Abstract
Accumulation of mis- and unfolded proteins during viral replication can cause stress in the endoplasmic reticulum (ER) and trigger the unfolded protein response (UPR). If unchecked, this process may induce cellular changes detrimental to viral replication. In the report, we investigated the impact of HSV-1 on the UPR during lytic replication. We found that HSV-1 effectively disarms the UPR in early stages of viral infection. Only ATF6 activation was detected during early infection, but with no upregulation of target chaperone proteins. Activity of the eIF2α/ATF4 signaling arm increased at the final stage of HSV-1 replication, which may indicate completion of virion assembly and egress, thus releasing suppression of the UPR. We also found that the promoter of viral ICP0 was responsive to ER stress, an apparent mimicry of cellular UPR genes. These results suggest that HSV-1 may use ICP0 as a sensor to modulate the cellular stress response.
Keywords: Herpes simplex virus, Endoplasmic reticulum stress, Unfolded protein response, ICP0, Viral mimicry
Introduction
The unfolded protein response (UPR) is an immediate cellular response necessary to maintain the equilibrium homeostasis of the ER. This natural adaptation relies on a combination of transcriptional and translational controls which help alleviate the protein burden caused by the accumulation of mis- and unfolded proteins in the ER. There are currently three well-established UPR signaling pathways, each represented by an ER-transmembrane sensor protein, ATF6, IRE1, or PERK. These sensor proteins are activated during ER stress and trigger a complex cascade of signals that activate downstream UPR-related genes (Fig. 1). Control over these cascades is mediated by ER stress-responsive proteins which bind to three known cis-acting elements in the promoter regions of UPR-related genes, i.e., ER stress–response element (ERSE), ER stress–response element II (ERSE-II), and unfolded protein response element (UPRE). Activation of these elements can lead to induction of chaperones, translational attenuation, ER-associated degradation (ERAD) of accumulated proteins, and ultimately cell death by apoptosis (reviewed in Bernales et al. 2006; Tabas and Ron 2011; Schroder 2008; Malhotra and Kaufman 2007; Mori 2009). Recently, a family of highly similar basic region leucine zipper (bZIP) proteins exemplified by CREB3/Luman (Liang et al. 2006; Raggo et al. 2002; Lu et al. 1997; Freiman and Herr 1997) has also been implicated in the UPR in specific cell types, including CREB3L1 (Chin et al. 2005; Omori et al. 2001), CREB3L2 (Storlazzi et al. 2003), CREB3L3 (Honma et al. 1999), and CREB3L4 (Cao et al. 2002; Stirling and O'Hare 2006; Qi et al. 2002; Stelzer and Don 2002; Nagamori et al. 2005).
Fig. 1.
Schematic illustration of the mammalian unfolded protein response. ER localized transmembrane proteins ATF6, IRE1, and PERK are activated in response to ER stress. ATF6 is cleaved in the Golgi releasing the N-terminus encoding a transcription factor, which transports into the nucleus to primarily induce ER chaperone genes (e.g., GRP78). IRE1 is activated by dimerization and auto-phosphorylation, mediating the splicing of XBP1 mRNA. Spliced XBP1 primarily induces expression of ERAD components (e.g., EDEM). PERK is also activated by dimerization and phosphorylation, which then phosphorylates eIF2α. Phosphorylated eIF2α causes global translational attenuation, but selectively activates ATF4 translation. In addition to the well-established three UPR branches described previously, CREB3 family proteins also have an ER-transmembrane domain that anchors them to the ER. Upon ER stress, these CREB3 proteins undergo regulated intramembrane proteolysis like ATF6 and translocate to the nucleus, thereby activating the downstream UPR genes. The CREB3/Luman protein appears to be primarily involved in ERAD
The UPR is triggered by factors such as lipid metabolism, differentiation of secretory cells, DNA damage, chemical insult, as well as viral infection (Schroder 2008). Viral activation of the UPR may be caused by the rapid synthesis of large amounts of viral proteins during infection. A number of viruses have evolved mechanisms to curtail or customize UPR signaling for their own benefit, including human cytomegalovirus (HCMV) (Isler et al. 2005; Buchkovich et al. 2008; Xuan et al. 2009), hepatitis C virus (HCV) (Tardif et al. 2002; Sir et al. 2008a, b), hepatitis B virus (HBV) (Li et al. 2007), Japanese encephalitis virus (JEV) (Su et al. 2002), bovine viral diarrhea virus (BVDV) (Jordan et al. 2002), Epstein-Barr virus (Freire et al. 2008; Lee and Sugden 2008), and adenovirus (Pahl et al. 1996). Manipulation of UPR pathways in infected cells often align with the specific needs of the virus at various stages of replication. This may involve the activation of UPR pathways that regulate molecular chaperone expression and increase folding capacity in the cell or repression of UPR pathways that trigger global translational attenuation and ER-associated degradation.
Herpes simplex virus type 1 (HSV-1) is a large (~152 kb) fast replicating DNA virus that produces over 70 viral proteins. The rapid production of large quantities of viral proteins during HSV replication may induce the UPR and consequently necessitate modulation of the cellular stress response. Indeed, a number of HSV-1 proteins have been shown to block phosphorylation of eIF2α, an important stress response mechanism of the cell, which leads to the attenuation of global protein synthesis (Cassady et al. 1998; He et al. 1996; Mulvey et al. 2006, 2007, 2003). Two kinases of eIF2α, PKR and PERK, are repressed by the viral proteins US11 (Cassady et al. 1998; Mulvey et al. 2003) and glycoprotein B (Mulvey et al. 2007), respectively. Another late protein γ134.5 promotes dephosphorylation of eIF2α by recruiting the cellular phosphatase PP1α (He et al. 1996). The outcome of this inhibition is a 1,000-fold increase in viral replication efficiency (Talloczy et al. 2006). To investigate whether and how HSV-1 manipulates components of the UPR, this report examined the impact of HSV-1 replication on the UPR throughout the course of infection.
Materials and methods
Materials
All restriction endonucleases and modifying enzymes were purchased from New England Biolabs. Oligonucleotides were purchased from Qiagen Operon, and Expand High Fidelity PCR DNA polymerase was purchased from Roche. All other reagents were obtained from Fisher Scientific unless otherwise noted.
Plasmids
The reporter plasmids pGL3-5 × UPRE-luciferase (gift from Ron Prywes, Columbia University), pGL3-3 × ERSE-luciferase (Tirasophon et al. 1998), and pGL3-3 × ERSE-II luciferase (Audas et al. 2008) contain five repeats of the UPRE sequence TGACGTG(G/A), three repeats of the ERSE sequence CCAAT-N9-CCACG, or three repeats of the ERSE-II sequence ATTGG-N-CCACG, respectively, linked to the coding sequence for firefly (Photinuspyralis) luciferase. The reporters pGL3-ICP0-luciferase and pGL3-ICP27-luciferase were created by PCR amplification of the sequence 1 kb upstream of the transcriptional start sites with primers containing additional restriction sites and inserted between the HindIII and KpnI sites of the reporter plasmid pGL3-Basic (Promega). The pRL-SV40 (Promega) contains the Renilla luciferase gene linked to the SV40 immediate-early promoter. The plasmids expressing ICP0 and ICP4 were PCR amplified from HSV-1 genomic DNA and inserted between the EcoRI and XhoI sites of the pFLAG-C vector (Audas et al. 2008).
Cell culture, transfection, and treatment
HeLa, Vero, U2OS, and IRE1+/+ and IRE1−/−mouse embryonic fibroblast (MEF) cells were grown in monolayer culture in Dulbecco's modified Eagle's medium (high glucose) supplemented with 10% (v/v) fetal bovine serum (Invitrogen), 100-IU/ml penicillin, and 100-μg/ml streptomycin. All cultures were maintained in a 5% CO2 humidified atmosphere at 37°C and passaged every 2–3 days. Cells were plated 24 h prior to transfection and allowed to grow to 50–60% confluency. Cells were transfected with DreamFect (OZ Biosciences) as per manufactures' instruction. The ER stressors, thapsigargin, tunicamycin, H2O2, dithiothreitol, and brefeldin A were used at concentrations of 300 nM, 2 μg/ml, 300 nM, 1 mM, and 1 μg/ml, respectively.
Viruses
The wildtype HSV-1 strains KOS and the ICP0 mutant strain 7134 (Cai and Schaffer 1989) were obtained from Karen Mossman at McMaster University. Wildtype (KOS) viral stocks were propagated in Vero cells and ICP0 null stocks (7134) in U2OS cells as described previously (Forrester et al. 1992; Yao and Schaffer 1995). Infections were carried out at a multiplicity of infection (MOI) of 10. One hour post-infection, media were replaced, and the cells were maintained at 37°C for the indicated times.
Luciferase assays
Cells were transfected as described previously. For luciferase assays involving viral infection, cells were infected 48 h post-transfection and harvested at the indicated time points. Cellular lysates were extracted, and dual luciferase assays were performed according to the manufacturer's protocols (Promega). Luciferase activity was measured using a Turner TD-20e Luminometer and calculated as relative luciferase activity (firefly luciferase/Renilla luciferase) to correct for transfection efficiency. Assays were independently repeated at least three times. Data are shown with standard errors relative to untreated, mock infected cells.
Western blotting
Lysates were harvested in Passive Lysis Buffer (Promega), quantified using the BCA protein assay (Pierce Biotechnology Inc., Rockford, IL) to normalize protein concentrations. Blots were probed with primary antibodies: eIF2α (sc-11386, Santa Cruz) at 1:1,000, ATF4 (sc-200, Santa Cruz) at 1:2,000, GRP78 (also known as BIP or HSPA5) (sc-13968, Santa Cruz) at 1:1,000, GRP94 (also called HSPC4) (sc-11402, Santa Cruz) at 1:1,000, phosphorylated eIF2α (44-728G, Biosource) at 1:1,000, ATF6 at 1:1,500 (IMG-273, Imgenex), M2 (A2220, Sigma) at 1:1,500, and β-actin (A5316, Sigma) at 1:2,000. Blots were visualized using ECL Plus (GE Healthcare) on a Typhoon 9400 Phosphorimager (GE Healthcare).
RNA analysis
Total RNA was isolated from HeLa cells with Trizol reagent (Invitrogen). Quantification of RNA was performed using the Nano Drop 1000 (ThermoScientific). Synthesis of cDNA was carried out using SuperScriptII reverse transcriptase (Invitrogen) and oligo(dT) (Roche). PCR amplification was performed using the following primer sets: ICP0 (5'-GTGCTGCGCCAAGAAAAT) and (5'-TCAACTCGCAGACACGACTC), ICP47 (5'-ATGTCGTGGGCCCTGG) and (5'-TCAACGGGTTACCGGATTAC), ICP6 (5'-TACGCCAGAGCATGATGAA) and (5'-CTGTTTTTAGTCCGCGCTTA), gC (5'-TGGAGTTTGTCTGGTTCGAG) and (5'-GTGACTGCCGTGGTGTTCTA), and XBP1 (5′-CTGGAACAGCAAGTGGTAGA) and (5′-CTGGGTCCTTCTGGGTAGAC)
qRT-PCR
Quantitative RT-PCR (qRT-PCR) analysis was carried out using SYBR Green PCR MasterMix (Applied Biosystems), and samples were analyzed using an ABI 7300 system. The intron-flanking primers used include: ATF4 (5'-CCCTCCAACAACAGCAAGGA) and (5'-ACCCAACAGGGCATCCAAG), CHOP (5'-CATCACCACACCTGAAAGCAGA) and (5'-TGGACAGTGTCCCGAAGGAG), EDEM (5'-GCACAGGCCGAAACCTCAT) and (5'-TGCTCTTTAAGGGCAGGGAG), GRP78 (5'-CAACCAAAGACGCTGGAACTATT) and (5'-CCCTCTTATCCAGGCCATAAGC), and GRP94 (5'-TCAGAGACATGCTTCGACGAA) and (5'-CTGACCGAAGCGTTGCTGT). Standard curves generated were analyzed using the ABI 7300 System Sequence Detection Software, version 1.2.2, and are shown relative to the mock infected cells. Data are presented as the averages of three independent repeats.
Results
HSV-1 infection activates UPR cis-acting enhancer elements
The UPR responsive elements UPRE, ERSE, and ERSE-II are key enhancer elements found in the promoters of numerous ER stress responsive genes and are commonly used as hallmark indicators of ER stress. Therefore, we sought to determine whether HSV-1 infection induces the UPR through these elements. HeLa cells were transiently transfected with the reporter plasmids pGL3-5 × UPRE-luciferase, pGL3-3 × ERSE-luciferase, or pGL3-3 × ERSE-II-luciferase and infected with a wildtype strain of HSV-1 (KOS). Cells treated with thapsigargin were used as a positive control. Infection with HSV-1 augmented UPRE, ERSE, and ERSE-II-mediated transcription throughout the course of the 24-h infection, with relative luciferase levels from ERSE and ERSE-II comparable to those of positive controls (Fig. 2).
Fig. 2.
HSV-1 induces transcription of ER stress-responsive elements. HeLa cells were transiently transfected with pGL3-5 × UPRE-luciferase (a), pGL3-3 × ERSE-luciferase (b), or pGL3-3 × ERSE-II-luciferase (c) and the control plasmid pRL-SV40. At 48 h post-transfection, cells were mock or HSV-1 (KOS) infected for the indicated times. Control cells were harvested after 8 h of thapsigargin treatment
ATF6 proteolysis is induced in early viral infection, but with no upregulation of ER chaperone proteins
As viral infection appears to activate the UPR, we next wanted to examine the effects of HSV-1 on the major UPR sensing pathways. ATF6 is activated by regulated intramembrane proteolysis, which liberates the transcriptionally active amino-terminus from the ER to stimulate transcription of several ER chaperone genes (Yoshida et al. 2000, 2001). To assay for ATF6 activation, western blotting was performed on cells transiently transfected with 3 × FLAG-ATF6 and infected with HSV-1 or treated with Tg. ATF6 was found to be proteolytically cleaved into its transcriptionally active form as early as 2 h post-infection, but was subsequently suppressed by the 8-h mark (Fig. 3a, first panel). Protein and mRNA expression levels of the ER-resident chaperones GRP78 and GRP94, the downstream targets of ATF6, were unchanged, indicating that these two genes were not affected by HSV-1 (Fig. 3). These results indicate that the ATF6 signaling arm of the UPR is impaired during HSV-1 infection.
Fig. 3.
ATF6 is proteolytically cleaved during the early stages of viral replication, but protein chaperones are not upregulated. a HeLa cells transiently transfected with ATF6 were untreated, HSV-1 (KOS) infected, or incubated with thapsigargin. Protein lysates were harvested at the indicated times and analyzed by western blotting. Antibodies specific for FLAG (M2), GRP78, GRP94, and β-actin were used. b qRT-PCR analysis of GRP78 and GRP94 expression levels in mock or HSV-1 (KOS)-infected HeLa cells for the indicated times
HSV-1 infection represses phosphorylation of eIF2α and reduces ATF4 expression
To determine whether the eIF2α/ATF4 arm of the UPR is affected by HSV-1, levels of eIF2α, phosphorylated eIF2α, and ATF4 were examined by western blotting. Though eIF2α expression remained constant throughout the course of infection (Fig. 4a, top panel), levels of the phosphorylated form and its downstream target protein ATF4 (Fig. 1) decreased from 2 to 8 h post-infection, only to be elevated by 24 h (Fig. 4a). At the mRNA level, both ATF4 and its downstream target CHOP followed a similar trend of early repression and late activation in response to HSV-1 infection, as assessed by quantitative RT-PCR (Fig. 4b). Activation of ATF4 and CHOP can lead to translational attenuation and apoptosis (Harding et al. 2000; Jiang et al. 2004; Lu et al. 2004; Vattem and Wek 2004); therefore, it seems logical that HSV-1 would inhibit these processes until the final stage of viral replication as apoptosis may aid in viral particle release.
Fig. 4.
Activation of the eIF2α/ATF4 arm in late stage of HSV-1 replication. a Proteins from mock, HSV-1 (KOS)-infected, or thapsigargin-treated HeLa cells were harvested at the indicated times, and lysates were analyzed by western blot. Antibodies specific for eIF2α, phosphorylated eIF2α, ATF4, and β-actin were used. b qRT-PCR analysis of ATF4 and CHOP expression levels in HeLa cells left untreated, thapsigargin treated, or HSV-1 (KOS) infected for the indicated times. P-values were standardized to untreated, mock infected cells (*p < 0.1; **p < 0.05)
The IRE1/XBP-1 branch of the UPR is repressed during HSV-1 infection
Next, we studied the effect of HSV-1 infection on the IRE1/XBP1 branch of the UPR. Utilizing semi-quantitative RT-PCR primers capable of distinguishing between the unspliced inactive form (XBP(u)) and the transcriptionally potent spliced form (XBP(s)) (Fig. 5a, upper panel), we screened wildtype and IRE1-knockout mouse embryonic fibroblast (MEF) cell line as these cells lack the spliceosome-independent enzymatic activity required to modify XBP1 mRNA. We found that in untreated IRE1+/+ cells, both variants are observed; however, upon HSV-1 infection, the spliced XBP1 isoform is no longer detectable, while the unspliced mRNA remained stable for up to 4 h, with a drastic decrease seen between 8 and 24 h post-infection (Fig. 5a). IRE1−/−cells showed no detectable XBP1 splicing during ER stress induction with Tg or HSV-1 infection, though the unspliced mRNA expression profile was similar to the wildtype cells (Fig. 5a, bottom panel). Next, we used quantitative RT-PCR to examine two downstream targets of XBP1: EDEM and Herp. In both cases, mRNA levels during HSV-1 infection were similar to those observed in mock infected cells and significantly lower than during Tg treatment (Fig. 5b). Together, these data demonstrate that the IRE1/XBP1 axis of the UPR is effectively silenced by HSV-1, preventing unfavorable effects such as ER-associated degradation.
Fig. 5.
The IRE1/XBP1 signaling arm of the UPR is silenced during HSV-1 replication. a A diagram depicting the IRE1-mediated excision of 26-bases from XBP1(u) mRNA, resulting in the generation, due to frameshifting, of the potent transcription factor XBP(s) (top panel) and semi-quantitative RT-PCR analysis of XBP1 expression levels in mock or HSV-infected IRE1+/+ and IRE1−/− MEF cells (bottom panel). Cells treated with thapsigargin for 8 h were used as a positive control for ER stress induction. b qRT-PCR analysis of EDEM and Herp mRNA in mock or HSV-1 (KOS)-infected HeLa cells for the indicated times. Cells treated with thapsigargin for 8 h were used as positive inducer of ER stress. c–e The IRE1/XBP-1 pathway is not essential for the activation of ER stress-responsive elements by HSV-1 infection. IRE1+/+ and IRE1−/− MEF cells were transiently transfected with pGL3-5 × UPRE-luciferase (c), pGL3-3 × ERSE-luciferase (d), or pGL3-3 × ERSE-II-luciferase (e) and the control plasmid pRL-SV40. At 48 h post-transfection, cells were mock or HSV-1 (KOS) infected for the indicated times
Although XBP1 has been shown to be the primary activator of the UPRE enhancer (Yoshida et al. 2003; Yamamoto et al. 2004, 2007), it is capable of mediating ER stress-induced transcription of all three cis-acting elements (Yoshida et al. 2003; Yamamoto et al. 2004, 2007). Therefore, we wanted to examine whether the IRE1/XBP1 branch of the UPR contributed to the activation of ER-responsive elements observed during HSV-1 infection (Fig. 2). Wildtype (IRE1+/+) and mutant (IRE1−/−) MEF cells were transfected with the ER stress-responsive reporter plasmids and infected with HSV-1. For each reporter tested, a similar activation profile was observed in the IRE1+/+ and IRE1−/− MEF cells, as expression steadily increased from 2 to 24 h post-infection, though the activation in IRE1−/− cells appears to be somewhat reduced (Fig. 5c–e), suggesting that IRE1 may play a role in achieving maximal UPR activation during HSV-1 infection. Together, these data demonstrate that although the IRE1/XBP1 axis of the UPR is effectively silenced, an unrelated factor activates these ER stress response elements during HSV-1 infection.
ICP0 activates UPR enhancer elements
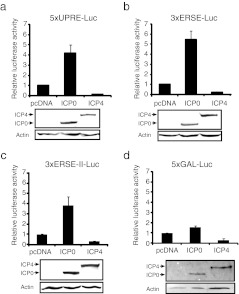
As HSV-1-mediated activation of UPR-responsive elements occurs at the earliest time points during infection (Figs. 2 and 5c–e), we were therefore interested in examining a potential role for ICP0 and ICP4, the only two immediate-early gene products known to have transcription factor capabilities (Everett 1988; Yao and Schaffer 1994). Using the ER stress-responsive reporter plasmids, we observed that ectopically expressing ICP0, but not ICP4, activated reporter gene expression (Fig. 6). To rule out the possibility that ICP0 might augment transcription non-specifically, a reporter containing 5 × GAL4 UAS was also included, showing minimal activation (Fig. 6d).
Fig. 6.
ICP0 activates ER stress-responsive elements. HEK293 cells were transfected with pGL3-5 × UPRE-luciferase (a), pGL3-3 × ERSE-luciferase (b), pGL3-3 × ERSE-II-luciferase (c), or pGL3-5 × Gal4 UAS-luciferase (d) and pRL-SV40 along with the expression plasmids pcDNA3.1, pFLAG-ICP0, or pFLAG-ICP4. Lysates were also analyzed by western blotting for the protein expression levels using antibodies against FLAG (M2) and β-actin
The potential role of ICP0 was further tested using the ICP0 null strain of HSV-1 (7134), which has the entire ICP0 open reading frame removed (Cai and Schaffer 1989). To confirm that both the ICP0 knockout and wildtype strains could elicit a productive infection, we used semi-quantitative RT-PCR analysis to show that both strains produced similar HSV-1 gene temporal transcription kinetics (Fig. 7a). Interestingly, in all cases where the ICP0 deficient virus was used, activation of UPRE, ERSE, and ERSE-II-responsive reporter plasmids was severely diminished when compared to the wildtype virus (Fig. 7b; at 24 dpi, p ≤ 4 × 10-5 for UPRE and ERSE and p < 0.03 for ERSE-II). Additionally, the timeline of ICP0 expression was found to coincide well with ER stress-responsive activation (Fig. 7).These results suggest that the viral ICP0 protein is the main factor triggering activation of the UPR enhancers during HSV-1 replication.
Fig. 7.
ICP0 is essential for activation of ER stress-responsive elements during HSV-1 infection. a HeLa cells were mock infected or infected with the wildtype (KOS) or ICP0-null (7134) strain of HSV-1. Total RNA was harvested at the indicated times and analyzed by semi-quantitative RT-PCR. b Wildtype MEF cells were transiently transfected with pGL3-5 × UPRE-luciferase, pGL3-3 × ERSE-luciferase, or pGL3-3 × ERSE-II-luciferase and the control plasmid pRL-SV40. At 48 h post-transfection, cells were left untreated, incubated with thapsigargin for 8 h, or infected with the wildtype (KOS) or ICP0-null (7134) strains of HSV-1 for the indicated times
The ICP0 promoter is responsive to ER stress
Re-establishment of lytic HSV-1 infection has been attributed to the receipt of stress-induced signaling by ICP0 (Everett 2000; Halford et al. 2001; Halford and Schaffer 2001; Leib et al. 1989). As the virus is capable of efficiently disarming the UPR, it likely possesses an early sensing mechanism to monitor the status of the cellular stress response. Therefore, we assayed whether ICP0 was responsive to various ER stressors including thapsigargin (Tg), tunicamycin (Tm), hydrogen peroxide (H2O2), dithiothreitol (DTT), and brefeldin A (Bref). Unlike the unresponsive control construct containing the promoter of ICP27, another immediate-early gene, the ICP0 promoter, showed a similar trend of responsiveness to ER stress when compared to the UPRE reporter (Fig. 8a).
Fig. 8.
The ICP0 promoter responds to ER stress and HSV-1 infection. a HeLa cells were transiently transfected with pGL3-5 × UPRE-luciferase, pGL3-ICP0-luciferase, or pGL3-ICP27-luciferase and the control plasmid pRL-SV40. At 24 h post-transfection, cells were treated for 8 h with thapsigargin (Tg), tunicamycin (Tm), H2O2, dithiothreitol (DTT), or brefeldin A, and luciferase assays were performed. b HeLa cells were transiently transfected with pGL3-ICP0-luciferase and the control plasmid pRL-SV40. At 48 h post-transfection, cells were mock infected or infected with wildtype (KOS) or ICP0-null (7134) strain of HSV-1 for the indicated times
Since the ICP0 promoter is responsive to ER stress (Fig. 8) and HSV-1 infection activates UPR responsive elements (Fig. 2), we sought to determine whether ICP0 is capable of auto-activation during viral infection. HeLa cells transiently transfected with the ICP0 promoter reporter construct were infected with wildtype (KOS) or ICP0-null (7134) virus. As expected, at 2 and 4 h post-infection, equal activation was observed in cells infected with wildtype and null strains, likely owing to the effect of VP16, the viral protein responsible for driving immediate-early genes activation during primary HSV-1 infection (Wysocka et al. 2003). However, between 8 and 24 h post-infection, cells infected with the wildtype strain showed a significantly greater activation of the ICP0 promoter (Fig. 8b).These results suggest that ICP0 can auto-activate itself through positive feedback during HSV-1 infection.
Discussion
Early disarmament of the UPR by HSV-1
Using a reporter assay, activation of the ER stress response elements UPRE, ERSE, and ERSE-II was detected throughout various stages of HSV-1 replication (Fig. 2); however, these potential gene activation events were not reflected in mRNA or protein levels of UPR signaling molecules. Our findings suggest that rapid induction of UPR gene expression is quickly aborted following viral infection, which may be explained by mRNA degradation triggered by the viral proteins virion host shutoff (vhs) and ICP27 (Laurent et al. 1998; Elgadi et al. 1999). Of the three well-studied UPR signaling branches, only ATF6 appeared to be activated by proteolysis in early stages of HSV-1 replication, though its downstream targets, the ER chaperones GRP78 and GRP94, were not upregulated (Fig. 3). This is likely due to the fact that prior to infection, a significant level of chaperones already exists in the cell. Activating the ATF6 signaling pathway to further increase the production of chaperones may induce ER stress responses that are harmful to HSV-1 replication, such as ERAD through crosstalk of UPR signal pathways.
Phosphorylation of eIF2α was also inhibited, likely causing the observed early repression of ATF4 and CHOP. Only at later stages of infection were these proteins activated (24 h post-infection, Fig. 4). The function of this inhibition is likely to prevent global translational attenuation and ensure that abundant viral mRNAs are translated by the cell after viral DNA replication. Release of this repression and induction of CHOP at the final stage of HSV-1 replication may trigger apoptosis, assisting the release of newly synthesized viral particles from the host cell. In fact, JEV and BVDV have been shown to trigger apoptosis in order to augment viral egress (Su et al. 2002).
For the IRE1/XBP1 signaling pathway, we found that XBP1 was inactive, and levels of the unspliced precursor actually diminished (Fig. 5a), consistent with previous findings (Mulvey et al.). XBP1 is primarily responsible for UPRE activation in many cell types and plays a key role in ER stress-associated protein degradation (ERAD). It is thus conceivable that the activation of XBP1 and expression of the ERAD-associated proteins Herp and EDEM are suppressed during viral infection to prevent viral protein degradation and facilitate mass production of the viral proteome.
Is ICP0 an ER stress sensor for HSV-1?
In this study, we discovered that ICP0 mimics cellular UPR genes as its promoter is responsive to ER stress (Fig. 8). This agrees with the previous finding that transcription from the ICP0 promoter is detected within 1 h of hyperthermic stress treatment (Thompson and Sawtell 2006). Thus, it is tempting to speculate that ICP0 may be an ER stress sensor for the virus. ICP0's ability to auto-activate itself via a positive feedback loop during HSV-1 replication may allow the ER stress signals intercepted by ICP0 to be quickly amplified. These signals could then be used by the virus to regulate replication kinetics during lytic infection and potentially trigger reactivation from latency. This hypothesis is supported by the fact that induction of cellular stress or infection at a high multiplicity of infection (MOI) overcomes the requirement of ICP0 in the reactivation from quiescent viral genomes (Preston 2007; Everett et al. 2004; Cai and Schaffer 1992; Chao et al. 1992; Stow and Stow 1986).
In summary, HSV-1 appears to use ICP0 as a sensor of the cellular ER stress response, while the virus efficiently disarms the UPR early in viral infection. This results in the prevention of translational attenuation and ER stress-induced protein degradation, in turn, creating a host cell environment that is conducive to viral gene expression and particle packaging.
Acknowledgement
We would like to thank Randal Kaufman for the IRE1−/− cells, Karen Mossman for the HSV-1 KOS and the 7134 strains, and Ron Prywesfor the pGL3-5 × UPRE-luciferase plasmid. This work was supported by funds from the Canadian Institutes for Health Research and Natural Sciences and Engineering Research Council of Canada.
Footnotes
Heather F. Burnett and Timothy E. Audas contributed equally to this work.
References
- Audas TE, Li Y, Liang G, Lu R. A novel protein, Luman/CREB3 recruitment factor, inhibits Luman activation of the unfolded protein response. Mol Cell Biol. 2008;28(12):3952–3966. doi: 10.1128/MCB.01439-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- Buchkovich NJ, Maguire TG, Yu Y, Paton AW, Paton JC, Alwine JC. Human cytomegalovirus specifically controls the levels of the endoplasmic reticulum chaperone BiP/GRP78, which is required for virion assembly. J Virol. 2008;82(1):31–39. doi: 10.1128/JVI.01881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai WZ, Schaffer PA. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J Virol. 1989;63(11):4579–4589. doi: 10.1128/jvi.63.11.4579-4589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Schaffer PA. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J Virol. 1992;66(5):2904–2915. doi: 10.1128/jvi.66.5.2904-2915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Ni X, Jiang M, Ma Y, Cheng H, Guo L, Ji C, Gu S, Xie Y, Mao Y. Molecular cloning and characterization of a novel human cAMP response element-binding (CREB) gene (CREB4) J Hum Genet. 2002;47(7):373–376. doi: 10.1007/s100380200053. [DOI] [PubMed] [Google Scholar]
- Cassady KA, Gross M, Roizman B. The herpes simplex virus US11 protein effectively compensates for the gamma1(34.5) gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2. J Virol. 1998;72(11):8620–8626. doi: 10.1128/jvi.72.11.8620-8626.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Yam WC, Chen LK, Lin-Chao S. Cloning of a functional Burkitt's lymphoma polypeptide-binding protein/78 kDa glucose-regulated protein (BiP/GRP78) gene promoter by the polymerase chain reaction, and its interaction with inducible cellular factors. Biochem J. 1992;286(Pt 2):555–559. doi: 10.1042/bj2860555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K-T, Zhou H-J, Wong C-M, Lee JM-F, Chan C-P, Qiang B-Q, Yuan J-G, Ng IO-l, Jin D-Y. The liver-enriched transcription factor CREB-H is a growth suppressor protein underexpressed in hepatocellular carcinoma. Nucl Acids Res. 2005;33(6):1859–1873. doi: 10.1093/nar/gki332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgadi MM, Hayes CE, Smiley JR. The herpes simplex virus vhs protein induces endoribonucleolytic cleavage of target RNAs in cell extracts. J Virol. 1999;73(9):7153–7164. doi: 10.1128/jvi.73.9.7153-7164.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett RD. Analysis of the functional domains of herpes simplex virus type 1 immediate-early polypeptide Vmw110. J Mol Biol. 1988;202(1):87–96. doi: 10.1016/0022-2836(88)90521-9. [DOI] [PubMed] [Google Scholar]
- Everett RD. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays. 2000;22(8):761–770. doi: 10.1002/1521-1878(200008)22:8<761::AID-BIES10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Everett RD, Boutell C, Orr A. Phenotype of a herpes simplex virus type 1 mutant that fails to express immediate-early regulatory protein ICP0. J Virol. 2004;78(4):1763–1774. doi: 10.1128/JVI.78.4.1763-1774.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992;66(1):341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiman RN, Herr W. Viral mimicry: common mode of association with HCF by VP16 and the cellular protein LZIP. Genes Dev. 1997;11(23):3122–3127. doi: 10.1101/gad.11.23.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire E, Oddo C, Frappier L, Prat-Gay G. Kinetically driven refolding of the hyperstable EBNA1 origin DNA-binding dimeric beta-barrel domain into amyloid-like spherical oligomers. Proteins. 2008;70(2):450–461. doi: 10.1002/prot.21580. [DOI] [PubMed] [Google Scholar]
- Halford WP, Schaffer PA. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J Virol. 2001;75(7):3240–3249. doi: 10.1128/JVI.75.7.3240-3249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford WP, Kemp CD, Isler JA, Davido DJ, Schaffer PA. ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J Virol. 2001;75(13):6143–6153. doi: 10.1128/JVI.75.13.6143-6153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/S1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- He B, Chou J, Liebermann DA, Hoffman B, Roizman B. The carboxyl terminus of the murine MyD116 gene substitutes for the corresponding domain of the gamma(1)34.5 gene of herpes simplex virus to preclude the premature shutoff of total protein synthesis in infected human cells. J Virol. 1996;70(1):84–90. doi: 10.1128/jvi.70.1.84-90.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma Y, K-y K, Mori T, Tanno Y, Tojo M, Kiyosawa H, Takeda J, Nikaido T, Tsukamoto T, Yokoya S, Wanaka A. Identification of a novel gene, OASIS, which encodes for a putative CREB/ATF family transcription factor in the long-term cultured astrocytes and gliotic tissue. Mol Brain Res. 1999;69(1):93–103. doi: 10.1016/S0169-328X(99)00102-3. [DOI] [PubMed] [Google Scholar]
- Isler JA, Skalet AH, Alwine JC. Human cytomegalovirus infection activates and regulates the unfolded protein response. J Virol. 2005;79(11):6890–6899. doi: 10.1128/JVI.79.11.6890-6899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, Wang X, Ron D, Cavener DR, Wek RC. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol. 2004;24(3):1365–1377. doi: 10.1128/MCB.24.3.1365-1377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R, Wang L, Graczyk TM, Block TM, Romano PR. Replication of a cytopathic strain of bovine viral diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J Virol. 2002;76(19):9588–9599. doi: 10.1128/JVI.76.19.9588-9599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent AM, Madjar JJ, Greco A. Translational control of viral and host protein synthesis during the course of herpes simplex virus type 1 infection: evidence that initiation of translation is the limiting step. J Gen Virol. 1998;79(Pt 11):2765–2775. doi: 10.1099/0022-1317-79-11-2765. [DOI] [PubMed] [Google Scholar]
- Lee DY, Sugden B. The LMP1 oncogene of EBV activates PERK and the unfolded protein response to drive its own synthesis. Blood. 2008;111(4):2280–2289. doi: 10.1182/blood-2007-07-100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leib DA, Coen DM, Bogard CL, Hicks KA, Yager DR, Knipe DM, Tyler KL, Schaffer PA. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63(2):759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Gao B, Ye L, Han X, Wang W, Kong L, Fang X, Zeng Y, Zheng H, Li S, Wu Z, Ye L. Hepatitis B virus X protein (HBx) activates ATF6 and IRE1-XBP1 pathways of unfolded protein response. Virus Res. 2007;124(1–2):44–49. doi: 10.1016/j.virusres.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Liang G, Audas TE, Li Y, Cockram GP, Dean JD, Martyn AC, Kokame K, Lu R. Luman/CREB3 induces transcription of the endoplasmic reticulum (ER) stress response protein Herp through an ER stress response element. Mol Cell Biol. 2006;26(21):7999–8010. doi: 10.1128/MCB.01046-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Yang P, O'Hare P, Misra V. Luman, a new member of the CREB/ATF family, binds to herpes simplex virus VP16-associated host cellular factor. Mol Cell Biol. 1997;17(9):5117–5126. doi: 10.1128/mcb.17.9.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167(1):27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Sem Cell Mol Biol. 2007;18(6):716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K (2009) Signaling pathways in the unfolded protein response: development from yeast to mammals. J Biochem. doi:10.1093/jb/mvp166 [DOI] [PubMed]
- Mulvey M, Poppers J, Sternberg D, Mohr I. Regulation of eIF2alpha phosphorylation by different functions that act during discrete phases in the herpes simplex virus type 1 life cycle. J Virol. 2003;77(20):10917–10928. doi: 10.1128/JVI.77.20.10917-10928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M, Arias C, Mohr I. Resistance of mRNA translation to acute endoplasmic reticulum stress-inducing agents in herpes simplex virus type 1-infected cells requires multiple virus-encoded functions. J Virol. 2006;80(15):7354–7363. doi: 10.1128/JVI.00479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M, Arias C, Mohr I. Maintenance of endoplasmic reticulum (ER) homeostasis in herpes simplex virus type 1-infected cells through the association of a viral glycoprotein with PERK, a cellular ER stress sensor. J Virol. 2007;81(7):3377–3390. doi: 10.1128/JVI.02191-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamori I, Yabuta N, Fujii T, Tanaka H, Yomogida K, Nishimune Y, Nojima H. Tisp40, a spermatid specific bZip transcription factor, functions by binding to the unfolded protein response element via the Rip pathway. Genes Cells. 2005;10(6):575–594. doi: 10.1111/j.1365-2443.2005.00860.x. [DOI] [PubMed] [Google Scholar]
- Omori Y, J-i I, Watanabe M, Komatsu T, Suzuki Y, Kataoka K, Watanabe S, Tanigami A, Sugano S. CREB-H: a novel mammalian transcription factor belonging to the CREB/ATF family and functioning via the box-B element with a liver-specific expression. Nucl Acids Res. 2001;29(10):2154–2162. doi: 10.1093/nar/29.10.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl HL, Sester M, Burgert HG, Baeuerle PA. Activation of transcription factor NF-kappaB by the adenovirus E3/19K protein requires its ER retention. J Cell Biol. 1996;132(4):511–522. doi: 10.1083/jcb.132.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston CM. Reactivation of expression from quiescent herpes simplex virus type 1 genomes in the absence of immediate-early protein ICP0. J Virol. 2007;81(21):11781–11789. doi: 10.1128/JVI.01234-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Fillion C, Labrie Y, Grenier J, Fournier A, Berger L, El-Alfy M, Labrie C. AIbZIP, a novel bZIP gene located on chromosome 1q21.3 that is highly expressed in prostate tumors and of which the expression is up-regulated by androgens in LNCaP human prostate cancer cells. Cancer Res. 2002;62(3):721–733. [PubMed] [Google Scholar]
- Raggo C, Rapin N, Stirling J, Gobeil P, Smith-Windsor E, O'Hare P, Misra V. Luman, the cellular counterpart of herpes simplex virus VP16, is processed by regulated intramembrane proteolysis. Mol Cell Biol. 2002;22(16):5639–5649. doi: 10.1128/MCB.22.16.5639-5649.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65(6):862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir D, Chen WL, Choi J, Wakita T, Yen TS, Ou JH. Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology. 2008;48(4):1054–1061. doi: 10.1002/hep.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir D, Liang C, Chen WL, Jung JU, Ou JH. Perturbation of autophagic pathway by hepatitis C virus. Autophagy. 2008;4(6):830–831. doi: 10.4161/auto.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer G, Don J. Atce1: a novel mouse cyclic adenosine 3',5'-monophosphate-responsive element-binding protein-like gene exclusively expressed in postmeiotic spermatids. Endocrinology. 2002;143(5):1578–1588. doi: 10.1210/en.143.5.1578. [DOI] [PubMed] [Google Scholar]
- Stirling J, O'Hare P. CREB4, a transmembrane bZip transcription factor and potential new substrate for regulation and cleavage by S1P. Mol Biol Cell. 2006;17(1):413–426. doi: 10.1091/mbc.E05-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storlazzi CT, Mertens F, Nascimento A, Isaksson M, Wejde J, Brosjo O, Mandahl N, Panagopoulos I. Fusion of the FUS and BBF2H7 genes in low grade fibromyxoid sarcoma. Hum Mol Genet. 2003;12(18):2349–2358. doi: 10.1093/hmg/ddg237. [DOI] [PubMed] [Google Scholar]
- Stow ND, Stow EC. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol. 1986;67(Pt 12):2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- Su HL, Liao CL, Lin YL. Japanese encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. J Virol. 2002;76(9):4162–4171. doi: 10.1128/JVI.76.9.4162-4171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13(3):184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talloczy Z, Virgin HWT, Levine B. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy. 2006;2(1):24–29. doi: 10.4161/auto.2176. [DOI] [PubMed] [Google Scholar]
- Tardif KD, Mori K, Siddiqui A. Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J Virol. 2002;76(15):7453–7459. doi: 10.1128/JVI.76.15.7453-7459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RL, Sawtell NM. Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo. J Virol. 2006;80(22):10919–10930. doi: 10.1128/JVI.01253-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12(12):1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101(31):11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka J, Myers MP, Laherty CD, Eisenman RN, Herr W. Human Sin3 deacetylase and trithorax-related Set1/Ash2 histone H3-K4 methyltransferase are tethered together selectively by the cell-proliferation factor HCF-1. Genes Dev. 2003;17(7):896–911. doi: 10.1101/gad.252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan B, Qian Z, Torigoi E, Yu D. Human cytomegalovirus protein pUL38 induces ATF4 expression, inhibits persistent JNK phosphorylation, and suppresses endoplasmic reticulum stress-induced cell death. J Virol. 2009;83(8):3463–3474. doi: 10.1128/JVI.02307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem (Tokyo) 2004;136(3):343–350. doi: 10.1093/jb/mvh122. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13(3):365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Yao F, Schaffer PA. Physical interaction between the herpes simplex virus type 1 immediate-early regulatory proteins ICP0 and ICP4. J Virol. 1994;68(12):8158–8168. doi: 10.1128/jvi.68.12.8158-8168.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F, Schaffer PA. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J Virol. 1995;69(10):6249–6258. doi: 10.1128/jvi.69.10.6249-6258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol. 2000;20(18):6755–6767. doi: 10.1128/MCB.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6alpha and 6beta that activates the mammalian unfolded protein response. Mol Cell Biol. 2001;21(4):1239–1248. doi: 10.1128/MCB.21.4.1239-1248.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K. A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell. 2003;4(2):265–271. doi: 10.1016/S1534-5807(03)00022-4. [DOI] [PubMed] [Google Scholar]