilized with 0.2% BSA/0.1% Triton X-100 solution for 30 min and then incubated overnight in a 1:100 dilution of Nrf2 antibody. After washing in phosphate buffered saline with tween 20 (PBST), the cover-slips were incubated for 1 h with FITC-conjugated goat anti-rabbit IgG antibody at room temperature. Where indicated, the nuclei of cells were then sequentially stained for 30 min with 10 μg/ml propidium iodide, washed three times in PBST, and mounted to slides. Cells were then analyzed for fluorescence using a fluorescence microscope (Zeiss, Germany).
Measurement of glutathione levels
The concentration of glutathione (GSH) was determined in whole cell lysate using dithionitrobenzoic acid (DTNB) method at 412 nm (Ellman 1959).
Superoxide dismutase activity assay
Superoxide dismutase (SOD) activity was measured based on the extent inhibition of amino blue tetrazolium formazan formation in the mixture of nicotinamide adenine dinucleotide, phenazine methosulfate, and nitroblue tetrazolium (NADH–PMS–NBT), according to the method of Kakkar et al. (1984). The assay mixture contained 0.1 ml of cell lysate, 1.2 ml of sodium pyrophosphate buffer (pH 8.3, 0.052 M), 0.1 ml of PMS (186 μM), 0.3 ml of NBT (300 μM), and 0.2 ml of NADH (750 μM). Reaction was started by the addition of NADH. After incubation at 30°C for 90 s, the reaction was stopped by the addition of 0.1 ml of glacial acetic acid. The reaction mixture was stirred vigorously with 4.0 ml of n-butanol. Color intensity of the chromogen in butanol was measured spectrophotometrically at 560 nm. One unit of SOD activity was defined as the amount of enzyme that caused 50% inhibition of NBT reduction per milligram of protein.
Catalase activity assay
Catalase (CAT) activity was measured by the method of Aebi (1984). Briefly, 200 μl of cell lysate was added to a cuvette containing 1.995 ml of 50 mM phosphate buffer (pH 7.0). Reaction was started by the addition of 1.0 ml of freshly prepared 30 mM H2O2. The rate of decomposition of H2O2 was measured spectrophotometrically at 240 nm.
Measurement of lipid peroxidation
Malondialdehyde (MDA) levels were measured by the double heating method (Draper and Hadley 1990). This method is based on spectrophotometric measurement of the purple color that is generated by the reaction of thiobarbituric acid (TBA) with MDA. Briefly, 0.5 ml of cell lysate was mixed with 2.5 ml of TCA (10% w/v) solution followed by boiling in a water bath for 15 min. After cooling to room temperature, the samples were centrifuged at 3,000 rpm for 10 min. Two milliliters of each sample supernatant was transferred to a test tube containing 1 ml of TBA solution (0.67% w/v). Each tube was then placed in boiling water for 15 min. After cooling to room temperature, the absorbance was measured at 532 nm with respect to the blank solution.
Statistical analysis
All data are the average of triplicate analysis. The data were recorded as means ± SEM. Analysis of variances was performed by ANOVA procedures. P < 0.05 was considered as significant difference (* or #P < 0.05, ** or ##P < 0.01 and *** or ###P < 0.001).
Results
TMDPT and TMDCPT improved neurite outgrowth in H2O2-exposed PC12 cells
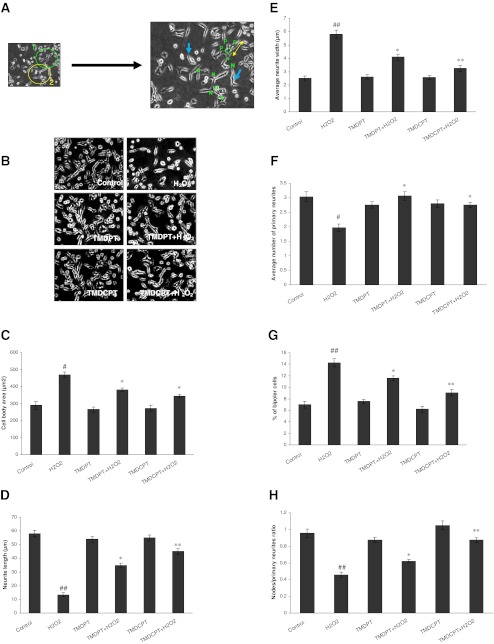
Cell body area, the length of the longest isolated neurites, and the average of neurite width were evaluated to monitor cell growth (Fig. 2a). As shown in Fig. 2b, c, average cell body area was increased in H2O2-exposed cultures, whereas pretreatment of cells with TMDPT and TMDCPT resulted in a significant decrease in cell body area. The effects of triazine derivatives on neurite length contrasted with the results of cell body area (Fig. 2d). Moreover, neurites exposed to 150 μM H2O2 were wider than those in control cultures. Because of variability along the length of the neurite, the total area of the neurite was divided by the neurite length to calculate average neurite width. This ratio was significantly lower in the presence of TMDPT and TMDCPT than in controls, after 24 h (Fig. 2e).
Fig. 2.
Effect of TMDPT and TMDCPT on H2O2-induced disruption of neurite outgrowth in differentiated PC12 cells. a The criteria of PC12 differentiation is shown on two neurons (left image) of a sample image. The “P” on the right image indicates the primary neuritis of a neuron 1. The yellow arrow shows the length of a neurite, extent elongated, and membrane-enclosed protrusions of cytoplasm. The green circle on the right image shows the cell body. Neurite width is not equal in all parts of the neurons, thus the average neurite width must be calculated by dividing cell body area to average neurite length. The blue arrows show bipolar cells. The letter “N” indicates the nodes, the sites at which individual neurites branched or separate neurites contacted each other. b NGF-differentiated PC12 cells were pretreated with TMDPT and TMDCPT (10 μM) in the presence of H2O2 (150 μM). Criteria were quantified at 24 h; c cell body area; d average neurite length; e average neurite width; f number of primary neurites per cell; g percent of bipolar cells; and h the ratio of nodes to primary neurites. #P < 0.05 and ##P < 0.01, significantly different from control cells. *P < 0.05 and **P < 0.01, significantly different from H2O2-treated cells
Specific parameters of morphological complexity were also measured. First, the numbers of primary neurites (>10 μm) emanating from individual cell bodies were measured. As shown in Fig. 2f, the numbers of primary neurites per cell body were decreased in H2O2-treated cells. In contrast, the proportion of cells with the very simple bipolar morphology and only two neurites was increased in H2O2-treated cells, compared to control cultures (Fig. 2g). Pretreatment of cells with TMDPT and TMDCPT increased the numbers of neurites per cell and thus decreased the proportion of bipolar cells (Fig. 2f, g). We also calculated the ratio of the total neurite branching nodes to the total number of primary neurites. This ratio was decreased in H2O2-treated cells, while it was increased significantly in the presence of TMDPT and TMDCPT (Fig. 2h). All the experiments were done in the presence of 10 μM TMDPT and TMDCPT, which was the most protective concentration (Irannejad et al. 2010).
TMDPT and TMDCPT decreased Hsp90 expression in PC12 cells
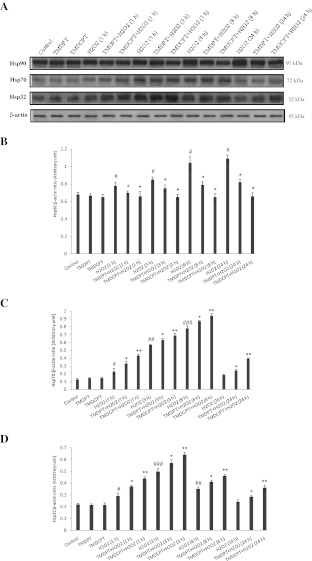
Classically, Hsp90 has been viewed as a molecular chaperone required for the late folding of signaling molecules (Mayer and Bukau 1999). Considering the recent data, the Hsp90 interaction network involves a wide range of biological processes including pathways associated with protein transport, cell metabolism, cell organization, and nuclear activities (Zhao et al. 2005). We studied the effect of H2O2 at different times (0, 1, 3, 8, and 24 h) on Hsp90 levels in PC12 cells. We found that in the presence of H2O2, Hsp90 levels increased and reached their highest level at 24 h (Fig. 3a, b). As shown in Fig. 3a, b, a 1.60-fold induction of Hsp90 was detected in H2O2-treated cells, after 24 h, while TMDPT and TMDCPT decreased the level of Hsp90 by 1.32- and 1.65-fold, respectively, compared to the H2O2-treated cells.
Fig. 3.
Hsp90, Hsp70, and Hsp32 levels in PC12 cells pretreated with TMDPT and TMDCPT. a PC12 cells were pretreated with triazine derivatives (10 μM) for 3 h and then exposed to H2O2 for 24 h. Twenty-microgram proteins were separated on SDS-PAGE, western blotted, probed with anti-Hsp90, anti-Hsp70, and anti-Hsp32 antibodies, and reprobed with anti-β-actin antibody (one representative western blot was shown; n = 3). The densities of Hsp90 (b), Hsp70 (c), and Hsp32 (d) bands were measured and the ratio was calculated. The mean of three independent experiments is shown. #P < 0.05, significantly different from untreated cells. *P < 0.05 and **P < 0.01, significantly different from H2O2-treated cells
TMDPT and TMDCPT increased Hsp70 expression in PC12 cells
A number of genes are upregulated in stress conditions, among which a set of HSPs play an important role in cytoprotection (Benarroch 2011). Stress-inducible Hsp70 is a prominent cytoprotective factor. When Hsp70 is upregulated by cellular stress, it inhibits the apoptosis induced by a wide range of insults (Yenari et al. 1999). We studied the effect of H2O2 at different times (0, 1, 3, 8, and 24 h) on Hsp70 levels in PC12 cells. Hsp70 levels increased and reached the highest level at 8 h, and then started to decrease in a way that after 24 h, its level was not significantly different from the control (Fig. 3a, d). To determine whether TMDPT and TMDCPT induce Hsp70, PC12 neurons were pretreated with 10 μM of triazine compounds for 3 h and then exposed to H2O2 for 24 h. As shown in Fig. 3a, c, H2O2 increased Hsp70 levels by 1.45-fold, compared to the control, whereas TMDPT and TMDCPT increased Hsp70 levels by 1.30- and 2.13-fold, respectively, compared to the H2O2-treated cells. These results indicate that TMDPT and TMDCPT could keep Hsp70 at high levels and thereby increase the ability of the cell to cope with the destructive effect of H2O2.
TMDPT and TMDCPT increased Hsp32 expression in PC12 cells
Another HSP that exerts cytoprotective and antioxidant effects is Hsp32 or hemeoxygenase-1 (HO-1) (Satoh et al. 2003). We studied the effect of H2O2 at different times (0, 1, 3, 8, and 24 h) on Hsp32 levels in PC12 cells. Hsp32 levels increased and it reached its highest level at 3 h, and then started to decrease such that after 24 h its level was not significantly different from that of the control (Fig. 3a, d). As shown in Fig. 3a, d, similar to Hsp70, the level of Hsp32 was increased by 1.17- and 1.49-fold in the presence of TMDPT and TMDCPT, respectively, compared to H2O2-treated cells at 24 h.
TMDPT and TMDCPT enhanced nuclear translocation of Nrf2 in PC12 cells
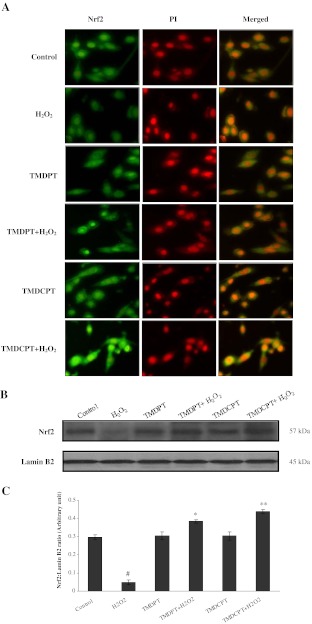
Nrf2 plays a key role in the adaptive response to oxidative and electrophilic stresses, maintaining cellular self-defense (Osburn and Kensler 2008). Since activated Nrf2 has a short life span, stabilization of Nrf2 is believed to be a valid strategy in protecting cells from oxidative-induced injury. It has been shown that following exposure to H2O2, a great increase in the nuclear level of Nrf2 followed by a decrease to basal or even lower levels occurs in PC12 cells (Tusi et al. 2010). Due to the short half-life of Nrf2, the ability of the cell to restore high levels of Nrf2 in the nucleus dictates its ability to increase cellular antioxidant capacity. To establish whether the Nrf2 pathway was involved in the cytoprotection exerted by these triazine derivatives in PC12 neurons, we determined the cellular location of Nrf2, in the presence and/or absence of TMDPT and TMDCPT, by fluorescent microscopy (Fig. 4a). We also studied the nuclear levels of Nrf2 by western blot analysis (Fig. 4b, c). As our results illustrate, a low level of Nrf2 was detected in PC12 cells treated for 24 h with H2O2, while pretreatment with triazine derivatives for 3 h significantly increased the Nrf2 nuclear level. This suggests that one of the mechanisms involved in the cytoprotective effect of these compounds is their ability to stabilize Nrf2 in the nucleus and the upregulation of the Nrf2-target genes.
Fig. 4.
Effect of TMDPT and TMDCPT on Nrf2 nuclear translocation in PC12 cells. Cells were grown in chamber slides and treated with/without triazine derivatives (10 μM) for 3 h, followed by exposure to H2O2 for 24 h. a Nrf2 subcellular localization was determined as described in “Materials and methods”. Immunofluorescent analysis was performed to measure the effects of triazine derivatives on nuclear level of Nrf2 in PC12 cells. b Twenty-microgram proteins were separated on SDS-PAGE, western blotted, probed with anti-Nrf2 antibody, and reprobed with anti-lamin B2 antibody (one representative western blot was shown; n = 3). c The densities of Nrf2 bands were measured and the ratio was calculated. The mean of three independent experiments is shown. #P < 0.05, significantly different from untreated cells. *P < 0.05 and **P < 0.01, significantly different from H2O2-treated cells
TMDPT and TMDCPT increased glutathione level in PC12 cells
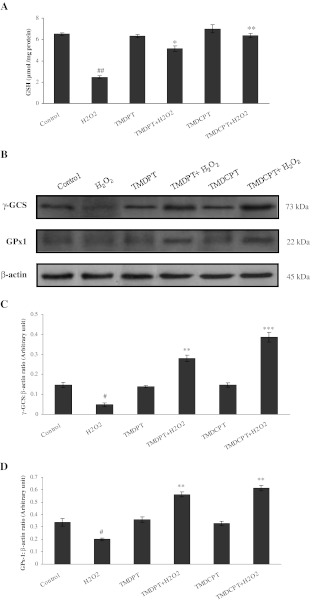
GSH participates in many biological processes including cellular defense against oxidative stress by reducing the disulfide linkage of proteins and other cellular molecules or by scavenging free radicals and reactive oxygen intermediates (Dickinson et al. 2003). γ-GCS is the key enzyme for GSH synthesis. From another perspective, GPx1 is an enzyme that is involved in the glutathione redox cycle and converts GSH to its oxidized form (GSSG). We found that in the presence of TMDPT and TMDCPT, the amount of GSH increased significantly (Fig. 5a) and this was due to the increase of γ-GCS levels (Fig. 5b, c). Moreover, our results showed that H2O2 significantly decreased GPx1 levels, whereas preincubation of the cells with triazine derivatives attenuated the decrease of GPx1 expression (Fig. 5b, d).
Fig. 5.
GSH, γ-GCS, and GPx1 levels in PC12 cells pretreated with TMDPT and TMDCPT. PC12 cells were pretreated with triazine derivatives (10 μM) for 3 h and then exposed to H2O2 for 24 h. a Glutathione level was measured by DTNB method. b Twenty-microgram proteins were separated on SDS-PAGE, western blotted, probed with anti-γ-GCS and anti-GPx1 antibodies, and reprobed with anti-β-actin antibody (one representative western blot was shown; n = 3). The densities of γ-GCS (c) and GPx1 (d) bands were measured and the ratio was calculated. The mean of three independent experiments is shown. #P < 0.05 and ##P < 0.01, significantly different from untreated cells. *P < 0.05, **P < 0.01, and ***P < 0.001, significantly different from H2O2-treated cells
TMDPT and TMDCPT mounted antioxidant enzyme activity and reduced lipid peroxidation in PC12 cells
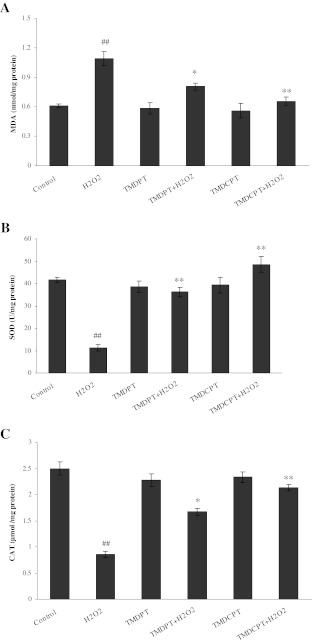
Lipid peroxidation has been implicated in the pathogenesis of various diseases by inducing disturbance of membrane organization, functional loss, and modification of proteins and DNA bases (Blair 2008). As shown in Fig. 6a, treatment of PC12 cells with 150 μM H2O2 increased MDA levels by 1.78-fold, while preincubation of the cells with TMDPT and TMDCPT markedly attenuated MDA levels by about 1.35- and 1.66-fold, respectively, compared to H2O2-treated cells.
Fig. 6.
Effects of TMDPT and TMDCPT on lipid peroxidation (a) and antioxidant enzyme activities, SOD (b), and CAT (c), in H2O2-treated PC12 cells. ##P < 0.01, significantly different from untreated cells. *P < 0.05 and **P < 0.01, significantly different from H2O2-treated cells
Several antioxidant mechanisms exist in cells that detoxify generated ROS and thus attenuate cell damage. Under oxidative stress conditions, SOD protects cells by degrading superoxide radicals into oxygen and hydrogen peroxide which is subsequently degraded by CAT activity (Sampayo et al. 2003). In PC12 cells treated with 150 μM H2O2, activities of SOD and CAT were decreased by about 3.70- and 2.94-fold, respectively. Pretreatment of cells with TMDPT and TMDCPT significantly attenuated the decrease of SOD activity by about 3.22- and 4.31-fold (Fig. 6b) and increased CAT activity by about 1.96- and 2.50-fold (Fig. 6c), respectively, compared to H2O2-treated cells.
Discussion
Apoptosis involves a range of intracellular mechanisms of “recognition, response, and removal” which results in physiological cell death, as well as pathophysiological massive loss of cells (Shimohama 2000). Although a diverse array of factors is capable of inducing apoptosis, dysregulation of homeostasis between generation and quenching of free radicals plays a crucial role in stimulating programmed cell death (Simon et al. 2000). Previously, we showed that TMDPT and TMDCPT possess neuroprotective and antiapoptotic effects through the reduction of caspase-3 level (Irannejad et al. 2010). Herein, to gain better understanding of the neuroprotective mechanisms of TMDPT and TMDCPT, we focused on molecules, HSPs and Nrf2, and analyzed alteration in oxidative stress markers in the presence of TMDPT and TMDCPT in differentiated PC12 cells.
The PC12 cell line has been widely used as a model for the study of the effect of compounds on neurotoxicity and neuronal differentiation (Greene and Tischler 1976). PC12 cells are derived from a rat adrenal medullary tumor and exhibit the phenotypic properties of adrenal chromaffin cells. However, in the presence of NGF, they cease proliferation, extend many broad sheet-like extensions called “neurites” which are neither axons, nor dendrites (Greene 1977). As a hallmark of neuronal differentiation, it is not surprising that many studies have evaluated neurite outgrowth in a PC12 cell model to assess the effect of toxicants and protective compounds (Radio and Mundy 2008).
In the present study, we found that H2O2 decreased neurite length, while in the presence of triazine derivatives, TMDPT and TMDCPT, the cells could restore the criteria of differentiation. The rescue of damaged neurons and the stimulation of neurogenesis are attractive strategies for the treatment of neurodegenerative diseases, because the lack of normal neurite outgrowth might reflect the dysfunction of molecules or proteins that are important for maintaining a normal neuronal process.
Oxidative stress induces a variety of cellular defense responses which are both genetically and non-genetically regulated. Interactions among these responses determine the fate of cells and their modulation could alter both function and survival of the cells. One of the known stress responses that provides a high level of resistance to subsequently more severe insults is the heat shock response (Richter et al. 2010). HSPs are necessary for the folding, assembly, and transport of proteins. They may also limit cell damage by preventing or stimulating the activity of sensor/effector molecules (Lanneau et al. 2008). Moreover, the heat shock response may provide time for other genetically regulated responses to occur. Inducible Hsp70 is one of the most important HSPs for maintenance of cell integrity during basal as well as pathophysiological conditions (Yenari et al. 1999). Several studies have shown that Hsp70 plays a role in mediating neuroprotection against cytotoxic stresses including oxidative stress (Ayala and Tapia 2008; Calabrese et al. 2004). It has been shown that Hsp70 increases the yield of native functional proteins by refolding damaged proteins and by stabilizing partially denatured proteins, which otherwise may aggregate and/or bind non-specifically to cellular proteins and interfere with their normal function (Young 2010). Hsp70 can also serve as a cytoplasmic “antioxidant” by protecting the sensitive sites of the target proteins (Papp et al. 2003). In addition to this chaperone activity, Hsp70 may directly target apoptosis by inhibiting caspase-3, thus suppressing apoptotic pathways (Li et al. 2000).
Hsp90 is another stress response protein involved in folding and stabilizing different native proteins, as well as mutant species (Mayer and Bukau 1999). Recently, the beneficial effects of Hsp70 induction and Hsp90 inhibition as a therapeutic approach in neurodegenerative diseases have been investigated (Ayala and Tapia 2008; Luo et al. 2008). Inhibition of Hsp90 results in the activation of heat shock factor-1 and, consequently, in the activation of protective stress-induced HSPs, such as Hsp70 (Luo et al. 2008). In this study, we found that the neuroprotective effect of TMDPT and TMDCPT was associated with decreased levels of Hsp90 and increased levels of Hsp70 in cellular events that occur as a consequence of oxidative stress. Thus, it seems that modulating the activities of molecular chaperones is one of the targets of these triazine compounds. To the best of our knowledge, this property has not been very often reported with neuroprotective compounds, yet it can be beneficial to the cells from various aspects. It also identifies the possibility for the development of HSP-based therapies for the treatment of neurodegenerative diseases. However, it should be mentioned that one of the great difficulties in the development of an HSP-based therapy for any disease that involves oxidative stress is the correct timing of HSP induction, which must occur within a relatively small therapeutic window. The time course results support the view that TMDPT and TMDCPT could increase Hsp70 and Hsp32 levels and decrease Hsp90 levels, at the right time, when their levels were deregulated by H2O2.
Hsp32 or HO-1 is another sensitive marker of cellular oxidative stress which is induced by various stimuli, including oxidative stress, and which exerts antioxidant, as well as neuroprotective properties (Satoh et al. 2003). In response to oxidative challenge, induction of Hsp32 via the Nrf2 pathway may protect cells through restoring a more favorable redox microenvironment by promoting the catabolism of pro-oxidant metalloporphyrins, such as heme, to bile pigments (biliverdin, bilirubin) with free radical scavenging capabilities (Tenhunen et al. 1968). Hsp32 expression is predominantly regulated at the transcriptional level, which is governed by AREs located at its promoter (Kobayashi and Yamamoto 2006).
One possible strategy for the development of neuroprotective drugs is to search for low molecular weight compounds that can regulate redox status and thereby counter oxidative damage. This study provides evidence that these compounds can afford neuroprotection through an increase of Hsp32 via the Nrf2 pathway. The pharmacological agents that have the ability to activate the Nrf2 signaling pathway hold great promise for therapeutic intervention in neurodegenerative disease (Calkins et al. 2009). In this study, we found that HSPs and Nrf2 are both involved in coping with environmental stress, although the present study did not address their mediatory or causal effects.
The key element in successful neuroprotection by these compounds is that they can activate the Nrf2 pathway at nontoxic concentrations. Many other electrophilic compounds cause systemic side effects and are not neuroprotective, probably because they also deplete critical reducing substances in the cell, such as GSH (Satoh et al. 2006). But this is not the case with our synthetic compounds. Recently, decreased Nrf2 transcriptional activity was also reported to cause age-related loss of GSH synthesis (Suh et al. 2004). Low molecular weight compounds can induce γ-GCS through activation of the ARE to increase GSH levels (Kobayashi and Yamamoto 2006). Thus, compounds that regulate the Nrf2 pathway may be promising candidates for neuroprotection against free radical stress through induction of γ-GCS as well as Hsp32, both of which prevent accumulation of ROS.
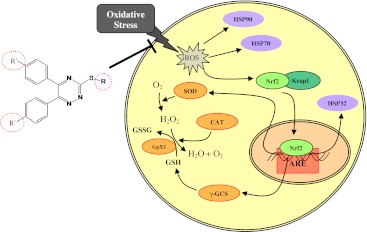
The glutathione redox cycle, along with catalase enzymes, detoxifies hydrogen peroxide generated by the activity of SOD. SOD is one of the key enzymes that catalyzes dismutation of the reactive superoxide form to hydrogen peroxide (Blair 2008). All together, these enzymatic and non-enzymatic antioxidants provide an efficient cascade to quench generated reactive species and insults (Scheme 1).
Scheme 1.
Mechanism of action of TMDPT and TMDCPT
One of the most important conclusions of this report is the relation between the structure and activity of triazine derivatives. Our data, in accordance with previous studies, suggest that a molecular scaffold including (a) a central triazine ring, (b) two aryl groups at positions 4 and 5 of the heterocyclic ring, and (c) a thioalkyl substitution at position 2 of triazine ring could have protective effects. Previously, we found that the length of the alkyl group affects the neuroprotective activity of triazine derivatives. It seems that there is an inverse correlation between the number of carbon atoms substituted on the thiol group and the neuroprotective effect of the compounds. In other words, an increase in the length of moiety, from methyl to n-butyl, decreases the protective effect of compounds against H2O2-induced cell death (Irannejad et al. 2010). In this study, we investigated the effect of different substitution patterns at the para position of aryl groups in restoring neurite outgrowth and antioxidant defense. We found that introducing a chloride group at the para-substitution of the aryl group increases the effect of TMDCPT, compared to TMDPT, while a fluoride group decreases the neuroprotective effect (data not shown). The obtained structure and activity relationship data may serve as guides for the design of future neuroprotective compounds. Conversely, although relatively small changes in structure appear to have large activity effects, it is not yet clear whether the differences in activity are due to changes in binding to putative protein targets or due to differences in stability, absorption, distribution, or metabolism (i.e., pharmacokinetics) of the compounds in our whole cell assay. To answer this question, further investigations are now in progress in our laboratory.
Acknowledgments
The authors thank Helen Neumann for editing the manuscript. The authors also thank Ms. Ghazaleh Rafatian for her help in preparing the manuscript. F. KH. and M. A. thank the National Elite Fund, Iran, for the Young Scientist Research Fellowship. This work was supported partially by Shahid Beheshti University of Medical Sciences Research Funds.
Conflict of interest statement
The authors have no conflict of interest.
Glossary
- AD
Alzheimer’s disease
- ARE
Antioxidant response element
- CAT
Catalase
- DTNB
Dithionitrobenzoic acid
- ECL
Electrochemiluminescence
- γ-GCS
Glutamylcysteine synthetase
- GPx1
Glutathione peroxidase-1
- HO-1
Hemeoxygenase-1
- HSP
Heat shock protein
- Keap1
Kelch-like ECH-associated protein 1
- MDA
Malondialdehyde
- NADH
Nicotinamide adenine dinucleotide
- NBT
Nitroblue tetrazolium
- NGF
Nerve growth factor
- Nrf2
NF-E2 related factor 2
- PBST
Phosphate buffered saline with tween 20
- PMS
Phenazine methosulfate
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- TBA
Thiobarbituric acid
- TCA
Trichloroacetic acid
- TMDCPT
3-Thiomethyl-5,6-(dichlorophenyl)-1,2,4-triazine
- TMDPT
3-Thiomethyl-5,6-(diphenyl)-1,2,4-triazine
References
- Aebi H. Catalase in vitro. Meth Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ansari N, Khodagholi F, Amini M. 2-Ethoxy-4,5-diphenyl-1,3-oxazine-6-one activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Eur J Pharmacol. 2011;658:84–90. doi: 10.1016/j.ejphar.2011.02.028. [DOI] [PubMed] [Google Scholar]
- Ayala GX, Tapia R. HSP70 expression protects against hippocampal neurodegeneration induced by endogenous glutamate in vivo. Neuropharmacology. 2008;55:1383–1390. doi: 10.1016/j.neuropharm.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Heat shock proteins: multiple neuroprotective functions and implications for neurologic disease. Neurology. 2011;76:660–667. doi: 10.1212/WNL.0b013e31820c3119. [DOI] [PubMed] [Google Scholar]
- Blair IA. DNA adducts with lipid peroxidation products. J Biol Chem. 2008;283:15545–15549. doi: 10.1074/jbc.R700051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Yatin SM, Varadarajan S, Koppal T. Amyloid beta-peptide-associated free radical oxidative stress, neurotoxicity, and Alzheimer’s disease. Methods Enzymol. 1999;309:746–768. doi: 10.1016/S0076-6879(99)09050-3. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Stella AM, Butterfield DA, Scapagnini G. Redox regulation in neurodegeneration and longevity: role of the heme oxygenase and HSP70 systems in brain stress tolerance. Antioxid Redox Signal. 2004;6:895–913. doi: 10.1089/ars.2004.6.895. [DOI] [PubMed] [Google Scholar]
- Calkins MJ, Johnson DA, Townsend JA, Vargas MR, Dowell JA, Williamson TP, Kraft AD, Lee JM, Li J, Johnson JA. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal. 2009;11:497–508. doi: 10.1089/ars.2008.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DA, Moellering DR, Iles KE, Patel RP, Levonen AL, Wigley A, Darley-Usmar VM, Forman HJ. Cytoprotection against oxidative stress and the regulation of glutathione synthesis. Biol Chem. 2003;384:527–537. doi: 10.1515/BC.2003.061. [DOI] [PubMed] [Google Scholar]
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Meth Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-I. [DOI] [PubMed] [Google Scholar]
- Eftekharzadeh B, Maghsoudi N, Khodagholi F. Stabilization of transcription factor Nrf2 by tBHQ prevents oxidative stress-induced amyloid beta formation in NT2N neurons. Biochimie. 2010;2:245–253. doi: 10.1016/j.biochi.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Frankel S, Concannon J, Brusky K, Pietrowicz E, Giorgianni S, Thompson WD, Currie DA. Arsenic exposure disrupts neurite growth and complexity in vitro. Neurotoxicology. 2009;30:529–537. doi: 10.1016/j.neuro.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Greene LA. A quantitative bioassay for nerve growth factor (NGF) activity employing a clonal pheochromocytoma cell line. Brain Res. 1977;133:350–353. doi: 10.1016/0006-8993(77)90770-3. [DOI] [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad H, Amini M, Khodagholi F, Ansari N, Tusi SK, Sharifzadeh M, Shafiee A. Synthesis and in vitro evaluation of novel 1,2,4-triazine derivatives as neuroprotective agents. Bioorg Med Chem. 2010;18:4224–4230. doi: 10.1016/j.bmc.2010.04.097. [DOI] [PubMed] [Google Scholar]
- Iwashita A, Maemoto T, Nakada H, Shima I, Matsuoka N, Hisajima H. A novel potent radical scavenger, 8-(4-fluorophenyl)-2-((2E)-3-phenyl-2-propenoyl)-1,2,3,4-tetrahydropyrazolo[5,1-c] [1,2,4]triazine (FR210575), prevents neuronal cell death in cultured primary neurons and attenuates brain injury after focal ischemia in rats. J Pharmacol Exp Ther. 2003;307:961–968. doi: 10.1124/jpet.103.056572. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- Kalmar B, Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev. 2009;61:310–318. doi: 10.1016/j.addr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Kim W, Kim Y, Min J, Kim DJ, Chang YT, Hecht MH. A high-throughput screen for compounds that inhibit aggregation of the Alzheimer’s peptide. ACS Chem Biol. 2006;1:461–469. doi: 10.1021/cb600135w. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 2006;46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Kutuk O, Basaga H. Aspirin prevents apoptosis and NFkappaB activation induced by H2O2 in HeLa cells. Free Radic Res. 2003;37:1267–1276. doi: 10.1080/10715760310001616005. [DOI] [PubMed] [Google Scholar]
- Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12:743–761. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem. 2000;275:25665–25671. doi: 10.1074/jbc.M906383199. [DOI] [PubMed] [Google Scholar]
- Li J, Johnson D, Calkins M, Wright L, Svendsen C, Johnson J. Stabilization of Nrf2 by tBHQ confers protection against oxidative stress-induced cell death in human neural stem cells. Toxicol Sci. 2005;83:313–328. doi: 10.1093/toxsci/kfi027. [DOI] [PubMed] [Google Scholar]
- Luo W, Rodina A, Chiosis G. Heat shock protein 90: translation from cancer to Alzheimer’s disease treatment? BMC Neurosci. 2008;9(Suppl 2):S7. doi: 10.1186/1471-2202-9-S2-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MP, Bukau B. Molecular chaperones: the busy life of Hsp90. Curr Biol. 1999;9:R322–R325. doi: 10.1016/S0960-9822(99)80203-6. [DOI] [PubMed] [Google Scholar]
- Mullick P, Khan SA, Begum T, Verma S, Kaushik D, Alam O. Synthesis of 1,2,4-triazine derivatives as potential anti-anxiety and anti-inflammatory agents. Acta Pol Pharm. 2009;66:379–385. [PubMed] [Google Scholar]
- Osburn WO, Kensler TW. Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutat Res. 2008;659:31–39. doi: 10.1016/j.mrrev.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SK, Singh A, Singh A, Nizamuddin Antimicrobial studies of some novel quinazolinones fused with [1,2,4]-triazole, [1,2,4]-triazine and [1,2,4,5]-tetrazine rings. Eur J Med Chem. 2009;44:1188–1197. doi: 10.1016/j.ejmech.2008.05.033. [DOI] [PubMed] [Google Scholar]
- Papp E, Nardai G, Söti C, Csermely P. Molecular chaperones, stress proteins and redox homeostasis. Biofactors. 2003;17:249–257. doi: 10.1002/biof.5520170124. [DOI] [PubMed] [Google Scholar]
- Radio NM, Mundy WR. Developmental neurotoxicity testing in vitro: models for assessing chemical effects on neurite outgrowth. Neurotoxicology. 2008;29:361–376. doi: 10.1016/j.neuro.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Rand MD, Dao JC, Clason TA. Methylmercury disruption of embryonic neural development in Drosophila. Neurotoxicology. 2009;30:794–802. doi: 10.1016/j.neuro.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Cell Mol Life Sci. 1962;18:571–573. doi: 10.1007/BF02172188. [DOI] [Google Scholar]
- Sampayo JN, Gill MS, Lithgow GJ. Oxidative stress and aging—the use of superoxide dismutase/catalase mimetics to extend lifespan. Biochem Soc Trans. 2003;31:1305–1307. doi: 10.1042/BST0311305. [DOI] [PubMed] [Google Scholar]
- Satoh T, Baba M, Nakatsuka D, Ishikawa Y, Aburatani H, Furuta K, Ishikawa T, Hatanaka H, Suzuki M, Watanabe Y. Role of heme oxygenase-1 protein in the neuroprotective effects of cyclopentenone prostaglandin derivatives under oxidative stress. Eur J Neurosci. 2003;17:2249–2255. doi: 10.1046/j.1460-9568.2003.02688.x. [DOI] [PubMed] [Google Scholar]
- Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc Natl Acad Sci U S A. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimohama S. Apoptosis in Alzheimer’s disease—an update. Apoptosis. 2000;5:9–16. doi: 10.1023/A:1009625323388. [DOI] [PubMed] [Google Scholar]
- Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/A:1009616228304. [DOI] [PubMed] [Google Scholar]
- Söti C, Csermely P. Protein stress and stress proteins: implications in aging and disease. J Biosci. 2007;32:511–515. doi: 10.1007/s12038-007-0050-z. [DOI] [PubMed] [Google Scholar]
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. A dual activity of ROS and oxidative stress on adult neurogenesis and Alzheimer’s disease. Cent Nerv Syst Agents Med Chem. 2010;10:16–21. doi: 10.2174/187152410790780172. [DOI] [PubMed] [Google Scholar]
- Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusi SK, Ansari N, Amini M, Amirabad AD, Shafiee A, Khodagholi F. Attenuation of NF-κB and activation of Nrf2 signaling by 1,2,4-triazine derivatives, protects neuron-like PC12 cells against apoptosis. Apoptosis. 2010;15:738–751. doi: 10.1007/s10495-010-0496-6. [DOI] [PubMed] [Google Scholar]
- Welch WJ. The role of heat-shock proteins as molecular chaperones. Curr Opin Cell Biol. 1991;3:1033–1038. doi: 10.1016/0955-0674(91)90125-I. [DOI] [PubMed] [Google Scholar]
- Yenari MA. Heat shock proteins and neuroprotection. Adv Exp Med Biol. 2002;513:281–299. doi: 10.1007/978-1-4615-0123-7_10. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Giffard RG, Sapolsky RM, Steinberg GK. The neuroprotective potential of heat shock protein 70 (HSP70) Mol Med Today. 1999;5:525–531. doi: 10.1016/S1357-4310(99)01599-3. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- Young JC. Mechanisms of the Hsp70 chaperone system. Biochem Cell Biol. 2010;88:291–300. doi: 10.1139/O09-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, Krogan N, Cagney G, Mai D, Greenblatt J, Boone C, Emili A, Houry WA. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120:715–727. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Zhao F, Wu T, Lau A, Jiang T, Huang Z, Wang XJ, Chen W, Wong PK, Zhang DD (2009) Nrf2 promotes neuronal cell differentiation. Free Radic Biol Med 47:867–879 [DOI] [PMC free article] [PubMed]
- Zhu X, Su B, Wang X, Smith MA, Perry G. Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci. 2007;64:2202–2210. doi: 10.1007/s00018-007-7218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]