Abstract
Synaptic plasticity is thought to be the basis of learning and memory, but it is mostly studied on the timescale of mere minutes. This review discusses synaptic consolidation, a process that enables synapses to retain their strength for a much longer time (days to years), instead of returning to their original value. The process involves specific plasticity-related proteins, and depends on the dopamine D1/D5 receptors. Here, we review the research on synaptic consolidation, describing electrophysiology experiments, recent modeling work, as well as behavioral correlates.
Keywords: Synaptic tagging, Synaptic consolidation, Synaptic plasticity, Model, Behavior, Electrophysiology, Review
Memory consolidation
Memories are stored for different amounts of time. For example, people remember for a whole day where they parked their car, without being able to remember where they parked it a month ago. On the other hand, they perfectly remember where they parked at their wedding. Thus, selected memories are stored for a very long time, a process that is called memory consolidation.
Memories are thought to be stored in the connections between neurons called synapses, whose strength can be changed by learning. Every new memory changes the synapse strengths, which in turn alters previously stored memories. This phenomenon is puzzling, because some memories appear to be stored for an entire life-time. One explanatory hypothesis is relying on the fact that memories can be stored (in synaptic strengths) in different parts of the brain: it is plausible that important memories are transfered from one brain area to a different one that is better protected from changes induced by new incoming memories. There is some biological evidence that memories are stored in the medial temporal lobe. In particular, during consolidation, memories that are first stored in the hippocampus are transfered to other areas of the cortex (Kirwan et al. 2008; Smith and Squire 2009). This transfer can happen during replay events while resting and sleeping (Wilson and McNaughton 1994; Diba et al. 2007). The hypothesis is reinforced by the famous patient HM (Scoville and Milner 1957) whose hippocampus was removed following epilepsy in the medial temporal lobe. HM retained old memories from before his surgery, but he could barely acquire any new long-term memories.
This review will describe an additional, less-known mechanism of memory consolidation, which happens at the synapse level. Synapses can be plastic, which means that their strength can vary. A change in synaptic strength can last for different lengths of time: we speak about short-term plasticity when the change lasts up to a few minutes, early-long-term plasticity when it lasts up to a few hours and late-long-term plasticity when it lasts beyond the experiment’s duration (which is often about 10 h) but is thought to last much longer even, possibly a life-time. This last type of plasticity is also called synaptic consolidation or maintenance. This process allows relevant memories to be consolidated within a single synapse, so that new memories can no longer alter previously consolidated ones.
The remainder of this article will describe the mechanism of synaptic consolidation, as shown by synaptic tagging experiments. First, the phenomenology will be described, together with the key slice experiments. Then the two existing models of synaptic consolidation will be presented and compared. Finally, the link to behavior will be discussed, followed by a link to reinforcement learning and open computational questions.
Background on synaptic plasticity
Synaptic plasticity is typically induced by pairing the activity of the pre- and postsynaptic spike in a slice preparation. The first experimental evidence of synaptic plasticity was found by presynaptic stimulation using an extracellular electrode leading to long-term potentiation (LTP) (Bliss and Lomo 1973) and long-term depression (LTD) (Lynch et al. 1977). The change of plasticity was measured typically for half an hour to one hour. Synaptic plasticity was also induced by stimulating the presynaptic cells while depolarizing the postsynaptic membrane potential (Artola et al. 1990; Ngezahayo et al. 2000). More recently, synaptic plasticity was found to depend on the precise timing of the spikes, typically by inducing pairs of pre- and postsynaptic spike, i.e., spike-timing dependent plasticity (STDP). In some brain areas a presynaptic spike induced before a postsynaptic spike leads to LTP, whereas a postsynaptic spike followed by a presynaptic one leads to LTD (Bi and Poo 1998; Froemke and Dan 2002). However, plasticity does not only depend on pair interactions of spikes (Markram et al. 1997; Sjöström et al. 2001; Froemke et al. 2006; Pfister et al. 2006; Clopath and Gerstner 2010; Clopath et al. 2010). Despite being motivated largely by memory (often implicitly assumed to last very long), all those experiments only measure the synaptic weight for up to one hour after induction.
Numerous models of synaptic plasticity have been developed, first rate-based (Hebb 1949; Oja 1982; Bienenstock et al. 1982) then spike-based (Gerstner and Abbott 1997; Song et al. 2000; Gütig et al. 2003; Pfister and Gerstner 2006; Clopath and Gerstner 2010). Functional implications of synaptic plasticity (Gerstner et al. 1996; Roberts and Bell 2000; Legenstein et al. 2005; Guyonneau et al. 2005; Gerstner and Kistler 2002; Clopath et al. 2010) assume that plasticity induction lasts for as long as no new induction is elicited, which is not always the case, as shown below.
Synaptic consolidation experiments
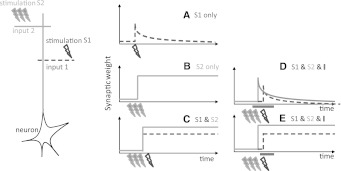
Frey et al. (Frey and Morris 1997) separated long-term plasticity into (1) early-long-term plasticity (eLTP/D, P for potentiation, D for depression) that lasts 2–3 h and (2) late-long-term plasticity (lLTP/D) that lasts for more than 10 h, supposedly days, years. Figure 1 schematically describes their experiments. In hippocampal slices they presynaptically stimulated a weak tetanus (21 pulses at 100 Hz) with an extracellular electrode, and recorded the weight change (panel A). The synaptic weight after the induction is potentiated by about 50% but slowly decays back to its original weight within about 3 h. If however, a strong tetanus (3 times 100 pulses at 100 Hz) is induced presynaptically (panel B), the weight is potentiated by about 80% and it lasts for the length of the recording, i.e., 10 h (See Frey and Morris 1997: Figure 4 and Sajikumar and Frey 2004a for the experimental results). The mechanism postulated by Frey and Morris is the theory of synaptic tagging and capture (Reymann and Frey 2007): Following a weak stimulation, many synapses undergo the early-phase of synaptic plasticity, these synapses also are tagged. The early-phase of plasticity and the tag decay. Note that the synaptic weight is a compound of several synapses due to the extracellular stimulation of several presynaptic neurons connected to one postsynaptic neuron and the several contact points between two neurons. Following a strong stimulation, the synapses also undergo the early-phase and the tagging. In this scenario however, more tags have been set due to the strong stimulation. This allows plasticity-related proteins to be synthesized so that the synapses that are tagged will be consolidated. For more details about the biophysics, please read (Redondo and Morris 2011) for review and (Sajikumar et al. 2005), describing the biophysical encoding of tags.
Fig. 1.
Schematic of synaptic tagging experiments. a Synaptic weight change resulting of a presynaptic extra-cellular stimulation of a weak tetanus (21 pulses at 100 Hz), b strong tetanus (3 times 100 pulses at 100 Hz), c weak tetanus in the S1 pathway (dashed line) and strong tetanus in the S2 pathway (solid line), d strong tetanus in the S2 pathway followed 1 h later by a weak tetanus in the S1 pathway. Plasticity-related-protein synthesis inhibitor is applied during a period that starts 25 min before the induction of the strong tetanus, and lasts until 1 h after the weak tetanus induction (horizontal bar). e. Same as (d) but the inhibitor is applied during a period that starts 25 min after the strong tetanus, and lasts until 1 h after the weak tetanus. See Frey and Morris (1997), Sajikumar and Frey (2004a) for details
Finally, the two different induction protocols were fused into a single experiment (panel C, see Frey and Morris 1997, Sajikumar and Frey 2004a): In one pathway (S1), a weak induction is induced whereas in another pathway (S2), a little bit before or after, a strong induction is induced. Both the weights from the S1 and the S2 pathways are potentiated and consolidated. This outcome is well explained by the synaptic tagging and capture theory, since the strong pathway allows plasticity-related proteins to be synthesized, which in turn allows the consolidation of all the synapses that have been tagged (independently of whether it comes from the S1 or the S2 pathway). Similarly, tagging experiments can induce (1) eLTD by weak low frequency stimulation (2) lLTD by strong low frequency stimulation (3) lLTD by a weak low frequency stimulation in one pathway and lLTD by a strong low frequency stimulation in the other pathway (4) lLTD and lLTP by weak low frequency stimulation in one pathway and strong tetanus in the other (Sajikumar and Frey 2004a). This last protocol is called cross-tagging, and leads to consolidation in all different combinations of a strong and a weak induction for depression or potentiation.
Synaptic consolidation depends on plasticity-related proteins, since consolidation can be prevented by application of plasticity-related proteins synthesis inhibitor (Frey and Morris 1997; Sajikumar et al. 2005). For example, a strong tetanus in one pathway applied 1 h before a weak tetanus leads to consolidation. But this consolidation fails if the application of the inhibitor starts before the first induction, and lasts until 1 h after the second induction (see Fig. 1d). However, if the inhibitor is applied only after the first induction (see Fig. 1e), both pathways are consolidated (see Frey and Morris 1997: Figure 2 for more details). Thus, the plasticity-related proteins synthesized from the first induction are also available to the second pathway. Finally, synaptic consolidation depends on dopamine D1/D5 receptors (Sajikumar and Frey 2004a; Frey et al. 1990; Navakkode et al. 2007).
Models of synaptic consolidation
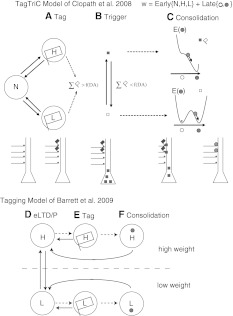
Synaptic consolidation has long been neglected in computational neuroscience; only recently, two models of synaptic tagging were designed (Clopath et al. 2008; Barrett et al. 2009), both of which will be described and compared in this section. The TagTriC model (Clopath et al. 2008) is separated into three processes (see Fig. 2a–c):
Tagging: The synapse strength is the sum of two parts, the weight of the early-phase and the weight of the late-phase. The early-weight is a stochastic three-states variable (Petersen et al. 1998; O’Connor et al. 2005), it can be in neutral state, high state or low state. If the early-weight is in high state or low state, it is automatically tagged. The state of the early-weight switches from neutral to high with a probability given by a Hebbian rule for the induction of plasticity (Clopath et al. 2008, 2010). It decays back to neutral with a certain constant transition probability.
Trigger: If the number of tags exceeds a threshold, plasticity-related proteins are synthesized. The threshold depends on the phasic dopamine level, such that if dopamine is high, the threshold is lowered. Thus, extracellular stimulation increases phasic dopamine signal which in turn lowers the threshold.
Consolidation: The late-weight of the synapse is a continuous variable with two stable states. If plasticity-related proteins are available and if the synapse is tagged, the late-weight will go to the upper stable state if eLTP was induced, lower if eLTD was induced.
Please see Clopath et al. (2008) for the mathematical details. This model allows to describe the synaptic tagging experiment shown in Fig. 1, as well as several pharmacological experiments (Frey and Morris 1997; Sajikumar and Frey 2004a).
Fig. 2.
Models of synaptic consolidation. a–c TagTriC model by Clopath et al. (2008). The synaptic weight is a sum of the early-weight and the late-weight. The three phases of the model. a Tag: The early-weight can be in the neutral (N), low (L) or high (H) state. If in the H or L state, a tag is set (flag). b Trigger: If the number of tags exceed a threshold as of function of dopamine (DA), plasticity-related proteins are synthesized (squares). c Consolidation: If the synapse is tagged and plasticity-related proteins are available, the late-weight (circle) goes to the elevated stable state if the early-weight is in H or the low stable state if the early-weight is in L. E stands for the energy landscape of the late-weight, showing the two stable states unless the tag and the plasticity-related proteins are presents, then E only has one stable state. d–f Consolidation Model by Barrett et al. (2009). Each synapse has 2 possible states with 3 meta-states each, so 6 states in total. d The synapse can be in the early-state without tag, e early-state with the tag, or f consolidated state, each for both a low weight or a high weight. See original papers for details
The consolidation model of Barrett et al. (Barrett et al. 2009) is theoretically very similar, with some differences in its implementation (see Fig. 2d–f). A synapse has one of two states (weak or low) but each state additionally has one of three meta-states: early-phase, tagged or consolidated. As in TagTriC, synapses are stochastic and their synaptic weight is a compound of several synapses. The transitions between states are a function of the protocol (weak tetanus or strong tetanus). Thus, induction of plasticity is not modeled, nor is a mechanism of plasticity-related proteins synthesis designed, in contrast to the TagTriC model. Nevertheless, the model dissociates the tag state of the eLTP/D state, which seems to be the case in slice experiments where tags are reset (Sajikumar and Frey 2004b), experiments that are not directly captured by the TagTriC model.
Behavioral tagging
Behavioral correlates of synaptic consolidation were recently shown in three different studies (Moncada and Viola 2007; Ballarini et al. 2009; Wang et al. 2010). The first study by Moncada and Viola (2007) used a protocol of inhibitory avoidance training (see Fig. 3). A rat is placed on an elevated platform, if it goes down, it receives a foot-shock. The rat learns not to go down from the platform. However, the next day, the rat has forgotten and thus goes down from the platform again (receiving the foot-shock). The rat seems to have undergone the early-phase of plasticity without the consolidation. If however, the rat is placed in a novel environment before the inhibitory avoidance training, a situation which is known to release dopamine (Li et al. 2003; Kentros et al. 2004), the rat learns to stay on the platform and still remembers this after 24 h. The interpretation is that the rat went through an early-phase of plasticity, which was consolidated; similar to the tagging experiments (Fig. 1c). Time-scales are in agreement with those of the synaptic tagging experiments (Frey and Morris 1997), e.g., the decay time constant of the early-phase of plasticity, or the maximum time between the novel environment and the inhibitory avoidance training for consolidation.
Fig. 3.
Behavioral tagging. a Inhibitory avoidance training, followed by a couple of hours of memory. b Inhibitory avoidance training paired with novel experience followed by more than 24 h of memory. See Moncada and Viola (2007) for details
This consolidation can be blocked pharmacologically under inhibition of the dopamine receptors D1/D5 or if plasticity-related protein synthesis are inhibited. If the rat is trained on a inhibitory avoidance training but with a strong foot-shock, the rat remembers more than 24 h after, therefore leading to consolidation. This is similar to the strong tetanus induction of the slices experiment (Fig. 1b). Another study by Viola’s group showed that this behavioral tagging also happens with different learning tasks (spatial object recognition, contextual fear conditioning and conditioned taste aversion) (Ballarini et al. 2009).
Another behavioral tagging experiment was done by Wang et al. (2010), using an everyday task instead of a fear conditioning task. A rat needs to find food at different locations and remembers these food location the next day. As in the studies described above, the rat only retains the location memory for several hours, unless it has had some novel experience, in which case the memories are consolidated. Similar time-scales and pharmacological results have been found.
A final behavioral correlate is a phenomenon that humans experience often. Most people will remember the exact place they were at during the September 11 attack, or the wine they drank at their wedding. Thus they tend to remember a lot of details if a strong event happens at the same time. Again, this is similar to the tagging experiment (Fig. 1c), where a weak induction alone does not lead to consolidation unless paired with a strong induction in another pathway.
Discussion
A few open questions and future directions are listed here and three of them are explained in more detail. Experimentally, it is still unclear if the standard slice plasticity experiments (see “Background on synaptic plasticity” section), for example the STDP experiments, undergo consolidation or not. The slices are usually kept for half an hour which is not long enough to see the late-phase of plasticity. On the model side, experiments of synaptic tagging have been reproduced but not yet the behavior experiments. On a higher level, it is not clear how this synaptic consolidation is linked to the consolidation by transferring memories from different brain areas typically happening during rest and sleep (Wilson and McNaughton 1994; Diba et al. 2007).
Relation to reinforcement learning
Reinforcement learning (Sutton and Barto 1998) is a framework where learning is guided by a reward. When the pre- and postsynaptic neurons are coactive, an eligibility trace is increased, otherwise it decays. This eligibility trace can be seen as a memory of this pre-post coactivation. Only when a reward is given, the weights are changed in proportion to their eligibility trace. This framework has similarities with the synaptic tagging theory: (a) The early-phase of plasticity is similar to the eligibility trace, although the time-scales can be different; (b) in the reinforcement learning framework, the weights only change in the presence of a reward. In the consolidation models, the weights are consolidated in the presence of phasic dopamine. Also, the data of Schultz et al. (1997), shows that the activity of the dopaminergic neurons (phasic activation) are coding for unexpected reward. Thus, consolidation in both frameworks depends on dopamine. In the future, it would be interesting to unify or distinguish these two concepts with more experiments and modeling.
Functional implications
An open question of synaptic consolidation theory is its function, which is a question to be addressed first by a modeling point of view. An important computational question in neuroscience is how can an animal learn quickly and remember for a long time. As memory is thought to be stored in synaptic weights, they should be very plastic in order to quickly encode new memories, but not too plastic so as to avoid erasing old memories. This paradox is called the palimpsest paradox (Nadal et al. 1986; Amit and Fusi 1994). One study that investigated this problem is the cascade model (Fusi et al. 2005). The way to overcome this problem was to design synapses that have several meta-states related to different plasticity levels. The synaptic tagging framework offers a similar solution. Indeed, not all the memories are stored the same way but only the important ones are stored in a highly non plastic state. Memory capacity and life-time in a network where the synapses follow one of the two models of consolidation remain promising directions of future research.
Modeling behavioral tagging
It is not clear whether the models for synaptic consolidation can reproduce the behavior experiment described above. The model would have to assume that novel environment exposure would lead to a phasic dopamine activation which would lower the triggering threshold of the model and thus allow for consolidation. Encouragingly, the time-scales of plasticity for synaptic tagging and behavioral tagging are similar. If the model is successful, it will provide additional evidence that behavioral tagging and synaptic tagging might be the same mechanism.
Conclusion
This paper motivated the importance of studying plasticity at very long time-scales, and reviewed the state of the art of synaptic consolidation at each level: electrophysiological experiments, different models and behavior correlates. Finally, the discussed open questions are providing a few pointers for promising future research.
Acknowledgments
This work was funded in part by the Agence Nationale de la Recherche grant ANR-08-SYSC-005. We thank Tom Schaul for helpful input.
References
- Amit D, Fusi S. Learning in neural networks with material synapses. Neural Comput. 1994;6:957–982. doi: 10.1162/neco.1994.6.5.957. [DOI] [Google Scholar]
- Artola A, Bröcher S, Singer W. Different voltage dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347:69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- Ballarini F, Moncada D, Martinez MC, Alen N, Viola H. Behavioral tagging is a general mechanism of long-term memory formation. Proc Natl Acad Sci USA. 2009;106:14599–14604. doi: 10.1073/pnas.0907078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A, Billings G, Morris R, Rossum M. State based model of long-term potentiation and synaptic tagging and capture. PLoS Comp Biol. 2009;5(1):e1000259. doi: 10.1371/journal.pcbi.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982;2(1):32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T, Lomo T. Long-lasting potentation of synaptic transmission in the dendate area of anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:351–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopath C, Gerstner W (2010) Voltage and spike timing interact in stdp: a unified model. Frontiers in synaptic neuroscience doi:10.3389/fnsyn.2010.00025 [DOI] [PMC free article] [PubMed]
- Clopath C, Vasilaki E, Buesing L, Gerstner W. Connectivity reflects coding: a model of voltage-based spike-timing-dependent-plasticity with homeostasis. Nat Neurosci. 2010;13:344–352. doi: 10.1038/nn.2479. [DOI] [PubMed] [Google Scholar]
- Clopath C, Ziegler L, Vasilaki E, Büsing L, Gerstner W (2008) Tag-trigger-consolidation: a model of early and late long-term-potentiation and depression. PLoS Comput Biol 4(12):e1000248. doi:10.1371/journal.pcbi.1000248 [DOI] [PMC free article] [PubMed]
- Diba K, Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci. 2007;10:1241–1242. doi: 10.1038/nn1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris R. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the ca1 region of rat hippocampal slices. Brain Res. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- Froemke R, Dan Y. Spike-timing dependent plasticity induced by natural spike trains. Nature. 2002;416:433–438. doi: 10.1038/416433a. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Tsay I, Raad M, Long J, Dan Y. Contribution of individual spikes in burst-induced long-term synaptic modification. J Neurophysiol. 2006;95:1620–1629. doi: 10.1152/jn.00910.2005. [DOI] [PubMed] [Google Scholar]
- Fusi S, Drew P, Abbott L. Cascade models of synaptically stored memories. Neuron. 2005;45:599–611. doi: 10.1016/j.neuron.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Gerstner W, Abbott LF. Learning navigational maps through potentiation and modulation of hippocampal place cells. J Comput Neurosci. 1997;4:79–94. doi: 10.1023/A:1008820728122. [DOI] [PubMed] [Google Scholar]
- Gerstner W, Kempter R, Hemmen J, Wagner H. A neuronal learning rule for sub-millisecond temporal coding. Nature. 1996;383(6595):76–78. doi: 10.1038/383076a0. [DOI] [PubMed] [Google Scholar]
- Gerstner W, Kistler WK. Spiking neuron models. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Gütig R, Aharonov S, Rotter S, Sompolinsky H. Learning input correlations through nonlinear temporally asymmetric Hebbian plasticity. J Neurosci. 2003;23(9):3697–3714. doi: 10.1523/JNEUROSCI.23-09-03697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyonneau R, VanRullen R, Thorpe S. Neurons tune to the earliest spikes through stdp. Neural Comput. 2005;17(4):859–879. doi: 10.1162/0899766053429390. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior. New York: Wiley; 1949. [Google Scholar]
- Kentros CG, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42:283–295. doi: 10.1016/S0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Wixted JT, Squire LR. Activity in the medial temporal lobe predicts memory strength, whereas activity in the prefrontal cortex predicts recollection. J Neurosci. 2008;28:10541–10548. doi: 10.1523/JNEUROSCI.3456-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legenstein R, Naeger C, Maass W. What can a neuron learn with spike-timing dependent plasticity. Neural Comput. 2005;17:2337–2382. doi: 10.1162/0899766054796888. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Lynch G, Dunwiddie T, Gribkoff V. Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature. 1977;266:737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postysnaptic AP and EPSP. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Moncada D, Viola H. Induction of long-term memory by exposure to novelty requires protein synthesis: evidence for a behavioral tagging. J Neurosci. 2007;27(28):7476–7481. doi: 10.1523/JNEUROSCI.1083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal J-P, Toulouse G, Changeux J-P, Dehaene S. Networks of formal neurons and memory palimpsests. Europhys Lett. 1986;1:349–381. doi: 10.1209/0295-5075/1/10/008. [DOI] [Google Scholar]
- Navakkode S, Sajikumar S, Frey J. Synergistic requirements for the induction of dopaminergic D1/D5-receptor-mediated LTP in hippocampal slices of rat CA1 in vitro. Neuropharmacology. 2007;52:1547–1554. doi: 10.1016/j.neuropharm.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Ngezahayo A, Schachner M, Artola A. Synaptic activation modulates the induction of bidirectional synaptic changes in adult mouse hippocamus. J Neurosci. 2000;20:2451–2458. doi: 10.1523/JNEUROSCI.20-07-02451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor D, Wittenberg G, Wang S-H. Graded bidirectional synaptic plasticity is composed of switch-like unitary events. Proc Natl Acad Sci USA. 2005;102:9679–9684. doi: 10.1073/pnas.0502332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oja E. A simplified neuron as a principal component analyzer. J Math Biol. 1982;15:267–273. doi: 10.1007/BF00275687. [DOI] [PubMed] [Google Scholar]
- Petersen C, Malenka R, Nicoll R, Hopfield J. All-or-none potentiation of ca3-ca1 synapses. Proc Natl Acad Sci USA. 1998;95:4732–4737. doi: 10.1073/pnas.95.8.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister J-P, Gerstner W. Triplets of spikes in a model of spike timing-dependent plasticity. J Neurosci. 2006;26:9673–9682. doi: 10.1523/JNEUROSCI.1425-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister J-P, Toyoizumi T, Barber D, Gerstner W. Optimal spike-timing dependent plasticity for precise action potential firing in supervised learning. Neural Comput. 2006;18:1309–1339. doi: 10.1162/neco.2006.18.6.1318. [DOI] [PubMed] [Google Scholar]
- Redondo R, Morris R. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12(1):17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- Reymann K, Frey J. The late maintenance of hippocampal LTP: requirements, phases,synaptic tagging, late associativity and implications. Neuropharmacology. 2007;52:24–40. doi: 10.1016/j.neuropharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Roberts P, Bell C. Computational consequences of temporally asymmetric learning rules: II. Sensory image cancellation. Comput Neurosci. 2000;9:67–83. doi: 10.1023/A:1008938428112. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Frey J. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol Learn Mem. 2004;82:12–25. doi: 10.1016/j.nlm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Frey J. Resetting of synaptic tags is time- and activity dependent in rat hippocampal ca1 in vitro. Neuroscience. 2004;129:503–507. doi: 10.1016/j.neuroscience.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Navakkode S, Sacktor T, Frey J. Synaptic tagging and cross-tagging: the role of protein kinase Mζ in maintaining long-term potentiation but not long-term depression. J Neurosci. 2005;25:5750–5756. doi: 10.1523/JNEUROSCI.1104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague R. A neural substrate for prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sjöström P, Turrigiano G, Nelson S. Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron. 2001;32:1149–1164. doi: 10.1016/S0896-6273(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Smith CN, Squire LR. Medial temporal lobe activity during retrieval of semantic memory is related to the age of the memory. J Neurosci. 2009;29:930–938. doi: 10.1523/JNEUROSCI.4545-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Miller K, Abbott L. Competitive Hebbian learning through spike-time-dependent synaptic plasticity. Nat Neurosci. 2000;3:919–926. doi: 10.1038/78829. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatr. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R, Barto A. Reinforcement learning: an introduction. Cambridge: MIT Press; 1998. [Google Scholar]
- Wang S, Redondo R, Morris R. Relevance of synaptic tagging and capture to the persistence of long-term potentiation and everyday spatial memory. Proc Natl Acad Sci USA. 2010;107(45):19537–19542. doi: 10.1073/pnas.1008638107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]