Abstract
Weight gain and dysfunction of glucose and lipid metabolism are well-known side effects of atypical antipsychotic drugs (AAPD). Here, we address the question whether a heat-shock protein (HSP) co-inducer, insulin sensitizer drug candidate, BGP-15, can prevent AAPD-induced glucose, lipid, and weight changes. We also examined how an AAPD alters HSP expression and whether BGP-15 alters that expression. Four different experiments are reported on the AAPD BGP-15 interventions in a human trial of healthy men, a rodent animal model, and an in vitro adipocyte cell culture system. Olanzapine caused rapid insulin resistance in healthy volunteers and was associated with decreased level of HSP72 in peripheral mononuclear blood cells. Both changes were restored by the administration of BGP-15. In Wistar rats, weight gain and insulin resistance induced by clozapine were abolished by BGP-15. In 3T3L1 adipocytes, clozapine increased intracellular fat accumulation, and BGP-15 inhibited this process. Taken together, our results indicate that BGP-15 inhibits multiple metabolic side effects of atypical antipsychotics, and this effect is likely to be related to its HSP co-inducing ability.
Keywords: HSP, BGP-15, Hydroxylamines, Schizophrenia, Heat-shock proteins, Diabetes, Metabolic syndrome, Weight gain, Insulin resistance, Mitochondria, Insulin signaling, Atypical antipsychotic drugs, Therapy, Side effects, Membranes, HSP inducers, HSP72
Introduction
The atypical antipsychotic drugs are mainstays in the treatment of various psychiatric disorders including schizophrenia and acute mania in bipolar disorder. Metabolic side effects, insulin resistance, obesity, and dyslipidemia have been attributed to these newer, second-generation agents like clozapine (Clozaril), olanzapine (Zyprexa), and risperidone (Risperdal). The metabolic risks associated with various atypical antipsychotics are outlined in the American Diabetes Association–American Psychiatric Association Consensus Guidelines Panel (ADA et al. 2004).
In a recent meta-analysis, 15,767 schizophrenic patients without diabetes on atypical antipsychotic drugs (AAPD) therapy were studied. One third of newly appearing diabetes was attributed to use of olanzapine during the 1-year period of the study (Lambert et al. 2006). Furthermore, the average weight gains after 10-week treatments with clozapine and olanzapine were 3.99 and 3.51 kg, respectively (Allison et al. 1999). The mechanisms responsible for these side effects are poorly understood and are not prevented by any of the agents that are routinely used to treat type 2 diabetes (Baptista et al. 2006; Dwyer and Donohoe 2003). In addition to promoting weight gain, metabolic syndrome, and diabetes, the side effects of atypical antipsychotics result in poor compliance and interfere with effective treatment of psychiatric disease.
In the past decade, a new paradigm for understanding insulin resistance, metabolic syndrome, and diabetes has emerged, suggesting that loss of intracellular heat-shock proteins (HSPs) in insulin-sensitive tissues (skeletal muscle, fat, liver, heart) plays a key role in promoting insulin resistance, weight gain, and the development of the metabolic syndrome. (Hooper and Hooper 2005, 2009). Indeed, inducing HSPs via genetic modification, heat, or use of an HSP co-inducer, BGP-15, improves insulin signaling, increases mitochondrial function, and is associated with weight loss (Chung et al. 2008; Gupte et al. 2009a, b). Therefore, we have explored whether administration of BGP-15 could reverse the adverse metabolic effects of antipsychotic medications.
Previously, we observed that BGP-15 administration could improve insulin sensitivity in insulin-resistant subjects (Literáti-Nagy et al. 2009). We then examined whether BGP-15 could restore insulin sensitivity in healthy subjects administered olanzapine. Indeed, the double-blind, parallel, placebo-controlled phase I study found that BGP-15 administration prevented olanzapine induction of insulin resistance. BGP-15 had no significant effect on the pharmacokinetics of olanzapine and on olanzapine-induced weight gain. No side effects were observed with BGP-15 (Literáti-Nagy et al. 2010). We now report unpublished data from that study which examined reversal of olanzapine-induced reduction in HSP72 protein expression in peripheral monocytes. We also report data from animal and in vitro studies that support the concept that BGP-15 can limit the untoward side effects of AAPD—weight gain, insulin resistance, adipocyte fat accumulation.
BGP-15 administration reverses the drop in HSP72 protein expression in peripheral mononuclear blood cells
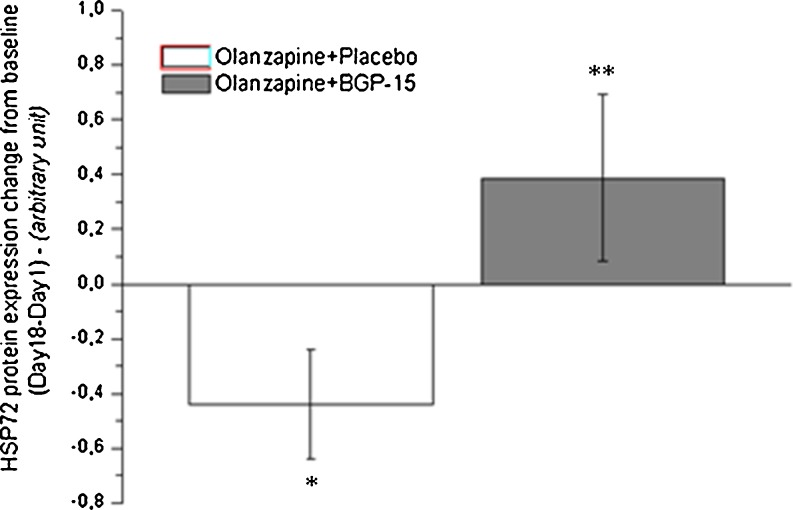
Blood drawn at the beginning and end of the olanzapine BGP-15 human study mentioned above (Literáti-Nagy et al. 2010) was examined to determine the effect of olanzapine alone and olanzapine combined with BGP-15 on monocyte HSP72 expression. The study was double blind, parallel, and placebo controlled. Two groups of healthy volunteers were enrolled and randomized to either 10 mg olanzapine and placebo or 10 mg olanzapine and 400 mg BGP-15 for 17 days. In order to meet the studies’ inclusion criteria, subjects had to be between 18 and 55 years of age; females were postmenopausal, surgically sterile, or had undergone hysterectomy, had to have a body mass index between 23 and 28 and fasting glucose level below 5.6 mmol/l. Exclusion criteria included first-degree family history of type 1 or type 2 diabetes mellitus, significant comorbid illness, previous exposure to antipsychotic medication or current use of recreational drugs, pregnancy, pursuing sports on a professional level, smoking, and abuse of alcohol and drugs. Due to safety reasons, a 5-mg initial dose (one time/day) of olanzapine was administered for 3 days and then raised up to 10 mg (Fig. 1).
Fig. 1.
Olanzapine and placebo reduced HSP72 protein expression from baseline values. (paired t test, *p < 0.05). After 18 days, the olanzapine and BGP-15 treatment group was higher than the olanzapine and placebo group (Mann–Whitney, **p < 0.05)
BGP-15 administration improves clozapine-induced insulin resistance in rats
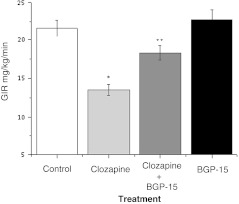
The aim of this experiment was to evaluate whether BGP-15 can improve insulin sensitivity after a chronic (1 month) clozapine treatment. The experiments were carried out with female Wistar rats (Charles River) weighing 210–230 g, housed in a room with controlled temperature at 23–25°C and humidity of 50–70%, fed commercial laboratory chow, and allowed free access to chow and to tap water ad libitum. After acclimatization to the laboratory conditions for 1 week, the animals were divided into four groups of six animals each. Groups were treated either with saline (control group), 10 mg/kg clozapine alone for 2 months, or 10 mg/kg clozapine (2 months) in combination with 20 mg/kg BGP-15 during the second month of clozapine treatment (1 month) or 20 mg/kg BGP-15 alone for 2 months. Insulin sensitivity was determined by hyperinsulinemic euglycemic glucose clamp. Human regular insulin was infused at a constant rate (5–12 mU/kg/min) via a catheter inserted into one of the jugular veins over 120 min. This insulin infusion rate was adjusted to procedure 100 ± 5 μU/ml in steady state in each species. Blood samples (0.2 ml) were taken from an arterial cannula introduced into one of the external carotid arteries for blood glucose concentration at 10-min intervals. Blood glucose concentration was maintained constant (5.5 ± 0.5 mmol/l) by a variable rate of glucose infusion. When blood glucose has stabilized for at least 20 min, we defined this condition as steady state. The glucose infusion rate during steady state was used to characterize insulin sensitivity (Fig. 2).
Fig. 2.
The effect of BGP-15 on insulin resistance provoked by clozapine. *Significantly different from the control, p < 0.05. **Significantly different from the clozapine-treated group, p < 0.05; ANOVA, Bonferroni post hoc test
BGP-15 administration blocks clozapine induced weight gain in rats
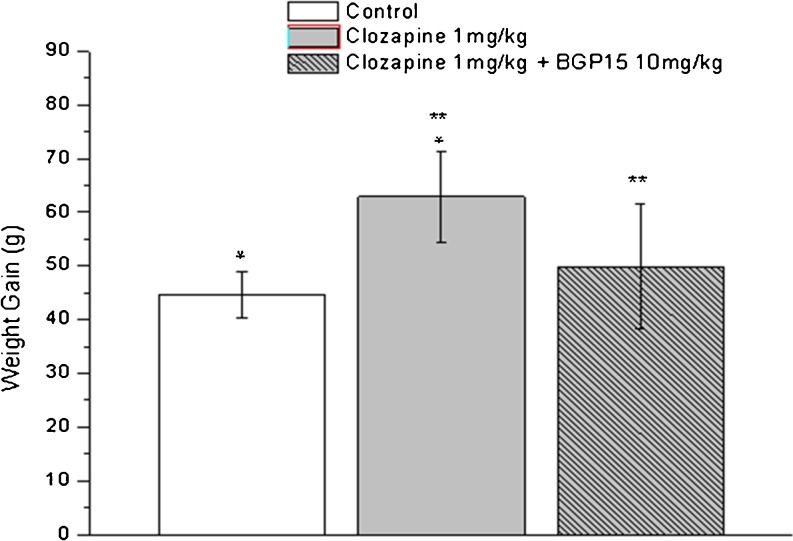
The purpose of this study was to find whether BGP-15 administration can cancel the weight gain induced by clozapine. Adult female Wistar rats (Charles River) weighing 210–230 g were housed in a room with controlled temperature at 23–25°C and humidity of 50–70%, fed commercial laboratory chow, and allowed free access to tap water ad libitum. After acclimatization to the laboratory conditions for 1 week, the animals were divided into three groups of eight animals each. Ten milligrams per kilogram clozapine-treated rats are somnolent and gain less weight (Cooper et al. 2005); therefore, in this study, the groups were treated either 1 mg/kg clozapine alone or 1 mg/kg clozapine in combination with 10 mg/kg BGP-15 or with saline (control group) by oral gavage for 35 days. Body weights were measured weekly (Fig. 3).
Fig. 3.
Change in body weight in Wistar rats after 35 days of oral clozapine administration. Bars represent means ± SD. *Difference between rats treated with placebo compared with clozapine (p < 0.05). **Difference between rats treated with clozapine compared with clozapine-BGP-15 combination (p < 0.05). Statistical analyses were performed by one-way ANOVA and Bonferroni post hoc test. p < 0.05 was taken as statistically significant
BGP-15 administration reduces fat accumulation induced by clozapine in cultured adipocytes
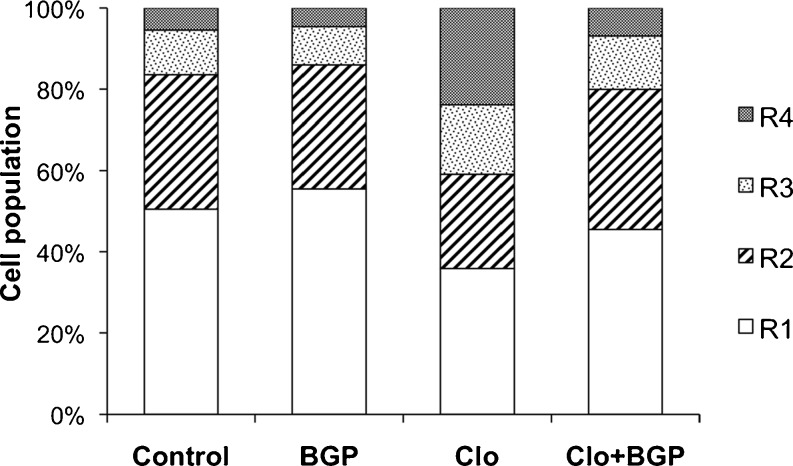
In this study, we addressed whether BGP-15 administration reduces fat accumulation induced by clozapine in cultured adipocytes. Adipogenesis of preadipocytes in culture has been frequently used to study the molecular basis and effect of drugs on fat cell conversion. Cells were analyzed for their granularity using flow cytometry as in Lee et al. (2004). The scatter plot of cytometric FSC and side scatter (SSC) reflects the cell diameter and granular structures within the cell, respectively. We divided into regions based on their SSC (granularity) values (R1, R2, R3, and R4 with increasing granularity). The scatter plot showed that after adipogenic induction, the 3T3-L1 adipocyte scatter plot have become increasingly heterogeneous in their granularity, and clozapine-induced increased fat accumulation was clearly visible. Clozapine (13 μM) significantly increased the cell populations in the R3 and R4 regions (see Fig. 4), where cells contain greater granularity, suggesting that more cells were adipogenically induced to store fat. BGP-15 blocked the lipogenic effects of clozapine.
Fig.4.
Effect of BGP15 on the clozapine-induced fat accumulation in 3T3-L1 adipocytes. 3T3-L1 preadipocytes were cultured in Dulbecco’s modified eagle’s medium containing 10% (v/v) calf serum. Adipogenesis was induced as in (Giri et al. 2006), briefly, on day 0 (2 days after 3T3-L1 preadipocytes reached confluence), control cells were given only the above medium, and the adipogenetic differentiation started by adding insulin (1.7 μmol/L), IBMX (0.5 mmol/L), and DEX (1 μmol/L). At the same time, 13 μM clozapine and 10 μM BGP-15 were added to the cells. Three days later, cells were briefly rinsed twice with prewarmed 0.25% trypsin–EDTA and then incubated for 5 min at 37°C. Cells were then gently resuspended in phosphate-buffered saline (PBS), washed twice with PBS, resuspended in cold PBS, and kept on ice prior to flow cytometric analysis (FACS Calibur, Beckton Dickinson). A scatter plot of 3T3-L1 cells was gated into four regions (as in Lee et al. 2004) based on their granularity distribution. The R1 region was gated to include the majority of control cells (where adipogenesis was not induced, exactly as in Lee et al. (Fig. 2) on the SSC scale. Regions R2 to R4 were gated to include the remaining range of SSC, and each region contained an equal range of SSC
Discussion
The exact mechanism by which the HSP co-inducer hydroxylamines (Vígh et al. 1997) can amplify the expression of HSPs is not well understood, but it seems that these compounds interfere with membrane hyperstructures via their highly specific lipid interactions, sufficient to enhance stress signals that activate hsp genes (Török et al. 2003.) In fact, a vast amount of data support that HSPs can be induced through remodeling cell membranes (Vigh et al. 1998, 2007; Balogh et al. 2005, 2010; Nagy et al. 2007). Our most recent observations show that BGP-15 is also able to remodel cholesterol-enriched lipid platforms (“lipid rafts”), similar to non-lethal heat priming or membrane stress conditioning initiated by non-proteotoxic benzyl alcohol, and were obligate for the generation and transmission of stress signals. BGP-15 activation of HSP expression involves the Rac1 signaling cascade and inhibition of the early-phase acetylation of heat-shock factor 1 (HSF1), thus prolonging duration of HSF1 binding to heat-shock elements and amplifying the net HSP response to stress (Gombos et al. 2011).
The data presented in these diverse studies support the concept that an HSP co-inducer like BGP-15 can ameliorate the negative side effects of AAPDs. BGP-15 could be an ideal agent to be used in conjunction with AAPDs. We have previously demonstrated that it did not alter the pharmacodynamics of olanzapine and has a good safety profile in humans (Literáti-Nagy et al. 2010). Another bioactive compound, alpha lipoic acid, both increases HSPs (Gupte et al. 2009a, b) and limits weight gain from AAPDs in patients with schizophrenia, supporting the concept that HSP induction can reverse negative AAPD metabolic effects (Kim et al. 2008).
Insulin signaling and intracellular HSP expression are intrinsically tied to one another (Hooper and Hooper 2009). The observation that olanzapine lowered HSP72 in PMBC is intriguing and further supports the concept that intracellular HSPs and the metabolic abnormalities of type 2 diabetes are intertwined with intracellular HSPs. Furthermore, the pharmacological activation of HSP72 by BGP-15 which improves AAPD insulin resistance, weight gain, and fat accumulation supports a key role for HSPs in carbohydrate and lipid metabolism. Additionally, ER stress in adipose tissue, liver, and pancreas plays a role in the pathogenesis of type 2 diabetes (Mandl et al. 2009). Relevantly, we note that diabetes and acetaminophen toxicity both result in ER stress and that BGP-15 has positive therapeutic effects on both conditions (Nagy et al. 2010).
Our results demonstrate that the combined use of BGP-15 with AAPD can limit the development of metabolic side effects including insulin resistance, weight gain, and dyslipidemia that reduces treatment-related risks thus increasing the therapeutic compliance of schizophrenic patients. Considering the novel mode and multiple activity of action BGP-15, it represents a promising new drug candidate to control the metabolic side effects of atypical antipsychotics.
Acknowledgments
Funding was in part from grants from the Hungarian National Scientific Research Foundation, OTKA NN 76716.
Contributor Information
Philip L. Hooper, Email: phoopermd@gmail.com
László Vígh, Email: icbl@icbl2006.hu.
References
- Allison DB, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156:1686. doi: 10.1176/ajp.156.11.1686. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. American Psychiatric Association. American Association of Clinical Endocrinologists. North American Association for the Study of Obesity Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:267–272. doi: 10.4088/JCP.v65n0219. [DOI] [PubMed] [Google Scholar]
- Balogh G, Horváth I, Nagy E, Hoyk Z, Benkõ S, Bensaude O, Vígh L. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 2005;272:6077–6086. doi: 10.1111/j.1742-4658.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- Balogh G, Péter M, Liebisch G, Horváth I, Török Z, Nagy E, Maslyanko A, Benkő S, Schmitz G, Harwood JL, Vígh L. Lipidomics reveals membrane lipid remodelling and release of potential lipid mediators during early stress responses in a murine melanoma cell line. Biochim Biophys Acta. 2010;1801:1036–1047. doi: 10.1016/j.bbalip.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Baptista T, Martínez J, Lacruz A, Rangel N, Beaulieu S, Serrano A, Arapé Y, Martinez M, Mendoza S, Teneud L, Hernández L. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double bind placebo-controlled trial. Can J Psychiatry. 2006;51:192–196. doi: 10.1177/070674370605100310. [DOI] [PubMed] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GD, Pickavance LC, Wilding JP, Halford JC, Goudie AJ. A parametric analysis of olanzapine-induced weight gain in female rats. Psychopharmacology (Berl) 2005;181:80–89. doi: 10.1007/s00213-005-2224-4. [DOI] [PubMed] [Google Scholar]
- Dwyer DS, Donohoe D. Induction of hyperglycemia in mice with atypical antipsychotic drugs that inhibit glucose uptake. Pharmacol Biochem Behav. 2003;75:255–260. doi: 10.1016/S0091-3057(03)00079-0. [DOI] [PubMed] [Google Scholar]
- Giri S, Rattan R, Haq E, Khan M, Yasmin R, Won J, Key L, Singh AK, Singh I. ICAR inhibits adipocyte differentiation in 3T3L1 and restores metabolic alterations in diet-induced obesity mice model. Nutr Metab (Lond) 2006;3:31. doi: 10.1186/1743-7075-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombos I, Crul T, Piotto S, Güngör B, Török Z, Balogh G, Péter M, Slotte PJ, Pilbat AN, Hunya A, Tóth N, Literati-Nagy Z, Vigh L, Jr, Glatz A, Brameshuber, M, Schütz GJ, Hevener A, Febbraio MA, Horváth I, Vígh L (2011) Membrane-lipid therapy in operation: the HSP co-inducer BGP-15 activates stress signal transduction pathways by remodeling plasma membrane rafts. PLoS ONE 6(12):e28818 [DOI] [PMC free article] [PubMed]
- Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes. 2009a;58:567–578. doi: 10.2337/db08-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Morris J, Gorres BK, Geiger PC. Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats. J Appl Physiol. 2009b;106:1425–1434. doi: 10.1152/japplphysiol.91210.2008. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Hooper JJ. Loss of defense against stress: diabetes and heat shock proteins. Diabetes Technol Ther. 2005;7:204–208. doi: 10.1089/dia.2005.7.204. [DOI] [PubMed] [Google Scholar]
- Hooper PL, Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones. 2009;14:113–115. doi: 10.1007/s12192-008-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Park DW, Choi SH, Kim JJ, Cho HS. A preliminary investigation of alpha-lipoic acid treatment of antipsychotic drug-induced weight gain in patients with schizophrenia. J Clin Psychopharmacol. 2008;28:138–146. doi: 10.1097/JCP.0b013e31816777f7. [DOI] [PubMed] [Google Scholar]
- Lambert BL, Cunningham FE, Miller DR, Dalack GW, Hur K. Diabetes risk associated with use of olanzapine, quetiapine, and risperidone in veterans health administration patients with schizophrenia. Am J Epidemiol. 2006;164:672–681. doi: 10.1093/aje/kwj289. [DOI] [PubMed] [Google Scholar]
- Lee YH, Chen SY, Wiesner RJ, Huang YF. Simple flow cytometric method used to assess lipid accumulation in fat cells. J Lipid Res. 2004;45:1162–1167. doi: 10.1194/jlr.D300028-JLR200. [DOI] [PubMed] [Google Scholar]
- Literáti-Nagy B, Kulcsár E, Literáti-Nagy Z, Buday B, Péterfai E, Horváth T, Tory K, Kolonics A, Fleming A, Mandl J, Korányi L. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res. 2009;41:374–380. doi: 10.1055/s-0028-1128142. [DOI] [PubMed] [Google Scholar]
- Literáti-Nagy B, Péterfai E, Kulcsár E, Literáti-Nagy Z, Buday B, Tory K, Mandl J, Sümegi B, Fleming A, Roth J, Korányi L. Beneficial effect of the insulin sensitizer (HSP co-inducer) BGP-15 on olanzapine-induced metabolic disorders. Brain Res Bull. 2010;83:340–344. doi: 10.1016/j.brainresbull.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Mandl J, Mészáros T, Bánhegyi G, Hunyady L, Csala M. Endoplasmic reticulum: nutrient sensor in physiology and pathology. Trends Endocrinol Metab. 2009;20:194–201. doi: 10.1016/j.tem.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Nagy E, Balogi Z, Gombos I, Akerfelt M, Björkbom A, Balogh G, Török Z, Maslyanko A, Fiszer-Kierzkowska A, Lisowska K, Slotte PJ, Sistonen L, Horváth I, Vígh L. Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. Proc Natl Acad Sci USA. 2007;104:7945–7950. doi: 10.1073/pnas.0702557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G, Szarka A, Lotz G, Dóczi J, Wunderlich L, Kiss A, Jemnitz K, Veres Z, Bánhegyi G, Schaff Z, Sümegi B, Mandl J. BGP-15 inhibits caspase-independent programmed cell death in acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 2010;243:96–103. doi: 10.1016/j.taap.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Török Z, Tsvetkova NM, Balogh G, Horváth I, Nagy E, Pénzes Z, Hargitai J, Bensaude O, Csermely P, Crowe JH, Maresca B, Vigh L. Heat shock protein co-inducers with no effect on protein denaturation specifically modulate the membrane lipid phase. Proc Natl Acad Sci USA. 2003;100:3131–3136. doi: 10.1073/pnas.0438003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vígh L, Literáti PN, Horváth I, Török Z, Balogh G, Glatz A, Kovács E, Boros I, Ferdinándy P, Farkas B, Jaszlits L, Jednákovits A, Korányi L, Maresca B. Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat Med. 1997;3:1150–1154. doi: 10.1038/nm1097-1150. [DOI] [PubMed] [Google Scholar]
- Vígh L, Maresca B, Harwood J. Does the membrane’s physical state control the expression of heat shock and other genes? Trends Biochem Sci. 1998;23:369–373. doi: 10.1016/S0968-0004(98)01279-1. [DOI] [PubMed] [Google Scholar]
- Vígh L, Horváth I, Maresca B, Harwood J. Can the stress protein response be controlled by ‘membrane-lipid therapy’? Trends Biochem Sci. 2007;32:357–363. doi: 10.1016/j.tibs.2007.06.009. [DOI] [PubMed] [Google Scholar]