Abstract
CD47 is a ubiquitously expressed transmembrane protein that, through signaling mechanisms mediated by signal regulatory protein alpha (SIRPα1), functions as a biological marker of ‘self-recognition’. We showed previously that inflammatory cell attachment to polymeric surfaces is inhibited by the attachment of biotinylated recombinant CD47 (CD47B). We test herein the hypothesis that CD47-modified blood conduits can reduce platelet and neutrophil activation under clinically relevant conditions. We appended a poly-lysine tag to the C-terminus of recombinant CD47 (CD47L) allowing for covalent linkage to the polymer. SIRPα1 expression was confirmed in isolated platelets. We then compared biocompatibility between CD47B and CD47L functionalized polyvinyl chloride (PVC) surfaces and unmodified control PVC surfaces. Quantitative and Qualitative analysis of blood cell attachment to CD47B and CD47L surfaces, via scanning electron microscopy, showed strikingly fewer platelets attached to CD47 modified surfaces compared to control. Flow cytometry analysis showed that activation markers for neutrophils (CD62L) and platelets (CD62P) exposed to CD47 modified PVC were equivalent to freshly acquired control blood, while significantly elevated in the unmodified PVC tubing. In addition, ethylene oxide gas sterilization did not inhibit the efficacy of the CD47 modification. In conclusion, CD47 modified PVC inhibits both the adhesion and activation of platelets and neutrophils.
1. Introduction
Aberrant biocompatibility between blood and the polymeric biomaterials that comprise the blood conduits used in such clinical procedures as cardiopulmonary bypass and renal dialysis are associated with post-procedural complications [1,2]. Investigators have attempted to address this issue by modifying the blood contacting surfaces with various moieties such as heparin, thrombotic inhibitors, and self assembled monolayers of alkylthiols [3–5]. As these technologies are in various states of development, a therapeutic strategy to address the untoward effects observed when large volumes of blood are exposed to synthetic surfaces remains an unmet need in biomaterials research.
We previously described and characterized a surface modification in which recombinant human CD47, a ubiquitously expressed transmembrane protein that functions as a molecular marker of self, was biotinylated (CD47B) and appended to avidin modified polyvinyl chloride (PVC) and polyurethane (PU) surfaces [6]. We further demonstrated, using protein specific antibodies, that the anti-inflammatory properties of CD47 functionalized polymeric surfaces were mediated by surface immobilized CD47 and Signal Regulatory Protein alpha 1 (SIRPα1) [6], a transmembrane protein expressed in cells of myeloid origin that is the cognate receptor of CD47 [7]. Work by others has largely defined the anti-inflammatory properties of CD47 mediated SIRPα1 signaling, via src homology region 2 containing phosphatase 1 (SHP-1) and SHP-2 secondary messengers interacting with the immune receptor tyrosine inhibitory motif (ITIM) of SIRPα1, within the context of its cytoskeletal effects [8, 9]. Thus, in our previous studies, we largely defined biocompatibility as a reduced affinity of inflammatory cells for the CD47-modified surfaces [6]. However, we did observe that the expression of CD18, a surface marker of neutrophil activation was significantly reduced when whole blood was perfused over the luminal surface of CD47 modified blood conduits [6]. Based upon these observations, we test herein the hypothesis that the anti-inflammatory capacity of CD47 functionalized surfaces extends beyond inhibiting inflammatory cell attachment to synthetic surfaces and that CD47-SIRPα interactions can alter the inflammatory activation state of blood cells.
Understanding the signaling relationship between CD47 functionalized surfaces and platelets is essential to applying this strategy to effectively inhibit the inflammatory response observed when large volumes of blood are exposed to synthetic surfaces. Platelets are important mediators of the acute inflammatory response observed in clinically used blood conduits [10–12]. To date, the effects of CD47- SIRPα1 signaling in platelets were never investigated. In these current investigations we begin to profile the platelet response to CD47 functionalized surfaces. Specifically, we ascertain the possibility of SIRPα1 mediated signaling mechanisms functioning in platelets and characterize the surface expression of proinflammatory markers of platelet activation when whole blood is exposed to CD47 modified blood conduits.
In addition to the biological response of CD47 modified surface, this current work begins to address the pragmatic challenges associated with the potential clinical application of CD47 modified blood conduits. Our previous attachment strategy for appending CD47 to polymeric surfaces was based on covalently binding avidin to the polymer surface via photoactivation chemistry and then appending biotinylated CD47B to the avidin functionalized surface [6] via Avidin-Biotin affinity binding. Although this attachment proved effective in reducing the affinity of inflammatory cells for CD47 functionalized surfaces under in vitro, ex vivo, and long term in vivo studies, there exists a concern that the avidin-biotin system may not be appropriate for all applications, due to potential avidin immunogenicity [13]. Thus, in the present studies, we also tested the hypothesis that molecular modifications can be made to the extracellular domain of recombinant CD47 that allows it to be covalently immobilized onto polymeric surfaces, via thiol chemistry, whereupon it can effectively enhance biocompatibility under dynamic conditions. This approach would eliminate the need for initially immobilizing avidin to the surface, and would hypothetically increase the resiliency of the CD47 protein by covalently attaching the recombinant protein to the biomaterial surface. In these studies, we compared, with respect to anti-inflammatory and durability endpoints, our original biotinylated CD47B with our recently developed lysine tagged (CD47L) attachment strategy.
Our goals of these studies were: 1. Investigate a chemical attachment strategy to directly link recombinant CD47 to polymeric surfaces using a lysine tag methodology, 2. Study the biologic response of platelets and neutrophils to CD47 functionalized PVC surfaces versus unmodified surfaces, 3. Assess the retention and effectiveness of the CD47 modification upon functionalized PVC surfaces following biophysical challenges as well as ethylene oxide gas sterilization.
2. Materials and Methods
2.1. Materials
Clinical grade polyvinyl chloride tubing conduits were acquired from Terumo Cardiovascular Systems (Ann Arbor, MI). A FITC-conjugated mouse monoclonal antibody directed against CD47 (Clone: B6H12) was purchased from BD Biosciences (Franklin Lakes, NJ). Ultrapure avidin was purchased from Life Technologies (Carlsbad, CA). N-Succinimidyl 3[2-pyridyldithio]-propionate (SPDP), Tris(2-carboxyethyl)phosphine hydrochloride (TCEP), and all other chemicals and solvents, unless otherwise specified, were purchased from Sigma Aldrich (St. Louis, MO).
2.2. Recombinant CD47 Protein Production and Purification
We used a modification of the previously published methodology to produce recombinant human CD47 from human whole blood [6]. Briefly, the extracellular domain of human CD47 (GenBank Accession Number NM_174708) was amplified from human cDNA using gene specific primers. Forward Primer: 5′ – ATAAGCTTATGTGGCCCCTGG – 3′. Reverse Primer: 5′ –GCGGATCCTCTGGTTCAACCCT – 3′. The PCR conditions were as follows: 95°C for 30 seconds denaturation, 59°C for 45 seconds annealing, and 72°C for 1 minute extension for a total of 35 cycles.
The PCR product was ligated into a thymidine/adenine vector containing the rat CD4 domains 3 and 4 (rCD4D3+4) coding sequence. Either a biotin coding sequence (B), required for the subsequent biotinylation of the final recombinant protein, or poly-lysine coding sequence (L) was cloned into the vector at the three prime end of the rat CD4 sequence to aid in protein purification and appendage to the polymeric surfaces. The expression cassette (5′ - hCD47-rCD4d3+4-Biotin – 3′ or 5′ – hCD47-rCD4d3+4-Poly-Lysine – 3′) was then subcloned into pcDNA5-FRT (Invitrogen, Carlsbad, CA) by digesting vector and insert with Hind III and BamHI and then ligating using T4 DNA ligase. All vectors were sequenced by the Children’s Hospital of Philadelphia Research Institute Nucleic Acid Facility and confirmed to be free of coding errors and in-frame for accurate protein production. The pcDNA5-FRT-hCD47-rCD4d3+4-Biotin (hCD47B) and pcDNA5-FRT-hCD47-rCD4d3+4-Poly-Lysine (hCD47L) were co-transfected with pOG44, used to transiently provide the recombinase enzyme required for integration of the hCD47-rCD4d3+4 into the genomic DNA of the host cell, into CHO Flp-In cells to allow for genomic integration of the expression cassette. Recombinant protein was isolated from the cell culture medium, concentrated, desalted, and purified using an avidin protein purification column or anti-CD47 amino-link protein purification column both obtained from Thermo Fisher Scientific (Wilmington, DE). Protein concentration and purity were analyzed by the Bradford assay and SDS-PAGE Western blot analysis.
2.3. Casting PVC Films
PVC was dissolved in dimethylacetamide, and then solvent cast as films with thickness ranging from 159 and 220 μm as used in prior studies [6]. Films were cast onto flow streamer slides specifically for the Flex-Flow parallel plate shear delivery system by Flex-Cell International (Hillsborough, NC) and subsequently used in shear studies as previously described [14].
2.4. Appending of Poly-Lysine Tagged Recombinant Proteins to Synthetic Polymeric Surfaces
PVC surfaces were reacted with 2-pyridyldithio-, benzophenone- and carboxy-modified polyallylamine (PDT-BzPh) and reduced with TCEP to obtain a thiol reactive surface, as previously described [6]. The poly-lysine tail of the recombinant CD47L protein was reacted with SPDP for one hour to form thiol-reactive groups. SPDP was removed via a desalting protein cut off column and the poly-lysine CD47 protein was incubated with the synthetic surface overnight at 4°C. Figure 1 is provided as a graphic illustration of the chemistry described in this section. Confirmation of recombinant protein attachment was determined by conjugation with a FITC-tagged antibody specific for human CD47 (BD Biosciences, Franklin Lakes, NJ). Where indicated, blood conduits were sterilized by ethylene oxide (EtO) gas sterilization by the Children’s Hospital of Philadelphia Instrument Sterilization Facility.
Fig. 1.
Schematic Representation of Chemistry Utilized to Append Recombinant CD47 Protein to Synthetic Biomaterials. Thiolated surfaces were provided by reacting the polymeric surface with a water soluble polymer, PDT-BzPh, photo-reacted, and reduced using TCEP as detailed in Materials and Methods. To append the recombinant CD47 poly-lysine protein, the protein was first reacted with SPDP to generate a thiol reactive site which then is able to bind to the activated synthetic biomaterials surface.
2.5. Analysis of Recombinant CD47 Poly-Lysine Appendage with Synthetic Polymeric Surfaces
PVC films, modified with either CD47B or CD47L (6 μg/cm2), were exposed to shear flow of 1X phosphate buffered saline for two hours at physiologically relevant levels of shear stress (twenty dynes/cm2), using the computer controlled Flex-Cell Streamer instrument [14]. Control films were not exposed to shear conditions. Following the protocol, films were stained with FITC-conjugated antibody specific for CD47. Briefly, the polymeric films were blocked using 5% bovine serum albumin (BSA) in 1X tris buffered saline with tween-20 (TBST) for one hour at room temperature. Following blocking, the films were stained with 100 μg FITC-conjugated CD47 antibody in 5% BSA 1X TBST for one hour at room temperature. Films were washed three times in 1X TBST and imaged by fluorescence microscopy. In order to estimate the concentration of recombinant hCD47 protein, the antibody was titrated to form a standard curve, which was then used to determine the concentration of recombinant hCD47 protein on the polymeric surfaces. To quantify results, the fluorescence intensity was collected for each film and correlated to a standard curve in order to determine the approximate protein concentration in micrograms.
2.6. Chandler Loop Analysis of CD47-Modified Polymeric Blood Conduits
PVC tubing (1/4″) was modified with either CD47B or CD47L. CD47B was attached to the polymeric surface using thiol reactive avidin, as previously described [6]. Whole blood isolated aseptically from healthy volunteers, per an IRB protocol approved by the University of Pennsylvania IRB after informed consent, was either kept as control or loaded into PVC, CD47B modified PVC, or CD47L modified PVC tubing. Blood was perfused over the polymeric surfaces for three hours to simulate the effect of whole blood interaction with the conduit polymeric surface and blood samples were withdrawn for flow cytometry analysis. As detailed below, at the termination of the protocol, whole blood was collected for flow cytometry analysis and scanning electron microscopy (SEM) was performed on the tubing.
2.7. Removal of Adsorbed Cells From PVC Blood Conduits
Using a previously described protocol [15–18], we gently removed attached cells from the PVC surface using a buffer composed of 10 mM HEPES, 0.15 M NaCl, and 0.01M EDTA. The buffer was added to the PVC tubing after washing three times with 1X PBS to remove non-adherent cells. As detailed below, the collected cells were analyzed for the presence of protein surface markers of inflammation via flow cytometry and SEM analysis was performed on the tubing to determine the extent of cellular displacement. As previously established, this procedure does not alter the activation state of the detached cells [15–18].
2.8. Flow Cytometic Analysis of Biomarker Expression
Antibodies utilized for flow cytometry included anti-CD62L (DREG-56) FITC, anti-CD62P (AK-4) APC, anti-CD47 (B6H12) FITC, and anti-SIRPα1 (SE7C2) FITC all from BD Biosciences (Franklin Lakes, NJ). Whole blood (5 μL) was added to staining buffer (200 μL; 1X phosphate buffer saline (PBS) with 1% BSA and 0.01% sodium azide) and stained with indicated antibodies for one hour on ice. Compensation beads were utilized for each fluorophore (BD Biosciences, Franklin Lakes, NJ) and fluorescence minus one (FMO) samples were utilized to set accurate gates. Cells were analyzed on a BD LSRII flow cytometer (BD Biosciences, Franklin Lakes, NJ) using FACS Diva software. Compensation and gating analysis was completed using Tree Stars FloJo Software Suite (Ashland, OR).
2.9. Scanning Electron Microscopy
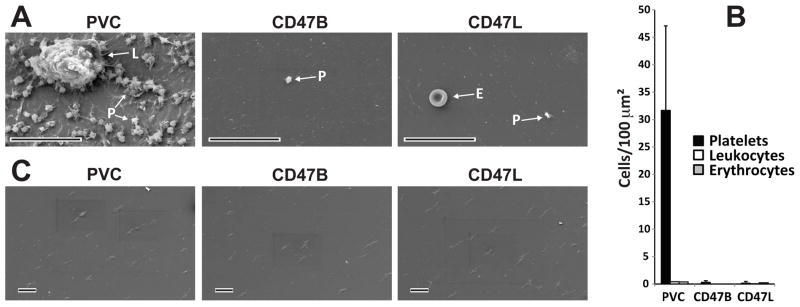
Following the Chandler Loop protocol, or the cell detachment protocol described above, tubing was rinsed in PBS and processed for SEM as previously described [19,20]. Briefly, adherent cells were affixed to the surfaces via a solution of 4% glutaraldehyde in Hepes Buffered Saline. Samples were dehydrated in ethanol, dried with hexamethyldisilazane, and sputter coated with gold-palladium. Samples were examined in a Phillip/FEI XL20 scanning electron microscope (FEI, Hillsboro, OR). For quantitative analysis, six randomly chosen pieces, approxiamately 2 × 3 mm of each type of surface were examined. After scanning of the entire surface area, 2 random images 62.2 × 45.53 μm each were recorded. All cells in each image were counted and the results were plotted as mean ± standard error per sample (Figure 3).
Fig. 3.
Scanning electron microscopy analysis of CD47 modified and control surfaces following ex vivo analysis. Human whole blood was perfused over unmodified, clinical grade blood conduits or CD47 modified, via biotinylated CD47 (CD47B) or lysine modified CD47 (CD47L), surfaces. (A) Representative image shows extensive platelet (P) attachment and spreading onto unmodified surfaces. Leukocytes (L) were also observed on unmodified surfaces. In contrast, only an occasional platelet or erythrocyte (E) was noted on CD47 modified surfaces. Bar equals 25μm. (B) Quantitative analysis shows significantly p < 0.05 more platelets were present on SEM fields from unmodified blood conduits compared to CD47 modified blood conduit surfaces. (C) SEM analysis shows complete removal of cells from the luminal surface of blood conduits following the detachment protocol detailed in Materials and Methods. All scale bars equal 25μm
2.10. Magnetic Purification of Primary Human Platelets
Human blood was collected after informed consent, per an IRB protocol from the Children’s Hospital of Philadelphia, from healthy, aspirin-free volunteers in 0.129 M sodium citrate supplemented with 300 nM carbacyclin (Biomol, Farmingdale, NY) to prevent platelet activation. To isolate platelets, 10 ml of whole blood was centrifuged at 200g for 15 minutes at room temperature to obtain platelet-rich plasma and contaminating leukocytes were removed using anti-CD45 magnetic beads (Miltenyi Biotec, Auburn, CA). In short, the platelet-rich plasma was incubated on ice with anti-CD45 magnetic beads for 30 minutes to allow anti-CD45 to bind any contaminating leukocytes. The cells and platelets were then passed through a magnetic column and the flow through contained purified (leukocyte-free) platelets for use in these experiments.
2.11. Western Blot Analysis
Whole cell lysates of purified platelets were prepared using ice cold RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA) and quantitated using Bradford assay (Fisher Scientific, Pittsburgh, PA). Proteins (30 μg) were separated by sodium dodecylsulfate-polyacrylamide (SDS-PAGE) electrophoresis using 4–15% gels (Biorad, Hercules, CA) followed by blotting to a PVDF membrane (Life Technologies, Carlsbad, CA). Blocking and antibody incubation steps were completed using 5% non-fat dry milk in 1X TBST (10 mM pH 7.5 Tris-HCl), 100 mM NaCl, and 0.1% Tween 20). Goat polyclonal anti-SIRPα1 (C-20) and anti-goat-HRP antibodies were used for primary and secondary, respectively, following manufacturer specifications. As a control for equal loading, mouse monoclonal anti-GAPDH (0411) antibody and anti-mouse-HRP were utilized following manufacturer specifications. All antibodies utilized for Western blotting were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Detection was completed using the ECL Plus kit from GE Biosciences (Piscataway, NJ) and visualized with auto-radiography film
2.12. Semi-Quantitative Real-Time PCR of Platelet RNA
Platelets were isolated as described above. RNA was isolated using TriZol reagent (Life Technologies, Carlsbad, CA) following manufacturer recommended protocol and converted to cDNA using a reverse transcriptase kit (Qiagen, Frederick, MD). Real-time PCR was completed on the ABI 7500 and samples were run on a 1% agarose gel. Conditions for the PCR procedure were as follows: 95°C for 15 seconds denaturation, 60°C for 30 seconds annealing, and 72°C for 30 seconds extension for a total of 30 cycles. Primers were purchased from IDT Technologies and pre-designed using sequences confirmed to cross exon-exon boarders. β2 Integrin Forward Primer: 5′ –GATGACAAACGACTGCTCCT – 3′. β2 Integrin Reverse Primer: 5′ –TCACCTACGACTCCTTCTGC – 3′. SIRPα1 Forward Primer: 5′ –CATTTGTGTCCTGTGTTATTTCTCT – 3′. SIRPα1 Reverse Primer = 5′ –ACCTCGTCCGAATCAGACA – 3′
2.13. Statistical Analysis
Data were calculated as means ± standard deviation (SD). Student’s t-test was used to determine the significance of differences. Statistical significance was noted with * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 as compared to the control.
3. Results
3.1. Attachment of Recombinant CD47 Poly-Lysine to Synthetic Surfaces
Recombinant CD47 was attached to the PVC surfaces using thiol chemistry. The soluble polymer PDT-BzPh, which we have previously described [6], was attached to the polymeric surface. After ultraviolet photo-activation, PDT groups were reduced with TCEP. CD47B appendage to synthetic surfaces has been previously described by our laboratory [6]. Recombinant CD47L was reacted with the bifunctional cross linker SPDP resulting in covalent binding of CD47L to the thiol reactive surface and thereby immobilizing the recombinant protein on the surface of the polymer.
3.2. SIRPα1 Expression in Human Platelets
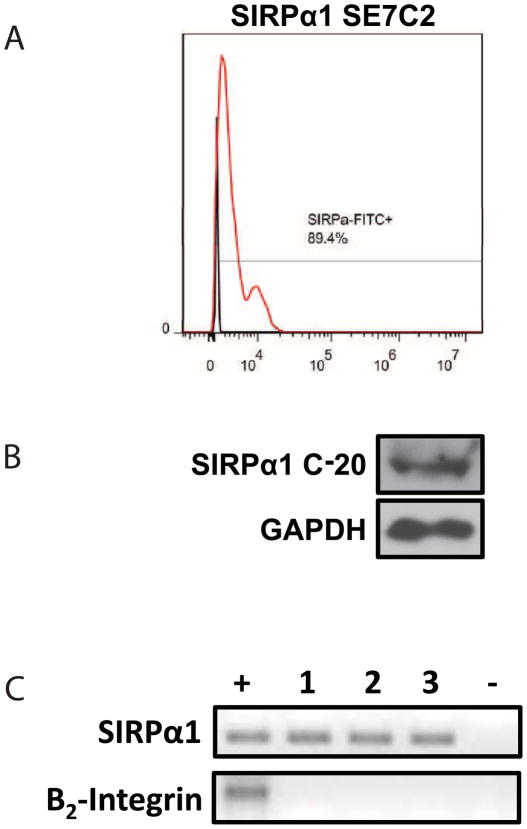
It has been previously documented that platelets express CD47 on their surface [21]. However, it has never been demonstrated that SIRPα1 is expressed in platelets. To begin to understand the mechanism regulating the inhibitory properties of CD47 functionalized surfaces to platelet activation, we characterized SIRPα1 expression in platelets using flow cytometry, Western blotting, and PCR. Flow cytometry using magnetically purified platelets was completed using an antibody for SIRPα1 (SEC72) that binds to the extracellular domain of SIRPα1. As shown in Figure 2A, a shift in the histogram as compared to unstained platelets demonstrates nearly 90% of the purified platelets expressed SIRPα1 on the surface. Since membrane receptors can be shed into the blood serum and thereafter taken up by platelets, we tested an antibody against the intracellular SIRPα1 domain. Purified platelets were lysed in RIPA buffer and analyzed by western blotting (Figure 2B). The SIRPα1 (C-20) antibody exclusively measures the intracellular domain of SIRPα1 and demonstrated a strong band at the correct molecular weight for SIRPα1. In addition, reverse transcription PCR confirms detection of RNA for SIRPα1 in purified human platelets (Figure 2C). The lack of white blood cell contamination was confirmed by the absence of the β2-integrin in platelet suspension [22]. Together, these findings demonstrate that human platelets do express SIRPα1 and the interaction between SIRPα1 and CD47 on the modified surfaces may be responsible for the significantly decreased number of platelets adhered to the modified blood conduits.
Fig. 2.
SIRPα1 is expressed in human platelets. (A) SIRPα1 expression was demonstrated in purified human platelets by flow cytometry using anti-human SIRPα1 antibody (SE7C2). (B) Representative immunoblots from purified platelet lysates for SIRPα confirmed SIRPα1 (C-20) expression in purified human platelets. (C) Semi-quantitative real-time PCR of reverse transcribed RNA isolated from purified human platelets. N = 3 separate experiments with different donors.
3.3. Attachment of Neutrophils and Platelets to CD47-Modified Surfaces
Since neutrophil and platelets are the initial cellular blood components to interact with polymeric blood conduits [23–25], we assessed the effects of CD47 immobilization upon neutrophil and platelet attachment and activation to CD47-functionalized surfaces. Clinical grade PVC tubing, modified with CD47L or CD47B, was exposed to whole blood using a Chandler Loop Apparatus [6], for three hours. At the end of the study, samples were examined by SEM as detailed above.
As shown in Figure 3A and 3B, the differences between the unmodified and CD47 modified surfaces were quite dramatic. On the unmodified surfaces, there was a large population of platelets. Nearly all of the platelets were activated as shown by the presence of their stellate morphology and pseudopod extensions. Often these platelets were attached to a thin layer of already spread platelets. In addition to the presence of platelets, activated leukocytes were also observed on the unmodified PVC surface. In contrast, there was little evidence of platelet attachment on the CD47 modified surfaces, and no leukocytes were observed (Figure 3A and 3B). Interestingly the few platelets that were observed on CD47 modified surfaces did not appear to be as spread on the polymer’s surface compared to those platelets adhered to the unmodified surface.
To better understand the activation state of the blood cells attached to the CD47 and control surfaces we employed a previously characterized procedure [15–18], as detailed above, to remove cells without eliciting an immune response. The detachment of cells on the unmodified and CD47 modified surfaces was confirmed using SEM (Figure 3C). As observed, the procedure resulted in complete removal of blood cells following the Chandler Loop procedure.
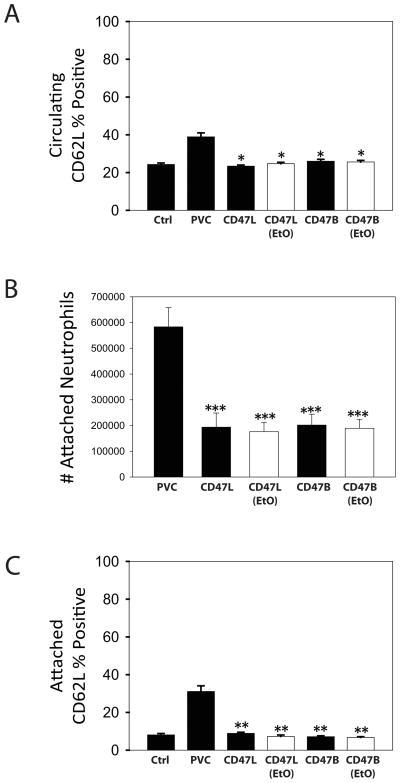
Surface expression of CD62L and CD62P, markers for neutrophil and platelets activation respectively, were measured using flow cytometry. CD47 immobilization significantly reduced the number of circulating activated platelets (Figure 4A) and neutrophils (Figure 5A) in whole blood cycled through the Chandler Loop apparatus as compared to the PVC surfaces. The total number of attached platelets (Figure 4B) and neutrophils (Figure 5B) were significantly reduced as compared to PVC. Attached platelets (Figure 4C) and neutrophils (Figure 5C) showed a significant decrease in CD62P and CD62L surface expression compared to unmodified PVC surfaces. These data show that the hemocompatibility conferred by the CD47 modification, was maintained irrespective of immobilization strategy or ethylene oxide sterilization.
Fig. 4.
Platelet Activation and Attachment to CD47 Modified PVC. Chandler Loop studies were utilized to evaluate immune cell interactions with modified polymeric biomaterials. Whole blood was loaded into unmodified PVC, CD47L, CD47L EtO sterilized, CD47B, or CD47B EtO sterilized blood conduits. As a control, blood was also set aside and not exposed to the blood conduits. CD62P, a marker of activated platelets, was utilized as a cell surface marker to evaluate the activation state of the platelets circulating in the whole blood (A) or attached to the PVC surface (C). Whole blood was analyzed by staining with an anti-CD62P APC-conjugated antibody and measured using flow cytometry. (B) Attached cells were gently removed from the luminal blood conduit surface as detailed in the materials and methods section and stained using an anti-CD62P APC-conjugated antibody and measured using flow cytometry. Number of attached platelets was obtained by counting the total number of anti-CD62P APC positive cells using the flow cytometer. * indicates P < 0.05; ** indicates P < 0.01; and *** indicates P < 0.001 as compared to the control. Data are the mean ± standard deviation from N = 5 separate experiments with different donors.
Fig. 5.
Neutrophil Activation and Attachment to CD47 Modified PVC. Chandler Loop studies were utilized to evaluate immune cell interactions with modified polymeric biomaterials. Whole blood was loaded into unmodified PVC, CD47L, CD47L EtO sterilized, CD47B, or CD47B EtO sterilized blood conduits. Blood from the same donor that was collected immediately prior to the initialization of the study, but not exposed to the blood conduits, was utilized as a control. CD62L, a marker of activated neutrophils, was utilized as a cell surface marker to evaluate the activation state of the neutrophils circulating in the whole blood (A) or attached to the PVC surface (C). Whole blood was analyzed by staining with an anti-CD62L FITC-conjugated antibody and measured using flow cytometry. (B) Attached cells were gently removed from the luminal blood conduit surface as detailed in the materials and methods section and stained using an anti-CD62L FITC-conjugated antibody and measured using flow cytometry. Number of attached neutrophils was obtained by counting the total number of anti-CD62L FITC positive cells using the flow cytometer. * indicates P < 0.05; ** indicates P < 0.01; and *** indicates P < 0.001 as compared to the control. Data are the mean ± standard deviation from N = 5 separate experiments with different donors.
3.4. Biophysical Characterization of CD47 Modified PVC
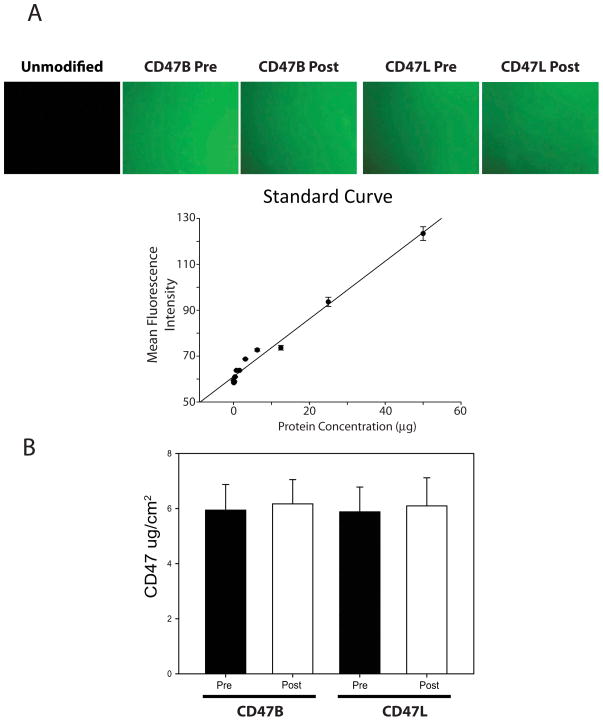
We confirmed successful immobilization of CD47L using a fluorescent tagged antibody against the extracellular portion of the CD47 protein. Shown in Figure 6A, fluorescent antibody staining of CD47L functionalized surfaces showed a uniform field of green fluorescence consistent with a complete appending of CD47L to PVC surfaces. Quantitative analysis of the fields using fluorometric analysis showed an average surface concentration of 6 μg/cm2 (Figure 6B), which was consistent with previous studies from our laboratory for CD47B [6].
Fig. 6.
Recombinant CD47 protein appended to the surface of the polymeric biomaterial is resistant to shear conditions. PVC films attached to glass microscopy slides were either modified by attaching CD47B (Using an avidin linkage) or CD47L. One of each of the slides was set aside to serve as the before shear control and the other slide was loaded into the Flow Cell Streamer and exposed to 20 dynes/cm2 of shear force for 2 hours. (A) Representative micrographs demonstrate unmodified, pre and post CD47B or CD47L fluorescence. (B) CD47 concentration was measured by correlating the fluorescence intensity of an anti-CD47 FITC-tagged antibody. The values of the fluorescence intensity were correlated to a standard curve made of known antibody concentrations. Data are the mean ± standard deviation from N = 3 separate experiments.
As the surfaces of polymeric blood conduits are exposed to hemodynamic shear stress, we tested the retention of the CD47 modification under physiological levels of fluid-induced shear. PVC coated glass microscopy slides were modified by the attachment of 6 μg/cm2 of recombinant CD47L or, via prior attachment of avidin, CD47B. Measurements of CD47 protein levels on the surface of the polymer were taken before and after two hours of venous levels (20 dynes/cm2) of laminar shear. The results (Figure 6) showed that CD47 was largely retained on the polymeric surface irrespective of immobilization strategy. This suggests that the surface modification of polymers with recombinant CD47 would withstand the hemodynamic conditions experienced by clinically used blood conduits.
4. Discussion
4.1. CD47 modified PVC blood contacting surfaces
At present, addressing the aberrant biocompatibility issues observed when blood is exposed to synthetic surfaces is an unmet need in the field of cardiovascular biomaterials. Polymeric blood contacting materials, such as the PVC tubing used in these studies, are widely used in medical devices such as cardiopulmonary bypass circuits, renal dialysis, and extracorporal membrane oxygenators [11,26,27]. Unfortunately, it has been widely documented that such devices elicit an acute inflammatory response that greatly contributes to the pathophysiology associated with their usage [23,25,28,29]. Modifying blood conduits with the extracellular domain of recombinant CD47 represents a viable strategy to fabricate a hemocompatible luminal blood-contacting surface. In support of a role for recombinant CD47 functionalized surfaces as a therapeutic strategy to fabricate clinically useful biomaterials we show the following: 1. Platelet and neutrophil activation and adhesion is reduced when whole blood is exposed to CD47 modified tubing. 2. Recombinant CD47 can be covalently linked to polymeric tubing used clinically for cardiopulmonary bypass (CPB). 3. CD47 functionalized surfaces can be sterilized with ethylene oxide, without reduction in anti-inflammatory properties, and are also retained following hemodynamic forces.
Strategies to fabricate a biocompatible blood conduit have largely centered on establishing a bioinert surface or appending bioactive moieties to the blood contacting surfaces. We recently shown that CD47 modified surfaces significantly reduced inflammatory cell attachment and activation, as per CD18 expression, compared to clinical blood conduits modified with poly(2-methoxyethylacrylate) (PMEA), a bioinert amphiphilic surface coating manufactured by Terumo [6]. These previous studies strongly support the role of a bioactive strategy to establish biocompatibility in clinically used blood conduits.
The newly discovered role of CD47 in platelet signaling suggests that CD47 immobilized surfaces may confer similar anti-thrombotic properties as heparin modified blood conduits. At present direct comparisons between bioactive, anti-thrombotic surfaces, such as heparin or peptide based thrombin inhibitors, and CD47 modified surfaces have not yet been made. Interestingly, previous results from studies looking at surfaces modified with the thrombin inhibitor, D-Phe-Pro-Arg-chloromethylketone (PPACK), showed that the surface modification seemed to elicit an elevated inflammatory response [3]. In contrast, our CD47 has been shown to effectively reduce the inflammatory response in in vitro, in vivo, and ex vivo conditions. In addition, despite decades of research and clinical trials, the benefits of heparin coated surfaces for clinical applications remains undecided [24,30–32]. We contend that CD47 modified surfaces, with their demonstrated ability to inhibit both inflammatory cells response and platelet activation, may confer a high level of biocompatibility for clinical applications.
4.2. CD47 Signaling in Inflammatory Cells
Our previous results largely focused on the ability of CD47 functionalized surfaces to resist inflammatory cell attachment to polymeric surfaces [6]. The present paper strongly supports the claim that the inhibitory effects of CD47 mediated signaling in inflammatory cells extend beyond the protein’s canonical function as an inhibitor of phagocytosis [8,9,33]. The finding, presented herein, that platelets express SIRPα1, strongly suggests an inhibitory role for SIRPα1 in platelet function. To that end, we show that the immune inhibitory properties of CD47 modified surfaces extend to platelets, by demonstrating that CD62P, a marker of platelet activation, was significantly reduced in blood exposed to CD47 functionalized PVC blood conduits (Figure 4). A likely mechanism for this observation is that immobilized recombinant CD47 on the PVC surface directly interacts with SIRPα1 on the platelet surface. In support of this argument, SHP-2, the signal mediator of SIRPα1 is expressed and functional in platelets [34]. Although a direct CD47-SIRPα1 signaling mechanism seems likely, several alternative mechanisms must also be considered. For example, CD47 is also a ligand for thrombospondin 1 (TSP-1), an adhesive glycoprotein involved in platelet aggregation, angiogenesis, and tumorigenesis [35–37]. As, TSP-1 CD47 interactions tend to be pro-thrombogenic and enhance platelet activation, the TSP-1 mediated inhibition of platelets is not likely [37–41]. Nevertheless, an indirect mechanism where CD47 exposure inhibits the release or expression of pro-thombogenic cytokines and/or cell surface proteins cannot be discounted from our current results. As a result of these investigations, future efforts will be made to understand CD47 signaling mechanisms in platelets.
Similar to the CD62P, Figure 5 shows that neutrophil activation, as determined by CD62L surface expression levels, was significantly reduced compared to control PVC blood conduits, when CD47 was immobilized on the PVC surface. These observations are consistent with our previous results demonstrating that blood exposed to CD47 functionalized surfaces had a significant reduction in CD18, a surface marker of neutrophil activation, expression [6]. Taken together, these data strongly suggest that SIRPα1 signaling mechanisms extend beyond simply preventing inflammatory cell binding.
4.3. Attached inflammatory cells on PVC surface
SEM analysis showed that the few platelets attached to the CD47 modified surfaces did not appear to be as activated as those attached to unmodified surfaces. However, microscopic analysis is restricted to a relatively small area of the entire blood conduit. Thus, in these studies to supplement our SEM results we used a previously reported technique [15–18] to profile the inflammatory cells (neutrophils and platelets) following the Chandler Loop run. This procedure has been used to characterize the inflammatory states of neutrophils following dialysis and cardiopulmonary bypass [15–18]. By all accounts, this technique has little impact upon neutrophil activation. However, its effect upon platelets was never examined. We show here (Figure 4C) that platelets detached, via EDTA treatment, has significantly lower levels of CD62P surface expression compared to platelets eluted from unmodified PVC surfaces. There was a slight, albeit statistically insignificant, increase in platelets detached from CD47L compared to control platelets. Similar results are seen from the detached neutrophil studies (Figure 5C). These data are consistent with the SEM results and strongly support the use of this detachment protocol as a methodology to analyze inflammatory cell attachment to blood conduits.
4.4. Lysine tagged CD47 versus Biotinylated CD47
In our previous studies, biotinylated CD47 was appended to avidin modified PVC and PU surfaces [6]. In this current work we examined and characterized a direct, covalent attachment strategy for immobilizing CD47 to polymer surfaces. The addition of ten additional lysines on the C-terminal end of the recombinant CD47 provided easily accessible, thiol-reactive groups via reacting the NH2 of the lysine residues of with the bifunctional SPDP. As shown in Figure 5, this strategy was effective in covalently linking the recombinant protein to the thiolated surface provided by the PDT-BzPh photoactivatable crosslinker. The use of poly-lysine tagged CD47 replaces the additional fabrication steps of immobilizing avidin and biotinylating recombinant CD47, thereby reducing the cost and time of the material modification.
The availability of these ten additional lysine residues was also hypothesized to preserve CD47 functionality by reducing the probability that SPDP would react with the native lysine residues within the primary structure of CD47. Direct comparison studies examining the anti-inflammatory capacity of CD47L and CD47B showed minimal differences between the two strategies. These data strongly suggest that the addition of the poly-lysine tag and the subsequent immobilization strategy had no adverse effects upon CD47 physiology. Thus, the use of CD47L provides an additional linkage strategy, where avidin usage may not be appropriate due to potential allergic reactions, to render synthetic surfaces biomimetic [13].
Irrespective of attachment strategy, we have demonstrated that the binding of recombinant CD47 to the polymeric surface is quite robust. For example, CD47 was retained following exposure to 20 dynes/cm2 shear flow. Furthermore, we tested the standard sterilization practice for PVC blood conduits, ethylene oxide gas sterilization, to evaluate the functional activity of CD47 modified surfaces before and after ethylene oxide gas sterilization. We found that the ethylene oxide gas sterilized conduits modified with recombinant CD47 perform equally as well as the non-sterilized CD47 modified conduits. Taken together, these data strongly support the claim that CD47 functionalized surfaces can function as a practical solution for addressing the hemocompatibility issues currently observed in clinically used polymeric blood conduits.
5. Conclusion
Modification of the blood-contacting surfaces of biomaterials with CD47 could be clinically beneficial by preventing neutrophil and platelet activation in vivo. These investigations have demonstrated proof of concept with a number of findings including thiol based attachment of CD47 to PVC surfaces, versus our previous report of avidin-biotin affinity linking. Immobilized CD47 retains binding and activity despite ethylene oxide sterilization and exposure to high shear flow. Most importantly, both neutrophil and platelet attachment as well as activation are inhibited with CD47 modified PVC. The likely mechanism responsible for CD47-mediated platelet effects is via the presence of SIRPα1 expression on platelets; this is reported herein for the first time.
Acknowledgments
This research was supported in part by an American Heart Association Scientist Development Grant (S.J.S.), the National Institute of Health Grants R01-HL090605 (R.J.L.), and T32-HL007915 (M.J.F. and R.J.L.) and HL090774 (J.W.W.). This research was also supported by the William J. Rashkind Endowment of The Children’s Hospital of Philadelphia. The authors would like to thank Yuri I. Veklich, Irina N. Chernysh, and Chandrasekaran Nagaswami of the Department of Cell and Developmental Biology, at the University of Pennsylvania, for their expertise and assistance with SEM.
Footnotes
Conflict of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bruck SD. Medical applications of polymeric materials. Med Prog Technol. 1982;9:1–16. [PubMed] [Google Scholar]
- 2.Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD. The role of complement in biomaterial-induced inflammation. Mol Immunol. 2007;44:82–94. doi: 10.1016/j.molimm.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Maitz MF, Sperling C, Werner C. Immobilization of the irreversible thrombin inhibitor D-Phe-Pro-Arg-chloromethylketone: a concept for hemocompatible surfaces? J Biomed Mater Res A. 2010;94:905–12. doi: 10.1002/jbm.a.32780. [DOI] [PubMed] [Google Scholar]
- 4.Sperling C, Salchert K, Streller U, Werner C. Covalently immobilized thrombomodulin inhibits coagulation and complement activation of artificial surfaces in vitro. Biomaterials. 2004;25:5101–13. doi: 10.1016/j.biomaterials.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Weber N, Wendel HP, Ziemer G. Hemocompatibility of heparin-coated surfaces and the role of selective plasma protein adsorption. Biomaterials. 2002;23:429–39. doi: 10.1016/s0142-9612(01)00122-3. [DOI] [PubMed] [Google Scholar]
- 6.Stachelek SJ, Finley MJ, Alferiev IS, Wang F, Tsai RK, Eckells EC, et al. The effect of CD47 modified polymer surfaces on inflammatory cell attachment and activation. Biomaterials. 2011;32:4317–26. doi: 10.1016/j.biomaterials.2011.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Beek EM, Cochrane F, Barclay AN, van den Berg TK. Signal regulatory proteins in the immune system. J Immunol. 2005;175:7781–7. doi: 10.4049/jimmunol.175.12.7781. [DOI] [PubMed] [Google Scholar]
- 8.Tsai RK, Discher DE. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180:989–1003. doi: 10.1083/jcb.200708043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai RK, Rodriguez PL, Discher DE. Self inhibition of phagocytosis: the affinity of ‘marker of self’ CD47 for SIRPalpha dictates potency of inhibition but only at low expression levels. Blood Cells Mol Dis. 2010;45:67–74. doi: 10.1016/j.bcmd.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark SC. Lung injury after cardiopulmonary bypass. Perfusion. 2006;21:225–8. doi: 10.1191/0267659106pf872oa. [DOI] [PubMed] [Google Scholar]
- 11.Gawaz MP, Mujais SK, Schmidt B, Blumenstein M, Gurland HJ. Platelet-leukocyte aggregates during hemodialysis: effect of membrane type. Artif Organs. 1999;23:29–36. doi: 10.1046/j.1525-1594.1999.06289.x. [DOI] [PubMed] [Google Scholar]
- 12.Gorman JH, 3rd, Edmunds LH., Jr Blood anesthesia for cardiopulmonary bypass. J Card Surg. 1995;10:270–9. doi: 10.1111/j.1540-8191.1995.tb00609.x. [DOI] [PubMed] [Google Scholar]
- 13.Scott D, Nitecki DE, Kindler H, Goodman JW. Immunogenicity of biotinylated hapten-avidin complexes. Mol Immunol. 1984;21:1055–60. doi: 10.1016/0161-5890(84)90115-9. [DOI] [PubMed] [Google Scholar]
- 14.Stachelek SJ, Alferiev I, Connolly JM, Sacks M, Hebbel RP, Bianco R, et al. Cholesterol-modified polyurethane valve cusps demonstrate blood outgrowth endothelial cell adhesion post-seeding in vitro and in vivo. Ann Thorac Surg. 2006;81:47–55. doi: 10.1016/j.athoracsur.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 15.Tabuchi N, Shibamiya A, Koyama T, Fukuda T, Oeveren WvW, Sunamori M. Activated leukocytes adsorbed on the surface of an extracorporeal circuit. Artif Organs. 2003;27:591–4. doi: 10.1046/j.1525-1594.2003.07050.x. [DOI] [PubMed] [Google Scholar]
- 16.Grooteman MP, Bos JC, van Houte AJ, van Limbeek J, Schoorl M, Nube MJ. Mechanisms of intra-dialyser granulocyte activation: a sequential dialyser elution study. Nephrol Dial Transplant. 1997;12:492–9. doi: 10.1093/ndt/12.3.492. [DOI] [PubMed] [Google Scholar]
- 17.Grooteman MP, Nube MJ, Bos JC, van Limbeek J, Schoorl M, van Houte AJ. Ex vivo elution of hemodialyzers. An additional criterion for the assessment of bioincompatibility. Blood Purif. 1996;14:421–30. doi: 10.1159/000170295. [DOI] [PubMed] [Google Scholar]
- 18.Grooteman MP, Nube MJ, van Limbeek J, Schoorl M, van Houte AJ. Lymphocyte subsets in dialyser eluates: a new parameter of bioincompatibility? Nephrol Dial Transplant. 1996;11:1073–8. [PubMed] [Google Scholar]
- 19.Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, et al. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol. 2011;57:1359–67. doi: 10.1016/j.jacc.2010.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisel JW, Nagaswami C. Computer modeling of fibrin polymerization kinetics correlated with electron microscope and turbidity observations: clot structure and assembly are kinetically controlled. Biophys J. 1992;63:111–28. doi: 10.1016/S0006-3495(92)81594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsson M, Bruhns P, Frazier WA, Ravetch JV, Oldenborg PA. Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood. 2005;105:3577–82. doi: 10.1182/blood-2004-08-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasirer-Friede A, Kahn ML, Shattil SJ. Platelet integrins and immunoreceptors. Immunol Rev. 2007;218:247–64. doi: 10.1111/j.1600-065X.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 23.Edmunds LH., Jr Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 1998;66:S12–6. doi: 10.1016/s0003-4975(98)00967-9. discussion S25–8. [DOI] [PubMed] [Google Scholar]
- 24.Groom RC. Pediatric cardiopulmonary bypass devices: trends in device use for cardiopulmonary bypass and postcardiotomy support. ASAIO J. 2005;51:525–9. doi: 10.1097/01.mat.0000180399.24938.a5. [DOI] [PubMed] [Google Scholar]
- 25.Pintar T, Collard CD. The systemic inflammatory response to cardiopulmonary bypass. Anesthesiol Clin North America. 2003;21:453–64. doi: 10.1016/s0889-8537(03)00039-7. [DOI] [PubMed] [Google Scholar]
- 26.Draaisma AM, Hazekamp MG. Coated versus noncoated circuits in pediatric cardiopulmonary bypass. ASAIO J. 2005;51:663–4. doi: 10.1097/01.mat.0000176954.24275.2e. [DOI] [PubMed] [Google Scholar]
- 27.Eisses MJ, Geiduschek JM, Jonmarker C, Cohen GA, Chandler WL. Effect of polymer coating (poly 2-methoxyethylacrylate) of the oxygenator on hemostatic markers during cardiopulmonary bypass in children. J Cardiothorac Vasc Anesth. 2007;21:28–34. doi: 10.1053/j.jvca.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Hill GE. The inflammatory response to cardiopulmonary bypass. Int Anesthesiol Clin. 1996;34:95–108. doi: 10.1097/00004311-199603420-00009. [DOI] [PubMed] [Google Scholar]
- 29.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715–20. doi: 10.1016/s0003-4975(02)04701-x. [DOI] [PubMed] [Google Scholar]
- 30.Harig F, Meier C, Hakami L, Strasser R, Bretzger J, Munch F, et al. Does the additional use of heparin-coated extracorporeal circuits (ECC) optimize the effect of modified ultrafiltration (MUF) in pediatric perfusion? Thorac Cardiovasc Surg. 2006;54:168–72. doi: 10.1055/s-2005-872863. [DOI] [PubMed] [Google Scholar]
- 31.Losel-Sadee H, Alefelder C. Heparin-bonded expanded polytetrafluoroethylene graft for infragenicular bypass: five-year results. J Cardiovasc Surg (Torino) 2009;50:339–43. [PubMed] [Google Scholar]
- 32.Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21:232–44. doi: 10.1016/s1010-7940(01)01099-5. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian S, Parthasarathy R, Sen S, Boder ET, Discher DE. Species- and cell type-specific interactions between CD47 and human SIRPalpha. Blood. 2006;107:2548–56. doi: 10.1182/blood-2005-04-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamao T, Noguchi T, Takeuchi O, Nishiyama U, Morita H, Hagiwara T, et al. Negative regulation of platelet clearance and of the macrophage phagocytic response by the transmembrane glycoprotein SHPS-1. J Biol Chem. 2002;277:39833–9. doi: 10.1074/jbc.M203287200. [DOI] [PubMed] [Google Scholar]
- 35.Isenberg JS, Roberts DD, Frazier WA. CD47: a new target in cardiovascular therapy. Arterioscler Thromb Vasc Biol. 2008;28:615–21. doi: 10.1161/ATVBAHA.107.158154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–5. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 37.Kaczorowski DJ, Billiar TR. Targeting CD47: NO limit on therapeutic potential. Circ Res. 2007;100:602–3. doi: 10.1161/01.RES.0000261609.44977.25. [DOI] [PubMed] [Google Scholar]
- 38.Chung J, Gao AG, Frazier WA. Thrombspondin acts via integrin-associated protein to activate the platelet integrin alphaIIbbeta3. J Biol Chem. 1997;272:14740–6. doi: 10.1074/jbc.272.23.14740. [DOI] [PubMed] [Google Scholar]
- 39.Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, et al. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood. 2008;111:613–23. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isenberg JS, Shiva S, Gladwin M. Thrombospondin-1-CD47 blockade and exogenous nitrite enhance ischemic tissue survival, blood flow and angiogenesis via coupled NO-cGMP pathway activation. Nitric Oxide. 2009;21:52–62. doi: 10.1016/j.niox.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD. Thrombospondin-1/CD47 blockade following ischemia-reperfusion injury is tissue protective. Plast Reconstr Surg. 2009;124:1880–9. doi: 10.1097/PRS.0b013e3181bceec3. [DOI] [PMC free article] [PubMed] [Google Scholar]