Abstract
Alveolar macrophages (AMs) are the first immune cells to respond to an invading pathogen and coordinate the inflammatory response within the lungs. Studies suggest that macrophages exhibit age-related deficiencies in Toll-like receptor (TLR) function; however, the impact of this dysfunction during pneumonia, the leading cause of infectious death in the elderly, and the underlying mechanisms responsible remain unclear. We examined disease severity in young, mature, and aged BALB/cBy mice following intratracheal infection with the Gram-positive bacteria Streptococcus pneumoniae (Spn). Both mature and aged mice failed to clear bacteria and as a result had increased mortality, tissue damage and vascular leakage. Early production of TNFα, IL-1β, and IL-6 during pneumonia declined with age and was associated with an inability of isolated AMs to respond to pneumococcal cell wall (CW) and ethanol-killed Spn ex vivo. Total levels of TLR1 were unaffected by age and TLR2 surface expression was slightly yet significantly increased on aged AMs suggesting that intracellular TLR signaling defects were responsible for the age-related decline in cytokine responsiveness. Following infection of isolated AMs with live Spn, a significant age-related decline in TLR2-induced phosphorylation of p65 NFκB, JNK and p38 MAPK, and an increase in ERK phosphorylation was observed by immunoblotting. These data are the first to demonstrate that TLR2-dependent recognition of Spn by aged AMs is impaired and is associated with a delayed pro-inflammatory cytokine response in vivo along with enhanced susceptibility to pneumococcal pneumonia.
Keywords: Aging, pneumonia, macrophages, alveolar macrophages, Streptococcus pneumoniae, Toll-like receptor, Cell signaling, Tumor necrosis factor α, Interleukin-6, MAPK, p65, p38, JNK, ERK
1. INTRODUCTION
Streptococcus pneumoniae (S. pneumoniae) is the leading cause of community-acquired pneumonia (CAP) and a leading cause of bacteremia and meningitis for those greater than 65 years of age. Worldwide, the mortality-rate associated with CAP exceeds 20% but may be as high as 40% for those within nursing homes (Loeb 2004; Maruyama et al., 2010). Routine vaccination of the elderly with Pneumovax® 23 (Merck & Co. Inc.) has reduced mortality rates related to invasive pneumococcal diseases such as bacteremia and meningitis but has had little effect on the incidence of pneumonia (French et al., 2000; Jackson et al., 2003; Johnson et al., 2000; Whitney et al., 2003). Healthy immunocompetent adults rarely succumb to pneumococcal diseases indicating that age-related changes in the immune system contribute to their enhanced susceptibility for life-threatening infections. Numerous reviews detail the functional decline of both the innate and adaptive immune response with age (Boyd and Orihuela 2011; Mahbub et al., 2011; Weksler and Szabo 2000; Weng 2006). Nonetheless, little is known regarding how aging impacts the innate immune response within the lungs, in particular, during pneumonia.
During an infection Toll-like receptors (TLRs) activate the innate immune response by recognizing conserved microbial components known as pathogen-associated molecular patterns (PAMPs). Binding of a TLR to its cognate PAMP initiates an intracellular signaling cascade through cytoplasmic intermediates including Myd88, IRAK-4 and IRAK-1, and TRAF6 that ultimately result in Nuclear Factor Kappa B (NFκB) and Mitogen-activated Protein Kinase (MAPK) activation which enhances the transcription and translation of cytokines, chemokines, and antimicrobial peptides (Akira and Sato 2003; Takeda et al., 2003). Studies have shown that heterodimers of TLR1/2 and homodimers of TLR4 and TLR9 recognize pneumococcal PAMPs, however, only TLRs 1/2 and 4 play a role in the early inflammatory response to S. pneumoniae (Albiger et al., 2007; Knapp et al., 2004; Malley et al., 2003). Briefly, TLR1/2 recognizes triacylated lipopeptides such as lipoteichoic acid found in the pneumococcal cell wall (Schroder et al., 2003; Yoshimura et al., 1999). TLR4 recognizes the pneumococcal toxin pneumolysin as well as Gram-negative bacterial lipopolysaccharide (LPS) (Malley et al., 2003; Srivastava et al., 2005). TLR9 recognizes unmethylated CpG motifs in bacterial and viral DNA (Barber 2011). While TLR2 also heterodimerizes with TLR6 to recognize diacylated lipoproteins (Schenk et al., 2009), to date no published studies have shown that TLR6 plays a role in the immune response to S. pneumoniae. Intriguingly, TLR2-deficient young mice exhibit a delayed inflammatory response but are no more susceptible to pneumococcal pneumonia than WT mice (Echchannaoui et al., 2002; Knapp et al., 2008; Knapp et al., 2004). Likewise, young TLR4-deficient mice are only modestly susceptible to pneumococcal pneumonia and meningitis (Benton et al., 1997; Branger et al., 2004; Klein et al., 2008). Nonetheless, Myd88-deficient mice were exquisitely susceptible to pneumococcal pneumonia suggesting that individual TLRs have redundant roles during infection and that TLR/IL-1R signaling is absolutely required for surviving pneumococcal infections (De Nardo et al., 2009). The latter notion is supported by the high incidence of invasive pneumococcal disease and associated mortality for human children with IRAK-4 deficiencies (Ku et al., 2007).
Resident alveolar macrophages (AMs) are the first immune cells to encounter an aspirated infectious microorganism and play an important role in the recruitment of immune cells and coordination of the inflammatory response within the lungs (Arredouani et al., 2004). Importantly, assorted ex vivo studies indicate that murine splenic and peritoneal macrophages as well as human peripheral monocytes exhibit a decline in the production of the pro-inflammatory cytokines TNFα, IL-6, and IL-12 when stimulated with purified and specific TLR agonists (Boehmer et al., 2004; Boehmer et al., 2005; Chelvarajan et al., 2006; Fallah et al., 2011; Mahbub et al., 2012; Renshaw et al., 2002; van Duin et al., 2007). However, these studies have yielded contrasting results as to the molecular mechanism(s) responsible for this age-related defect. While some investigators have shown decreased TLR mRNA expression and/or reduced TLR surface expression (Chelvarajan et al., 2006; Renshaw et al., 2002; van Duin et al., 2007), other investigators observed no changes in TLR surface expression and instead detected reduced expression and phosphorylation of the MAPK JNK and p38 following stimulation with LPS (Boehmer et al., 2004; Boehmer et al., 2005). Most recently, decreased macrophage pro-inflammatory cytokine production with age has been attributed to increased phosphatidyl inositol 3-kinase (PI3K)-Akt signaling pathway activity (Fallah et al., 2011). This pathway activates glycogen synthase kinase-3 (GSK3) which can suppress inflammation through inhibition of NFκB and MAPK and increases production of anti-inflammatory IL-10 (Wang et al., 2011). Therefore, to date no clear consensus exists regarding the mechanism(s) responsible for reduced responsiveness to TLR stimulation by macrophages with increased age. Importantly, whether TLR dysfunction occurs in alveolar macrophages, the first immune cells to contact aspirated microorganisms in the lungs, has not yet been examined, although pneumonia is the leading cause of infectious death in the elderly (Gorina and Lentzer 2008).
Herein, we report that the early production of TNFα and IL-6 is reduced during pneumococcal pneumonia in mature and aged mice and is associated with increased severity of disease and an age-related inability of AMs to respond to Gram-positive cell wall components. We demonstrate that this decline was TLR2-dependent and associated with decreased NFκB activation and reduced JNK and p38 phosphorylation, but with elevated ERK phosphorylation; findings that support the notion of common upstream inhibition through GSK3. This study is the first to describe an age-related decline in TLR-induced cell signaling for alveolar macrophages and in response to a Gram-positive pathogen. It therefore sheds light on the full impact and mechanisms of age-associated dysregulated macrophage function on susceptibility to pneumococcal CAP, the leading cause of infectious disease in the elderly.
2. METHODS
2.1 Mice
Young (4–5 months), mature (10–12 months) and aged (19–21 months) female Balb/cBy mice were purchased from the National Institute on Aging Aged Rodent Colony at Harlan Sprague Dawley, Inc. (Indianapolis, IN). Male C57Bl/6 TLR2 knockouts (KO) 2 months of age were obtained through a materials transfer agreement with Dr. Shizou Akira, Osaka University, Japan. Wildtype (WT) C57Bl/6 mice were bred at The University of Texas Health Science Center at San Antonio under Specific Pathogen Free conditions. All experiments were performed in compliance with approved Institutional Animal Care and Use Committee protocols at the University of Texas Health Science Center at San Antonio.
2.2 Mouse infection studies
S. pneumoniae serotype 4, strain TIGR4 was grown in Todd Hewitt Broth (Sigma, St. Louis MO) at 37° C in 5% CO2 (Tettelin et al., 2001). At mid-logarithmic phase (OD620 = 0.5; corresponds to 1.0 × 108 CFU/ml), pneumococci were centrifuged and suspended in phosphate buffered saline (PBS). Animals were lightly anesthetized using vaporized 2.5% isoflurane and 100 µl containing 1.0 × 105–7 CFU in PBS was delivered intranasally or intratracheally by forced inhalation (Hinojosa et al., 2009). In all instances the infectious dose delivered was confirmed by extrapolation of the infectious dose from colony counts the next day. At designated times mice were sacrificed and bacterial burden in the lungs and blood determined; bacterial titers in the lungs was assessed per gram of homogenized tissue. Excised lungs were paraffin-embedded and 5 µm sections stained with Hematoxylin and Eosin (H&E). Tissue sections were scored for pathologic changes in a blind manner based on lung consolidation and lymphocyte infiltration. Collection of bronchoalveolar lavage fluid (BALF) was performed by surgical visualization of the trachea, insertion of a 0.18 gauge angiocatheter (BD Biosciences), and repeated flushing of the lungs with 0.5 ml ice-cold PBS until a total volume of 3 ml was obtained. Total cells counts were done using a hemocytometer. Differential cell counts were determined by cytospin analysis of BALF cells.
Determination of vascular leakage was done by measurement of albumin in the BALF by ELISA (Bethyl Laboratories, Inc., Montgomery, TX).
2.3 Cytokine analysis
Determination of mouse TNFα, IL-1β, IL-6 and IL-10 concentrations in lung homogenates or cell culture supernatants was performed using BD OptEIA Mouse ELISA kits (BD Biosciences, San Diego, CA). ELISAs were performed following the manufacturer’s instructions.
2.4 Isolation and ex vivo stimulation of alveolar macrophages
Mice were asphyxiated using isoflurane and bronchoalveolar lavage performed. BALF was passed through a 40 µM cell strainer to remove debris, centrifuged, and the pelleted cells suspended in ice-cold PBS containing 0.5% fetal bovine serum (FBS). Those with macrophage morphology were counted using a hemocytometer. Cells were suspended in DMEM with 10% FBS and 1% penicillin/streptomycin and seeded in a 96-well tissue culture plate at 105 cells per well. Following 1 hour of adherence at 37° C in 5% CO2, non-adherent cells were removed by gently washing and the remaining AMs were allowed to rest overnight.
AMs were stimulated with medium containing either ethanol-killed S. pneumoniae equivalent to 1.0 × 106 CFU/ml, recombinant pneumolysin at 0.25 µg/ml, 1.0 × 106 CFU equivalents of purified pneumococcal cell wall, or exposed to medium alone for 16 hours. Pneumolysin was a gift from Tim Mitchell (University of Glasgow, United Kingdom) and cell wall was purified as previously described (Tuomanen et al., 1986); both were confirmed to be endotoxin free using a Limulus Amebocyte test (Associates of Cape Cod Inc., Falmouth, MA). When indicated, AMs were pretreated with either 10 µM of the p38 MAPK inhibitor SB203580 in DMSO (Cell Signaling Technology), an equivalent amount of DMSO, 10 µg/ml of TLR2 blocking antibody (clone TL2.1) (eBioScience, San Diego CA), or an isotype IgG1 control antibody (clone, MOPC-21) (eBioScience) for 1 hour prior to stimulation. Supernatants were collected and stored at −20° C for cytokine analysis.
2.5 Flow Cytometric Analysis
Cell suspensions from BALF were washed in ice-cold FACS staining buffer and incubated for 15 minutes with Fc Block (eBioscience). Cells were incubated with monoclonal antibody against F4/80-APC (clone, BM8), TLR1-PE (clone, TR23), TLR2-PE (clone, T2.5), both, or the corresponding isotype-matched control antibodies (eBioscience) for surface staining. Resting AMs have been phenotypically described as CD11c+, CD11b−, F4/80+ and MHCII low (Kirby et al., 2009). For this reason we used the macrophage specific marker F4/80 and excluded dendritic cells that are CD11c+ and neutrophils that are CD11b+. F4/80 positive cells were gated and analyzed for TLR2 and TLR1 surface expression. A total of 50,000 cells were analyzed on an LSRII instrument (BD Biosciences). Data analysis was performed using FlowJo software (Tree Star, Ashland, OR).
2.6 Immunoblotting
AMs were isolated and plated at 1.0 × 105/96-well as described above. AMs were infected with 1.0 × 106 CFU of S. pneumoniae for 15 minutes or media alone. Following stimulation, cells were lysed in RIPA buffer (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, 0.01 M NaH2PO4) containing 1% (vol/vol) protease inhibitor cocktail and 1% (vol/vol) phosphatase inhibitor cocktail (Sigma). Total protein concentration was determined by bicinchoninic acid protein assay. For each sample, 2–4 µg of protein was boiled in SDS-sample buffer for 10 minutes followed by brief centrifugation. Samples were loaded onto 10% SDS polyacrylamide gels, separated, and then transferred to polyvinylidene diflouride membranes using standard methods. Immunoblot analysis was performed using commercially available monoclonal antibodies for p65, phosphorylated (p)-p65, p38 MAPK, p-p38 MAPK, JNK, p-JNK, ERK, p-ERK (all from Cell Signaling), and rabbit polyclonal anti-TRAF6 (Santa Cruz Biotechnology). Detection was performed using goat anti-rabbit horse-radish peroxidase-conjugated secondary antibodies and Super-Signal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL). Relative protein levels were determined by comparative densitometry analysis of Western blot bands using a Molecular Imager Gel Doc XR System (BioRad, Hercules, CA). To ensure that equal amounts of protein had been probed, membranes were stripped and the amount of actin determined using rabbit anti-actin antibodies (Bethyl laboratories, Inc., Montgomery, TX).
2.8 Repeat measures and statistical analysis
For each experimental cohort biological samples were collected on at least 2 different days. All data are expressed as mean ± SEM. For survival analyses a Kaplan-Meier Log Rank Survival Test was used. For comparisons between aged cohorts statistical analysis was performed using a One-way ANOVA or two-tailed Student’s t test as indicated. P values < 0.05 were considered significant and indicated by a single asterisk; P < 0.001 is indicated by double asterisks.
3. RESULTS
3.1 Susceptibility to Pneumococcal Infection Increases Gradually with Age
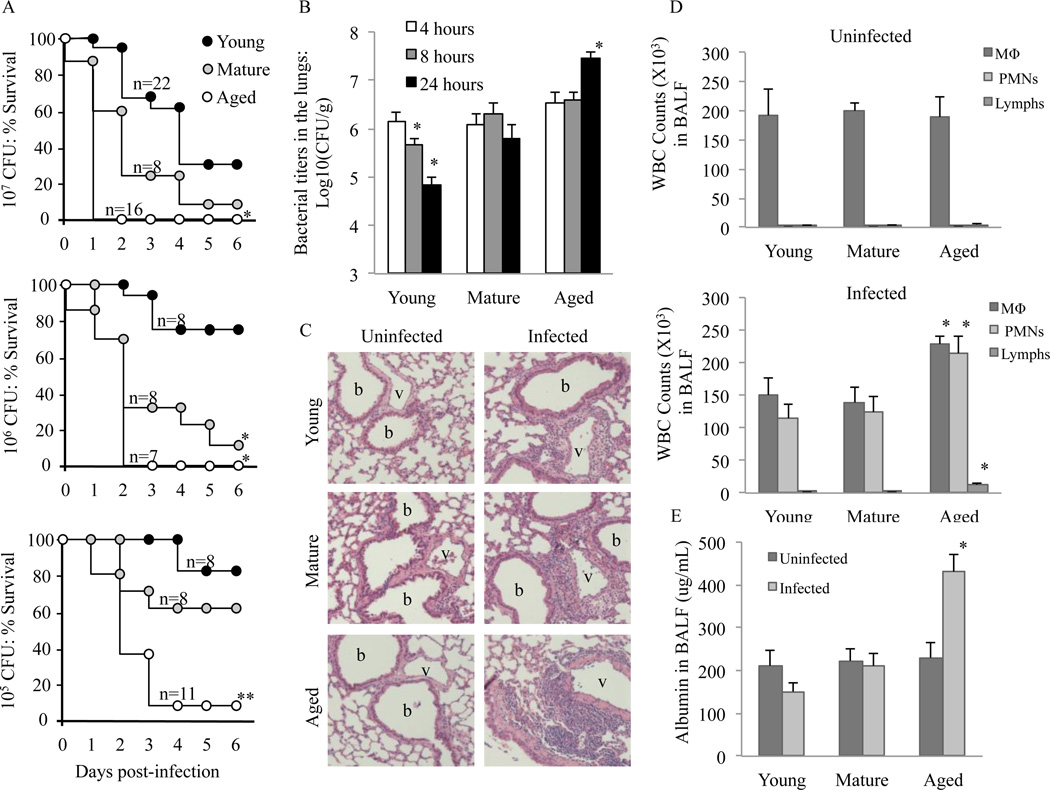
Similar to elderly humans, aged Balb/cBy mice are highly susceptible to infection with S. pneumoniae (Hinojosa et al., 2009). To ascertain whether susceptibility increased gradually or had a sudden onset with advanced age, we examined survival of young, mature, and aged mice following intranasal challenge with 107, 106, and 105 CFU of S. pneumoniae (Figure 1A). Aged mice experienced higher mortality rates than mature and young mice at each infectious dose. Mature mice had intermediate susceptibility whereas young mice were the most resistant. In contrast to aged mice, which had mortality rates >90%, for mature and young mice only the cohorts infected with >106 and >107 CFU, respectively, had mortality rates that exceeded 50%. In agreement with the latter, young mice infected intratracheally with S. pneumoniae were able to reduce bacterial titers in their lungs from 4 to 24 hours post-infection. In contrast, infected mature mice maintained a steady number of bacteria and aged mice experienced an increase in bacterial load. After 24 hours infected mature mice and aged mice had 10 (P=0.01) and 400-fold (P<0.01) more bacteria in their lungs, respectively, than young mice (Fig. 1B).
FIGURE 1.
Increased severity of pneumococcal pneumonia with advanced age. A) Survival of young, mature, and aged Balb/cBy mice following intranasal infection with increasing doses (105, 106 or 107 CFU) of S. pneumoniae. Statistical significance was determined using a Kaplan-Meier Log Rank Survival Test. B) Median bacterial titers in the lungs of young (n=6–8), mature (n=5) and aged (n=6) mice at 4, 8 and 24 hours after intratracheal infection. C) Representative H&E lung sections from uninfected and infected young (n=4), mature (n=4) and aged (n=4) mice 12 hours after intratracheal infection. Original magnification, 400× D) Differential cell counts of inflammatory cells in BALF of uninfected mice (n =3 per cohort) and 24 hours after infection (n=6 per cohort) as determined by cytospin analysis. E) In the same animals, vascular permeability was assessed by determining albumin protein levels in isolated BALF. Data are presented as the mean ± SEM. Statistical comparisons were performed against the values for the young cohort. P<0.05 were considered significant. A single asterisk denotes P< 0.05.
Given the dramatic differences in bacteria burden and survival, we examined the degree of acute lung injury that occurred during pneumonia; as early as 12 hours following intratracheal infection with 105 CFU, lung sections from aged mice showed worsened lung consolidation with increased perivascular and peribronchial inflammatory cell infiltrates compared to young and mature mice (Figure 1C). Differential cell counts of cells in the BALF at 24 hours confirmed a significant increase in monocytes (P=0.04), neutrophils (P=0.02) and lymphocytes (P<0.01) in aged mice compared to young (Figure 1D). No significant differences in cell counts were observed between the young and mature cohorts. Finally, aged, but not mature mice, had significantly increased vascular leakage compared to young mice as assessed by the amount of albumin present within collected BALF (P<0.01) (Figure 1E). Thus pneumococcal disease in mature and aged mice was more severe and for aged mice characterized by an inability to restrain bacterial replication and limit vascular leakage within the lungs.
3.2 Aging is associated with a gradual decline in the production of pro-inflammatory cytokines during early pneumococcal pneumonia
We have previously shown that aged mice produce significantly less IL-6 and TNFα following intratracheal challenge of purified pneumococcal cell wall (CW) and pneumolysin when compared to young mice (Hinojosa et al., 2009). To extend our original findings with CW to include a mature cohort we also challenged young, mature, and aged mice with CW and determined the cytokine response (Fig. 2A). Six hours post-challenge with CW, aged mice had a strong trend towards reduced production of TNFα (P =0.06) and significantly less IL-6 (P=0.03) and IL-1β (P<0.01) in whole lung homogenates. Mature mice had an intermediate capacity to respond with a trend towards reduced IL-6 (P=0.16) and IL-1β (P=0.09) production. To determine whether this age-related deficiency also occurred during live infection, a previously unexplored topic, we examined the cytokine response in lung homogenates 4 hours post-intratracheal infection with viable S. pneumoniae (Fig. 2B). We chose 4 hours to limit any contribution by neutrophil-derived cytokines and because in aged mice bacterial numbers eventually increased whereas young mice after 4 hours had begun to clear the infection (Fig. 1B). Thus, we examined the impact of age on the resident lung cell cytokine response to an equivalent live bacterial load. Following intratracheal challenge with 106 CFU, no differences in TNFα production were apparent between age groups, whereas for IL-6 and IL-1β production mature (IL-6 P=0.02; IL-1β P<0.01) and aged (IL-6 P=0.04; IL-1β P<0.01) mice had significantly less cytokines compared to young mice. Thus, we observed a step-wise age-related decline in the early production of IL-6 and IL-1β but not TNFα in response to Gram-positive cell wall and during pneumococcal pneumonia.
FIGURE 2.
Aged mice produce less pro-inflammatory cytokines during early pneumococcal pneumonia. A) Levels of TNFα, IL-6 and IL-1β were determined in young, mature, and aged mice 6 hours following intratracheal challenge of mice with 107 equivalents of pneumococcal cell wall (CW) (n=5/cohort). B) Levels of the same cytokines 4 hours after intratracheal challenge with 106 CFU S. pneumoniae (Spn; n=4–6/cohort). For all samples cytokine levels in whole lung homogenates were determined by ELISA. Data are presented as the mean ± SEM. Statistical analyses were performed using a One-way ANOVA. Asterisks indicate a statistically significant difference (p <0.05) versus the young cohort.
Importantly, we did not detect IL-10 in any of these lung samples (data not shown). This observation was consistent with a separate analysis for IL-10 using lung samples from mice infected with S. pneumoniae for 24 hours. Despite bacterial titers exceeding 107 CFU/gram, aged mice (n=5) had only 23.4 ± 6.3 SEM pg/mg of IL-10 in their lungs. Young mice (n=5) had 9.7 ± 5.0 SEM pg/mg of IL-10 with lung bacterial titers at 104–5 CFU/gram. Thus, physiologically relevant levels of IL-10 were not present in the lungs during early or acute pneumonia. Of note, we detected IL-10 in the blood of the 24-hour infected mice (Young IL-10: 315 ± 44 SEM pg/mg; Aged IL-10 = 1768 ± 822 SEM), thereby indicating that a distinct cytokine response was occurring in the lungs versus the periphery.
3.3 AMs from mature and aged mice are impaired in their production of TNFα and IL-6 when challenged with S. pneumoniae products
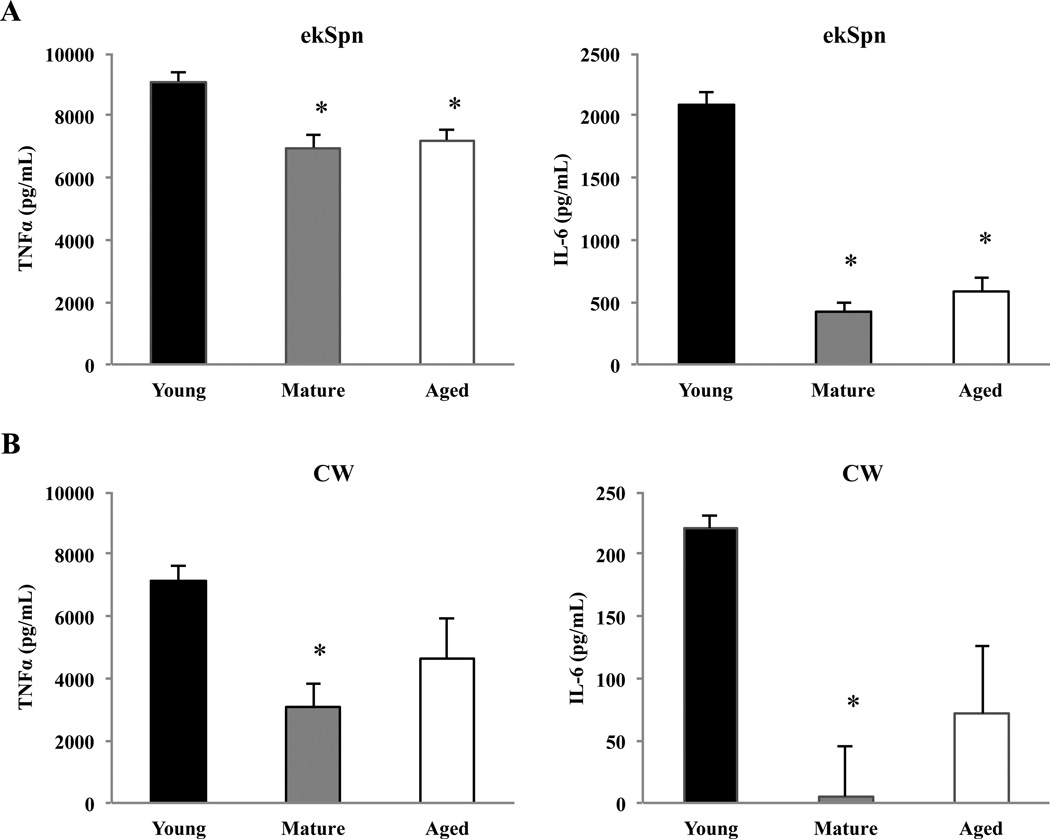
AMs are the most abundant immune cell within healthy lungs and are thought to be the primary producers of TNFα and a significant source of IL-6 during early infection (Xu et al., 2008). To determine whether AMs were responsible for the diminished IL-6 response observed for S. pneumoniae, AMs were isolated from BALF of young, mature, and aged mice and stimulated for 16 hours ex vivo with a sublytic dose of pneumolysin, pneumococcal cell wall (CW), or ethanol-fixed pneumococci (ekSpn). The culture supernatants were subsequently collected and analyzed by ELISA for cytokine production. Ex vivo stimulation of AMs with pneumolysin failed to elicit an IL-6 or TNFα response in all age groups examined (data not shown). Greater amounts of pneumolysin were not used due to its potent cytotoxic effects that would be confounding due to the release of Damage-associated Molecular Pattern Molecules that also trigger cytokine production (Hirst et al., 2004). Conversely, ekSpn elicited a robust TNFα response in AMs from young mice that was slightly but significantly diminished in AMs from mature (P<0.01) and aged animals (P<0.01) (Fig. 3A). In particular, a dramatic age-related reduction in the production of IL-6 was observed; AMs from mature (P<0.01) and aged mice (P<0.01) produced approximately 25% the IL-6 levels of AMs from young mice (Fig. 3A). AMs exposed to CW had a similar muted response with AMs from mature animals producing less TNFα and IL-6 compared to those from young. Aged AM trended towards reduced TNFα (P=0.08) and IL-6 (P=0.07) (Fig. 3B). Thus, isolated AMs from mature and aged mice were impaired in their ability to produce TNFα and IL-6 in response to S. pneumoniae products and presumably were partly responsible for the muted cytokine response observed in vivo. Of note, unlike in vivo experiments (Fig 2), IL-1β levels for all samples were below the level of detection (data not shown). This is most likely because IL-1β secretion requires Caspase-1 cleavage and purified cell wall and ekSpn would not activate the inflammasome (van de Veerdonk et al., 2011).
FIGURE 3.
Alveolar macrophages from mature and aged mouse lungs produce less TNFα and IL-6 ex vivo. AMs were isolated from young (n=5), mature (n=5) and aged (n=5) mice and challenged with A) ethanol-killed pneumococci (ekSpn;106 CFU) or B) pneumococcal cell wall (CW; 106 CFU equivalents) for 16 hours and levels of TNFα and IL-6 were determined by ELISA. Data are presented as the mean ± SEM. Statistical analyses were performed using a One-way ANOVA. Asterisks indicate a statistically significant difference (p <0.05) versus the young cohort.
3.4 The muted cytokine response of AMs is not due to reduced TLR2 surface expression
Studies have reported reduced TLR1 and TLR4 surface expression on peripheral monocytes and splenic macrophages as one mechanism responsible for the age-dependent decrease in cytokine response to purified PAMPs (Renshaw et al., 2002; van Duin et al., 2007). Thus a reduction in TLR2 or TLR1 surface levels, which together detect triacylated lipopeptides present in S. pneumoniae, could account for the observed muted cytokine response by AMs. To verify that the observed cytokine response by AMs was TLR2-dependent, isolated AMs from young mice were treated with a TLR2 blocking antibody or an isotype-matched control antibody prior to stimulation with CW or ekSpn. We determined that both TNFα and IL-6 production following stimulation with pneumococcal components were entirely TLR2 dependent (Fig. 4A). Similar results were obtained after stimulating AMs from C57BL/6 TLR2 KO and WT controls (Supplemental Figure 1).
FIGURE 4.
Alveolar macrophages from aged mice exhibit a modest increase in TLR2 surface levels. A) Balb/c AM from young (n=3), mature (n=3) and aged (n=3) mice were pretreated with blocking antibody for TLR2 or an isotype-matched control antibody and stimulated for 16 hours with ethanol-killed pneumococci (ekSpn) or purified cell wall (CW). B–D) F4/80 positive alveolar macrophages were gated and analyzed for TLR1 and TLR2 surface expression by flow cytometry. B) Representative histogram showing TLR2 surface expression on AMs from young, mature and aged mice compared to isotype-matched control antibody staining. C) TLR2 mean fluorescence intensity values for each mouse analyzed. D) A representative histogram of TLR1 AM surface expression from young, mature and aged mice compared to isotype-matched control antibody staining. E) Uninfected AMs from young, mature and aged mice (n=6/group) were analyzed for total levels of TLR1 by Western blot. Young and mature samples were run on the same gel, while aged samples were run on a separate gel. Western blots were developed at the same time for the same length of time. The same membranes were stripped and reprobed for actin (n=6 per age group). Statistical comparisons were performed against the values for the young cohort. Asterisks indicate a statistically significant difference (p <0.05) versus the young cohort using a two-tailed Student’s t-test.
We next isolated total cells in the BALF from young, mature and aged mice and determined the relative surface expression of TLR2 using flow cytometry. Importantly, the percentage of the total isolated cells that stained positive for TLR2 did not significantly change with age (data not shown). Using the macrophage specific marker F4/80 to gate AMs, we determined that AMs from aged mice had slightly increased surface expression of TLR2 compared to those isolated from both young (P=0.05) and mature mice (P<0.01) (Fig. 4B, C). We also sought to determine whether the expression of TLR1 changed with age. Following several attempts using different antibodies we were unable to detect TLR1 on the surface of unstimulated AMs in any age group by flow cytometry (Fig. 4D), however Western blot analysis of AM whole cell lysate determined that total levels of TLR1 do not change with age (Fig. 4E). Taken together these data indicate that the reduced capacity of AMs to respond to TLR1/2-specific pneumococcal PAMPs (i.e. triacylated lipoteichoic acid) was not the result of a reduction in TLR1 total levels or TLR2 surface expression and instead that altered intracellular signaling following TLR engagement might be responsible.
3.5 Phosphorylation of the p65, JNK and p38 MAPK are decreased in aged AMs following infection with S. pneumoniae
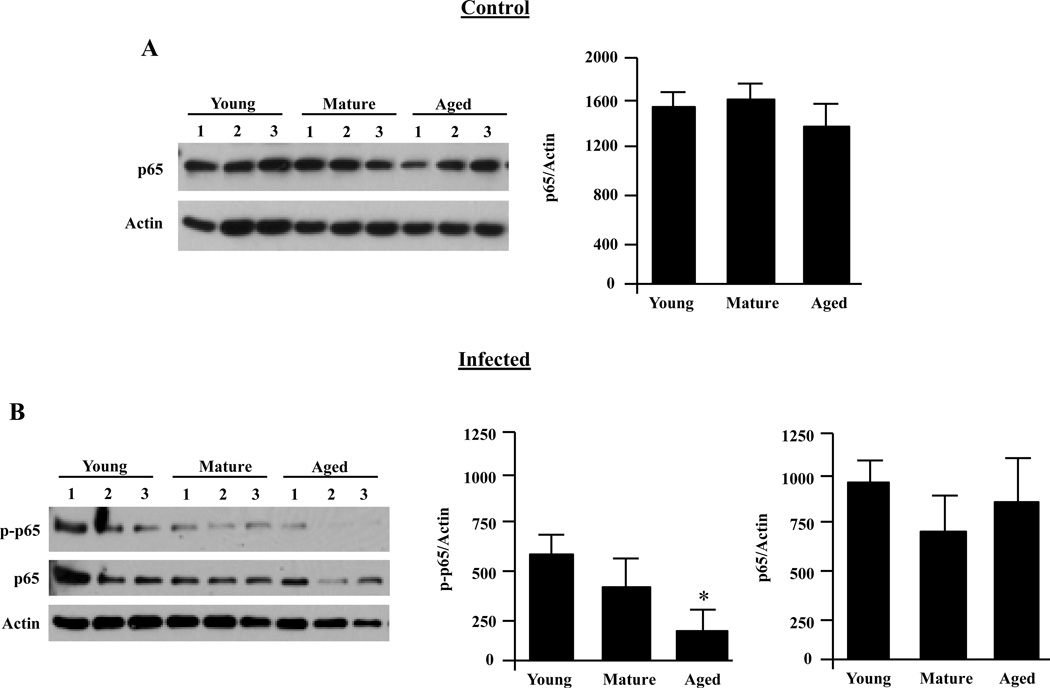
To determine whether the reduced production of IL-6 by AMs was associated with diminished NFκB activation, we examined total p65 levels prior to infection and levels of phosphorylated (p)-p65 following a 15-minute infection with live S. pneumoniae using Western blot analysis. Total protein levels of p65 in uninfected AMs did not change with age (Fig. 5A) or following infection (Fig 5B). In contrast, AMs from aged mice had significantly reduced levels of p-p65 post-infection compared with AMs from young mice (P=0.01) (Fig. 5B). AMs from mature mice exhibited a strong trend towards reduced phosphorylation (P=0.08).
FIGURE 5.
Alveolar macrophage NFκB activation decreases with age following infection. AMs were isolated from young, mature and aged mice and stimulated with S. pneumoniae at an MOI of 10 for 15 minutes or with media alone. A) Representative Western blot and corresponding densitometric analysis for p65 total protein levels in unstimulated AMs showing that total levels of p65 do not change with age. B) Western blot for phosphorylated p65 and corresponding densitometric analyses following infection with S. pneumoniae. The same membranes were stripped and reprobed for total p65 and actin. Immunoblots shown are representative, showing only 3 of the 6 mice used in the quantitative analysis. Graphs are the mean of two separate experiments (total n=6/cohort). Asterisks indicate a statistically significant difference (p <0.05) versus the young cohort using a two-tailed Student’s t-test. Note that direct comparison of uninfected and infected AM p65 levels is not appropriate due to the fact that image shown are from different immunoblots.
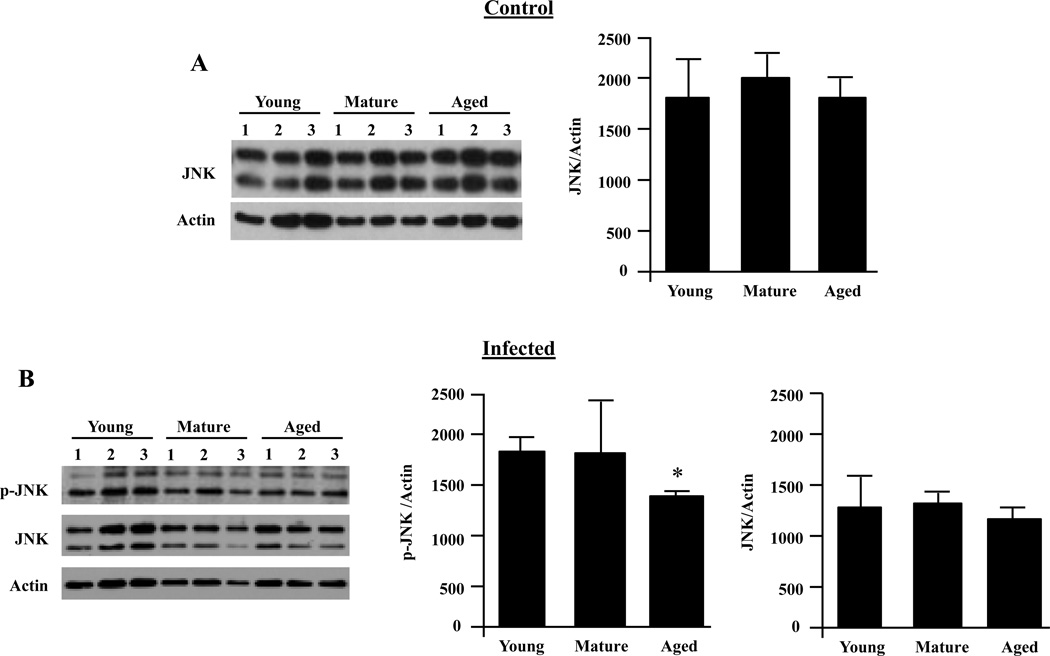
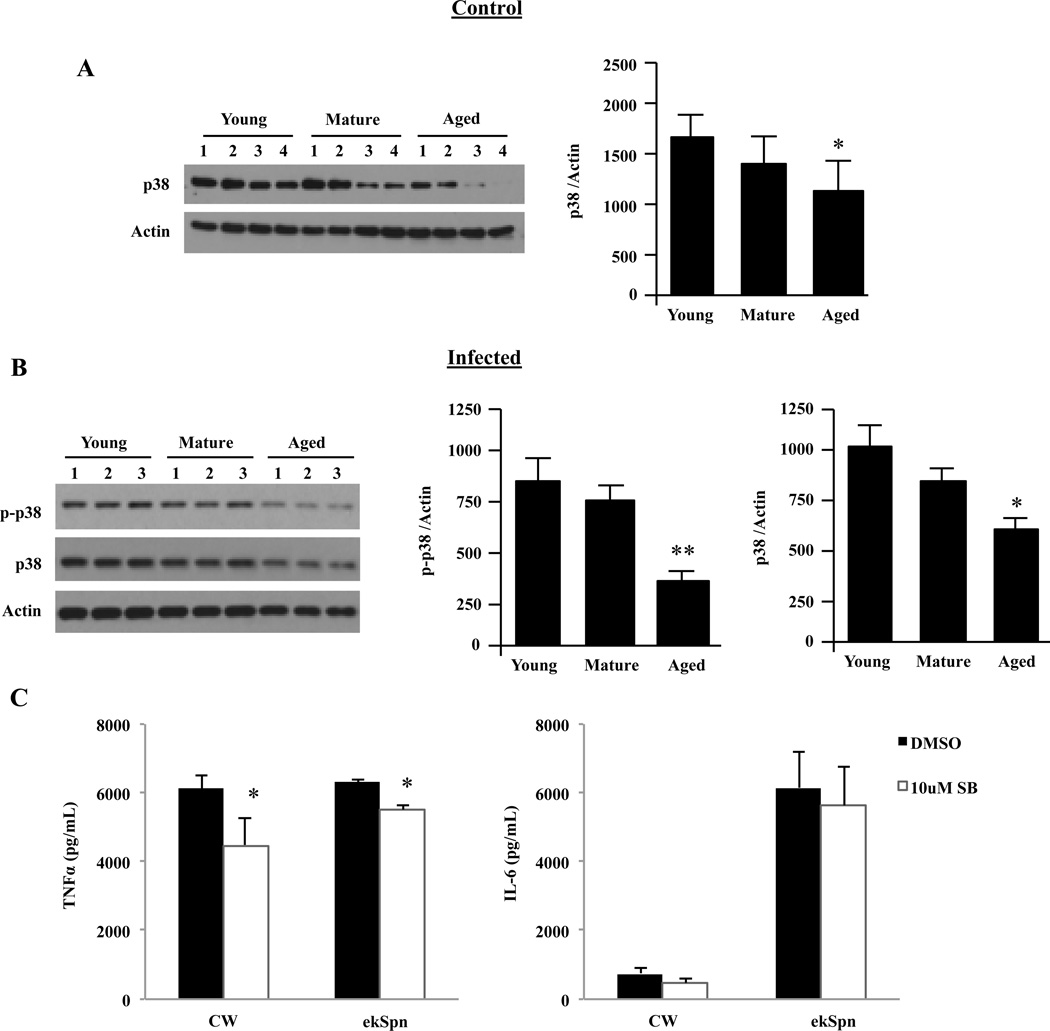
Previous studies had shown that splenic and peritoneal macrophages from aged mice express lower levels of total p38 MAPK and JNK with proportionate decreases in p-p38 and p-JNK following stimulation with LPS (Boehmer et al., 2004). Therefore, we next examined whether decreases in JNK or p38 also occurred for AMs. Total levels of JNK did not change with age (Fig. 6A and B); however, p-JNK was modestly decreased in aged AMs following infection (P=0.04) (Fig. 6B). Resting AMs from aged mice had significantly decreased total basal levels of p38 compared to their young counterparts (P<0.02) (Fig. 7A). Following infection, AMs from aged mice had significantly reduced p-p38 (P<0.01) (Fig. 7B). When we examined the ratio of p-p38 to total levels of p38 in infected cells it was determined that the ratio for young AMs was 1.6 while mature and aged AMs had ratios of 0.8 and 0.7, respectively. This suggested a defect in phosphorylation that was in addition to the reduction in total protein levels of p38 MAPK.
FIGURE 6.
Alveolar macrophage JNK activation decreases with age following infection. AMs were isolated from young, mature and aged mice and stimulated with S. pneumoniae at an MOI of 10 for 15 minutes or with media alone. A) Representative Western blot and corresponding densitometric analysis for JNK total protein levels in unstimulated AMs showing that total levels of JNK do not change with age (n=6/cohort). B) Western blot for phosphorylated JNK and corresponding densitometric analyses following infection with S. pneumoniae. The same membranes were stripped and reprobed for total JNK and actin. Immunoblots shown are representative, showing only 3 of the 6 mice used in the quantitative analysis. Graphs are the mean of two separate experiments (total n=6/cohort). Asterisks indicate a statistically significant difference (p <0.05) versus the young cohort using a two-tailed Student’s t-test. Note that direct comparison of uninfected and infected AM JNK levels is not appropriate due to the fact that image shown are from different immunoblots.
FIGURE 7.
Alveolar macrophage p38 activation decreases with age following infection. AMs were isolated from young, mature and aged mice and stimulated with S. pneumoniae at an MOI of 10 for 15 minutes or with media alone. A) Representative Western blot and corresponding densitometric analysis for p38 total protein levels in unstimulated AMs showing that total levels of p38 decline with age (n=6/cohort). B) Western blot for phosphorylated p38 and corresponding densitometric analyses following infection with S. pneumoniae. The same membranes were stripped and reprobed for total p38 and actin (n=6 per age group). Immunoblots shown are representative, showing only 3 of the 6 mice used in the quantitative analysis. Graphs are the mean of two separate experiments (total n=6/cohort). Single asterisks indicate a statistically significant difference (p <0.05) versus the young cohort using a two-tailed Student’s t-test. Double asterisk denotes p <0.01. C) AMs isolated from young mice (n=6) were pretreated with 10 µM of the p38 MAPK inhibitor SB203580 or an equivalent concentration of DMSO for 1 hour prior to stimulation with pneumococcal cell wall (CW; 106 CFU equivalents) or ethanol-killed pneumococci (ekSpn; 106 CFU) for 16 hours. Levels of TNFα and IL-6 were determined by ELISA. Note, inhibition of p38 MAPK kinase activity did not reduce IL-6 production. Data are presented as the mean ± SEM. Asterisks indicate a statistically significant difference (p <0.05) versus the DMSO treated macrophages using a two-tailed Student’s t-test. Note that direct comparison of uninfected and infected AM p38 levels is not appropriate due to the fact that image shown are from different immunoblots.
Administration of a p38 MAPK inhibitor (SB203580) has been shown to significantly reduce the production of TNFα, IL-6, and IL-8 following infection of human biopsied lung specimens with S. pneumoniae (Xu et al., 2008). Thus to examine whether inhibition of p38 MAPK alone could account for the reduced cytokine response in AMs, young AMs were pretreated with SB203580 for 1 hour prior to and during stimulation with CW or ekSpn. SB203580 reduced TNFα production but had no effect on IL-6 production (Fig. 7C). These data indicate that total protein levels and age-related decreases in p38 phosphorylation do not solely explain the decrease in IL-6 production by AMs.
3.6 Phosphorylation of ERK is increased in aged alveolar macrophages following infection with S. pneumoniae
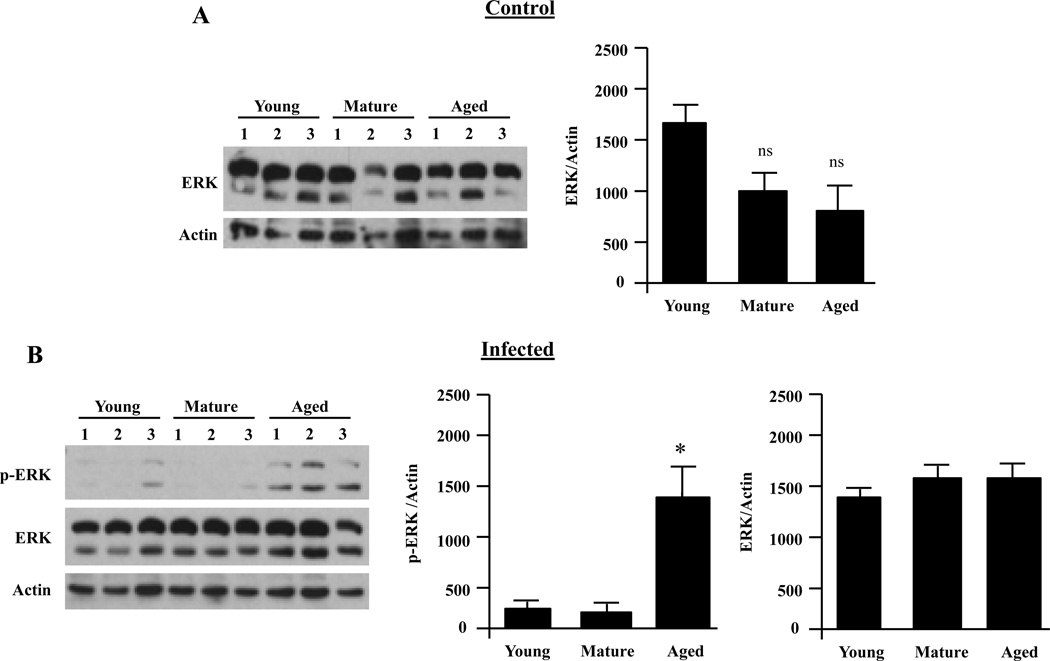
In contrast to diminished levels of p-p38 and p-JNK we detected elevated levels of p-ERK following infection of aged AMs with live S. pneumoniae (P=0.02) (Fig. 8). Interestingly, no differences were observed for p-ERK between the mature and young cohorts, suggesting enhanced ERK phosphorylation was exclusive to aged mice. Total protein levels of ERK did not differ between age groups.
FIGURE 8.
Alveolar macrophage ERK activation increases with age following infection. AMs were isolated from young, mature and aged mice and stimulated with S. pneumoniae at an MOI of 10 for 15 minutes or with media alone. A) Representative Western blot and corresponding densitometric analysis for ERK total protein levels in unstimulated AMs showing that total levels of ERK do not change with age (n=4/cohort). B) Western blot for phosphorylated ERK and corresponding densitometric analyses following infection with S. pneumoniae. The same membranes were stripped and reprobed for total ERK and actin (n=3 per age group). Asterisks indicate a statistically significant difference (p <0.05) versus the young cohort using a two-tailed Student’s t-test. Note that direct comparison of uninfected and infected AM ERK levels is not appropriate due to the fact that image shown are from different immunoblots.
4. DISCUSSION
While other investigators have examined age-related defects in macrophage cytokine production following exposure to purified TLR agonists ex vivo, this is the first study to examine the TLR response in vivo and in response to live S. pneumoniae, a Gram-positive pathogen and a leading cause of infectious death in the elderly, as well as with alveolar macrophages, the primary resident immune cells within the lungs. We report that during very early pneumococcal pneumonia, mature and aged mice exhibit an inability to mount a robust pro-inflammatory cytokine response that is associated with later failure to clear or limit bacterial replication and increased mortality. We go on to demonstrate that AMs are in part responsible for the decreased production of TNFα and IL-6 levels that was observed. To provide a potential mechanism for this muted response, we investigated the possibility that TLR levels or signaling was altered in an age-dependent fashion and as a result report that mature and aged AM have decreased phosphorylation of p65, JNK, and p38.
van der Poll and colleagues have published several studies identifying the pro-inflammatory cytokines required for efficient host defense to S. pneumoniae including but not limited to TNFα, IL-1β, and IL-6 (van der Poll et al., 1997), all of which we observed to be reduced in mature and aged mice infected with S. pneumoniae. TNFα functions to enhance phagocytic killing and the production of epithelial-derived cytokines and chemokines. TNFα receptor deficient mice or those passively immunized with anti-TNFα showed reduced bacterial killing and dramatically decreased survival following infection with S. pneumoniae (Elizur et al., 2008; van der Poll et al., 1997). TNFα production was significantly decreased when we examined mature and aged AMs ex vivo, however, this defect was less apparent following live infection. Although AMs have been reported to be the primary producers of TNFα, this observation suggests other cells in the lungs such as mast cells or dendritic cells might also be contributing to the TNFα response in vivo during early pneumococcal pneumonia.
We observed that production of IL-1β was reduced following CW stimulation as well as during early pneumococcal pneumonia in mature and aged mice compared to young. Levels of IL-1β secreted following ex vivo stimulation of AMs with pneumococcal components were below the limit of detection. As indicated, this is most likely due to the dual requirement for both NFκB and inflammasome activation, which would not be provided by stimulation with a single pneumococcal PAMP (van de Veerdonk et al., 2011). In vivo the pore-forming toxin pneumolysin would serve as the second signal necessary to activate the inflammasome (McNeela et al., 2010). Whether and how aging might affect activation of the inflammasome has not been examined and warrants further investigation. The most striking deficiency of mature and aged AMs was in their production of IL-6. IL-6 is a potent inducer of the acute-phase response in the liver and has been shown to act directly on recruited neutrophils to enhance their killing capacity within the lungs (Sutherland et al., 2008). Similar to aged mice, IL-6 gene deficient mice had uncontrolled bacterial outgrowth and were exquisitely susceptible to pneumococcal pneumonia (van der Poll et al., 1997). Interestingly, a study of hypoxia-induced lung injury found that prior administration of recombinant IL-6 intratracheally prevented reactive oxygen species (ROS)-mediated cytotoxicity (Kolliputi and Waxman 2009). During infection, S. pneumoniae produces large amounts of H2O2 (Braun et al., 2002). Therefore, aged mice in addition to failing to control bacterial replication may also be more susceptible to bacterial and macrophage-derived ROS during infection due to a reduction in IL-6. Importantly, we did not test for IL-12, a pro-inflammatory cytokine produced by macrophages and dendritic cells that is important for T-cell differentiation, the activities of natural killer cells, and development of humoral immunity or any chemokines (Helms et al., 2010; Metzger et al., 1996). Thus, future studies are warranted to determine the full impact of aging on the complete spectrum of cytokines and chemokines that are produced in response to pathogens.
In vivo we detected no IL-10 in the lungs of young or aged mice following pneumococcal challenge after 4 or 24 hours. This was in stark contrast to the high levels of IL-10 that were observed in the blood after 24 hours. This observation was consistent with and highlights the previously described notion of immune compartmentalization for IL-10 during pneumococcal pneumonia; which is interestingly bacterial pathogen specific (McConnell et al., 2010). Importantly, this observation strongly suggests that the anti-inflammatory effects of IL-10, which have been shown to suppress macrophage function in neonatal splenic macrophages (Chelvarajan et al., 2007) and in the lungs following influenza infection (van der Sluijs et al., 2004), were not responsible for the muted cytokine response of AM in aged mice. Likewise, that increased IL-10 production, which has been reported for aged splenic macrophages and is concomitant with their decreased TNFα, IL-1β, and IL-6 secretion following TLR stimulation (Fallah et al., 2011), does not occur for AM.
Although TLRs 2, 4 and 9 detect assorted pneumococcal products, we determined that the early production of cytokines by AMs was exclusively TLR2-dependent. Published studies are conflicted on whether TLR levels are altered with age on macrophages. Renshaw et al. reported diminished mRNA expression of TLRs 1–9 in splenic macrophages from aged mice versus young controls. Likewise, studies with human peripheral monocytes from young and elderly volunteers have demonstrated reduced levels of TLR1 (Nyugen et al., 2010; van Duin et al., 2007). In contrast, Rottinghaus et al., found slightly increased TLR2 surface expression on CD11c+ lung cells (i.e. AMs and dendritic cells) from aged mice (Rottinghaus et al., 2010). In agreement with the latter, we observed that TLR2 surface expression was slightly increased on F4/80+ AMs. While we were unable to detect TLR1 on the surface of AMs by flow cytometry, Western blot analyses of whole cell lysates from isolated AMs determined that total levels of TLR1 did not change with age. As indicated above, one possible explanation for the discrepancy between these two studies is that peripheral monocytes are phenotypically different from AMs. A notion supported by the fact that AMs are CD11b− whereas peripheral monocytes are CD11b+ (Kirby et al., 2009). Nonetheless, our findings do not exclude the possibility that reduced TLR1 surface expression occurs for AMs in aged mice.
Although no changes in basal levels of TLR1 and TLR2 were observed, we found that phosphorylation of p65, JNK and p38 MAPK were lower in AMs from aged mice following live bacterial challenge. Our findings are in agreement with studies by Boehmer et al. that found reduced total levels of p38 in aged splenic and peritoneal macrophages from BALB/c mice (Boehmer et al., 2004). Importantly, while Boehmer et al. did not observe a p38 phosphorylation defect, in our experiments we determined that phosphorylation of p38 was reduced in addition to the decrease in total protein levels, thereby suggesting that phosphorylation by upstream MAPK kinases was delayed relative to young AMs. In support of this concept, AMs from aged mice also had significantly decreased levels of p-p65 and p-JNK, while total levels of p65 and JNK were constant. Thus, the general age-related decline in phosphorylation following TLR stimulation suggests that signaling defects may occur upstream to NFκB and MAPK activation. If so, this would be consistent with the recent report by Fallah et al., suggesting that age-dependent increased pI3K-Akt activation of GSK3 in macrophages may be responsible (Fallah et al., 2011). Briefly, one way GSK3 suppresses the innate immune response is by enhancing the expression A20 and IκBα, which target the NFκB and MAPK pathways (Park et al., 2011).
MAPK activation occurs following TLR stimulation as well as through external stimuli such as reactive oxygen species and cytokine stimulation (Cuadrado and Nebreda 2010). In particular, studies have shown that p38 MAPK serves to enhance cytokine mRNA stability by phosphorylation of MAPK activated protein kinase 2 (MK2). MK2 controls cytokine mRNA stability by the phosphorylation and inactivation of the RNA-binding protein Tristetraprolin, thereby preventing its binding to target genes and enhancement of mRNA degradation (Khabar 2007). Thus it is possible that the age-related decline in cytokine production is due in part to diminished cytokine mRNA stability. Genetic deletion and chemical inhibition of p38 MAPK activation with SB203580 has been shown to result in a significant reduction in the production of cytokines such as TNFα in vitro (Ronkina et al., 2010). While we determined that p38 activation was important in TNFα production, treatment of AMs with the p38 MAPK inhibitor SB203580 did not reduce IL-6 production from young AMs following stimulation with CW or ekSpn. Thus our findings support a differential role of p38 MAPK activation in regulating TNFα and IL-6 production in AMs.
In contrast to p38 and JNK, ERK phosphorylation was significantly increased in aged AMs compared to mature and young. Inhibition of ERK in macrophages has been shown to differentially affect their activation depending on the stimulus applied (Monier et al., 2002). For example, bone marrow-derived macrophages treated with the ERK1/2 inhibitors PD98059 or UO126 had increased IL-6 production following exposure to Plasmodium falciparum glycosylphosphatidylinositols (Zhu et al., 2005). In contrast, ERK inhibition significantly decreased the production of IL-6 following stimulation of monocytes and macrophages with the pneumococcal adhesin PspA or a novel protein EstA, respectively (Cao et al., 2010; Kang et al., 2009). However, it is currently not clear how these proteins are enhancing MAPK activation. One possible explanation for these discrepancies is cell- and stimulus-specific expression of inhibitory microRNAs. In HeLa cells, ERK has been shown to induce the expression of microRNA-365 which binds to the 3’-UTR of IL-6 and repress mRNA translation (Xu et al., 2011). Thus enhanced ERK activation might possibly explain the significant reduction in IL-6 production following infection. It seems likely that it is the combined reduction in p38 and JNK along with enhanced ERK activation that collectively influences the reduced cytokine response of AMs following infection with S. pneumoniae.
Interestingly, age-related TLR dysfunction is not restricted to macrophages and reduced TLR function has also been described for dendritic cells isolated from elderly humans and aged mice (Panda et al., 2010; Stout-Delgado et al., 2008). Importantly, reduced dendritic cell TLR function was correlated with decreased antibody responses in elderly humans following influenza immunization; thereby indicating that TLR dysfunction might also contribute to the age-related decline in vaccine efficacy (Panda et al., 2010). As the bone marrow milieu and the lungs have both been found to have higher levels of inflammatory mediators during aging (Lepperdinger 2011; Meyer et al., 1996; Meyer et al., 1998), the findings that both macrophages and dendritic cells exhibit impaired TLR function raises the possibility that TLR dysfunction may reflect a state of tolerization or desensitization during development or after tissue infiltration. In support of this notion, macrophage desensitization has been shown to occur in vitro following exposure to endotoxin and in vivo following resolution of respiratory influenza infection (Didierlaurent et al., 2008; Park et al., 2011; Xiong et al., 2011).
Finally, there are many factors that can account for the differences observed between our studies and those of others, with particular regards to TLR levels and MAPK phosphorylation. Differences in mouse strains used (C57BL/6 vs. BALB/c), species examined (mouse vs. humans), bacterial species, and procedural differences in the isolation and purification of macrophage populations, and quality of animal housing may all together affect experimental outcomes. Nonetheless, all the studies consistently report that macrophages isolated from aged mice and humans are impaired in the early production of pro-inflammatory cytokines following stimulation with certain purified PAMPs.
In summary, these findings add to the growing literature of age-related defects in macrophage function. Herein we provide direct evidence that TLR dysfunction by AMs occurs in vivo and affects the production of pro-inflammatory cytokines during early pneumococcal pneumonia. We show that TLR dysfunction begins as early as mid-life and encompasses reduced p65, p38 and JNK phosphorylation. If applicable to humans, age-related TLR signaling defects within AMs is a mechanism that helps explain the enhanced susceptibility of the elderly to pneumonia.
Highlights.
Age-enhanced susceptibility to bacterial pneumonia begins at midlife
Aged mice fail to respond to S. pneumoniae with a robust pro-inflammatory response
Aged mice alveolar macrophages (AM) produce less TNFα & IL-6 after TLR2 engagement
Total AM TLR1 levels were unaffected by age; TLR2 surface levels slightly increased
TLR2 engaged aged AM have reduced phosphorylation of NFkB and assorted MAPK
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Benjamin Daniel and Karla Moncada in the UTHSCSA Core Flow cytometry laboratory. The UTHSCSA Core Flow Cytometry Facility is supported by NIH grants CA54174, AG013319 and AG19316. ARB received support from NIH AG013319-14S2. MTB is supported by NIH AI057986. CJO is supported by NIH AG033274.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis. 2003;35:555–562. doi: 10.1080/00365540310015683. [DOI] [PubMed] [Google Scholar]
- Albiger B, Dahlberg S, Sandgren A, Wartha F, Beiter K, Katsuragi H, Akira S, Normark S, Henriques-Normark B. Toll-like receptor 9 acts at an early stage in host defence against pneumococcal infection. Cell Microbiol. 2007;9:633–644. doi: 10.1111/j.1462-5822.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, Kobzik L. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med. 2004;200:267–272. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber GN. Cytoplasmic DNA innate immune pathways. Immunol Rev. 2011;243:99–108. doi: 10.1111/j.1600-065X.2011.01051.x. [DOI] [PubMed] [Google Scholar]
- Benton KA, Paton JC, Briles DE. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb Pathog. 1997;23:201–209. doi: 10.1006/mpat.1997.0150. [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126:1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Boyd AR, Orihuela CJ. Dysregulated inflammation as a risk factor for pneumonia in the elderly. Aging Dis. 2011;2:487–500. [PMC free article] [PubMed] [Google Scholar]
- Branger J, Knapp S, Weijer S, Leemans JC, Pater JM, Speelman P, Florquin SR, van der Poll T. Role of toll-like receptor 4 in Gram-positive and Gram-negative pneumonia in mice. Infect Immun. 2004;72:788–794. doi: 10.1128/IAI.72.2.788-794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JS, Sublett JE, Freyer D, Mitchell TJ, Cleveland JL, Tuomanen EI, Weber JR. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J Clin Invest. 2002;109:19–27. doi: 10.1172/JCI12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Chen T, Gong Y, Ying B, Li D, Xu W, Zhang X, Wang L, Yin Y. Molecular mechanisms of the secretion of cytokines and chemokines from human monocytes activated by pneumococcal surface protein A (PspA): Roles of mitogen-activated protein kinases and NF-kappaB. Microb Pathog. 2010;48:220–229. doi: 10.1016/j.micpath.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelvarajan L, Popa D, Liu Y, Getchell TV, Stromberg AJ, Bondada S. Molecular mechanisms underlying anti-inflammatory phenotype of neonatal splenic macrophages. J Leukoc Biol. 2007;82:403–416. doi: 10.1189/jlb.0107071. [DOI] [PubMed] [Google Scholar]
- Chelvarajan RL, Liu Y, Popa D, Getchell ML, Getchell TV, Stromberg AJ, Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol. 2006;79:1314–1327. doi: 10.1189/jlb.0106024. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- De Nardo D, De Nardo CM, Nguyen T, Hamilton JA, Scholz GM. Signaling crosstalk during sequential TLR4 and TLR9 activation amplifies the inflammatory response of mouse macrophages. J Immunol. 2009;183:8110–8118. doi: 10.4049/jimmunol.0901031. [DOI] [PubMed] [Google Scholar]
- Didierlaurent A, Goulding J, Patel S, Snelgrove R, Low L, Bebien M, Lawrence T, van Rijt LS, Lambrecht BN, Sirard JC, Hussell T. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med. 2008;205:323–329. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J Infect Dis. 2002;186:798–806. doi: 10.1086/342845. [DOI] [PubMed] [Google Scholar]
- Elizur A, Adair-Kirk TL, Kelley DG, Griffin GL, Demello DE, Senior RM. Tumor necrosis factor-alpha from macrophages enhances LPS-induced clara cell expression of keratinocyte-derived chemokine. Am J Respir Cell Mol Biol. 2008;38:8–15. doi: 10.1165/rcmb.2007-0203OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallah MP, Chelvarajan RL, Garvy BA, Bondada S. Role of phosphoinositide 3-kinase-Akt signaling pathway in the age-related cytokine dysregulation in splenic macrophages stimulated via TLR-2 or TLR-4 receptors. Mech Ageing Dev. 2011;132:274–286. doi: 10.1016/j.mad.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French N, Nakiyingi J, Carpenter LM, Lugada E, Watera C, Moi K, Moore M, Antvelink D, Mulder D, Janoff EN, Whitworth J, Gilks CF. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355:2106–2111. doi: 10.1016/s0140-6736(00)02377-1. [DOI] [PubMed] [Google Scholar]
- Gorina Y, Lentzer H. Multiple causes of death in old age. Aging Trends. 2008;9 [PubMed] [Google Scholar]
- Helms MW, Prescher JA, Cao YA, Schaffert S, Contag CH. IL-12 enhances efficacy and shortens enrichment time in cytokine-induced killer cell immunotherapy. Cancer Immunol Immunother. 2010;59:1325–1334. doi: 10.1007/s00262-010-0860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinojosa E, Boyd AR, Orihuela CJ. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J Infect Dis. 2009;200:546–554. doi: 10.1086/600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst RA, Kadioglu A, O'Callaghan C, Andrew PW. The role of pneumolysin in pneumococcal pneumonia and meningitis. Clin Exp Immunol. 2004;138:195–201. doi: 10.1111/j.1365-2249.2004.02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Hanson CA, Mahoney LD, Shay DK, Thompson WW. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–1755. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- Johnson JC, Jayadevappa R, Baccash PD, Taylor L. Nonspecific presentation of pneumonia in hospitalized older people: age effect or dementia? J Am Geriatr Soc. 2000;48:1316–1320. doi: 10.1111/j.1532-5415.2000.tb02607.x. [DOI] [PubMed] [Google Scholar]
- Kang EH, Gebru E, Kim MH, Cheng H, Park SC. EstA protein, a novel virulence factor of Streptococcus pneumoniae, induces nitric oxide and pro-inflammatory cytokine production in RAW 264.7 macrophages through NF-kappaB/MAPK. Microb Pathog. 2009;47:196–201. doi: 10.1016/j.micpath.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Khabar KS. Rapid transit in the immune cells: the role of mRNA turnover regulation. J Leukoc Biol. 2007;81:1335–1344. doi: 10.1189/jlb.0207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby AC, Coles MC, Kaye PM. Alveolar macrophages transport pathogens to lung draining lymph nodes. J Immunol. 2009;183:1983–1989. doi: 10.4049/jimmunol.0901089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Obermaier B, Angele B, Pfister HW, Wagner H, Koedel U, Kirschning CJ. Innate immunity to pneumococcal infection of the central nervous system depends on Toll-like receptor (TLR) 2 and TLR4. Journal of Infectious Diseases. 2008;198:1028–1036. doi: 10.1086/591626. [DOI] [PubMed] [Google Scholar]
- Knapp S, von Aulock S, Leendertse M, Haslinger I, Draing C, Golenbock DT, van der Poll T. Lipoteichoic acid-induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. J Immunol. 2008;180:3478–3484. doi: 10.4049/jimmunol.180.5.3478. [DOI] [PubMed] [Google Scholar]
- Knapp S, Wieland CW, van 't Veer C, Takeuchi O, Akira S, Florquin S, van der Poll T. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J Immunol. 2004;172:3132–3138. doi: 10.4049/jimmunol.172.5.3132. [DOI] [PubMed] [Google Scholar]
- Kolliputi N, Waxman AB. IL-6 cytoprotection in hyperoxic acute lung injury occurs via suppressor of cytokine signaling-1-induced apoptosis signal-regulating kinase-1 degradation. Am J Respir Cell Mol Biol. 2009;40:314–324. doi: 10.1165/rcmb.2007-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku CL, von Bernuth H, Picard C, Zhang SY, Chang HH, Yang K, Chrabieh M, Issekutz AC, Cunningham CK, Gallin J, Holland SM, Roifman C, Ehl S, Smart J, Tang M, Barrat FJ, Levy O, McDonald D, Day-Good NK, Miller R, Takada H, Hara T, Al-Hajjar S, Al-Ghonaium A, Speert D, Sanlaville D, Li X, Geissmann F, Vivier E, Marodi L, Garty BZ, Chapel H, Rodriguez-Gallego C, Bossuyt X, Abel L, Puel A, Casanova JL. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204:2407–2422. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepperdinger G. Inflammation and mesenchymal stem cell aging. Curr Opin Immunol. 2011;23:518–524. doi: 10.1016/j.coi.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. Pneumonia in the elderly. Curr Opin Infect Dis. 2004;17:127–130. doi: 10.1097/00001432-200404000-00010. [DOI] [PubMed] [Google Scholar]
- Mahbub S, Brubaker AL, Kovacs EJ. Aging of the Innate Immune System: An Update. Curr Immunol Rev. 2011;7:104–115. doi: 10.2174/157339511794474181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res. 2012;32:18–26. doi: 10.1089/jir.2011.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci U S A. 2003;100:1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, D'Alessandro-Gabazza C, Nakayama S, Nishikubo K, Noguchi T, Takei Y, Gabazza EC. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004. doi: 10.1136/bmj.c1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell KW, McDunn JE, Clark AT, Dunne WM, Dixon DJ, Turnbull IR, Dipasco PJ, Osberghaus WF, Sherman B, Martin JR, Walter MJ, Cobb JP, Buchman TG, Hotchkiss RS, Coopersmith CM. Streptococcus pneumoniae and Pseudomonas aeruginosa pneumonia induce distinct host responses. Crit Care Med. 2010;38:223–241. doi: 10.1097/CCM.0b013e3181b4a76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, Moran B, Fitzgerald KA, Tschopp J, Petrilli V, Andrew PW, Kadioglu A, Lavelle EC. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 2010;6:e1001191. doi: 10.1371/journal.ppat.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger DW, Buchanan JM, Collins JT, Lester TL, Murray KS, Van Cleave VH, Vogel LA, Dunnick WA. Enhancement of humoral immunity by interleukin-12. Ann N Y Acad Sci. 1996;795:100–115. doi: 10.1111/j.1749-6632.1996.tb52659.x. [DOI] [PubMed] [Google Scholar]
- Meyer KC, Ershler W, Rosenthal NS, Lu XG, Peterson K. Immune dysregulation in the aging human lung. Am J Respir Crit Care Med. 1996;153:1072–1079. doi: 10.1164/ajrccm.153.3.8630547. [DOI] [PubMed] [Google Scholar]
- Meyer KC, Rosenthal NS, Soergel P, Peterson K. Neutrophils and low-grade inflammation in the seemingly normal aging human lung. Mech Ageing Dev. 1998;104:169–181. doi: 10.1016/s0047-6374(98)00065-7. [DOI] [PubMed] [Google Scholar]
- Monier RM, Orman KL, Meals EA, English BK. Differential effects of p38- and extracellular signal-regulated kinase mitogen-activated protein kinase inhibitors on inducible nitric oxide synthase and tumor necrosis factor production in murine macrophages stimulated with Streptococcus pneumoniae. J Infect Dis. 2002;185:921–926. doi: 10.1086/339483. [DOI] [PubMed] [Google Scholar]
- Nyugen J, Agrawal S, Gollapudi S, Gupta S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J Clin Immunol. 2010;30:806–813. doi: 10.1007/s10875-010-9448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, Allore HG, Montgomery RR, Shaw AC. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Park-Min KH, Chen J, Hu X, Ivashkiv LB. Tumor necrosis factor induces GSK3 kinase-mediated cross-tolerance to endotoxin in macrophages. Nat Immunol. 2011;12:607–615. doi: 10.1038/ni.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- Ronkina N, Menon MB, Schwermann J, Tiedje C, Hitti E, Kotlyarov A, Gaestel M. MAPKAP kinases MK2 and MK3 in inflammation: complex regulation of TNF biosynthesis via expression and phosphorylation of tristetraprolin. Biochem Pharmacol. 2010;80:1915–1920. doi: 10.1016/j.bcp.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Rottinghaus EK, Vesosky B, Turner J. TLR-2 independent recognition of Mycobacterium tuberculosis by CD11c+ pulmonary cells from old mice. Mech Ageing Dev. 2010;131:405–414. doi: 10.1016/j.mad.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder NWJ, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, Gobel UB, Weber JR, Schumann RR. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Bio Chem. 2003;278:15587–15594. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Henneke P, Visintin A, Morse SC, Martin V, Watkins C, Paton JC, Wessels MR, Golenbock DT, Malley R. The apoptotic response to pneumolysin is toll-like receptor 4 dependent and protects against pneumococcal disease. Infect Immun. 2005;73:6479–6487. doi: 10.1128/IAI.73.10.6479-6487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout-Delgado HW, Yang X, Walker WE, Tesar BM, Goldstein DR. Aging impairs IFN regulatory factor 7 up-regulation in plasmacytoid dendritic cells during TLR9 activation. J Immunol. 2008;181:6747–6756. doi: 10.4049/jimmunol.181.10.6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol. 2008;181:5598–5605. doi: 10.4049/jimmunol.181.8.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, Heidelberg J, DeBoy RT, Haft DH, Dodson RJ, Durkin AS, Gwinn M, Kolonay JF, Nelson WC, Peterson JD, Umayam LA, White O, Salzberg SL, Lewis MR, Radune D, Holtzapple E, Khouri H, Wolf AM, Utterback TR, Hansen CL, McDonald LA, Feldblyum TV, Angiuoli S, Dickinson T, Hickey EK, Holt IE, Loftus BJ, Yang F, Smith HO, Venter JC, Dougherty BA, Morrison DA, Hollingshead SK, Fraser CM. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- Tuomanen E, Hengstler B, Zak O, Tomasz A. Induction of meningeal inflammation by diverse bacterial cell walls. Eur J Clin Microbiol. 1986;5:682–684. doi: 10.1007/BF02013304. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997;176:439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- van der Sluijs KF, van Elden LJ, Nijhuis M, Schuurman R, Pater JM, Florquin S, Goldman M, Jansen HM, Lutter R, van der Poll T. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol. 2004;172:7603–7609. doi: 10.4049/jimmunol.172.12.7603. [DOI] [PubMed] [Google Scholar]
- van Duin D, Mohanty S, Thomas V, Ginter S, Montgomery RR, Fikrig E, Allore HG, Medzhitov R, Shaw AC. Age-associated defect in human TLR-1/2 function. J Immunol. 2007;178:970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- Wang H, Brown J, Martin M. Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine. 2011;53:130–140. doi: 10.1016/j.cyto.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler ME, Szabo P. The effect of age on the B-cell repertoire. J Clin Immunol. 2000;20:240–249. doi: 10.1023/a:1006659401385. [DOI] [PubMed] [Google Scholar]
- Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Qiu F, Piao W, Song C, Wahl LM, Medvedev AE. Endotoxin tolerance impairs IL-1 receptor-associated kinase (IRAK) 4 and TGF-beta-activated kinase 1 activation, K63-linked polyubiquitination and assembly of IRAK1, TNF receptor-associated factor 6, and IkappaB kinase gamma and increases A20 expression. J Biol Chem. 2011;286:7905–7916. doi: 10.1074/jbc.M110.182873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Droemann D, Rupp J, Shen H, Wu X, Goldmann T, Hippenstiel S, Zabel P, Dalhoff K. Modulation of the inflammatory response to Streptococcus pneumoniae in a model of acute lung tissue infection. Am J Respir Cell Mol Biol. 2008;39:522–529. doi: 10.1165/rcmb.2007-0328OC. [DOI] [PubMed] [Google Scholar]
- Xu Z, Xiao SB, Xu P, Xie Q, Cao L, Wang D, Luo R, Zhong Y, Chen HC, Fang LR. miR-365, a novel negative regulator of IL-6 gene expression, is cooperatively regulated by Sp1 and NF-kappaB. J Biol Chem. 2011 doi: 10.1074/jbc.M110.198630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: Recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- Zhu J, Krishnegowda G, Gowda DC. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: the requirement of extracellular signal-regulated kinase, p38, c-Jun N-terminal kinase and NF-kappaB pathways for the expression of proinflammatory cytokines and nitric oxide. J Biol Chem. 2005;280:8617–8627. doi: 10.1074/jbc.M413539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.