Abstract
CD4+ T helper cells are obligate regulators of inflammatory disease. An expanding cadre of T helper (Th) subsets, specialized for promoting particular types of inflammation, function through the secretion of a restricted set of cytokines. The latest addition to the list of subsets is the Th9 cell that secretes IL-9 as a signature cytokine and contributes to several classes of inflammatory disease. In this review we focus on recent advances in understanding the development of Th9 cells, and how Th9 cells contribute to the orchestration of disease.
Introduction
Naïve CD4+ T helper cells, after encountering specific antigen, become activated and differentiate into effector T helper subsets, each characterized by a distinct pattern of cytokine secretion and function. Th1 cells mediate immunity to intracellular pathogens, Th2 cells provide protection against extracellular parasites, and Th17 cells are involved in resistance to extracellular bacteria and fungal infections. Another effector subset, termed Th9, secretes IL-9 and may be involved in immune-mediated diseases ranging from autoimmunity to asthma. The biology of IL-9 has been recently reviewed [1–3]; this review is focused on discussing recent advances in our understanding of the development of IL-9-secreting T cells, and the functions of Th9 cells in vivo.
The pathway towards Th9 differentiation
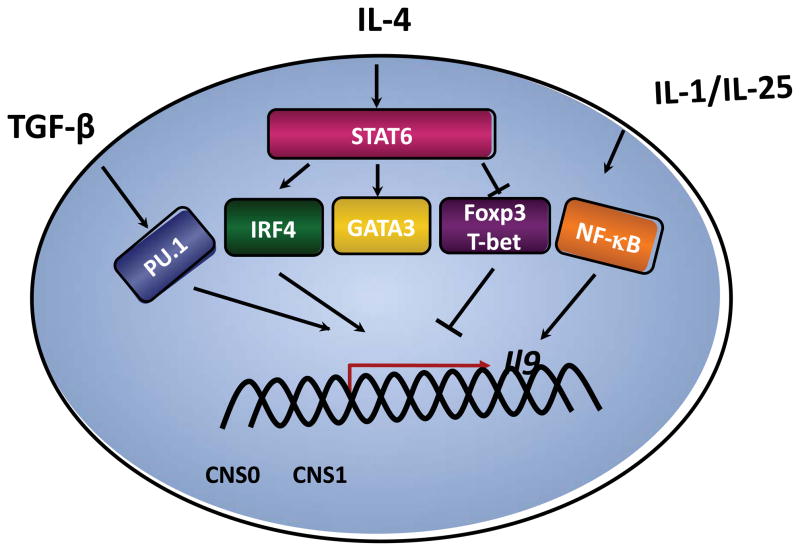
T helper cells secreting IL-9 are primed in response to TGF-β and IL-4 and are termed Th9 [4,5]. Both signals are required as cells that lack IL-4 or TGF-β signaling components fail to develop into IL-9-secreting cells [4–6]. Since Th9 cells require balanced signals from TGF-β and IL-4 [4–6], each cytokine likely leads to the induction of transcription factors that regulate IL-9 production, and the expression of other genes associated with the Th9 phenotype. The TGF-β signal, which induces Foxp3, also induces the expression of PU.1 [6](Figure 1). PU.1, an ETS family transcription factor, also identified as the spleen focus forming virus proviral integration site-1 (Sfpi1) is a key transcription factor in the Th9 developmental program [7••]. PU.1 is expressed in Th9 cells at higher amounts than in Th2 cells. PU.1 negatively regulates Th2 cell development, and ectopic expression of PU.1 enhances production of IL-9, at least partly by binding directly to the Il9 promoter [7••–9]. Naïve CD4+ T cells from PU.1-deficient mice, when cultured under Th9 conditions, had reduced production of IL-9 [7••].
Figure.
Transcriptional network in Th9 cells. Transcription factors including PU.1, downstream of TGF-β signals, and IL-4-activated STAT6 that promotes expression of GATA3 and IRF4, contribute to the expression of the Il9 gene in Th9 cells.
Th9 development is clearly dependent upon the IL-4-activated transcription factor STAT6 [4–6](Figure 1). Although STAT5, downstream of IL-2, can bind the Il9 promoter, STAT6 binds the Il9 gene poorly compared to other genes [6,10]. Thus, the IL-4 signal likely induces Il9 indirectly, through the regulation of additional transcription factors. IL-4 and STAT6 are required to repress expression of Foxp3, which induces a Treg phenotype and can repress IL-9 production [4–6,11,12]. IL-4 and STAT6 also repress expression of T-bet in Th9 cells, and T-bet likely cooperates with Runx3 in the repression of IL-9 in Th1 cells [6].
IL-4 and STAT6 also promote the expression of several factors common to both Th9 and Th2 cells including IRF4, c-maf and GATA3. STAT6 induces IRF4 that is required for Th9 development, in addition to its contributions to Th2 and Th17 differentiation [13–17••]. Naïve CD4+ T cells from IRF4-deficient mice, when cultured under Th9 conditions, exhibited substantial reduction in the IL-9 levels, compared with wild-type cells. IRF4 regulates Th9 development by binding to the Il9 promoter. Ectopic expression of c-maf repressed IL-9 production, suggesting that c-maf is not directly regulating Il9, and might regulate other Th9 genes such as IL-21 [6,18].
IL-4 and STAT6 are required for the induction of GATA3, a master regulator of the Th2 phenotype. In contrast to a detailed understanding of a role for GATA3 in Th2 cells [19], the role of GATA3 in Th9 development is complex. Although GATA3 is expressed in Th9 cells, albeit at lower levels than in Th2 cells, and it is required for the development of Th9 cells, ectopic expression of GATA3 decreased production of IL-9 [4–6]. Moreover, while GATA3 induces IL-4 and IL-13 when transduced into Stat6−/− Th2 cells, it did not induce IL-9 when transduced into Stat6−/− Th9 cultures [6] suggesting that it does not directly act on the Il9 gene. One possibility is that GATA3 plays a role in the STAT6-dependent repression of Foxp3 [20], although the recent description of a requirement for GATA-3 in Treg development makes this less likely [21,22]. Together, these results suggest that the requirement for GATA3 in Th9 development may be temporally confined, and that the amount of GATA3 present within Th9 cells is tightly controlled.
Other cytokines also regulate IL-9 production. Schmitt et al. demonstrated that IL-9 production from murine CD4+ T cells is IL-2 dependent and is inhibited by IFN-γ [23]. IL-25, a member of the IL-17 cytokine family, enhances IL-9 production in the presence of TGF-β and IL-4 through IL-17RB. IL-17RB is differentially expressed in T helper subsets with the highest expression in Th9 cells and both IL-4 and TGF-β significantly enhanced the expression of IL-17RB in activated T cells [24•]. In addition to TGF-β and IL-4, IL-1 family members promote IL-9 production from CD4+ T cells independently of IL-4 [25]. Each of these cytokines activates transcription factors including NF-κB, which bind to the Il9 promoter [26,27].
Naïve human CD4+ T cells also acquire a Th9 phenotype when differentiated in presence of TGF-β and IL-4 [7,17,28–31]. Among other inflammatory cytokines, IFN-α, IFN-β and IL-21 were potent enhancers of IL-9 production. Blocking IL-21 decreased IL-9, whereas IFNγ and IL-27 inhibited IL-9 production in a dose dependent manner [32]. TGF-β has also been shown to induce IL-9 production in human Th17 cells, and repeated stimulation under Th17 conditions, resulted in the co-expression of IL-17A and IL-9. As with their mouse counterparts, human Th9 cells require PU.1 and IRF4 for expression of IL-9 [7,17].
Functions of Th9 cells
Th9 cells are pro-inflammatory, but appear to function in a broad spectrum of autoimmune diseases and allergic inflammation. Their precise function likely depends upon the tissue microenvironment and other T helper cell cytokines that are present in the inflammatory milieu.
Th9 cells contribute to inflammation in several autoimmune disease models. Th9 cells induce inflammation in a T cell transfer colitis model [5]. Mice that received Th9 cells only, lost weight and developed a moderate colitis. Moreover, mice that received effector T cells together with Th9 cells developed a more severe colitis. A similar pro-inflammatory role of Th9 cells was demonstrated in an EAE model [33]. MOG-specific naïve CD4+ T cells were differentiated in vitro under Th1, Th2, Th17 and Th9 polarizing conditions before adoptive transfer. All mice that received Th9 cells developed severe EAE and lesions in the CNS. Cells in the CNS of Th9 recipients retained IL-9 producing capacity, but also produced IFN-γ. Although Th1, Th17 and Th9 cells induced EAE with similar severity, differences in CNS pathology suggested Th9 cells promote inflammation through distinct mechanisms [33]. In agreement with these results, treatment with anti-IL-9 antibody or IL-9 receptor deficiency ameliorates EAE, possibly by decreasing MOG-reactive Th1 and Th17 cells, and lymph node mast cell numbers [34•, 35•]. This is consistent with the ability of IL-9 to promote Th17 development and as a growth factor for mast cells [36–38].
The pathogenic ability of Th9 cells was further supported in an adoptive transfer model where Th9 cells specific for hen egg lysozyme (HEL) were transferred into recipient mice expressing HEL in the eye lens. Ocular inflammation developed in mice that received Th9 cells, although in this model the IL-9-secreting phenotype was not stable and cells recovered from the inflamed site produced primarily IFN-γ [39]. Moreover, anti-IL-9 did not protect these mice from disease, suggesting that the flexibility in cytokine production of the transferred cells, rather than IL-9 itself, was pathogenic [39].
Paradoxically, IL-9 might also promote Treg function. Elyaman et al. found that blocking IL-9 signaling with anti-IL-9 reverses nTreg-mediated suppression in vitro whereas addition of rIL-9 enhances Treg suppressive capacity [36]. Consistent with this, they observed that Il9r−/− mice developed more severe EAE than wild-type mice when immunized with MOG-peptide, and a higher frequency of Th1 and Th17 cells in the Il9r−/− mice both in the periphery and CNS as compared to WT mice. It is still not clear how the differences between Nowak et al. and Elyaman et al. can be reconciled [34,36]. There are modest protocol differences in the induction of EAE and source of reagents that might impact the types of Th or Treg cells induced and ultimately effect disease. The different outcome in these studies might indicate something important about the induction of IL-9-producing cells.
Th9 cells also contribute to allergic inflammation and disease. IL-9 is highly expressed in the lungs of asthmatic patients [40,41]. More recently our group and others have found that IL-9 production was significantly higher in T cells from atopic infants in comparison with a non-atopic group [7,29,42]. In mice, transgenic expression of IL-9 in the lungs induces an asthma-like response, and blocking IL-9 in an asthma model results in reduced airway inflammation [7,43,44]. Similarly blocking IL-9 in a chronic model of lung inflammation inhibits mastocytosis and airway remodeling [45]. Inflammation similar to allergic disease is also observed during helminthic parasite infection, and experiments with transgenic mice expressing a dominant negative TGF-βR demonstrated a requirement for Th9 cells in immunity to Trichuris muris [4].
There are several reports documenting a role of Th9 cells in the development of allergic airway disease (AAD). In an adoptive transfer model, Rag−/− recipients of either Th2 or Th9 cells developed severe asthma symptoms characterized by increased airway reactivity to methacholine, increased goblet cell metaplasia, and greater eosinophil infiltration after airway challenge. Administration of anti-IL-9 antibody resulted in a remarkable amelioration of AAD in Th9 cell recipients, whereas Th2-recipient Rag2−/− mice showed only slight improvement in AAD symptoms with antibody treatment [17]. The role of Th9 cells in an OVA/alum-induced allergic inflammation model was demonstrated in mice with a conditional deletion of PU.1 in T cells. These mice have normal Th2 and dendritic cell development, but have greatly diminished Th9 development. These mice not only exhibited less inflammation in lung but also demonstrated significantly less airway hyper-responsiveness in response to methacholine challenge compared to wild-type mice [7]. However, the role for Th9 cells may not be universal in all models. A recent report, using IL-9 reporter mice, confirmed that the primary IL-9-producing population in the OVA/alum model was CD4+ T cells. However, in a papain-induced airway inflammation model, innate lymphoid cells (ILCs) were the main source of IL-9 [46•]. Some T cell production of IL-9 was observed in this model, although IL-9 production was transient in both populations of cells.
Concluding remarks
The Th9 subset develops in response to combined signals from TGF-β and IL-4 among a cacophony of other cytokines in the extracellular milieu. The transcriptional network that regulates Th9 development includes TGF-β-induced Sfpi1, and IL-4-induced STAT6 that induces IRF4 as it represses Foxp3 and T-bet (Figure 1). Additional transcription factors, possibly downstream of these and additional cytokines, undoubtedly harmonize in efficient transcription of the Il9 gene.
IL-9 promotes inflammation by stimulating growth of hematopoietic cells, particularly mast cells, and the secretion of factors including chemokines that recruit additional cells to inflamed sites. Th9 cells are capable of promoting autoimmune inflammation, although whether Th9 cells are required as a source of IL-9 for autoimmune inflammation is still not clearly established. Among the obstacles to defining these functions is that lack of a more detailed understanding of sensitization conditions that prime IL-9-producing T cells. More evidence supports an important role for Th9 cells in allergic inflammation, but how Th9 cells contribute to allergic disease, and how they cooperate with Th2 cells in promoting inflammation is the focus of ongoing investigation. Moreover, whether the mechanisms of Th9 cells contributing to autoimmune and allergic inflammation are distinct has not been examined. The next steps in this area will be to define the orchestration of Th9 cells, and the direction by Th9 cells, in the symphony of inflammation.
Highlights.
Th9 cells are a T helper cell subset that secretes IL-9 as a signature cytokine
Th9 cell development requires transcription factors including PU.1, IRF4 and STAT6
Th9 cells promote inflammation in autoimmunity and allergic disease
Acknowledgments
The authors thank the Kaplan lab members for helpful input. Preparation of this article was supported by Public Health Service grant AI057459 and the Wells Center for Pediatric Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the last two years of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011;186:3283–3288. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neill DR, McKenzie AN. TH9 cell generation. TH9: the latest addition to the expanding repertoire of IL-25 targets. Immunol Cell Biol. 2010;88:502–504. doi: 10.1038/icb.2010.43. [DOI] [PubMed] [Google Scholar]
- 3.Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nat Rev Immunol. 2010;10:683–687. doi: 10.1038/nri2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 5.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goswami R, Jabeen R, Yagi R, Pham D, Zhu J, Goenka S, Kaplan MH. STAT6-Dependent Regulation of Th9 Development. J Immunol. 2012;188:968–975. doi: 10.4049/jimmunol.1102840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. Identified that the transcription factor, PU.1 is required for the Th9 phenotype in mouse and human cells. Together with [6] describes a transcriptional network in Th9 cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang HC, Zhang S, Thieu VT, Slee RB, Bruns HA, Laribee RN, Klemsz MJ, Kaplan MH. PU. 1 expression delineates heterogeneity in primary Th2 cells. Immunity. 2005;22:693–703. doi: 10.1016/j.immuni.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Chang HC, Han L, Jabeen R, Carotta S, Nutt SL, Kaplan MH. PU. 1 regulates TCR expression by modulating GATA-3 activity. J Immunol. 2009;183:4887–4894. doi: 10.4049/jimmunol.0900363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung MM, Chu YL, Fink JL, Wallace A, McGuire KL. IL-2- and STAT5-regulated cytokine gene expression in cells expressing the Tax protein of HTLV-1. Oncogene. 2005;24:4624–4633. doi: 10.1038/sj.onc.1208507. [DOI] [PubMed] [Google Scholar]
- 11.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Malley JT, Sehra S, Thieu VT, Yu Q, Chang HC, Stritesky GL, Nguyen ET, Mathur AN, Levy DE, Kaplan MH. Signal transducer and activator of transcription 4 limits the development of adaptive regulatory T cells. Immunology. 2009;127:587–595. doi: 10.1111/j.1365-2567.2008.03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 14.Lohoff M, Mittrucker HW, Prechtl S, Bischof S, Sommer F, Kock S, Ferrick DA, Duncan GS, Gessner A, Mak TW. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proc Natl Acad Sci U S A. 2002;99 :11808–11812. doi: 10.1073/pnas.182425099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rengarajan J, Mowen KA, McBride KD, Smith ED, Singh H, Glimcher LH. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J Exp Med. 2002;195:1003–1012. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahyi AN, Chang HC, Dent AL, Nutt SL, Kaplan MH. IFN regulatory factor 4 regulates the expression of a subset of Th2 cytokines. J Immunol. 2009;183:1598–1606. doi: 10.4049/jimmunol.0803302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, Gerlitzki B, Hoffmann M, Ulges A, Taube C, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. Demonstrates that IRF4 is required for human and mouse Th9 development. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan MH, Glosson NL, Stritesky GL, Yeh N, Kinzfogl J, Rohrabaugh SL, Goswami R, Pham D, Levy DE, Brutkiewicz RR, et al. STAT3-dependent IL-21 production from T helper cells regulates hematopoietic progenitor cell homeostasis. Blood. 2011;117:6198–6201. doi: 10.1182/blood-2011-02-334367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho IC, Tai TS, Pai SY. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol. 2009;9:125–135. doi: 10.1038/nri2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Ruckert B, Karagiannidis C, Lambrecht BN, Hendriks RW, Crameri R, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, Hall JA, Yagi R, Naik S, Bhairavabhotla R, et al. GATA3 controls Foxp3 regulatory T cell fate during inflammation in mice. J Clin Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kuhn R, Muller W, Palm N, Rude E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153:3989–3996. [PubMed] [Google Scholar]
- 24•.Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. Regulation of IL-9 expression by IL-25 signaling. Nat Immunol. 2010;11:250–256. doi: 10.1038/ni.1846. Demonstrates that Th9 cells express IL-25R and that IL-25 stimulates IL-9 production. IL-25 deficiency significantly reduces IL-9 production in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uyttenhove C, Brombacher F, Van Snick J. TGF-beta interactions with IL-1 family members trigger IL-4-independent IL-9 production by mouse CD4(+) T cells. Eur J Immunol. 2010;40:2230–2235. doi: 10.1002/eji.200940281. [DOI] [PubMed] [Google Scholar]
- 26.Stassen M, Muller C, Arnold M, Hultner L, Klein-Hessling S, Neudorfl C, Reineke T, Serfling E, Schmitt E. IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-kappa B is decisively involved in the expression of IL-9. J Immunol. 2001;166:4391–4398. doi: 10.4049/jimmunol.166.7.4391. [DOI] [PubMed] [Google Scholar]
- 27.Perumal NB, Kaplan MH. Regulating Il9 transcription in T helper cells. Trends Immunol. 2011;32:146–150. doi: 10.1016/j.it.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houssiau FA, Schandene L, Stevens M, Cambiaso C, Goldman M, van Snick J, Renauld JC. A cascade of cytokines is responsible for IL-9 expression in human T cells. Involvement of IL-2, IL-4, and IL-10. J Immunol. 1995;154:2624–2630. [PubMed] [Google Scholar]
- 29.Yao W, Tepper RS, Kaplan MH. Predisposition to the development of IL-9-secreting T cells in atopic infants. J Allergy Clin Immunol. 2011;128:1357–1360. e1355. doi: 10.1016/j.jaci.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beriou G, Bradshaw EM, Lozano E, Costantino CM, Hastings WD, Orban T, Elyaman W, Khoury SJ, Kuchroo VK, Baecher-Allan C, et al. TGF-beta induces IL-9 production from human Th17 cells. J Immunol. 2010;185:46–54. doi: 10.4049/jimmunol.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Putheti P, Awasthi A, Popoola J, Gao W, Strom TB. Human CD4 memory T cells can become CD4+IL-9+ T cells. PLoS One. 2010;5:e8706. doi: 10.1371/journal.pone.0008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong MT, Ye JJ, Alonso MN, Landrigan A, Cheung RK, Engleman E, Utz PJ. Regulation of human Th9 differentiation by type I interferons and IL-21. Immunol Cell Biol. 2010;88:624–631. doi: 10.1038/icb.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, Coyle AJ, Kasper LH, Noelle RJ. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206:1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Li H, Nourbakhsh B, Ciric B, Zhang GX, Rostami A. Neutralization of IL-9 ameliorates experimental autoimmune encephalomyelitis by decreasing the effector T cell population. J Immunol. 2010;185:4095–4100. doi: 10.4049/jimmunol.1000986. Together with [34•] demonstrates that Th9 cells contribute to autoimmune inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–583. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 38.Matsuzawa S, Sakashita K, Kinoshita T, Ito S, Yamashita T, Koike K. IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. J Immunol. 2003;170:3461–3467. doi: 10.4049/jimmunol.170.7.3461. [DOI] [PubMed] [Google Scholar]
- 39.Tan C, Aziz MK, Lovaas JD, Vistica BP, Shi G, Wawrousek EF, Gery I. Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. J Immunol. 2010;185:6795–6801. doi: 10.4049/jimmunol.1001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erpenbeck VJ, Hohlfeld JM, Volkmann B, Hagenberg A, Geldmacher H, Braun A, Krug N. Segmental allergen challenge in patients with atopic asthma leads to increased IL-9 expression in bronchoalveolar lavage fluid lymphocytes. J Allergy Clin Immunol. 2003;111:1319–1327. doi: 10.1067/mai.2003.1485. [DOI] [PubMed] [Google Scholar]
- 41.Shimbara A, Christodoulopoulos P, Soussi-Gounni A, Olivenstein R, Nakamura Y, Levitt RC, Nicolaides NC, Holroyd KJ, Tsicopoulos A, Lafitte JJ, et al. IL-9 and its receptor in allergic and nonallergic lung disease: increased expression in asthma. J Allergy Clin Immunol. 2000;105:108–115. doi: 10.1016/s0091-6749(00)90185-4. [DOI] [PubMed] [Google Scholar]
- 42.Bullens DM, Kasran A, Dilissen E, De Swert K, Coorevits L, Van Snick J, Ceuppens JL. In vivo maturation of T(H) cells in relation to atopy. J Allergy Clin Immunol. 2011;128:234–237. e237. doi: 10.1016/j.jaci.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 43.Temann UA, Laouar Y, Eynon EE, Homer R, Flavell RA. IL9 leads to airway inflammation by inducing IL13 expression in airway epithelial cells. Int Immunol. 2007;19:1–10. doi: 10.1093/intimm/dxl117. [DOI] [PubMed] [Google Scholar]
- 44.Cheng G, Arima M, Honda K, Hirata H, Eda F, Yoshida N, Fukushima F, Ishii Y, Fukuda T. Anti-interleukin-9 antibody treatment inhibits airway inflammation and hyperreactivity in mouse asthma model. Am J Respir Crit Care Med. 2002;166:409–416. doi: 10.1164/rccm.2105079. [DOI] [PubMed] [Google Scholar]
- 45.Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, Pegorier S, Brewah Y, Burwell TJ, Bjermer L, et al. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med. 2010;183:865–875. doi: 10.1164/rccm.200909-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. This report documents that in a papain-induced airway inflammation model, innate lymphoid cells (ILCs) are the main source of IL-9, contrasting the OVA/alum model where CD4+ T cells are the predominant source. The results highlight the importance of context in defining the source of IL-9. [DOI] [PMC free article] [PubMed] [Google Scholar]