Abstract
Objective
Faith Moves Mountains assessed the effectiveness of a faith-placed lay health advisor (LHA) intervention to increase Papanicolaou (Pap) test use among middle-aged and older women in a region disproportionately affected by cervical cancer and low screening rates (regionally, only 68% screened in prior 3 years).
Method
This community-based RCT was conducted in four Appalachian Kentucky counties (December 2005 – June 2008). Women aged 40–64 and overdue for screening were recruited from churches and individually randomized to treatment (n=176) or wait-list control (n=169). The intervention provided LHA home visits and newsletters addressing barriers to screening. Self-reported Pap test receipt was the primary outcome.
Results
Intention-to-treat analyses revealed that treatment group participants (17.6% screened) had over twice the odds of wait-list controls (11.2% screened) of reporting Pap test receipt post-intervention, OR=2.56, 95%CI: 1.03–6.38, p=0.04. Independent of group, recently screened participants (last Pap >1 but <5 years ago) had significantly higher odds of obtaining screening during the study than rarely or never screened participants (last Pap ≥5 years ago), OR=2.50, 95%CI: 1.48–4.25, p=0.001.
Conclusions
The intervention was associated with increased cervical cancer screening. The faith-placed LHA addressing barriers comprises a novel approach to reducing cervical cancer disparities among Appalachian women.
Keywords: cervical cancer screening, randomized controlled trial, faith-placed, Appalachia
Introduction
In nearly all cases, invasive cervical cancer (ICC) can be prevented through early detection and treatment (American Cancer Society [ACS], 2011a). Papanicolaou (Pap) tests and follow-up have dramatically decreased ICC mortality rates over the past 50 years (ACS, 2011a; Surveillance Epidemiology and End Results (SEER) Program, 2009). Nonetheless, 12,710 ICC diagnoses and 4,290 deaths were expected in 2011 (ACS, 2011a). This persistent mortality in light of the potential for prevention is concerning, especially among vulnerable groups.
Faith Moves Mountains (FMM) was a four-year community-based randomized controlled trial (RCT) testing the effectiveness of an intervention to reduce ICC burden in Appalachian Kentucky. The ICC incidence rate in Appalachia is 15 per 100,000–approximately 67% above the national average (Hopenhayn et al., 2005; Ries et al., 2005)—and only 68% of middle-aged and older women in the region have been screened for cervical cancer within the prior three years (Kentucky Department of Public Health, 2006). The intervention sought to increase screening among women ages 40–64 who (a) had not been screened for cervical cancer according to ACS guidelines in 2005 (Saslow et al., 2002), and (b) were considered hard-to-reach due to rural location, lack of resources, competing demands, and history of inadequate screening (Hall et al., 2002).
To address this ICC disparity, a culturally appropriate lay health advisor (LHA) intervention was delivered in collaboration with faith communities. Preliminary work demonstrated that middle-aged Appalachian women view churches as an acceptable environment for promoting positive health behavior (Campbell, 2007; Schoenberg et al., 2009). Moreover, churches are sustainable, central, and well-attended Appalachian institutions; the proportion of Appalachian residents reporting church affiliation is higher than observed nationally (Pew Forum on Religion & Public Life, 2008), and well over one-third report weekly church attendance (Campbell, 2007; Schoenberg et al., 2009). Recruitment efforts were centered in local faith-based institutions (henceforth “churches”). Significant efforts were made by study staff to build affiliations between FMM and each participating church, comprising the faith-placed nature of FMM. Delivered by LHAs (Earp & Flax, 1999; Eng et al., 1997; Salber, 1979), the intervention itself was based on the PRECEDE-PROCEED framework (Green & Kreuter, 1991). Elements of several models of health behavior (e.g., Social Cognitive Theory (Bandura, 1977) and the Health Belief Model (Rosenstock et al., 1988)) were integrated to facilitate remediation of participant-identified barriers to cervical cancer screening.
It was hypothesized that following intervention delivery, treatment group participants would be more likely to obtain Pap tests, compared to wait-list controls. This paper (a) presents results regarding the primary outcome, self-reported receipt of Pap tests, and (b) explores sociodemographic and health-related characteristics associated with study-wide screening.
Methods
Study Setting
Appalachia consists of 410 counties in 13 states encompassing 22 million people, or 8.3% of the total U.S. population. The region has notably high rates of poverty, isolation, and poor health (Appalachian Regional Commission [ARC], 2011). Appalachian Kentucky comprises 54 counties with socioeconomic indicators among the lowest in the nation. Table 1 highlights characteristics of the region and the four FMM counties.
Table 1.
Selected socioeconomic data: United States, Kentucky, Appalachian Kentucky, and four Faith Moves Mountains counties
| Population adults 25+ years, 2000 |
Per capita market income (USD), 2007 |
Poverty ratea (%), 2000 |
Completed high school (%), 2000 |
Completed College (%), 2000 |
|
|---|---|---|---|---|---|
| United States | 182,211,639 | 32,930 | 12.4 | 80.4 | 24.4 |
| Kentucky | 2,646,397 | 24,708 | 15.8 | 74.1 | 17.1 |
| Appalachian | 764,131 | 15,690 | 24.4 | 62.5 | 10.4 |
| Kentucky | |||||
| Harlan County | 22,041 | 12,178 | 32.5 | 58.7 | 8.9 |
| Knott County | 11,427 | 13,015 | 31.1 | 58.7 | 10.2 |
| Letcher County | 16,930 | 14,191 | 27.1 | 58.5 | 7.7 |
| Perry County | 19,596 | 16,825 | 29.1 | 58.3 | 8.9 |
Note. USD = United States Dollar.
Ratio of persons below 100% Federal Poverty Level to the total number of persons for whom poverty status has been determined.
Sources: (a) Appalachian Regional Commission, County Economics Status, Fiscal Year 2011. Retrieved July 29, 2010 from http://www.arc.gov/reports/region_report.asp?FIPS=21999&REPORT_ID=36 (b) Appalachian Regional Commission, Education - High School and College Completion Rates in Appalachia, 2000: Kentucky. Retrieved July 29, 2010 from http://www.arc.gov/reports/custom_report.asp?REPORT_ID=18
Harlan, Knott, Letcher, and Perry counties (Figure 1) are among the most economically distressed counties in Kentucky and the U.S. (Murray et al., 2006). They experience high poverty and unemployment rates, ranking in the lowest 10% of counties nationally on these indicators (ARC, 2011).
Figure 1.
Study location. The study location is circled and indicated in gray: Harlan, Knott, Letcher, and Perry counties, Kentucky, U.S.A. 2005–2008.
Participant Selection, Recruitment and Enrollment
Recruitment began in December 2005 and ended in June 2008. Church recruitment was challenging, and a probability sampling scheme for churches was replaced with snowball sampling procedures in which project staff personally contacted church representatives (Shelton et al., unpublished results). Of the 32 churches approached by project staff, 29 were ultimately recruited as sources for individual participants. Typical of the region, most participating churches were small (i.e., fewer than 50 adult female members).
Congregants were invited to an informational meeting conducted at the church. There, interested female attendees were screened for eligibility, including being 40–64 years old, speaking English, and being outside ACS guidelines at the time for cervical cancer screening (i.e., no Pap test within the prior 12 months) (Saslow et al., 2002). The age-based eligibility criterion was set considering that (a) at these ages, screening notably decreases, whereas vulnerability to cervical cancer increases (Freeman et al., 2005; Hall et al., 2002); and (b) participants over 64 years old would be Medicare-eligible, potentially skewing the distribution of health care access by age. The 2002 ACS recommendations were used for their stability (in the context of contemporaneous changes in screening procedures and guidelines) and their consistency with Healthy People 2010 goals (U.S. Department of Health and Human Services, 2000).
Eligible participants provided voluntary informed consent and completed a baseline questionnaire. Interviewers offered to administer all documents orally, mitigating limited literacy. Participants received $25 for completing the baseline assessment and any subsequent interviews. To support retention, thank-you letters and invitations to continue contributing to the study were sent to all participants following enrollment.
Study Design
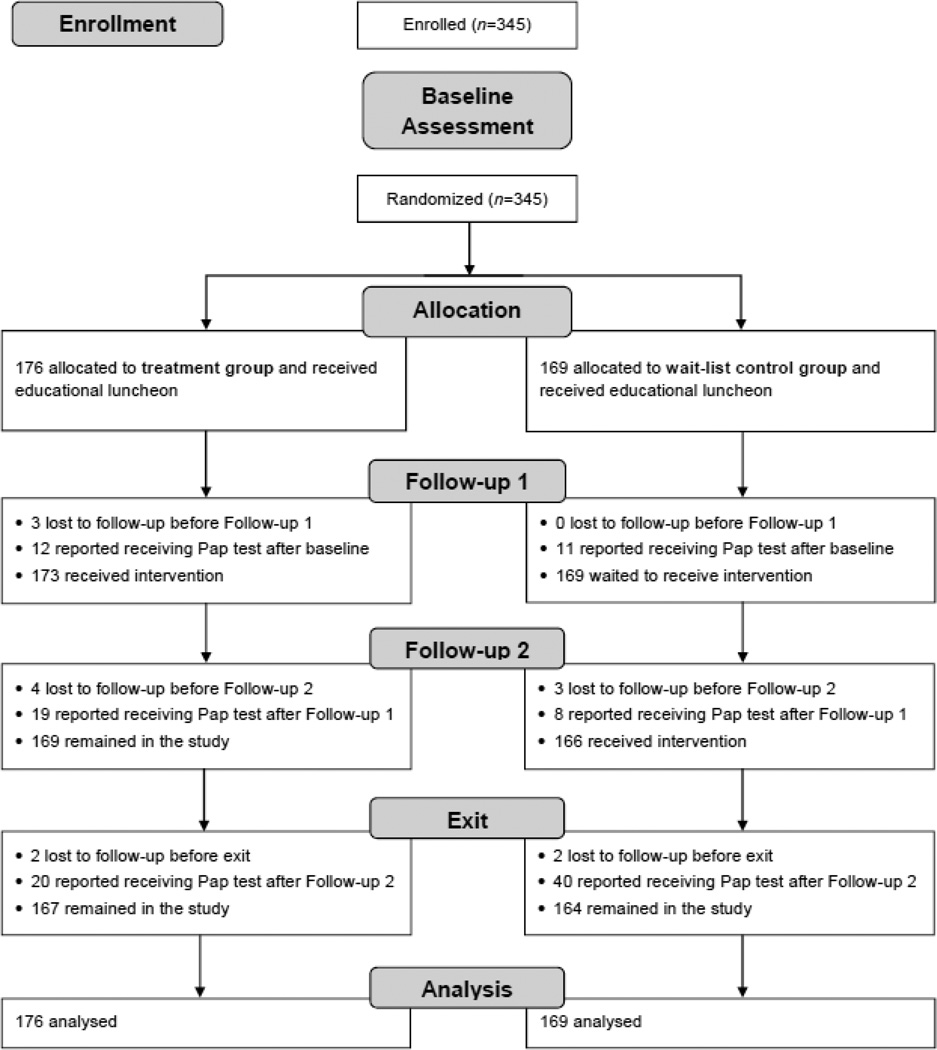
Figure 2 illustrates the design of this single-blind, two-armed RCT with a wait-list control group. Baseline data were collected regarding (a) sociodemographic and health-related characteristics; (b) history of cervical cancer screening; (c) cervical cancer screening knowledge, attitudes, and behaviors; and (d) perceived barriers to cervical cancer screening. Participants were subsequently randomized to treatment or wait-list control conditions. Data collectors and investigators were blind to group allocation throughout the study.
Figure 2.
Study flow diagram. Harlan, Knott, Letcher, and Perry counties, Kentucky, U.S.A. 2005–2008.
Next, all participants received an educational lunch program at the church, at which local project staff delivered information on cervical cancer screening and prevention. The educational program not only ensured that all participants had a basic level of awareness about the importance of screening before the individualized intervention was delivered (Kreuter & Wray, 2003), but also was intended to enhance study retention by promoting affiliation among each church’s participants. Approximately four months after baseline, Follow-up 1 was conducted with all participants, involving reassessment of knowledge, attitudes, and behaviors regarding cervical cancer screening. This served as a secondary baseline for those who were not screened following the educational luncheon offered to all participants.
One month after Follow-up 1, the treatment group received the intervention, described in detail below. Approximately four months after Follow-up 1, all participants completed Follow-up 2, and cervical cancer screening status was reassessed. The wait-list control group subsequently received the intervention. Both groups completed an Exit Interview approximately six months after Follow-up 2, including reassessment of cervical cancer screening status. The Exit Interview served as a long-term follow-up for the treatment group, and as a post-test following intervention delivery for the wait-list control group. See Figure 2 for an illustration of study flow.
Intervention
Ten LHAs were recruited, with most belonging to participating churches; all were residents of one of the four FMM counties. The LHAs were demographically similar to most participants (i.e., married, middle-aged, middle-to-lower socioeconomic status), with no professional health care background. All were trained by the study team about cervical cancer, Pap tests, local community resources, and screening determinants. Before delivering the intervention, LHAs completed three training sessions on human subjects protection, home visit procedures, and inclusion of tailored content regarding participants’ identified barriers into the newsletter template. The LHAs received feedback from the project team throughout the study and were retrained as necessary.
The tailored home visit and newsletter addressed the specific barriers identified by each participant. Prior to the home visit, the LHA reviewed the participant’s identified barriers from the baseline assessment. Possible barriers included predisposing (e.g., beliefs about cervical cancer and screening), enabling (e.g., health insurance coverage), and reinforcing (e.g., social support for screening) factors, as conceptualized in the PRECEDE/PROCEED framework (Green & Kreuter, 1991). The LHA prepared a tailored newsletter using a standard template, adding specific information regarding the participant’s barriers. For example, if a participant identified transportation as a barrier to screening, the LHA included information about local transportation options.
Home visits were designed to last approximately two hours. The LHA provided information about cervical cancer and Pap tests, then addressed each of the participant’s identified barriers to screening. Any additional issues brought up during home visits were also addressed. The tailored newsletter was left as a supportive reminder and informational source. The intervention was intended to: (a) affect the participant’s knowledge, beliefs, and attitudes regarding cervical cancer screening, depending on self-identified barriers; and (b) facilitate the participant’s scheduling and obtaining of a Pap test. No actual referrals or appointments were provided by the LHAs, as the project aim included enabling women to make their own screening arrangements.
Measures
All study measures were developed using data from in-depth interviews conducted prior to the trial (Schoenberg et al., 2006). Pilot-testing with 10 age-appropriate local women yielded an 88-item instrument. The primary outcome was self-reported Pap test receipt, obtained by asking participants to provide the approximate date of their most recent screening. Sociodemographic and health-related variables included age, race, income, educational level, perceived health status, and others. General knowledge, attitudes, behaviors, and perceived barriers regarding cervical cancer screening were also assessed, using culturally appropriate questions derived from developmental work in the region (Schoenberg et al., 2006).
Sample Size and Randomization
Previous research suggested that a difference of 12.5% in proportions of participants reporting Pap test receipt could be anticipated. Allowing for a 10% dropout rate, a sample size of 300 participants per group was planned to achieve 80% power to detect this difference with alpha set at .05 for two-tailed tests. Blocked randomization of individuals with variable block sizes ensured equal probabilities of group assignment. The computer-generated randomization scheme was carried out by a biostatistician to maintain concealment. Study arm assignment was conducted by project staff members uninvolved in questionnaire administration.
Data Analyses
Data analyses were completed in 2012. Descriptive statistics characterized the study sample, and bivariate tests (i.e., chi-square tests of independence for categorical and independent t-tests for continuous variables) assessed baseline group differences.
A logistic regression model was used to test the effect of the intervention on the primary outcome of obtaining a Pap test between baseline and Follow-up 2 (i.e., after the treatment group had completed the intervention and the wait-list control group had not), adjusting for the random effect of clustering within churches. An additional exploratory model added several participant characteristics selected post hoc, including age group, marital status, perceived health status, and baseline screening history. Baseline screening history was categorized into two groups to control for previously reported differences observed between recently screened (i.e., more than one year but less than five years ago) and rarely or never screened (i.e., five years ago or longer, or never) participants (Hatcher et al., 2011; Paskett et al., 2011). Likelihood ratio tests were used to assess improvements in model fit. A sensitivity analysis tested the effect of the intervention only among participants who remained unscreened at Follow-up 1, following the educational luncheon, using a logistic regression model adjusting for the random effect of church.
To evaluate the effect of the intervention in the wait-list control group, McNemar’s test for paired proportions was conducted with wait-list control participants only. This analysis compared the proportions of wait-list control participants reporting Pap tests at Follow-up 2 and Exit Interview (i.e., pre- and post-intervention).
Finally, secondary analyses examined associations between obtaining a Pap test at any time during the study and participants’ sociodemographic and health-related characteristics, controlling for effects of treatment group and church. Variables examined in these analyses appear in Table 4. Separate bivariate logistic regression analyses assessed associations between each characteristic and the primary outcome, controlling for treatment group (fixed effect) and church (random effect). Due to the exploratory nature of these analyses, no adjustments to alpha were made.
Table 4.
Associations between study-wide Pap test receipt and participant characteristics, adjusted for treatment group and church (n=345). Harlan, Knott, Letcher, and Perry counties, Kentucky, U.S.A. 2005–2008.
| Receipt of a Pap test |
||||||
|---|---|---|---|---|---|---|
| No (n=235) |
Yes (n=110) |
OR | 95% CI | |||
| Variables | n | (%) | n | (%) | ||
| Age (in years) | ||||||
| 40–44 | 43 | (62.3) | 26 | (37.7) | Ref. | |
| 45–49 | 45 | (65.2) | 24 | (34.8) | 0.87 | 0.43 – 1.75 |
| 50–54 | 51 | (62.2) | 31 | (37.8) | 1.00 | 0.52 – 1.94 |
| 55–59 | 63 | (79.7) | 16 | (20.3) | 0.41 | 0.20 – 0.86* |
| 60–64 | 33 | (71.7) | 13 | (28.3) | 0.64 | 0.29 – 1.44 |
| Race | ||||||
| White | 225 | (68.6) | 103 | (31.4) | Ref. | |
| Non-White | 10 | (58.8) | 7 | (41.2) | 1.54 | 0.57 – 4.17 |
| Marital status | ||||||
| Married/partnered | 147 | (69.7) | 64 | (30.3) | Ref. | |
| Separated/divorced/widowed | 73 | (63.5) | 42 | (36.5) | 1.31 | 0.81 – 2.11 |
| Never married | 15 | (79.0) | 4 | (21.1) | 0.61 | 0.20 – 1.92 |
| Education | ||||||
| Less than high school | 63 | (71.6) | 25 | (28.4) | Ref. | |
| High school graduate/GED | 94 | (69.6) | 41 | (30.4) | 1.08 | 0.59 – 1.95 |
| Some college | 52 | (65.8) | 27 | (34.2) | 1.31 | 0.68 – 2.54 |
| College graduate or more | 23 | (57.5) | 17 | (42.5) | 1.93 | 0.88 – 4.22 |
| Employed currently | 115 | (66.9) | 57 | (33.1) | 1.13 | 0.72 – 1.78 |
| Annual Household Income | ||||||
| < $10,000 | 56 | (65.9) | 29 | (34.1) | Ref. | |
| $10,000 – $30,000 | 70 | (66.0) | 36 | (34.0) | 1.01 | 0.55 – 1.84 |
| > $30,000 | 42 | (63.6) | 24 | (36.4) | 1.11 | 0.56 – 2.17 |
| Don’t know/refused | 67 | (76.1) | 21 | (23.9) | 0.60 | 0.31 – 1.17 |
| Perceived financial status | ||||||
| Struggle to meet needs | 117 | (65.7) | 61 | (34.3) | Ref. | |
| Just enough to get by | 90 | (70.3) | 38 | (29.7) | 0.79 | 0.48 – 1.29 |
| More than I need to live well | 16 | (80.0) | 4 | (20.0) | 0.46 | 0.15 – 1.45 |
| Don’t know/refused | 12 | (63.2) | 7 | (36.8) | 1.06 | 0.39 – 2.85 |
| Health insurance | ||||||
| Private | 83 | (74.8) | 28 | (25.2) | Ref. | |
| Employer-provided | 40 | (67.8) | 19 | (32.2) | 1.42 | 0.71 – 2.86 |
| Medicare | 22 | (61.1) | 14 | (38.9) | 1.89 | 0.85 – 4.19 |
| Medicaid | 80 | (63.5) | 46 | (36.5) | 1.71 | 0.97 – 2.99 |
| None | 10 | (76.9) | 3 | (23.1) | 0.90 | 0.23 – 3.50 |
| Perceived health status | ||||||
| Poor/fair | 105 | (68.2) | 49 | (31.8) | Ref. | |
| Good | 93 | (73.2) | 34 | (26.8) | 0.79 | 0.46 – 1.31 |
| Very good/excellent | 37 | (57.8) | 27 | (42.2) | 1.53 | 0.84 – 2.80 |
| Had last Pap smear | ||||||
| ≥ 5 years ago and never | 94 | (80.3) | 23 | (19.7) | Ref. | |
| > 1 year and < 5 years ago | 141 | (61.8) | 87 | (38.2) | 2.50 | 1.47 – 4.25*** |
Notes:
p < 0.05.
p < 0.001.
OR = estimated odds ratio, adjusted for treatment group (fixed effect) and church (random effect). CI = confidence interval. GED = General Education Development test. Percentages for each variable may not sum to 100% due to rounding.
Participants lost to follow-up were retained in analyses with the assumption that they were not screened; intent-to-treat principles were followed. Alpha was set at .05, and all tests were two-sided. Analyses were conducted using Intercooled Stata 10.1 (StataCorp, Inc.) and R: A Language and Environment for Statistical Computing (version 2.13.0) (R Foundation for Statistical Computing, 2011), including the package lme4 (Bates et al., 2011).
Results
Sample Characteristics
A total of 345 eligible women ages 40–64 from 29 churches were enrolled in the study, individually randomized to treatment (n=176) and wait-list control (n=169) groups. Of those, 14 (4.1%) were lost to follow-up, including 9 from the treatment group and 5 from the wait-list control group. The groups did not differ significantly on any baseline characteristics (Table 2).
Table 2.
Baseline sample characteristics (n=345). Harlan, Knott, Letcher, and Perry counties, Kentucky, U.S.A. 2005–2008.
| Variables | n | (%) |
|---|---|---|
| Age (in years) | ||
| 40–44 | 69 | (20.0) |
| 45–49 | 69 | (20.0) |
| 50–54 | 82 | (23.8) |
| 55–59 | 79 | (22.9) |
| 60–64 | 46 | (13.3) |
| Race | ||
| White | 328 | (95.1) |
| Black | 16 | (4.6) |
| American Indian | 1 | (0.3) |
| Marital status | ||
| Married/partnered | 211 | (61.2) |
| Separated/divorced | 87 | (25.2) |
| Widowed | 28 | (8.1) |
| Never married | 19 | (5.5) |
| Education | ||
| Less than high school | 88 | (25.7) |
| High school graduate or GED | 135 | (39.5) |
| Some college | 79 | (23.1) |
| College graduate or more | 40 | (11.7) |
| Employed currently | 172 | (49.9) |
| Annual household income | ||
| < $10,000 | 85 | (24.6) |
| $10,000 – $30,000 | 106 | (30.7) |
| > $30,000 | 66 | (19.1) |
| Don’t know/refused | 88 | (25.5) |
| Perceived financial status | ||
| Struggle to meet needs | 178 | (51.6) |
| Just enough to get by | 128 | (37.1) |
| More than I need to live well | 20 | (5.5) |
| Don’t know/refused | 19 | (5.5) |
| Health insurance | ||
| Private | 139 | (40.3) |
| Public | 95 | (27.5) |
| None | 111 | (32.2) |
| Perceived health status | ||
| Poor/fair | 154 | (44.6) |
| Good | 127 | (36.8) |
| Very good/excellent | 64 | (18.6) |
| Screening history | ||
| Never | 4 | (1.2) |
| ≥ 5 years ago | 113 | (32.8) |
| > 1 year and < 5 years ago | 228 | (66.1) |
Notes: Percentages for each variable may not sum to 100% due to rounding. No differences between groups were found at baseline. The categories Never and ≥ 5 years ago under Screening history were combined for analyses due to a low cell count in Never and to previous findings discriminating this combined group from those screened > 1 year and < 5 years ago (see Hatcher et al., 2011).
All participants described their ethnicity as non-Hispanic, and most (95.1%) described themselves as White, reflecting the overall demography of the four FMM counties (U.S. Census Bureau, 2010). Most were married (61.2%), and one-quarter (25.7%) had less than a high school education. Half (50.1%) were unemployed, and one-third (32.2%) were uninsured. Over half (54.6%) reported struggling to meet their needs financially. Fewer than one-fifth (18.6%) reported having very good or excellent health status.
Regarding Pap test history, 34.0% were rarely or never screened (last Pap test five years ago or more, or never), while 66.1% were recently screened (less than five but more than one year ago).
Primary Outcome: Obtaining a Pap Test at Follow-up 2
See Figure 2 for an illustration of participant flow through the study. Of the 345 participants, 23 (6.7%) reported obtaining a Pap test after the educational luncheon (i.e., at Follow-up 1), before the LHA intervention was delivered to the treatment group. Of these, 12 were in the treatment group and 11 were in the wait-list control group.
At Follow-up 2 (i.e., post- intervention), 19 additional treatment group participants and 8 additional wait-list controls reported obtaining a Pap test, for a total of 31 (18%) treatment group and 19 (11%) wait-list control group participants screened. Logistic regression modeling adjusting for the random effect of church demonstrated a significant intervention effect (Table 3). Compared to wait-list controls, the treatment group had over twice the odds of being screened, est. adj. OR = 2.56, 95%CI: 1.03–6.38, p = 0.04. An exploratory logistic regression model including other participant characteristics (i.e., age group, marital status, perceived health status, baseline screening history) revealed very similar treatment effects with no improvement in model fit.
Table 3.
Primary outcome: Odds ratios of Pap test receipt for treatment group versus wait-list control group at Follow-up 2 (n=345). Harlan, Knott, Letcher, and Perry counties, Kentucky, U.S.A. 2005–2008.
| Model | OR (95% CI) | p | |
|---|---|---|---|
| Model 1a: | Unadjusted (fixed effect of treatment only) | 2.43 (1.04–5.72) | 0.04 |
| Model 2b: | Adjusted for church (random effect) | 2.56 (1.03–6.38) | 0.04 |
| Model 3c: | Adjusted for church (random effect) and participant characteristics (fixed effects) |
2.73 (1.08–6.89) | 0.03 |
Notes. OR = odds ratio. CI = confidence interval.
Model 1 provides the raw OR for treatment effect, not adjusted for covariates.
Model 2 represents the primary outcome analysis, providing the adjusted OR for treatment effect accounting for participant clustering in churches.
Model 3 provides the adjusted OR for treatment effect accounting for participant clustering in churches as well as additional participant characteristics selected post hoc (age group, marital status, perceived health status, baseline screening status). A likelihood ratio test detected no improved fit for Model 3 versus Model 2 (p = 0.63).
The sensitivity analysis excluded the 23 participants who obtained Pap tests between baseline and Follow-up 1 (i.e., before the intervention was delivered). At Follow-up 2 (post-intervention), 19 (11.6%) of the 164 treatment group participants and 8 (5.1%) of the 158 wait-list controls still in need of cervical cancer screening reported obtaining a Pap test. Logistic regression results were nearly identical to results of the intent-to-treat primary outcome analysis (OR = 2.59, 95%CI: 1.04–6.46, p = 0.04).
At Exit Interview, 40 (25.3%) of the 158 wait-list control group participants still in need of screening at Follow-up 2 reported having obtained a Pap test post-intervention, compared to the 8 (5.1%) observed at Follow-up 2. McNemar’s test of paired proportions revealed this difference to be statistically significant, χ2(1df, n=158) = 21.3, p < 0.001. An additional 20 (13.8%) of 145 treatment group participants still in need of cervical cancer screening also reported obtaining a Pap test at Exit Interview.
Secondary Analyses: Factors Associated with Obtaining a Pap Test
Controlling for treatment group and church, age and baseline history of screening were significantly associated with obtaining a Pap test during the study (Table 4). Compared to women aged 40–44, those who were age 55–59 years were significantly less likely to be screened, est. adj. OR = 0.41, 95%CI: 0.20–0.86, p = 0.02. Recently screened participants had significantly higher odds of obtaining a Pap test during the study than rarely/never screened participants, est. adj. OR = 2.50, 95%CI: 1.47–4.25, p < 0.001.
Discussion
Faith Moves Mountains assessed the effectiveness of a faith-placed LHA intervention to increase Pap test use among middle-aged and older women in Appalachian Kentucky. The significant difference in the proportions of treatment versus wait-list control group participants who reported being screened at Follow-up 2 (i.e., after the treatment group had received the intervention and the wait-list control group had not) demonstrated the intervention’s effectiveness.
As with many community-based projects, FMM depended upon the efforts of local residents, often requiring a delicate balance of community needs and scientific procedures. For example, recruitment of churches and participants progressed more slowly than anticipated. Initial attempts to implement a probability sampling scheme proved futile, as randomly selected churches did not respond to “cold calls” and letters. While shifting to snowball sampling may have impacted generalizability of findings (Shelton et al., unpublished results), it was a necessary step to achieve acceptable participant enrollment. Despite the need for such trade-offs, a significant intervention effect was detected. This finding is important, particularly in the context of a community-based intervention to increase cervical cancer screening among vulnerable women in a disproportionately affected region of the U.S.
These results add to the literature supporting the effectiveness of individualized interventions via LHAs (e.g., Dignan et al., 1998; Green, 1977; Paskett et al., 2011). While a small percentage of participants were screened following the educational luncheon, nearly one-quarter of the sample obtained Pap tests after receiving the LHA intervention. Leveraging church connections to facilitate LHA-provided remediation of barriers to screening appears to be an effective strategy to increase Pap test use among Appalachian women outside of screening recommendations.
Overall, 31.9% of all FMM participants reported being screened for cervical cancer during the study period. This result is on the high end of rates reported in other community-based intervention studies targeting cervical cancer screening. A systematic review of 46 studies published from 1980 through 2001—all of which evaluated similar sociologic, cognitive, and behavioral interventions—reported increases in Pap test use rates ranging from 2.7% to 36.0% (Yabroff et al., 2003). In contrast to clinic-based intervention studies, which often report higher rates of Pap test use among participants (e.g., Paskett et al., 2011), community-based projects often yield lower screening rates than achieved by FMM in the current study.
Secondary analyses found that age and cervical cancer screening history were associated with Pap test receipt. Independent of treatment group, women in the second-to-oldest group were significantly less likely than those in the youngest group to report receiving a Pap test. Perceptions of reduced risk for cervical cancer with increased age may underlie this finding (Marlow et al., 2009), despite recommendations that screening not be discontinued until at least age 65 for most women (ACS, 2011b; U.S. Preventive Services Task Force, 2003).
In addition, controlling for treatment group, women who were furthest outside ACS screening guidelines had significantly lower odds of being screened during the study, compared to women who were more recently screened. The majority (80.3%) of rarely or never screened participants did not obtain Pap tests throughout the study. This group reported baseline attitudes and beliefs about cervical cancer and screening that differed from those reported by recently screened women (Hatcher et al., 2011); for example, compared to recently screened participants, higher proportions of rarely or never screened women believed that cervical cancer has symptoms and that screening causes worry. In addition, Hatcher and colleagues found that rarely or never screened women reported different barriers at baseline, compared to recently screened women. These included part-time employment, perceiving screening as too expensive, and lacking a usual health care source. Finally, at baseline, even a health provider’s direct recommendation was perceived as less influential by the rarely or never screened, compared to the recently screened (Hatcher et al., 2011). These differences may underlie the apparently reduced effectiveness of the intervention among rarely or never screened Appalachian women, who may be at the greatest risk of ICC. Future analyses will investigate barriers to screening still identified by rarely or never screened participants post-intervention, with the goal of refining the intervention to increase its impact among this vulnerable subgroup.
Study Limitations
This project had several limitations. First, the study employed a small, relatively homogenous sample from a limited geographical region. This concern is mitigated by representativeness of the sample to the demography of the central Appalachian region (U.S. Census Bureau, 2010). Another limitation is reliance on self-report data. However, studies have demonstrated 70% positive and 95% negative predictive value of recall for Pap tests (McGovern et al., 1998; McPhee et al., 2002). Additionally, recruitment of churches and individual participants progressed more slowly than anticipated, resulting in a non-random sample of churches as recruitment sites and a smaller sample size than planned. Despite these shortcomings, a significant treatment effect was still detected. For dissemination purposes, participant identification of barriers is an important aspect of the intervention. In this study, barriers were gleaned from the baseline assessment; in practice, barriers could be assessed prior to or at the beginning of the LHA home visit. However, this study did not address potential differences in the timing or approach to assessing barriers. Finally, unintended and unmeasured effects may have existed, related to the faith-placed nature of the project. Specifically, participants within a single church could be randomized to either the treatment or the wait-list control group, allowing potential contamination. Because this may have “watered down” the intervention effect, future efforts should consider measurement of contamination and a group-randomized design with churches as the unit of randomization.
Conclusions
Despite these limitations, the RCT design of FMM enabled the detection of a significant intervention effect. Results are notable for three additional reasons. First, since past behavior often predicts future behavior (Ouellette and Wood, 1998; Sutton, 2004; Weinstein, 2007), it is likely that the women who obtained Pap tests during FMM will maintain cervical cancer screening in the future. Second, the target population included hard-to-reach women. One-third of FMM participants were rarely or never screened, presenting significant challenges to behavior change. Despite the relative lack of behavior change within this subgroup, FMM was one of the first projects in the region to successfully recruit and enroll a substantial number of these women in research. Although recruitment of these unlikely research participants was successful, the intervention was least effective for them. However, the data provided by this vulnerable subgroup will supply valuable information for modifying the intervention. Finally, FMM developed an infrastructure for future projects, which will investigate the effects of community-based, faith-placed interventions to improve other health behaviors in this region.
To our knowledge, this project was the first to combine a LHA approach with faith-placed recruitment, tailored home visits, and tailored newsletters focused on participant-identified barriers to screening. As a novel strategy to reduce a recognized health disparity experienced by hard-to-reach Appalachian women, results of the current study support further efforts in this vein.
Acknowledgements
We acknowledge and thank all of Faith Moves Mountains’ community and scientific staff and participants. We also express our appreciation to the community members who have offered extensive support to this project. This project was approved by the University of Kentucky Medical Institutional Review Board on 3/8/2004.
Sponsor of Research
This research was supported by the National Cancer Institute (R01CA108696). The project was initiated and analyzed by the investigators.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Contributor Information
Yelena N. Tarasenko, Email: YTarasenko@cdc.gov.
Nancy E. Schoenberg, Email: nesch@uky.edu.
Brent J. Shelton, Email: bshelton@kcp.uky.edu.
Jennifer Hatcher-Keller, Email: jennifer.hatcher@uky.edu.
Mark B. Dignan, Email: mbdign2@email.uky.edu.
References
- American Cancer Society. Cancer Facts and Figures 2011. Atlanta: American Cancer Society; 2011a. [Accessed November 9, 2011]. from http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-029771.pdf. [Google Scholar]
- American Cancer Society. [Accessed January 20, 2012];American Cancer Society guidelines for the early detection of cancer. 2011b from http://www.cancer.org/Healthy/FindCancerEarly/CancerScreeningGuidelines/americancancer-society-guidelines-for-the-early-detection-of-cancer.
- Appalachian Regional Commission. [Accessed November 9, 2010];Research, maps, and data. 2011 from http://www.arc.gov/research/RegionalDataandResearch.asp.
- Bandura A. Social Learning Theory. Englewood Cliffs, New Jersey: Prentice Hall; 1977. [Google Scholar]
- Bates D, Maechler M, Bolker B. [Accessed November 9, 2011];Lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-41. 2011 from http://CRAN.R-project.org/package=lme4.
- Campbell MK, Hudson MA, Resnicow K, Blakeney N, Paxton A, Baskin M. Church-based health promotion interventions: evidence and lessons learned. Annu Rev Public Health. 2007;28:213–234. doi: 10.1146/annurev.publhealth.28.021406.144016. [DOI] [PubMed] [Google Scholar]
- Dignan MB, Michielutte R, Wells HB, Sharp P, Blinson K, Case LD, Bell R, Konen J, Davis S, McQuellon RP. Health education to increase screening for cervical cancer among Lumbee Indian women in North Carolina. Health Educ Res. 1998;13:545–556. doi: 10.1093/her/13.4.545. [DOI] [PubMed] [Google Scholar]
- Earp JL, Flax VL. What lay health advisors do: An evaluation of advisors’ activities. Can Pract. 1999;7:16–21. doi: 10.1046/j.1523-5394.1999.07104.x. [DOI] [PubMed] [Google Scholar]
- Eng E, Parker E, Harlan C. Lay health advisor intervention strategies: a continuum from natural helping to paraprofessional helping. Health Educ Behav. 1997;24:413–417. doi: 10.1177/109019819702400402. [DOI] [PubMed] [Google Scholar]
- Freeman HP, Wingrove BK. Excess cervical cancer mortality: A marker for low access to health care in poor communities. NIH Pub. No. 05-5282. Rockville, Maryland: National Cancer Institute; 2005. [Google Scholar]
- Green LW. Evaluation and measurement: Some dilemmas for health education. Am J Public Health. 1977;67:155–161. doi: 10.2105/ajph.67.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LW, Kreuter MW. Health promotion planning: An educational and environmental approach. Mountain View, California: Mayfield; 1991. [Google Scholar]
- Hall HI, Uhler RJ, Coughlin SS, Miller DS. Breast and cervical cancer screening among Appalachian women. Cancer Epidemiol Biomarkers Prev. 2002;11:137–142. [PubMed] [Google Scholar]
- Hatcher J, Studts C, Dignan M, Turner L, Schoenberg N. Predictors of cervical cancer screening for rarely or never screened rural Appalachian women. J Health Care Poor Underserved. 2011;22:176–193. doi: 10.1353/hpu.2011.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopenhayn C, Bush H, Christian A, Shelton BJ. Comparative analysis of invasive cervical cancer incidence rates in three Appalachian states. Prev Med. 2005;41:859–864. doi: 10.1016/j.ypmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Kentucky Department of Public Health. [Accessed January 18, 2012];Kentucky Behavioral Risk Factor Surveillance System 2006 Annual Report. 2006 from http://chfs.ky.gov/NR/rdonlyres/CAA859A6-4C7B-4A60-8ACC-CB2DBE859A85/0/Final2006Report.pdf.
- Kreuter MW, Wray RJ. Tailored and targeted health communication: Strategies for enhancing information relevance. Am J Health Behav. 2003;27:S227–S232. doi: 10.5993/ajhb.27.1.s3.6. [DOI] [PubMed] [Google Scholar]
- Marlow LAV, Waller J, Wardle J. The impact of human papillomavirus information on perceived risk of cervical cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:373–376. doi: 10.1158/1055-9965.EPI-08-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern PG, Lurie N, Margolis KL, Slater JS. Accuracy of self-report of mammography and pap smear in a low-income urban population. Am J Prev Med. 1998;14:201–208. doi: 10.1016/s0749-3797(97)00076-7. [DOI] [PubMed] [Google Scholar]
- McPhee SJ, Nguyen TT, Shema SJ, Nguyen B, Somkin C, Vo P, Pasick R. Validation of recall of breast and cervical cancer screening by women in an ethnically diverse population. Prev Med. 2002;35:463–473. doi: 10.1006/pmed.2002.1096. [DOI] [PubMed] [Google Scholar]
- Murray CJL, Kulkarni SC, Michaud C, Tomijilma N, Bulzacchelli MT. Eight Americas: Investigating mortality disparities across race, counties, and race-counties in the US. PLoS Med. 2006;3:1513–1524. doi: 10.1371/journal.pmed.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette JA, Wood JS. Habit and intention in everyday life: The multiple processes by which past behavior predicts future behavior. Psychol Bull. 1998;124:54–74. [Google Scholar]
- Paskett ED, McLaughlin JM, Lehman A, Katz ML, Tatum C, Oliveri JM. Evaluating the efficacy of lay health advisors for increasing risk-appropriate Pap test screening: a randomized controlled trial among Ohio Appalachian women. Cancer Epidemiol Biomarkers Prev. 2011;20:835–843. doi: 10.1158/1055-9965.EPI-10-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Forum on Religion & Public Life. [Accessed November 9, 2011];U.S. Religious Landscape Survey. Religious Affiliation: Diverse and Dynamic. 2008 from http://religions.pewforum.org/pdf/report-religious-landscape-study-full.pdf.
- R Development Core Team. [Accessed November 9, 2011];R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. 2011 from http://www.R-project.org/.
- Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ. In: SEER Cancer Statistics Review, 1975–2002. Edwards BK, editor. Bethesda, MD: National Cancer Institute; 2005. [Google Scholar]
- Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health Educ Q. 1988;15:175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- Salber E. The lay advisor as a community health resource. J Health Polit Policy Law. 1979;3:469–478. doi: 10.1215/03616878-3-4-469. [DOI] [PubMed] [Google Scholar]
- Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, Cohen C. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342–362. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- Schoenberg NE, Hatcher J, Dignan MB, Shelton B, Wright S, Dollarhide KF. Faith Moves Mountains: An Appalachian cervical cancer prevention program. Am J Health Behav. 2009;33:627–633. doi: 10.5993/ajhb.33.6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg NE, Hopenhayn C, Christian A, Knight E, Rubio A. An in-depth and updated perspective on determinants of cervical cancer screening among central Appalachian women. Women and Health. 2006;42:89–105. doi: 10.1300/j013v42n02_06. [DOI] [PubMed] [Google Scholar]
- Shelton BJ, Schoenberg NE, Dignan M, Dollarhide K, Hatcher J, Studts CR, van Meter E. Trials and tribulations of sampling faith-based groups for a cancer screening intervention: Lessons learned from the Faith Moves Mountains project. (unpublished results). [Google Scholar]
- Surveillance Epidemiology and End Results (SEER) Program. Cancer Statistics Review. Bethesda, Maryland: National Cancer Institute; 2009. [Google Scholar]
- Sutton SR. Determinants of health-related behaviors. In: Baum Sutton S., editor. Theoretical and Methodological Issues. London: The Sage Handbook of Health Psychology, Sage; 2004. [Google Scholar]
- U.S. Census Bureau. [Accessed November 9, 2011];State and county quick facts: Harlan, Knott, Letcher, and Perry Counties. 2010 from http://quickfacts.census.gov/qfd/states/21000.html.
- U.S. Department of Health and Human Services. [Accessed January 21, 2009];Healthy people 2010: Understanding and improving health. 2000 from http://www.health.gov/healthypeople/url/
- U.S. Preventive Services Task Force. [Accessed January 20, 2012];Screening for cervical cancer. 2003 at http://www.uspreventiveservicestaskforce.org/uspstf/uspscerv.htm.
- Weinstein ND. Misleading tests of health behavior theories. Ann Behav Med. 2007;33:1–10. doi: 10.1207/s15324796abm3301_1. [DOI] [PubMed] [Google Scholar]
- Yabroff KR, Mangan P, Mandelblatt J. Effectiveness of interventions to increase papanicolaou smear use. J Am Board Fam Pract. 2003;16:188–203. doi: 10.3122/jabfm.16.3.188. [DOI] [PubMed] [Google Scholar]