Abstract
Purpose
A device composed of extracellular matrix (ECM) was investigated as an inductive template in vivo for reconstruction of the TMJ disk following discectomy.
Methods
A scaffold material composed of porcine derived ECM was configured to mimic the shape and size of the TMJ. This device was implanted in a canine model of bilateral TMJ discectomy. Following discectomy, one side was repaired with an ECM scaffold material and the contralateral side was left empty as a control. At 6 months post-implantation the joint space was opened, the joints evaluated for signs of gross pathologic degenerative changes, and newly formed tissue was excised for histologic, biochemical, and biomechanical analysis.
Results
The results show that implantation of an initially acellular material supported the formation of site-appropriate, functional host tissue which resembled that of the native TMJ disk. Further, this prevented gross degenerative changes in the temporal fossa and mandibular condyle. No tissue formation and mild to severe gross pathologic changes were observed in the contralateral controls.
Conclusion
These results suggest that an ECM based bioscaffold may represent an off-the-shelf solution for TMJ disk replacement.
1.0 Introduction
Currently, no alloplastic alternatives exist to safely and effectively replace a degenerative, non-repairable temporomandibular joint (TMJ) disk in humans. Previous attempts to utilize alloplastic materials have resulted in unsatisfactory outcomes including increased joint pathology among other complications (1–4). Several autogenous tissues such as temporalis muscle, auricular cartilage, dermis, and abdominal adipose tissue have been used as replacement materials, but only short-term success has been reported (5–11). In addition, use of these tissues is associated with donor site morbidity, the eventual formation of scar tissue with resulting decreased range of motion of the mandible, and further joint pathology. Studies have documented reduction of joint pain after a discectomy without a replacement procedure, but these patients experience various degrees of subsequent degenerative change (12–14). Thus, identification of a suitable off-the-shelf disk replacement material would obviate associated donor site morbidity and avoid downstream degenerative changes of the condyle. Ideally, such a material would act as a template for cellular in-growth, integrate with surrounding host tissues, and eventually restore the native morphology and function of the TMJ disk.
Tissue engineering and regenerative medicine approaches to the reconstruction of injured or missing tissues typically include cell-based, scaffold-based, or signaling molecule-based strategies. Regardless of the initial strategy, all approaches are intended to promote the formation of a tissue or organ that restores normal or near-normal structure and function. When prepared and utilized appropriately, biologic scaffold materials composed of extracellular matrix (ECM) have been shown to be capable of promoting the de novo formation of new, site-appropriate, functional host tissues in a wide variety of preclinical and clinical applications (15–17). This type of “constructive remodeling” is in direct contrast to the default mammalian response observed following tissue injury or following the implantation of many commercially available surgical mesh materials, which generally includes scar tissue formation or a foreign body response.
A number of tissue engineering and regenerative medicine approaches to the replacement of the TMJ disk have been suggested (18–32). However, to date, these studies have focused primarily upon the selection of ideal cell sources, growth factors, and scaffold materials for the engineering of tissues that recapitulate the TMJ disk in vitro. Recently, a preliminary study reported in this journal showed that a device composed of decellularized porcine urinary bladder tissue scaffold (urinary bladder matrix, UBM) alone was capable of providing an effective interpositional material while serving as an inductive template for reconstruction of the TMJ disk in vivo (33). In this study, a device consisting of a powdered UBM “pillow” encapsulated within sheets of the same material was placed as an interpositional graft following discectomy in a canine model. The implanted material was observed to progressively remodel from three weeks to six months post-implantation and the newly formed host tissues resembled the native fibrocartilage of the TMJ disk in both gross and histologic morphology. This study, however, was limited by small sample size, a lack of mechanical testing data, and a lack of an appropriate negative control.
The objectives of the present study were to expand upon the characterization of the gross morphologic and histologic appearance of the newly formed tissues and, additionally, to evaluate the biomechanical properties and biochemical content of the remodeled tissues in ten animals at six months post-implantation in a model of bilateral discectomy.
2.0 Materials and Methods
2.1 Overview
A device consisting of particulate UBM was encased within sheets of UBM to provide an inductive and resorbable “pillow” of interpositional ECM material while mimicking the shape and size of the native canine TMJ disk as previously described (33). The device was implanted unilaterally in a canine model of bilateral TMJ discectomy and remodeling was assessed at six months post-implantation. Assessment was performed using gross morphologic examination, histologic and immunolabeling methods, compositional studies, and mechanical testing.
2.2 UBM Device Preparation
UBM was prepared from porcine bladders as previously described (78). Briefly, urinary bladders were harvested from market weight pigs (110–120 kg) immediately following sacrifice. The bladder tissue was rinsed in water to facilitate the removal of excess urine and the urothelial cell layer. Excess connective and adipose tissue was removed from the serosal surface of the bladder using scissors. The apex of the bladder was removed and the bladder was then split longitudinally from the apical opening to the neck of the bladder forming a rectangular sheet. The tunica serosa, tunica muscularis externa, tunica submucosa, and the majority of the muscularis mucosa were removed by mechanical delamination of the abluminal side of the bladder. The remaining tissue consisted of the basement membrane, tunica propria, and resident cells.
The tissue was then treated in a 0.1% peracetic acid/4% ethanol solution for two hours to initiate decellularization and disinfection of the tissue. Following treatment in the peracetic acid/ethanol solution, the tissue was repeatedly washed in phosphate buffered saline (PBS; pH 7.4) and water to remove cellular remnants and traces of the peracetic acid/ethanol solution and to return the pH of the material to 7.4. The remaining decellularized tissue was stored in water until used and represented the hydrated sheet form of UBM. A portion of the hydrated sheet form of UBM was frozen and lyophilized. The lyophilized sheet was cut into smaller pieces and comminuted using a Wiley mill with a #60 mesh screen. The comminuted UBM represented the particulate form of UBM.
A hard plastic mold was milled to create an oval-shaped depression with the approximate size of the desired central core of the TMJ device (10 mm × 14 mm oval, 2 mm depth) and a flat surface surrounding the depression to allow for the formation of a “pillow-like” core and a flat surrounding anchoring site. Two hydrated sheets of UBM were then cut to size and placed onto the mold. Following placement, the hydrated sheets were pressed to line the inside of the depression, creating a pocket into which particulate UBM was packed. Approximately 200–300 mg of the ECM powder was packed into the depression and two hydrated sheets were cut to size and placed over the top of the powder to create an enclosed core. The construct was subjected to a vacuum of at least 28 inches Hg until dry, forming a multilaminate pillow-like device. All constructs were then terminally sterilized using ethylene oxide prior to implantation.
2.3 Animal Model
Ten adult female mongrel dogs of approximately 15–20 kg were purchased from Marshall Bio-Resources USA. All animals were examined by a veterinarian prior to surgery and were found to be in good health and all animal procedures were performed in compliance with the 1996 National Institutes of Health “Guide for The Care and Use of Laboratory Animals” and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
2.4 Surgical Procedure
All animals were sedated with acepromazine (0.1–0.5 mg/kg body weight) prior to intubation and maintained in a surgical plane of anesthesia with isoflurane (1–5%). The surgical site was shaved then prepared using a betadine scrub prior to the placement of sterile drapes. An incision was made anterior to the tragus, preserving the local innervation and vasculature. The native disk was exposed and then completely excised. The same discectomy procedure was used on both experimental and control sides. Following excision of the native disk on the experimental side, the disk was replaced with a UBM device as described below. The contralateral side was left empty.
The UBM implants were hydrated in sterile saline (0.9%) for approximately 10 minutes prior to implantation as a replacement device for the native disk. The implants were placed such that the powder “pillow” was positioned between the temporal fossa and the condylar head. Three holes were created in the temporal fossa and the implants were secured to the temporal fossa using slow resorbing fixation sutures. Fixation sutures were also placed in the anterior and posterior aspects of the implant to adjacent soft tissue. The skin was closed using resorbable suture material.
2.5 Post-operative Care
Following the surgical procedure, the animals were recovered from anesthesia, extubated and monitored until resting comfortably in a sternal position. The animals were then monitored and the following parameters were recorded every 3 hours for the first 24 hours post surgery: pulse rate, strength of pulse, capillary refill time, respiratory rate and ability to maintain an open airway, urinary output, and defecation. Body temperature was measured and recorded every 12 hours. The animals were restricted to confinement housing (not more than 2–3 days) until stable, and were then placed in 10×14 ft runs and allowed free movement. Buprenorphine was administered (0.005 – 0.01 mg/kg body weight) for 5 days post-operatively and then as needed thereafter for pain management. The dogs were also given cephalexin (35 mg/kg body weight) for 5 days post-operatively. Animals were fed a soft diet for the first 5–7 days post-operatively and were returned to a normal hard diet thereafter.
2.6 Euthanasia and Sample Harvest
At six months post-implantation, the animals were sedated with acepromazine (0.1–0.5 mg/kg body weight), anesthetized using isoflurane (5%) and euthanized by intravenous administration of pentobarbital sodium (390 mg/4.5 kg body weight). Following euthanasia, the temporal fossa, the condylar head, and the interpositional material between the structures were excised separately in the following order: (1) the joint capsule was opened, (2) the mandibular condyle was excised, (3) the remodeled UBM device was excised, (4) the temporal fossa was removed.
Portions of the remodled UBM device from 5 of the 10 animals were retained for biochemical and biomechanical analysis and tissues from all animals were fixed in formalin for histologic analysis. In those animals for which tissues were subjected to biochemical and biomechanical testing, a portion of the tissue adjacent to the area used for biomechanical and biochemical testing was fixed for histologic analysis. Allocation of animals for biomechanical and biochemical testing was selected at random. Native menisci from 6 animals were harvested at the time of discectomy to serve as positive controls for biochemical and biomechanical analysis.
2.7 Gross Morphologic Examination
At the time of explant, the joint space of all animals was examined for signs of gross pathology including degeneration of the articulating surfaces of the temporal fossa and mandibular condyle. Assessment was performed both an oral and maxillofacial surgeon (W.L.C.) and a pathologist (S.F.B.). The surface of the temporal fossa and condylar head were evaluated for surface irregularities with the assistance of a surgical probe/elevator and documented with photographs. This evaluation was performed in both control no-implant and implant groups, and observations were compared against tissues observed at time of implant.
2.8 Histologic Evaluation
Formalin fixed tissues were embedded in paraffin, cut into 6 μm sections and mounted on glass slides. Sections were deparaffinized by immersion in xylenes followed by a graded series of ethanol. The slides were stained using hematoxylin and eosin or von Kossa stain, and were then dehydrated in ethanol and xylenes prior to coverslipping. The slides were evaluated by light microscopy.
2.9 Immunolabeling
Tissue sections were labeled using antibodies specific for CD68, CD31, and smooth muscle actin to determine the presence of macrophages, blood vessels, and myofibroblasts, respectively, within the remodeled UBM implant. Slides were deparaffinized by immersion in xylenes and a graded series of ethanol. Antigen retrieval was then performed by boiling slides in 10 mM citric acid monohydrate (pH 6.0) for 20 minutes. Following antigen retrieval, the slides were exposed to a solution consisting of TRIS buffered saline and 0.05% Tween 20. Slides were then washed in PBS (pH 7.4) three times for a total of 10 minutes and a solution of 3% H2O2 in methanol was applied for 30 minutes at room temperature to quench endogenous peroxidase activity. Slides were blocked in a solution consisting of 2% normal serum, 1% BSA, 0.1% Triton-X 100, and 0.05% Tween 20 in PBS for 30 minutes at room temperature. Primary antibodies were diluted in the blocking solution (1:250) and applied to the slides overnight at 4°C. Slides were washed in PBS, and peroxidase conjugated secondary antibodies diluted in blocking solution (1:100) were applied for 30 minutes at room temperature. The slides were washed in PBS and water prior to development using 4% diaminobenzadine substrate solution. Finally, slides were counterstained using hematoxylin, dehydrated using the reverse of the dewaxing procedure above, and coverslipped for examination under light microscopy.
2.10 Biochemical Content Analysis
Collagen and glycosaminoglycan (GAG) content of the remodeled UBM device were determined as a percentage of dry weight using a hydroxyproline assay and a sulfated GAG assay as per published protocols (79, 80). Water content was determined as the percentage of water per total tissue weight by allowing the tissue to become saturated in a 37°C water bath, measuring the weight of the sample, and then comparing to the weight of the sample following complete lyophilization. All values obtained for collagen, GAG, and water content were compared to values obtained for the pre-implantation UBM device and for native canine TMJ tissue.
2.11 Biomechanical Testing
Compressive mechanical properties (peak stress, equilibrium stress, percent stress relaxation, and tangent modulus) of the native menisci, the pre-implantation device, and remodeled UBM devices were tested using an MTS Insight mechanical testing apparatus per published protocols (81). Briefly, 4 mm cylindrical punches were taken from the center of the explanted samples and placed in a 37°C saline bath prior to testing. A preload of 0.1 N was applied for 30 minutes prior to preconditioning, which consisted of 10 cycles between 0–10% strain at a 9%/minute strain rate. Samples were then tested in unconfined compression to 10% strain. Samples were allowed 30 minutes to reach equilibrium and stress-relaxation behavior was determined. Mechanical properties data obtained for remodeled samples were compared to values obtained both for the pre-implantation UBM device and for native canine disk tissue.
2.12 Statistical Methods
Statistical significance of the mechanical properties and biochemical testing data were determined using a students T-test. P-values of p<0.05 were used to determine statistical significance.
3.0 Results
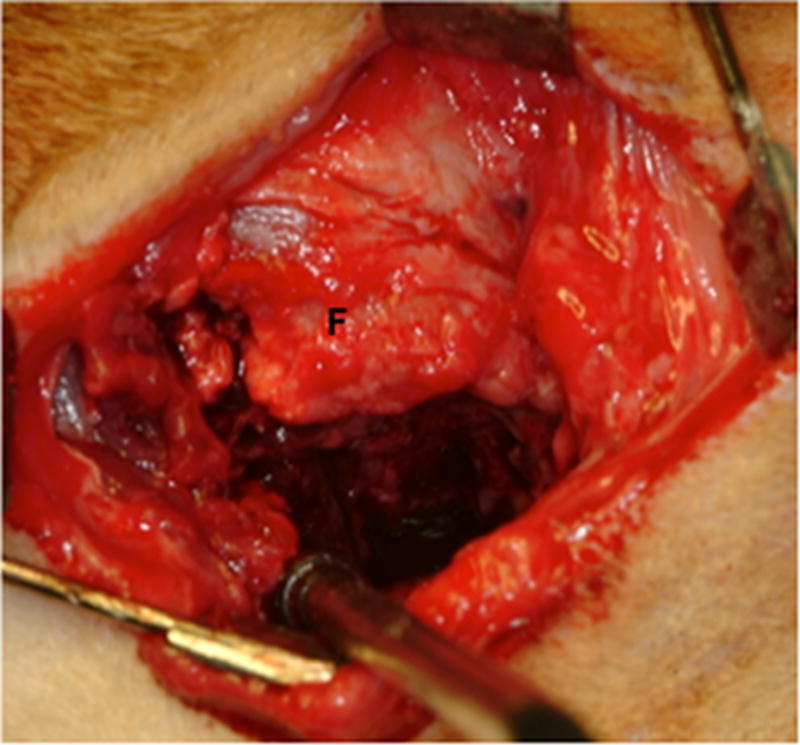
Ten female mongrel dogs were subjected to bilateral excision of the native TMJ disk. Animals were treated by unilateral replacement of the excised disk with an inductive biologic scaffold designed to mimic both the shape and size of the canine disk, leaving the contralateral side empty as a negative control. The device was composed of a pillow-like core of powdered porcine UBM encapsulated within sheets of the same material. Images of the explanted native meniscus and implanted UBM device are shown in Figure 1.
Figure 1.
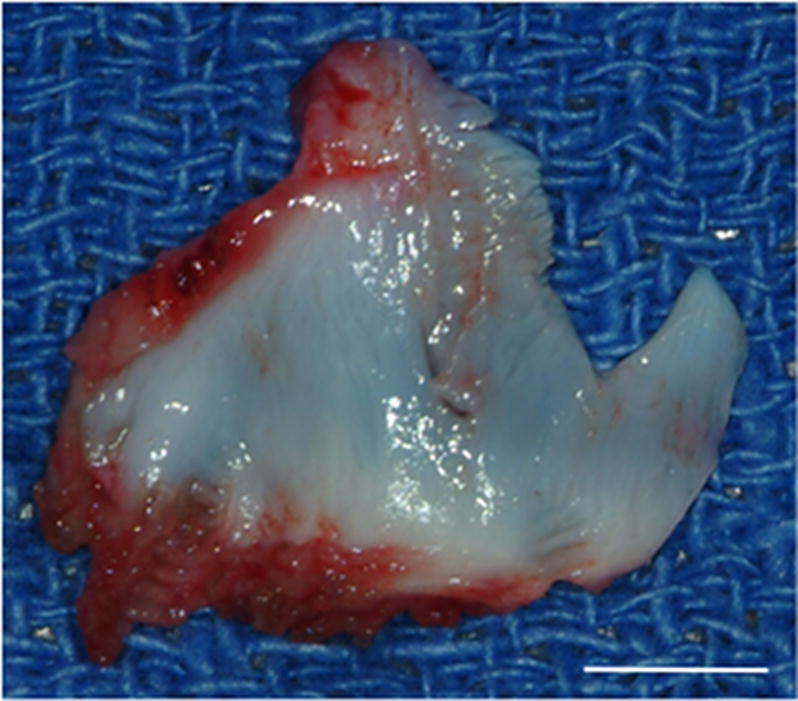
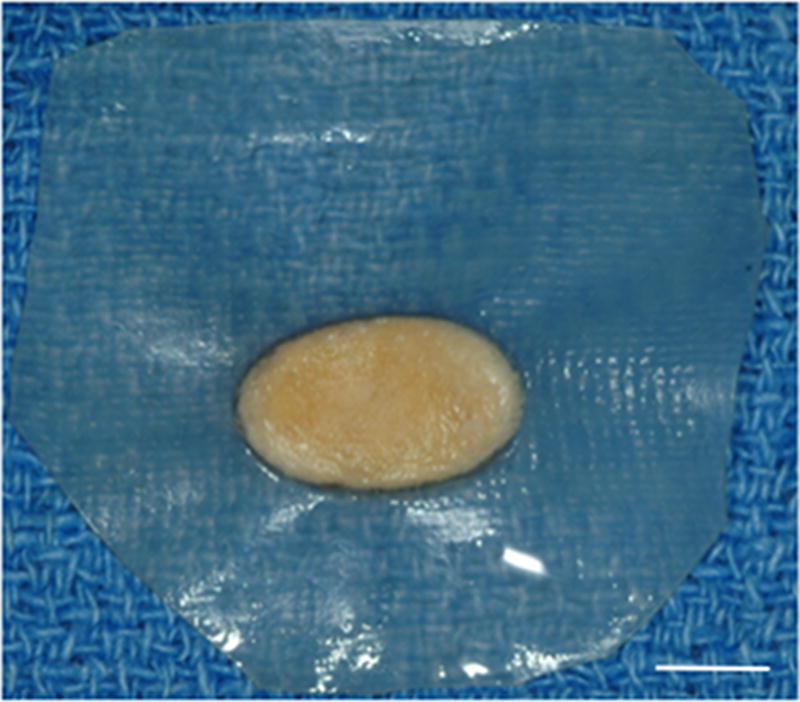
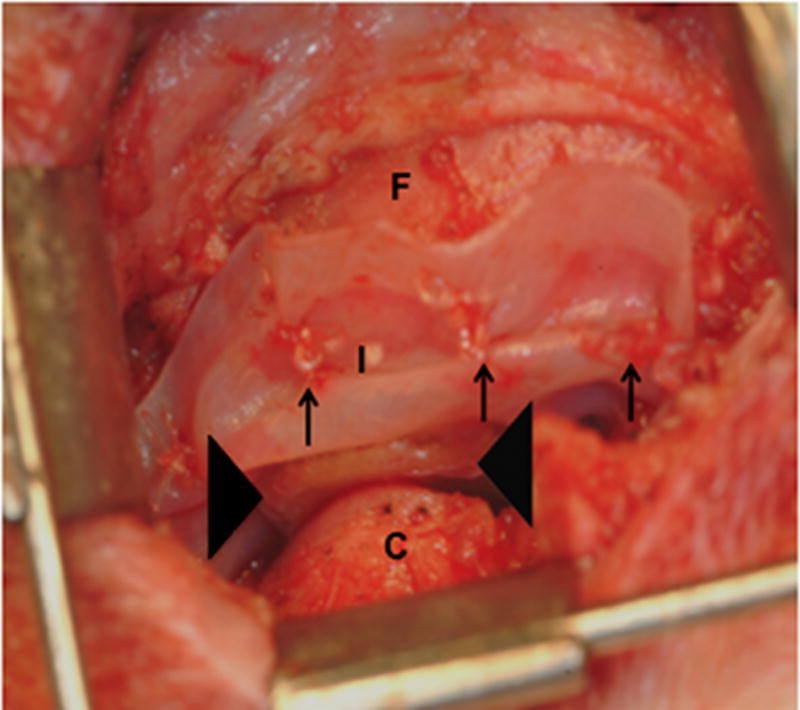
Images showing the surgical procedure. The native disk (A) is exposed and excised leaving the joint space empty. UBM implants (B) were hydrated and trimmed to size prior to placement between the condyle and fossa on the experimental side and attachment through the fossa (C). Note sutures through the anchoring site (arrows) and interpositional pillow-like core (arrowheads). The contralateral side was left devoid of a meniscal substitute. C = condyle, F = fossa, M = disk, and I = implanted UBM-ECM device. Scale bars in A and B = 5 mm.
All of the animals in this study survived the surgical procedure and lived until their predetermined sacrifice date without major complications. No animals were observed to exhibit signs of significant discomfort, and all animals gained weight throughout the study period. Two animals experienced dehiscence of the skin closure only without exposure of the UBM device. These animals were anesthetized and the surgical site repaired. Appetite and mastication were unaffected by the surgical procedure and all animals gained weight through the study period.
3.1 Gross Morphologic Findings
Gross morphologic examination of the experimental implantation site at six months post-implantation showed little to no gross pathologic changes in the articulating surfaces of the temporal fossa or the mandibular condyle following placement of the UBM device. The remodeled UBM device was not distinguishable from newly formed host tissue and was replaced with a structure that highly resembled the appearance of the fibrocartilage of the native TMJ disk in 9 of 10 animals. In 5 of 9 animals in which newly formed tissue was observed, varying degrees of loose highly vascular, thin and transparent connective tissue were present surrounding the meniscus, potentially acting as a nutrient supply to the remodeling tissues. In one animal, degradation of the UBM device without formation of new interpositional tissue was observed. In this animal, degenerative changes of the articulating surfaces of the condylar head and temporal fossa were present. An example of the gross morphologic appearance of the experimental remodeling site is provided in Figure 2.
Figure 2.
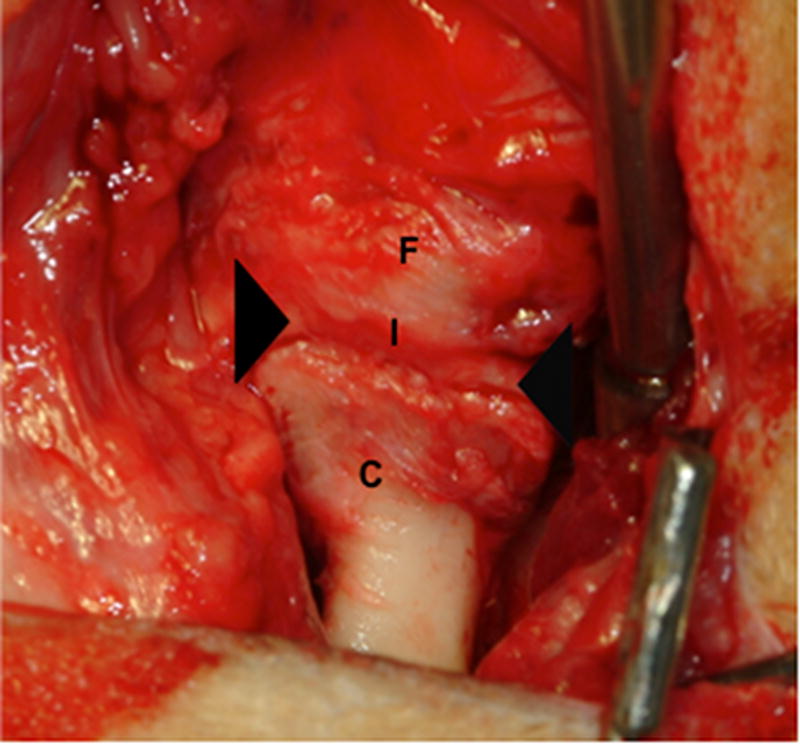
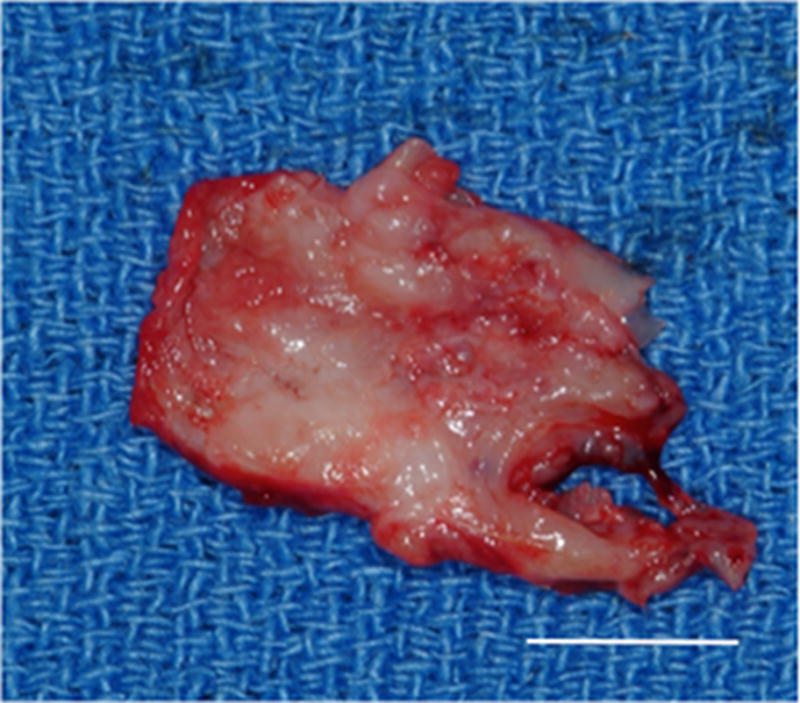
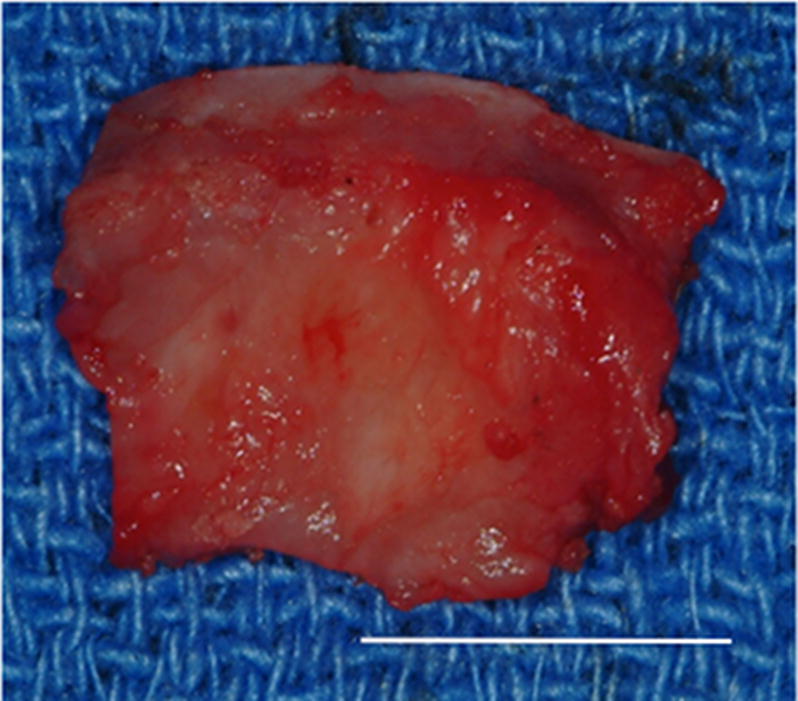
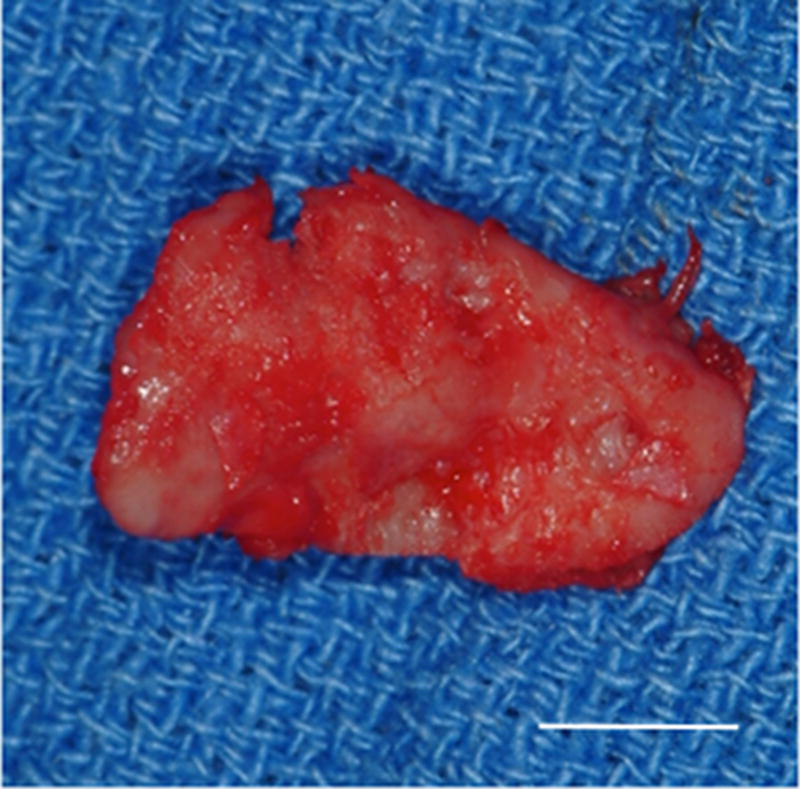
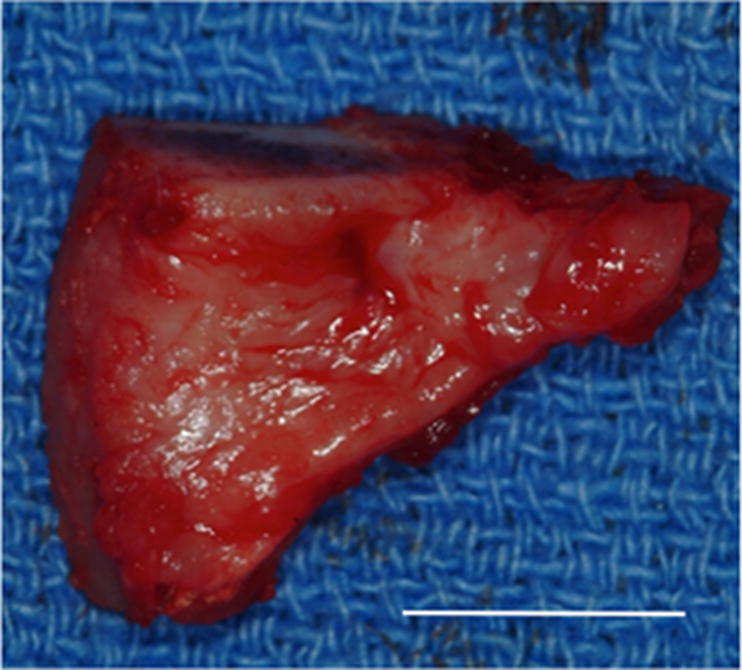
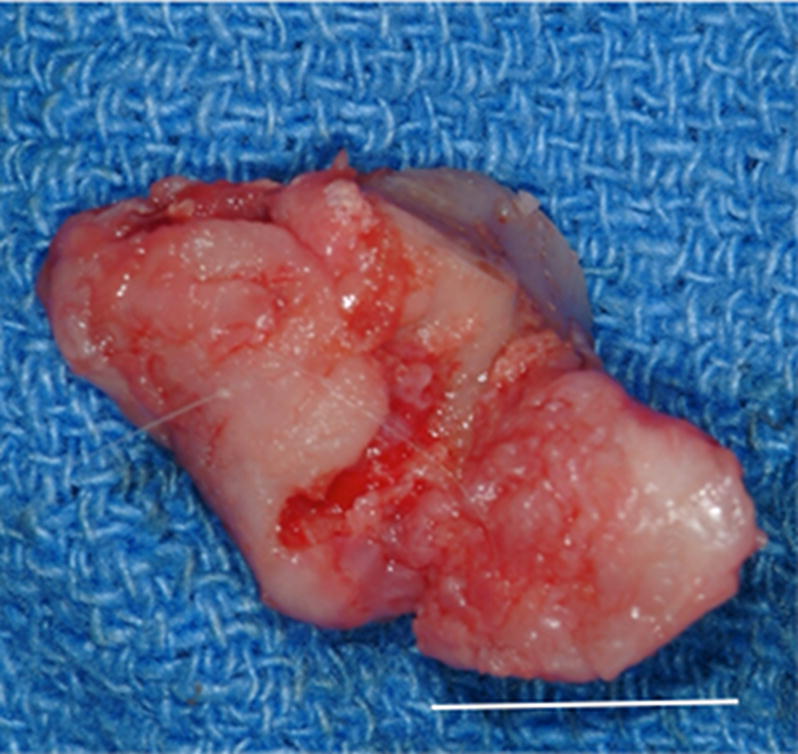
Images showing the gross morphologic appearance of the TMJ space at six months post-surgery. The experimental side (A) was characterized by the presence of interpositional material between the articulating surfaces of the fossa and condyle. Upon removal of the condyle, the newly formed tissue was removed and retained for histologic and mechanical testing. The newly formed tissue (B) is shown and was characterized by a white shiny appearance resembling that of the native tissue. The articulating portions of the fossa (C) and condyle (D) were characterized by a grossly normal looking articulating surface. No interpositional tissue formation was observed on the control sides. Upon removal of the condyle, mild to severe (E) degenerative changes in the surface of the fossa could be observed and were accompanied by the formation of varying degrees of granulation tissue and adhesions within the joint space. The excised fossa (F) and condylar (G) tissues were characterized by mild to severe gross pathologic changes in the articulating surface. C = condyle, F = fossa, I = newly formed interpositional tissue. Black arrowheads denote the location of the observed interpositional material in image A. Scale bars = 10 mm.
All control sites, which did not receive implantation of a UBM device, were characterized by grossly visible irregularities of the articulating surfaces of both the condylar head and the temporal fossa. Joint adhesions and fibrotic tissue formation characteristic of scarring were also observed in the control side of the majority of the animals examined. No regrowth of the excised disk was observed on the control side of any animal in the study. An example of the gross morphologic appearance of the control site is provided in Figure 2
3.2 Histopathologic Findings
At six months post-implantation, a sparse population of spindle shaped cells within an aligned matrix of collagenous tissue was observed throughout the bulk of the remodeled UBM scaffold. In one sample, von Kossa staining showed the formation of a small focus of calcified tissue within the remodeled UBM device; however, the calcification was limited to a small area outside of the area of articulation and was adjacent to the drill hole created in the temporal fossa for suture placement. Bundles of skeletal muscle were present within the remodeled scaffold, but only near the peripheral soft tissue attachment. These muscle bundles were surrounded by mature, well-organized collagenous extracellular matrix and highly resembled the pattern of muscular attachment present in the native TMJ disk attachment site. Figure 3 shows representative examples of the histologic appearance of the remodeled UBM devices and Figure 4 shows the area of calcification observed in one animal.
Figure 3.
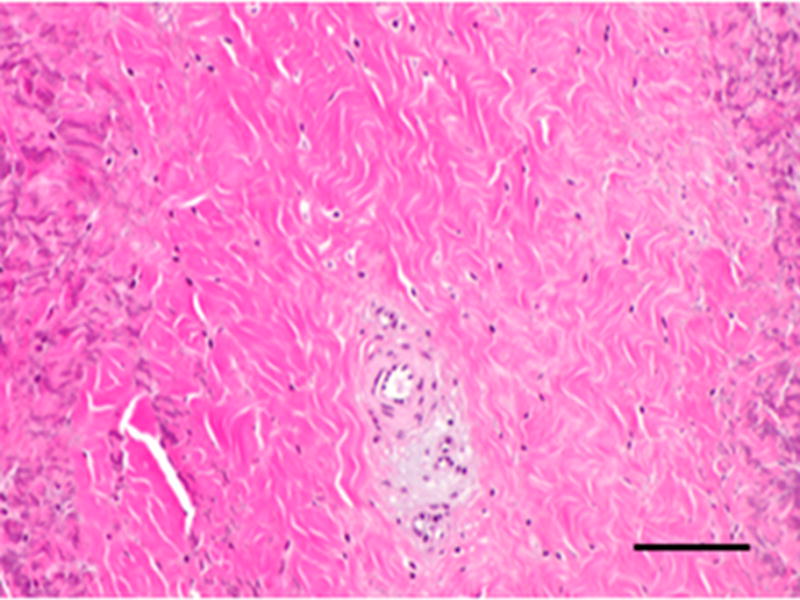
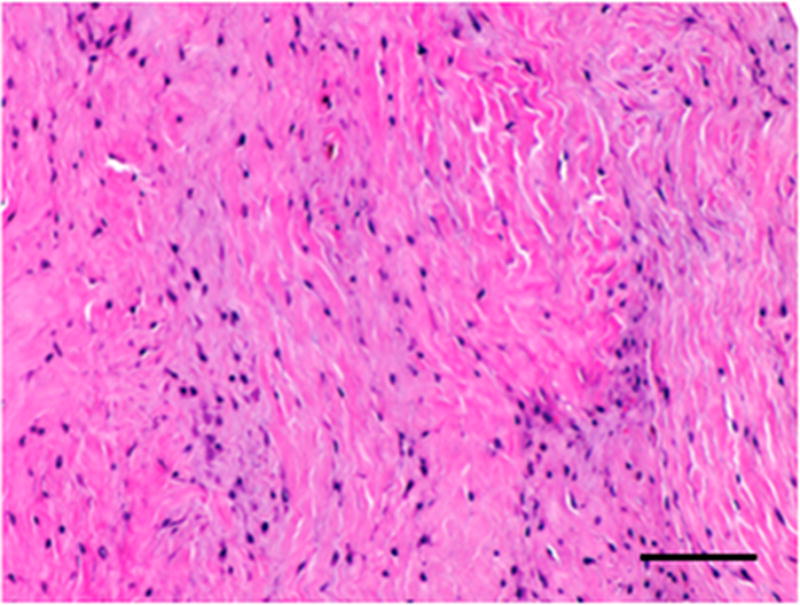
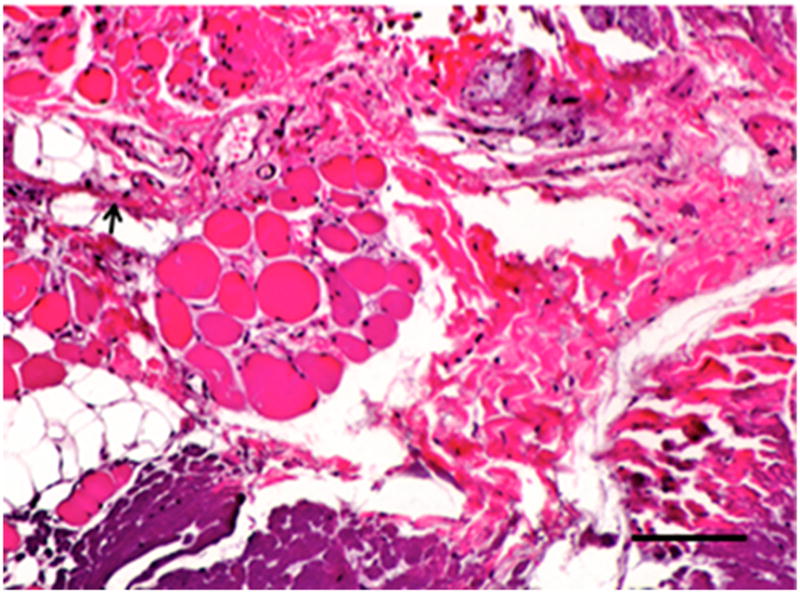
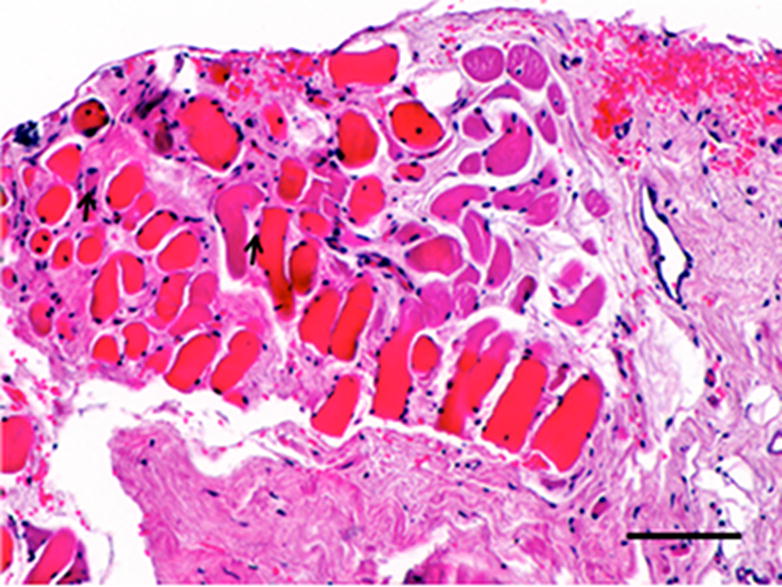
Histopathologic and immunolabeling findings. Microscopic views of hematoxylin and eosin staining of the bulk of the native (A) and remodeled tissues (B) showing a highly aligned collagenous matrix and spindle shaped cells. Microscopic views of hematoxylin and eosin staining of the peripheral muscular attachments of the native (C) and remodeled (D) tissues showing bundles of skeletal muscle within highly aligned collagenous matrix. CD31 and SMA labeling was observed only around the vascular structures observed primarily at the periphery of the tissues (not shown). All images are 20X original magnification.. Scale bar = 100 μm.
Figure 4.
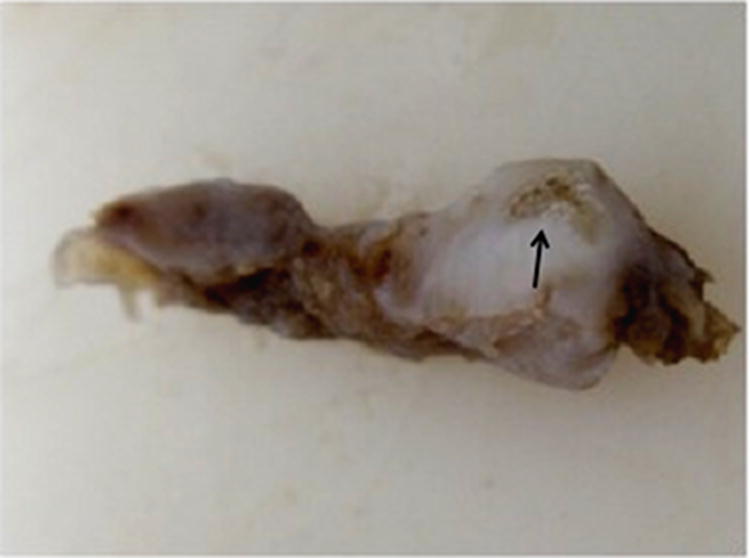
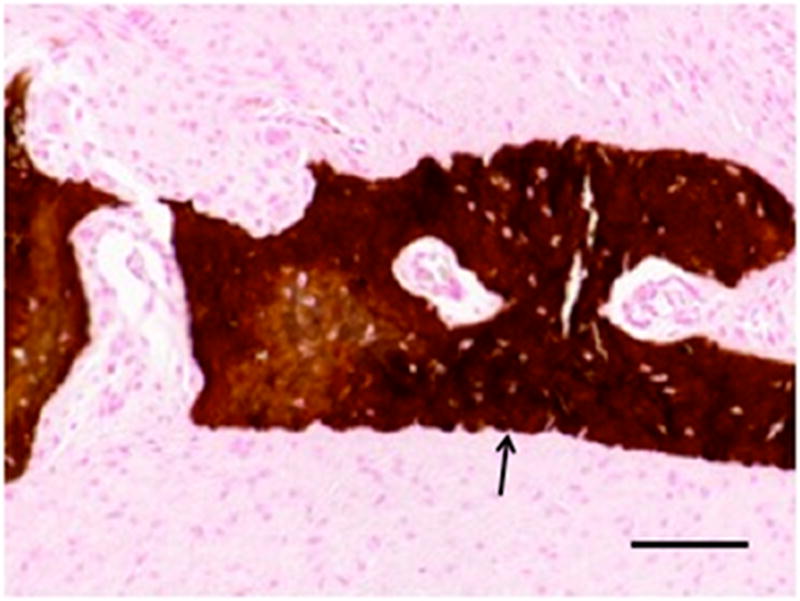
Area of calcification observed in remodeled tissues excised from one animal. Grossly identifiable calcification was observed outside of the area of articulation within the remodeled tissues in one animal (A). von Kossa staining was used to confirm the formation of calcified tissue (B). Calcification was not observed in other animals. Arrow denotes area of calcified tissue formation in A. Image B is 20X original magnification. Scale bar = 100 μm.
3.3 Immunolabeling
Studies have shown that macrophages play an important and potentially determinant role in the degradation and remodeling of ECM based scaffold materials following placement, and our preliminary study showed that there was a population of CD68+ (a macrophage marker) mononuclear cells which participated in the early host tissue remodeling response following implantation of the UBM device (33–36). No cells were observed to label positive for CD68 in any of the explanted UBM samples examined in the present study, suggesting that the remodeling process associated with the implantation of the UBM device was complete. Additional immunolabeling was performed to examine the presence of vascular structures within the remodeled UBM devices. Results showed that the tissue contained small vascular structures similar to those observed for native TMJ tissue. Lastly, samples were immunolabeled for smooth muscle actin, a marker of myofibroblasts and often indicative of scar tissue formation when present within sites of tissue remodeling. Smooth muscle actin positive cells were found only surrounding the vasculature within the remodeled UBM devices in a pattern consistent with the presence of vascular smooth muscle and were not present within the bulk of the remodeled material.
3.4 Biochemical Content
Quantitative evaluation of biochemical content showed that both the pre-implantation UBM device and the remodeled device had a collagen content which was similar to that observed in the native disk tissues. A similar pattern was observed for water content. The remodeled tissue was found to have a slightly higher (+0.5%) GAG content than the pre-implantation device and the native tissue. Results of the biochemical content testing are shown in Table 1.
Table 1.
Biochemical content.
| Water (%) | Glycosaminoglycan (% of dry weight) | Collagen (% of dry weight) | |
|---|---|---|---|
| Native | 77.63 ± 5.26 | 0.55 ± 0.11 | 61.95 ±17.40 |
| Pre-Implantation UBM | 69.47 ± 16.72 | 0.47 ± 0.23 | 59.71 ± 29.97 |
| Remodeled Tissue | 82.54 ± 2.09 | 1.03 ± 0.08* | 60.39 ± 18.68 |
Denotes a significant difference compared to native tissue with p <0.05. All values are presented as mean ± standard deviation
3.5 Biomechanical Properties
Biomechanical compression testing showed that the pre-implantation UBM device had a higher peak stress, equilibrium stress, and tangent modulus than did the native TMJ meniscus tissue and a lower percent relaxation. By six months post-implantation the remodeled UBM device had a maximum stress, equilibrium stress, tangent modulus, and percent relaxation which approached that of the native disk, suggesting that the UBM device had remodeled into a structure with site-appropriate compressive mechanical properties similar to those of the native disk tissue. While the remodeled UBM device had a slightly higher tangent modulus, the difference was not statistically significant. Results of biomechanical properties testing are shown in Table 2.
Table 2.
Mechanical testing.
| Peak Stress (kPa) | Equilibrium Stress (kPa) | Stress Relaxation (%) | Tangent Modulus (kPa) | |
|---|---|---|---|---|
| Native | 2.68 ± 0.57 | 1.46 ± 0.35 | 45.35 ±9.48 | 23.45 ±6.01 |
| Pre-Implantation UBM | 16.38 ± 5.78* | 11.29 ± 4.23* | 31.28 ± 2.20* | 179.96 ± 48.29* |
| Remodeled Tissue | 3.71 ± 1.69 | 1.81 ± 0.65 | 48.10 ± 16.47 | 50.72 ± 32.10 |
Denotes a significant difference compared to native tissue with p <0.05. All values are presented as mean ± standard deviation
4.0 Discussion
The present study showed that an initially acellular biologic scaffold composed of porcine ECM served as an inductive template for constructive remodeling following discectomy. This remodeling process resulted in the formation of multiple tissue types (vasculature, muscle, connective tissue, fibrocartilage) that were arranged in a site-appropriate manner. No signs of gross pathologic changes in the articulating surfaces of the mandibular condyle or the articulating fossa were observed following implantation of the UBM device, further suggesting that the remodeled tissue also acted as an effective interpositional material. The remodeled UBM devices were similar to those of the native TMJ disk from gross morphologic, histologic, biochemical and biomechanical perspectives. Briefly, the histologic appearance of the remodeled UBM device consisted of elongated fibroblast-like cells within a highly aligned matrix of collagen and site-appropriate soft tissue attachments at the periphery of the implanted material. In pilot studies, no statistically significant differences in the number of cells present within the remodeled ECM device at 6 months post-implantation as compared to native tissue were observed (33). Similar cell counts were not performed in the present study. Histologic images showed that the tissue formed within the remodeled UBM device in the present study may have been slightly hypercellular as compared to native tissue. However, the cells stained negative for markers indicative of inflammation (CD68) and scar tissue formation (SMA). Biochemical testing showed that the collagen and water content of the remodeled UBM device was similar to that observed for native tissues. GAG content was found to be slightly higher in the remodeled UBM device than in the pre-implantation UBM implant and the native tissue. However, though statistically significant, this difference represented only a small percentage of the biochemical content of the remodeled UBM device and biomechanical testing indicated that the compressive mechanical properties which were similar to those of the native tissues.
In the absence of a UBM implant, re-growth of the excised disk did not occur and significant gross pathologic changes in the surfaces of both the condyle and fossa were observed. These results suggest that discectomy without replacement in the canine model leads to many of the same degenerative changes that have been observed in other animal models as well as in human patients following discectomy (33, 37, 38). Adipose tissues, dermis and cartilage have all been used as interpositional grafts following discectomy with some degree of clinical success (5–11). However, donor site morbidity, fibrosis, and necrosis of the implanted tissues have been observed. The inductive ECM scaffold based approach described in the present study therefore provides a potential improvement upon the currently available treatments for reconstruction following discectomy.
While the exact mechanisms by which ECM scaffold materials are capable of promoting the type of constructive remodeling observed in the present study have not been fully described, a number of key elements have been identified. These critical events include the modulation of the host innate immune response towards a more regulatory and pro-remodeling phenotype, the rapid degradation of the scaffold material with the release of biologically active matricryptic peptides, the recruitment of a variety of stem and progenitor cells to the site of implantation, and the site-appropriate response to mechanical stimuli.
The implantation of both synthetic and biologic materials is invariably associated with a robust macrophage response beginning as early as two day post-implantation and continuing for many months depending on the nature of the implanted material (39). The presence of such a response is generally interpreted as chronic inflammation with associated granulation tissue formation and downstream encapsulation of the material with little to no functional recovery. However, it has been shown that non-crosslinked, acellular ECM based scaffold materials such as that used in the present study are capable of promoting the formation of new functional tissues without encapsulation or scarring, even in the presence of a robust, mononuclear cell dominated host innate immune response (35, 36, 40–42). Indeed, a preliminary study showed that the implantation of a UBM device in the TMJ joint space following discectomy elicited a CD68+ mononuclear cell response which persisted to varying degrees for up to two months post-implantation, but did not inhibit the formation of tissue which highly resembled that of the native TMJ with time (33). This dichotomy of outcomes (i.e. encapsulation vs. constructive tissue remodeling) suggests that phenotypic differences in the host cells which respond following implantation may exist.
A number of recent studies have described phenotypic differences in the macrophage population that responds following tissue injury (43–47). Briefly, macrophage phenotype has been broadly characterized as M1, or “classically” activated, and M2, or “alternatively” activated, mimicking the Th1/Th2 nomenclature (48, 49). Effective transition from a pro-inflammatory M1 type response to a more permissive M2 type macrophage response has been shown to be desirable for the functional recovery of a number of tissues following injury (43–45, 50). Acellular, non-crosslinked ECM scaffold materials have been shown to modulate the default response towards a more M2 type macrophage response as early as three days post-implantation (34, 35). The exact mechanisms by which this early transition affects tissue remodeling remains a subject of ongoing study; however it is increasingly clear that modulation of the default hose response to tissue injury is essential for the success of tissue engineering and regenerative medicine based approaches to tissue reconstruction.
While acellular, non-crosslinked ECM scaffold materials have been shown to modulate the host innate immune response resulting in constructive remodeling, the remodeling outcome associated with poorly decellularized or chemically crosslinked scaffold materials has been less desirable (42). Materials which have been inefficiently decellularized or chemically crosslinked have been shown to elicit a primarily M1 type macrophage response and result in degradation of the material without functional recovery or encapsulation, respectively (35, 41). The exact mechanisms by which chemical crosslinking affects the ability of ECM based scaffold materials to modulate the host response and to promote constructive remodeling is unclear but chemical crosslinking has been shown to change the ligand landscape and surface topology of the native material, prevent the degradation of the scaffold material, and inhibit the subsequent release of bioactive matricryptic peptides which may be contained within (36, 41, 42, 51).
ECM scaffolds such as the one used in the present study have been shown to degrade rapidly upon implantation (36, 52–54). A number of small peptides generated during this rapid degradation have potent antibacterial effects and chemotactic and mitogenic activity for a variety of cells, including local and marrow derived progenitor cell populations (55–61). The recruitment of these cells is thought to play a crucial role in facilitating the formation of new, site-specific functional host tissues. The exact phenotype of the cells recruited to the site of remodeling in the present study was not investigated; however, previous studies have shown that cells recruited to sites of ECM scaffold remodeling express an array of markers associated with both multipotent stem cell populations and mononuclear cells (61, 62). It is thought that these cells remain within the site of tissue remodeling in the long term and contribute to the observed constructive remodeling response. The biochemical and biomechanical milieu of the implantation site can have a determinant impact upon the phenotype of the cells participating in the remodeling process and the type and quality of the tissue which is eventually formed (63–67).
ECM scaffolds have been derived from a number of different sources and the source tissue can affect the phenotype of infiltrating cells in complex tissues and organs such as lung and liver (68–71). However, the parent tissue source of many ECM scaffolds does not appear to be a critical factor for the constructive remodeling of many tissues For example, the UBM material used in the present has been shown capable of supporting the formation of not only urinary bladder, but also trachea, esophagus, and myocardium, among others (63, 72–74). This diversity of constructive outcomes with a single source ECM scaffold suggests that tissue remodeling is critically dependent upon site-specific microenvironmental niche conditions. A number of studies have shown the effects of biomechanical stimulation upon the phenotype of progenitor cell populations and it is likely that local biomechanical cues, in addition to biochemical cues, influence the outcome of tissue remodeling (75–77). In the absence of mechanical cues, tissue remodeling following the placement of ECM scaffold materials has often resulted in degradation of the scaffold material without constructive and functional remodeling and instead, replacement of the bioscaffold with non-functional scar tissue. In the present study, it is likely that the unique mechanical environment, multiple tissue types present, and complex biochemical milieu of the synovium and TMJ space contributed to the observed site-appropriate patterns and spatial distributions of muscle, connective, vascular, and fibrocartilaginous tissue formation. The fate of infiltrating cells, and the specific role of biochemical signals and biomechanical stimuli which lead to site-appropriate remodeling remain a subject of ongoing investigation.
Few significant complications were observed in the present study. Two animals experienced dehiscence of the surgical site; however, this was not likely related to the implant itself and was repaired without incident. In one animal, a small area of calcified tissue formation was observed within the remodeled UBM device. It is speculated that this area of calcification was due to the surgical procedure, in which the device was placed within the joint space prior to creation of the attachment sites by drilling through the temporal fossa. Placement of the device prior to drilling of the bone likely resulted in bone fragments and bone cells being deposited within the device in this animal prior to closure and initiation of the remodeling process. In one animal, formation for new discal tissue was not observed. While the exact reason for degradation of the scaffold material without formation of new tissue in this case is unknown, it is possible that the UBM fixation sutures may have failed, allowing the implant to migrate from the joint space. Lastly, the present study was limited in that it included only gross morphologic evaluation of the articulating surface of both the condylar head and the temporal fossa. Future studies may include more in-depth analysis of the histologic architecture of the articulating surfaces and radiographic imaging. However, it is clear from the present study that the implanted UBM device protected the articulating surfaces from the grossly visible pathologic changes which occurred in the discectomy alone control group.
In conclusion, the results of the present study suggest that a biologic ECM scaffold material derived from xenogeneic urinary bladder can serve as an inductive and effective substrate for the formation of new, site-appropriate, functional TMJ meniscus tissue. The UBM device may represent an “off-the-shelf” option for reconstruction of the TMJ disk following discectomy.
Acknowledgments
A portion of this study was funded by Biomet Microfixation (Jacksonville, Florida) and by a grant from the NIH (5R01 AR053603 03 - SFB).
Footnotes
All work was performed at the University of Pittsburgh.
6.0 Disclosure: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7.0 References
- 1.Alonso A, Kaimal S, Look J, Swift J, Fricton J, Myers S, et al. A quantitative evaluation of inflammatory cells in human temporomandibular joint tissues from patients with and without implants. J Oral Maxillofac Surg. 2009;67:788–96. doi: 10.1016/j.joms.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira JN, Ko CC, Myers S, Swift J, Fricton JR. Evaluation of surgically retrieved temporomandibular joint alloplastic implants: pilot study. J Oral Maxillofac Surg. 2008;66:1112–24. doi: 10.1016/j.joms.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fricton JR, Look JO, Schiffman E, Swift J. Long-term study of temporomandibular joint surgery with alloplastic implants compared with nonimplant surgery and nonsurgcal rehabilitation for painful temporomandibular joint disc displacement. J Oral Maxillofac Surg. 2002;60:1400–11. doi: 10.1053/joms.2002.36091. discussion 11-2. [DOI] [PubMed] [Google Scholar]
- 4.Dolwick MF, Aufdemorte TB. Silicone-induced foreign body reaction and lymphadenopathy after temporomandibular join arthroplasty. Oal Surg Oral Medtroral Pathol. 1985;59:449–52. doi: 10.1016/0030-4220(85)90079-9. [DOI] [PubMed] [Google Scholar]
- 5.Dimitroulis G. A critical review of interpositional grafts following temporomandibular joint discectomy with an overview of the dermis-fat graft. Int J Oral Maxillofac Surg. 2011;40:561–8. doi: 10.1016/j.ijom.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Dimitroulis G. The use of dermis grafts after discectomy for internal derangement of the temporomandibular joint. J Oral Maxillofac Surg. 2005;63:173–8. doi: 10.1016/j.joms.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 7.Matukas VJ, Lachner J. The use of autologous auricular cartilage for temporomandibular joint disc replacement: a preliminary report. J Oral Maxillofac Surg. 1990;48:348–53. doi: 10.1016/0278-2391(90)90429-6. [DOI] [PubMed] [Google Scholar]
- 8.Meyer RA. The autogenous dermal graft in temporomandibular joint disc surgery. J Oral Maxillofac Surg. 1988;46:948–54. doi: 10.1016/0278-2391(88)90332-1. [DOI] [PubMed] [Google Scholar]
- 9.Pogrel MA, Kaban LB. The role of a temporalis fascia and muscle flap in temporomandibular joint surgery. J Oral Maxillofac Surg. 1990;48:14–9. doi: 10.1016/0278-2391(90)90173-y. [DOI] [PubMed] [Google Scholar]
- 10.Thyne GM, Yoon JH, Luyk NH, McMillan MD. Temporalis muscle as a disc replacement in the temporomandibular joint of sheep. J Oral Maxillofac Surg. 1992;50:979–87. doi: 10.1016/0278-2391(92)90059-9. discussion 87–8. [DOI] [PubMed] [Google Scholar]
- 11.Dimitroulis G. Macroscopic and Histologic Analysis of Abdominal Dermis-Fat Grafts Retrieved From Human Temporomandibular Joints. J Oral Maxillofac Surg. 2011 doi: 10.1016/j.joms.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 12.McKenna SJ. Discectomy for the treatment of internal derangements of the temporomandibular joint. J Oral Maxillofac Surg. 2001;59:1051–6. doi: 10.1053/joms.2001.26682. [DOI] [PubMed] [Google Scholar]
- 13.Krug J, Jirousek Z, Suchmova H, Cermakova E. Influence of discoplasty and discectomy of the temporomandibular joint on elimination of pain and restricted mouth opening. Acta Medica (Hradec Kralove) 2004;47:47–53. [PubMed] [Google Scholar]
- 14.Nyberg J, Adell R, Svensson B. Temporomandibular joint discectomy for treatment of unilateral internal derangements--a 5 year follow-up evaluation. Int J Oral Maxillofac Surg. 2004;33:8–12. doi: 10.1054/ijom.2002.0453. [DOI] [PubMed] [Google Scholar]
- 15.Badylak SF, Brown BN, Gilbert TW, Daly KA, Huber A, Turner NJ. Biologic scaffolds for constructive tissue remodeling. Biomaterials. 2011;32:316–9. doi: 10.1016/j.biomaterials.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Almarza AJ, Athanasiou KA. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 2004;10:1787–95. doi: 10.1089/ten.2004.10.1787. [DOI] [PubMed] [Google Scholar]
- 19.Almarza AJ, Athanasiou KA. Effects of hydrostatic pressure on TMJ disc cells. Tissue Eng. 2006;12:1285–94. doi: 10.1089/ten.2006.12.1285. [DOI] [PubMed] [Google Scholar]
- 20.Bean AC, Almarza AJ, Athanasiou KA. Effects of ascorbic acid concentration on the tissue engineering of the temporomandibular joint disc. Proc Inst Mech Eng H. 2006;220:439–47. doi: 10.1243/09544119JEIM51. [DOI] [PubMed] [Google Scholar]
- 21.Detamore MS, Athanasiou KA. Motivation, characterization, and strategy for tissue engineering the temporomandibular joint disc. Tissue Eng. 2003;9:1065–87. doi: 10.1089/10763270360727991. [DOI] [PubMed] [Google Scholar]
- 22.Detamore MS, Athanasiou KA. Structure and function of the temporomandibular joint disc: implications for tissue engineering. J Oral Maxillofac Surg. 2003;61:494–506. doi: 10.1053/joms.2003.50096. [DOI] [PubMed] [Google Scholar]
- 23.Detamore MS, Athanasiou KA. Evaluation of three growth factors or TMJ disc tissue engineering. Ann Biomed Eng. 2005;33:383–90. doi: 10.1007/s10439-005-1741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS One. 2008;3:e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elder BD, Eleswarapu SV, Athanasiou KA. Extraction techniques for the decellularization of tissue engineered articular cartilage constructs. Biomaterials. 2009;30:3749–56. doi: 10.1016/j.biomaterials.2009.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grayson WL, Frohlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, et al. Regenerative Medicine Special Feature: Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 107:3299–304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johns DE, Athanasiou KA. Design characteristics for temporomandibular joint disc tissue engineering: learning from tendon and articular cartilage. Proc Inst Mech Eng H. 2007;221:509–26. doi: 10.1243/09544119JEIM158. [DOI] [PubMed] [Google Scholar]
- 28.Johns DE, Athanasiou KA. Improving culture conditions for temporomandibular joint disc tissue engineering. Cells Tissues Organs. 2007;185:246–57. doi: 10.1159/000102173. [DOI] [PubMed] [Google Scholar]
- 29.Johns DE, Wong ME, Athanasiou KA. Clinicaly relevant cell sources for TMJ disc engineering. J Dent Res. 2008;87:548–52. doi: 10.1177/154405910808700609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lumpkins SB, McFetridge PS. Regional variations in the viscoelastic compressive properties of the temporomandibular joint disc and implications toward tissue engineering. J Biomed Mater Res A. 2009;90:784–91. doi: 10.1002/jbm.a.32148. [DOI] [PubMed] [Google Scholar]
- 31.Lumpkins SB, Pierre N, McFetridge PS. A mechanical evaluation of three decellularization methods in the design of a xenogeneic scaffold for tissue engineering the temporomandibular joint disc. Acta Biomater. 2008;4:808–16. doi: 10.1016/j.actbio.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Lazebnik M, Detamore MS. Hyaline cartilage cells outperform mandibular condylar cartilage cells in a TMJ fibrocartilage tissue engineering application. Osteoarthritis Cartilage. 2009;17:346–53. doi: 10.1016/j.joca.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Brown BN, Chung WL, Pavlick M, Reppas S, Ochs MW, Russell AJ, et al. Extracellular Matrix as an Inductive Template for Temporomandibular Joint Meniscus Reconstruction: A Pilot Study. J Oral Maxillofac Surg. 2011 doi: 10.1016/j.joms.2011.02.130. [DOI] [PubMed] [Google Scholar]
- 34.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage Phenotype as a Determinant of Biologic Scaffold Remodeling. Tissue Eng. 2008 doi: 10.1089/ten.tea.2007.0264. In Press. [DOI] [PubMed] [Google Scholar]
- 35.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–91. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valentin JE, Stewart-Akers AM, Gilbert TW, Badylak SF. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng Part A. 2009;15:1687–94. doi: 10.1089/ten.tea.2008.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martini DT, De Campos Boldrini S, De Vasconcellos Fontes RB, Liberti EA. Ultrastructural study of the temporomandibular joint after unilateral meniscectomy in Wistar rats. J Oral Rehabil. 2006;33:722–8. doi: 10.1111/j.1365-2842.2006.01647.x. [DOI] [PubMed] [Google Scholar]
- 38.Tong AC, Tideman H. A comparative study on meniscectomy and autogenous graft replacement of the Rhesus monkey temporomandibular joint articular disc--Part I. Int J Oral Maxillofac Surg. 2000;29:140–5. [PubMed] [Google Scholar]
- 39.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valentin JE, Turner NJ, Gilbert TW, Badylak SF. Functional skeletal muscle formation with a biologic scaffold. Biomaterials. 2011;31:7475–84. doi: 10.1016/j.biomaterials.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–42. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 42.Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88:2673–86. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 43.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–44. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, Rosenthal N, et al. A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci U S A. 2009;106:17475–80. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2011;298:R1173–87. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellon T, Martinez V, Lucendo B, Del Peso G, Castro MJ, Aroeira LS, et al. Alternative activation of macrophages in human peritoneum: implications for peritoneal fibrosis. Nephrol Dial Transplant. 2011 doi: 10.1093/ndt/gfq771. [DOI] [PubMed] [Google Scholar]
- 47.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–26. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 49.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Deonarine K, Panelli MC, Stashower ME, Jin P, Smith K, Slade HB, et al. Gene expression profiling of cutaneous wound healing. J Transl Med. 2007;5:11. doi: 10.1186/1479-5876-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown BN, Barnes CA, Kasick RT, Michel R, Gilbert TW, Beer-Stolz D, et al. Surface characterization of extracellular matrix scaffolds. Biomaterials. 2010;31:428–37. doi: 10.1016/j.biomaterials.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilbert TW, Stewart-Akers AM, Badylak SF. A quantitative method for evaluating the degradation of biologic scaffold materials. Biomaterials. 2007;28:147–50. doi: 10.1016/j.biomaterials.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 53.Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89:621–30. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 54.Record RD, Hillegonds D, Simmons C, Tullius R, Rickey FA, Elmore D, et al. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials. 2001;22:2653–9. doi: 10.1016/s0142-9612(01)00007-2. [DOI] [PubMed] [Google Scholar]
- 55.Beattie AJ, Gilbert TW, Guyot JP, Yates AJ, Badylak SF. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng Part A. 2009;15:1119–25. doi: 10.1089/ten.tea.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brennan EP, Reing J, Chew D, Myers-Irvin JM, Young EJ, Badylak SF. Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng. 2006;12:2949–55. doi: 10.1089/ten.2006.12.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brennan EP, Tang XH, Stewart-Akers AM, Gudas LJ, Badylak SF. Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells. J Tissue Eng Regen Med. 2008;2:491–8. doi: 10.1002/term.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li F, Li W, Johnson S, Ingram D, Yoder M, Badylak S. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium. 2004;11:199–206. doi: 10.1080/10623320490512390. [DOI] [PubMed] [Google Scholar]
- 59.Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15:605–14. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 60.Tottey S, Corselli M, Jeffries EM, Londono R, Peault B, Badylak SF. Extracellular matrix degradation products and low-oxygen conditions enhance the regenerative potential of perivascular stem cells. Tissue Eng Part A. 2011;17:37–44. doi: 10.1089/ten.tea.2010.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zantop T, Gilbert TW, Yoder MC, Badylak SF. Extracellular matrix scaffolds are repopulated by bone marrow-derived cells in a mouse model of achilles tendon reconstruction. J Orthop Res. 2006;24:1299–309. doi: 10.1002/jor.20071. [DOI] [PubMed] [Google Scholar]
- 62.Agrawal V, Johnson SA, Reing J, Zhang L, Tottey S, Wang G, et al. Epimorphic regeneration approach to tissue replacement in adult mammals. Proc Natl Acad Sci U S A. 2010;107:3351–5. doi: 10.1073/pnas.0905851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boruch AV, Nieponice A, Qureshi IR, Gilbert TW, Badylak SF. Constructive Remodeling of Biologic Scaffolds is Dependent on Early Exposure to Physiologic Bladder Filling in a Canine Partial Cystectomy Model. J Surg Res. 2009 doi: 10.1016/j.jss.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 64.Hodde JP, Badylak SF, Shelbourne KD. The effect of range of motion on remodeling of small intestinal submucosa (SIS) when used as an Achilles tendon repair material in the rabbit. Tissue Eng. 1997;3:27–37. [Google Scholar]
- 65.Turner NJ, Yates AJ, Jr, Weber DJ, Qureshi IR, Stolz DB, Gilbert TW, et al. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng Part A. 2010;16:3309–17. doi: 10.1089/ten.TEA.2010.0169. [DOI] [PubMed] [Google Scholar]
- 66.Lantz GC, Badylak SF, Hiles MC, Coffey AC, Geddes LA, Kokini K, et al. Small intestinal submucosa as a vascular graft: a review. J Invest Surg. 1993;6:297–310. doi: 10.3109/08941939309141619. [DOI] [PubMed] [Google Scholar]
- 67.Sandusky GE, Jr, Badylak SF, Morff RJ, Johnson WD, Lantz G. Histologic findings after in vivo placement of small intestine submucosal vascular grafts and saphenous vein grafts in the carotid artery in dogs. Am J Pathol. 1992;140:317–24. [PMC free article] [PubMed] [Google Scholar]
- 68.Lin P, Chan WC, Badylak SF, Bhatia SN. Assessing porcine liver-derived biomatrix for hepatic tissue engineering. Tissue Eng. 2004;10:1046–53. doi: 10.1089/ten.2004.10.1046. [DOI] [PubMed] [Google Scholar]
- 69.Sellaro TL, Ravindra AK, Stolz DB, Badylak SF. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng. 2007;13:2301–10. doi: 10.1089/ten.2006.0437. [DOI] [PubMed] [Google Scholar]
- 70.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565–80. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 72.Gilbert TW, Gilbert S, Madden M, Reynolds SD, Badylak SF. Morphologic assessment of extracellular matrix scaffolds for patch tracheoplasty in a canine model. Ann Thorac Surg. 2008;86:967–74. doi: 10.1016/j.athoracsur.2008.04.071. discussion –74. [DOI] [PubMed] [Google Scholar]
- 73.Badylak SF, Kochupura PV, Cohen IS, Doronin SV, Saltman AE, Gilbert TW, et al. The use of extracellular matrix as an inductive scaffold for the partial replacement of functional myocardium. Cell Transplant. 2006;15 (Suppl 1):S29–40. doi: 10.3727/000000006783982368. [DOI] [PubMed] [Google Scholar]
- 74.Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO, et al. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87–97. doi: 10.1016/j.jss.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Altman GH, Horan RL, Martin I, Farhadi J, Stark PR, Volloch V, et al. Cell differentiation by mechanical stress. FASEB J. 2002;16:270–2. doi: 10.1096/fj.01-0656fje. [DOI] [PubMed] [Google Scholar]
- 76.Matziolis G, Tuischer J, Kasper G, Thompson M, Bartmeyer B, Krocker D, et al. Simulation of cell differentiation in fracture healing: mechanically loaded composite scaffolds in a novel bioreactor system. Tissue Eng. 2006;12:201–8. doi: 10.1089/ten.2006.12.201. [DOI] [PubMed] [Google Scholar]
- 77.Nieponice A, Maul TM, Cumer JM, Soletti L, Vorp DA. Mechanical stimulation induces morphological and phenotypic changes in bone marrow-derived progenitor cells within a three-dimensional fibrin matrix. J Biomed Mater Res A. 2007;81:523–30. doi: 10.1002/jbm.a.31041. [DOI] [PubMed] [Google Scholar]
- 78.Brown B, Lindberg K, Reing J, Stolz DB, Badylak SF. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12:519–26. doi: 10.1089/ten.2006.12.519. [DOI] [PubMed] [Google Scholar]
- 79.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–8. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 80.Woessner JJ. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Archives of Biochemistry and Biophysics. 1961:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 81.Hagandora CKCTW, Almarza AJ. A Comparison of the Mechanical Properties of the Goat Temporomandibular Joint Disc to the Mandibular Condylar Cartilage in Unconfined Compression. Journal of Dental Biomechanics. 2011 doi: 10.4061/2011/212385. [DOI] [PMC free article] [PubMed] [Google Scholar]


