SUMMARY
Assessment of oxyhemoglobin saturation in patients with sickle cell disease (SCD) is vital for prompt recognition of hypoxemia. The accuracy of pulse oximeter measurements of blood oxygenation in SCD patients is variable, partially due to carboxyhemoglobin (COHb) and methemoglobin (MetHb), which decrease the oxygen content of blood. This study evaluated the accuracy and reliability of a non-invasive pulse co-oximeter in measuring COHb and MetHb percentages (SpCO and SpMet) in children with SCD. We hypothesized that measurements of COHb and MetHb by non-invasive pulse co-oximetry agree within acceptable clinical accuracy with those made by invasive whole blood co-oximetry. Fifty children with SCD-SS underwent pulse co-oximetry and blood co-oximetry while breathing room air. Non-invasive COHb and MetHb readings were compared to the corresponding blood measurements. The pulse co-oximeter bias was 0.1% for COHb and −0.22% for MetHb. The precision of the measured SpCO was ±2.1% within a COHb range of 0.4–6.1%, and the precision of the measured SpMet was ±0.33% within a MetHb range of 0.1–1.1%. Non-invasive pulse co-oximetry was useful in measuring COHb and MetHb levels in children with SCD. Although the non-invasive technique slightly overestimated the invasive COHb measurements and slightly underestimated the invasive MetHb measurements, there was close agreement between the two methods.
Keywords: sickle cell anemia, pediatrics, oximetry, blood gas analysis, hemoglobins
INTRODUCTION
Sickle cell disease (SCD) is an inherited disorder in which the predominance of sickle hemoglobin in erythrocytes leads to chronic hemolytic disease and vaso-occlusive complications. Oxyhemoglobin desaturation (OHD) is a well-documented phenomenon in patients with SCD.1–7 The OHD is attributed to hypoxemia and a low arterial partial pressure of oxygen (PaO2), the rightward shift of the oxyhemoglobin dissociation curve, and elevated levels of dyshemoglobins.8–14 Carboxyhemoglobin (COHb) and methemoglobin (MetHb), elevated in the presence of intravascular hemolysis, are dysfunctional forms of hemoglobin incapable of transporting oxygen, resulting in decreased arterial oxygen content.10–14 In addition to chronic anemia in SCD, the contribution of dyshemoglobins to decreased arterial oxygen content needs to be considered when evaluating patients with SCD, as COHb and MetHb may be more important in causing OHD than a low PaO2.8,15–18
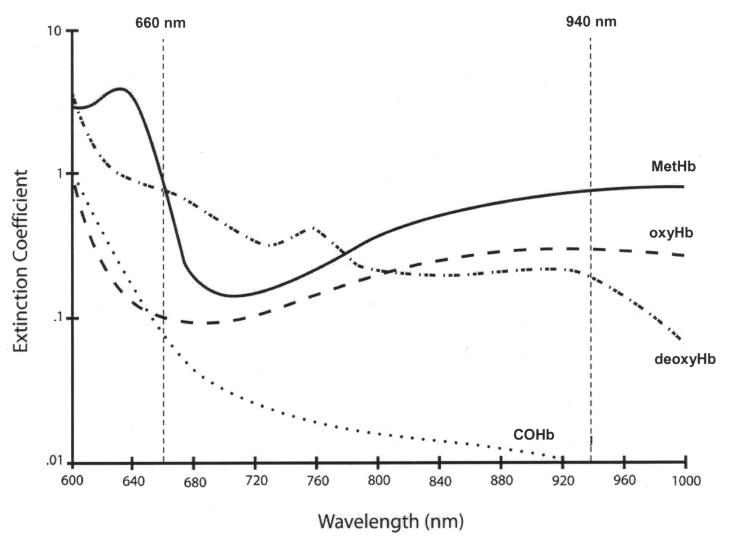
Most studies of OHD in children and adults with SCD have relied on non-invasive pulse oximeters, indirectly estimating the functional arterial oxyhemoglobin saturation (SaO2) by measuring the absorbance of light transmitted through well-perfused tissue, displayed as SpO2. The functional arterial oxyhemoglobin saturation, SaO2, is the ratio of oxyhemoglobin (oxyHb) to the sum of oxyHb and deoxyhemoglobin (deoxyHb), measured via co-oximetry. Since most pulse oximeters measure light absorbance at only two wavelengths of light, 660 and 940 nm (Figure 1), they only measure two light absorbers in blood, oxyHb and deoxyHb, and are incapable of distinguishing dyshemoglobins from oxyHb or deoxyHb.19 With elevated COHb and MetHb in the blood of patients with SCD, conventional pulse oximeters are subject to serious errors.20–22 The validity of two-wavelength pulse oximeters in SCD has been questioned, as many have found SpO2 to consistently overestimate the fractional arterial oxyhemoglobin saturation, FaO2Hb, the ratio of oxyHb to the sum of oxyHb, deoxyHb, COHb, and MetHb, measured via co-oximetry.8,16–18,23 To calculate the FaO2Hb, the most accurate reflection of true oxygen content and delivery to the tissues, it is currently necessary to measure arterial blood concentrations of oxyHb, deoxyHb, COHb, and MetHb by performing invasive arterial blood gas analysis with a laboratory blood co-oximeter, which spectrophotometrically measures light transmission through a blood sample at four or more discrete wavelengths of light, to distinguish oxyHb from deoxyHb, COHb, and MetHb.24–26
Fig 1.
Light absorbance (extinction coefficient) versus wavelength for oxyHb, deoxyHb, COHb, and MetHb. Conventional pulse oximeters use 2 wavelengths of light, red (660 nm) and infrared (940 nm), to measure the absorbance of oxyHb and deoxyHb.
From Barker SJ, Curry J, Redford D, Morgan S. Measurement of carboxyhemoglobin and methemoglobin by pulse oximetry: a human volunteer study. Anesthesiology 2006; 105:892–7 [Fig. 5 (p. 895)]. With kind permission from Wolters Kluwer Health.
Recently, an FDA approved non-invasive pulse co-oximeter has been developed to estimate percentages of COHb and MetHb, displayed as SpCO and SpMet. The pulse co-oximeter uses a fingertip sensor with 8 distinct wavelengths of light to non-invasively measure COHb and MetHb by spectrophotometry. As shown previously, in children with SCD, the partial pressure of oxygen at which hemoglobin is 50% saturated with oxygen (P50), as measured by spectrophotometry, was similar to that measured by manometric methods, suggesting that spectrophotometry is accurate in patients with SCD.8 Earlier studies have demonstrated a strong agreement between non-invasive SpCO and SpMet levels and measurements performed by blood co-oximetry in healthy adult volunteers.20 The pulse co-oximeter has been evaluated in clinical studies, but not in patients with SCD.27–29 This study evaluated the accuracy and reliability of the multi-wavelength pulse co-oximeter to non-invasively measure COHb and MetHb percentages in children with SCD. We hypothesized that in children with SCD, measurements of COHb and MetHb by non-invasive pulse co-oximetry agree with those made by invasive whole blood co-oximetry within an acceptable clinical accuracy of 3% for COHb and 1% for MetHb.
MATERIALS AND METHODS
This study was approved by The Children’s Hospital of Philadelphia Institutional Review Board (No. 2007-6-5188). All subjects eligible for participation in this study were enrolled after obtaining informed consent from their parents and, when appropriate, assent or consent from the subject.
Study Design
Fifty African American children with SCD-SS, 2–18 years old, not receiving chronic blood transfusions or hydroxyurea, participated in this prospective study. Subjects were recruited over a one year period from the Comprehensive Sickle Cell Center at The Children’s Hospital of Philadelphia. Subjects were clinically at baseline and studied at steady state, defined as a three month interval since the last red blood cell transfusion and one month since the last acute chest syndrome or painful episode. None of the subjects required supplemental oxygen and none smoked.
At the time of testing, while awake and breathing room air, each subject had an appropriately sized multi-wavelength fingertip sensor placed on the 3rd digit of the right hand and attached to a pulse co-oximeter (Radical-7 Rainbow SET Pulse CO-Oximeter; Masimo Corporation; Irvine, CA). Calibration and measurement techniques were performed according to published protocols.30 Measurements were recorded every two seconds for two minutes and internally stored in the pulse co-oximeter. Measurements were also obtained for two minutes using the 4th digit of the right hand, 3rd digit of the left hand, and 4th digit of the left hand, the best perfused digits.30 The averages of the two second measurements obtained over the two minute recording intervals from each digit were used in the data analysis.
Immediately after the non-invasive measurements were performed, a sample of arterial blood (n=34) was anaerobically drawn from the radial artery into a pre-heparinized 1-mL syringe and placed on ice. Arterial blood samples were not obtained in 16 subjects because arterial puncture was technically unsuccessful in 10 subjects and 6 subjects refused arterial puncture. These 16 subjects had venous samples drawn. Blood samples were analyzed within 10 minutes using a pre-calibrated and quality-controlled Rapidlab 1265 blood gas analyzer and co-oximeter (Siemens Medical Solutions Diagnostics; Tarrytown, NY) according to standard laboratory practice.31 Blood gas analysis of the arterial blood samples provided values of pH, PaO2, PaCO2, and calculated values of SaO2 and FaO2Hb. Blood co-oximeter analysis of the arterial (n=34) and venous (n=16) samples provided spectrophotometric values of total hemoglobin and percentages of COHb and MetHb. The blood COHb and MetHb percentages of each subject were compared with the average values of SpCO and SpMet obtained from all 4 fingers using the pulse co-oximeter.
Statistical Analysis
Statistical analyses were performed using SPSS (Chicago, IL) and MedCalc for Windows, version 9.5.0.0 (Mariakerke, Belgium) software. A two-tailed p-value < 0.05 was the criterion for statistical significance. Descriptive statistics were calculated for the variables measured. Statistical analysis of agreement, comparing calculated bias and precision, between the non-invasive and invasive measurements was performed using the Bland and Altman technique.32,33 This analysis involved comparing and plotting the differences between measurements obtained from the non-invasive and invasive techniques against the average value of the two measurements. The bias was the mean of the differences between the values measured by each technique, and the precision was the standard deviation of the measurement differences, an indicator of measurement uncertainty. We defined bias so that a positive difference (bias) indicated a non-invasive measurement overestimating the comparison invasive measurement.
Linear regression analysis was used to examine the relationship between oxyhemoglobin saturation and dyshemoglobin measurements. Sample size estimation was based on testing the two techniques for equivalence in measuring COHb and MetHb. We assumed the mean differences between non-invasive and invasive COHb and MetHb measurements were 1.25 (common standard deviation of 2) and 0.25 (common standard deviation of 0.45), respectively. We further assumed that differences in COHb measurements between 0 and 2.5 and differences in MetHb measurements between 0 and 0.75 were clinically insignificant and within published uncertainties for the pulse co-oximeter (SpCO: ±3% and SpMet: ±1%).30
RESULTS
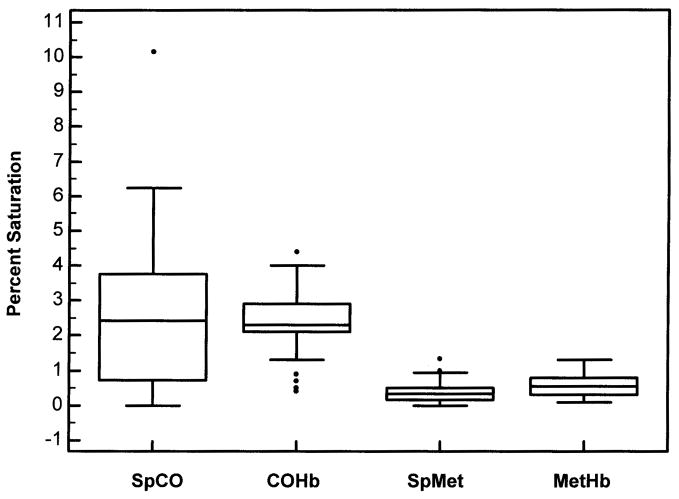
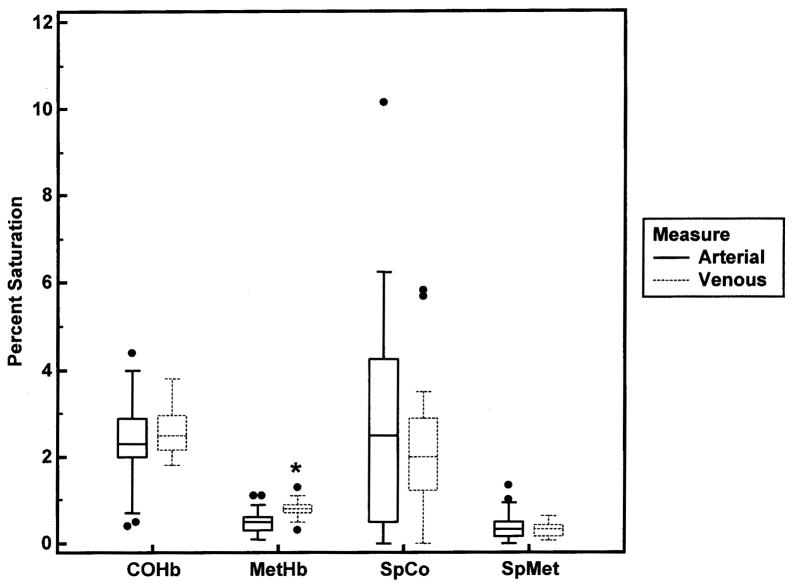
The average age of the 50 subjects was 8.3±5.0 (mean±SD) years and 23 were females. Pulse co-oximetry and blood co-oximetry results are shown in Table 1. The mean SpO2 was 97% with six subjects’ SpO2 ≤ 93%. All 34 subjects (68%) who had arterial blood obtained, including the 6 with SpO2 ≤ 93%, were normoxemic (PaO2 > 70 mm Hg) in room air. The percent of dyshemoglobins measured by the pulse co-oximeter (SpCO and SpMet) and by the laboratory blood co-oximeter (COHb and MetHb) are displayed in Figure 2. There was no significant difference in COHb values measured from arterial and venous blood; however, there was a small but statistically significant difference in MetHb values measured with arterial and venous blood samples (p=0.002) using the Wilcoxon two sample test (Figure 3). The pulse co-oximeter values of SpCO and SpMet were not statistically different in subjects who had arterial or venous blood drawn.
TABLE 1.
Results of the Measurements Analyzed (n=50)
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| SpO2 (%) | 97 ± 2.7 | 89 | 100 |
| SaO2 (%)a | 95 ± 3.1 | 88 | 99 |
| FaO2Hb (%)a | 93 ± 3.5 | 85 | 98 |
| PaO2 (mmHg)a | 92.5 ± 9.2 | 76.5 | 109.1 |
| SpCO (%) | 2.5 ± 2.1 | 0 | 10.2 |
| COHb (%) | 2.4 ± 0.8 | 0.4 | 4.4 |
| SpMet (%) | 0.4 ± 0.3 | 0 | 1.3 |
| MetHb (%) | 0.6 ± 0.3 | 0.1 | 1.3 |
| Hgb (g/dL) | 8.3 ± 1.2 | 5.9 | 11.2 |
Subjects with arterial blood samples (n=34)
Fig 2.
Box-and-whisker plots for the dyshemoglobin measurements. The lower, middle, and upper lines of the box represent the 25%ile, 50%ile, and 75%ile, respectively. The vertical lines span ±1.5 times the interquartile range above and below the upper and lower quartiles, respectively. The measurements are: SpCO, pulse co-oximeter measurement of carboxyhemoglobin; COHb, laboratory co-oximeter measurement of carboxyhemoglobin; SpMet, pulse co-oximeter measurement of methemoglobin; MetHb, laboratory co-oximeter measurement of methemoglobin.
Fig 3.
Box-and-whisker plots for the dyshemoglobin measurements obtained via arterial (n=34) and venous (n=16) blood samples. Symbols as in Figure 2. *p=0.002.
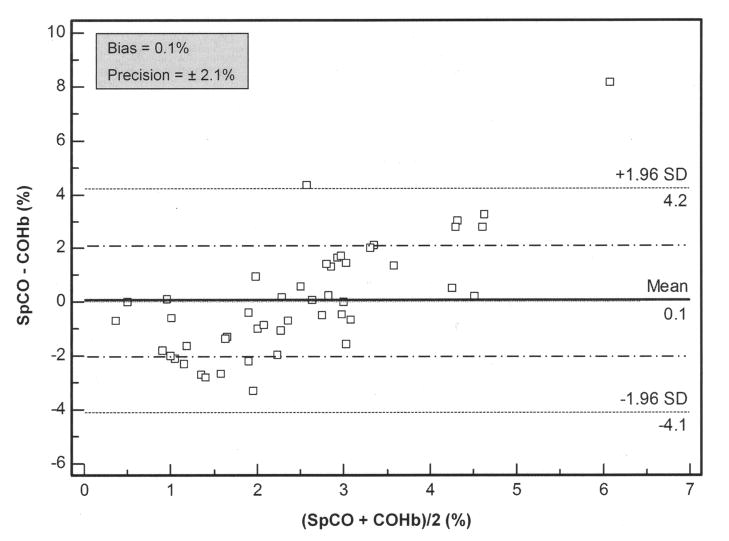
The mean SpCO was 2.5±2.1% (95% CI, 1.9 to 3.1%) and mean COHb was 2.4±0.8% (95% CI, 2.2 to 2.7%). There was greater variability in the non-invasive SpCO measurements compared to the invasive COHb measurements (Figure 2). Utilizing the Bland and Altman analysis (Figure 4), the pulse co-oximeter slightly overestimated COHb with a bias of 0.1% (95% CI, −0.5 to 0.7%), within average COHb levels of 0.4–6.1%. As shown in Figure 4, the magnitude of the SpCO measurement affected the bias, such that at average COHb values <2%, the SpCO underestimated the COHb and at average COHb values >4%, the SpCO overestimated the COHb. However, the measurement precision of SpCO was ±2.1%.
Fig 4.
Bland-Altman bias plot of measurement differences, SpCO − COHb, against the mean of the measurements, (SpCO + COHb)/2, for carboxyhemoglobin (n = 50). COHb and SpCO are expressed as percentage saturation. Lines show values of bias (solid) ± precision (long/short dash). Lines marked with a mean ± 1.96 SD represent the limits of agreement, between which 95% of the bias measurements lie.
The mean SpMet was 0.4±0.3% (95% CI, 0.3 to 0.5%) and mean MetHb was 0.6±0.3% (95% CI, 0.5 to 0.7%) with similar variability in the non-invasive and invasive measurements (Figure 2). The pulse co-oximeter slightly underestimated MetHb with a bias of −0.22% (95% CI, −0.3 to −0.1%), within average MetHb levels of 0.1 – 1.1% (Figure 5). The measurement precision of SpMet was ±0.33%.
Fig 5.
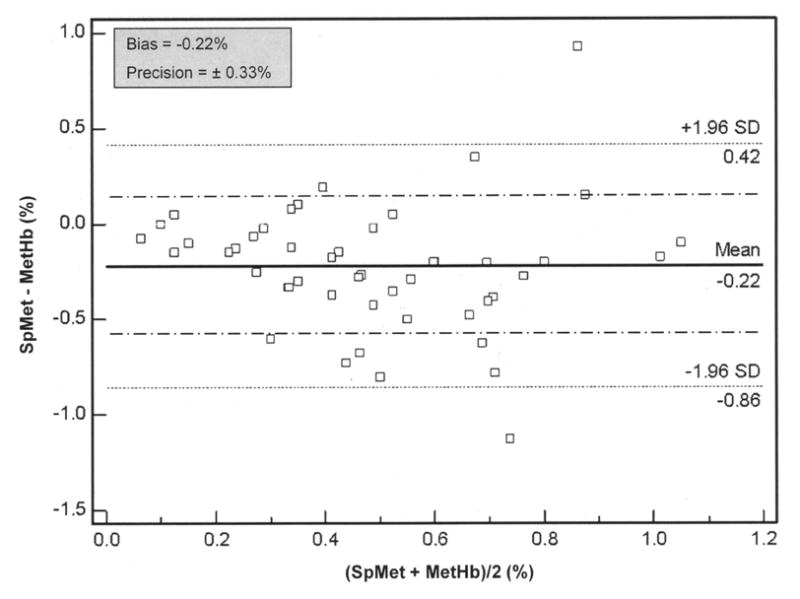
Bland-Altman bias plot of measurement differences, SpMet − MetHb, against the mean of the measurements, (SpMet + MetHb)/2, for methemoglobin (n = 50). MetHb and SpMet are expressed as percentage saturation. Lines show values of bias (solid) ± precision (long/short dash). Lines marked with a mean ± 1.96 SD represent the limits of agreement, between which 95% of the bias measurements lie.
While the bias and precision of the SpCO and SpMet measurements were within acceptable clinical accuracy (3% and 1%, respectively), Lin’s concordance correlations were low (0.14 and 0.27, respectively).
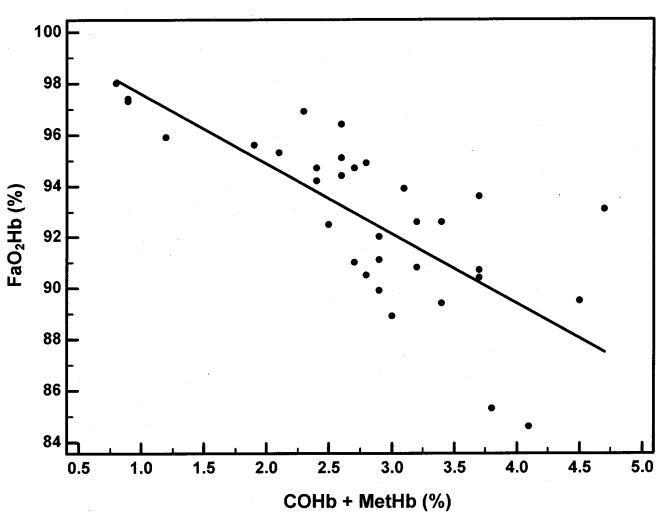
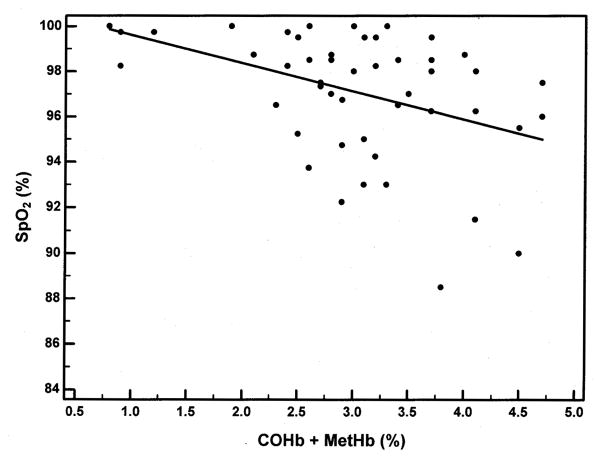
Regression analysis was performed to examine the relationship of FaO2Hb by co-oximetry in the subjects who had arterial blood obtained (Figure 6) and SpO2 by pulse oximetry in all subjects (Figure 7) with the sum of the dyshemoglobin measurements by co-oximetry. The regression equation when comparing the FaO2Hb and COHb + MetHb was y = 100.31-2.72x. Both the y-intercept (p<0.0001) and the slope (p<0.0001) were statistically significant, and the R2 was 0.55. The regression equation when comparing the SpO2 and COHb + MetHb was y = 100.87-1.24x. Both the y-intercept (p<0.0001) and the slope (p=0.003) were statistically significant, and the R2 was 0.17.
Fig 6.
Arterial fractional oxyhemoglobin saturation (FaO2Hb) as a function of dyshemoglobinemia (COHb + MetHb). The regression equation was y = 100.31-2.72x. The y-intercept (p<0.0001) and the slope (p<0.0001) were statistically significant. There was a negative correlation between the independent and dependent variables (R2 = 0.55).
Fig 7.
Pulse oximetry oxyhemoglobin saturation (SpO2) as a function of dyshemoglobinemia (COHb + MetHb). The regression equation was y = 100.87-1.24x. The y-intercept (p<0.0001) and the slope (p=0.003) were statistically significant. There was a negative correlation between the independent and dependent variables (R2 = 0.17).
DISCUSSION
SCD is a blood disorder characterized by adherent, rigid, abnormally-shaped erythrocytes that occlude blood vessels and compromise blood and oxygen supply to tissues and organs. Although the causes of tissue hypoxia in patients with SCD are complex, hypoxemia is recognized as a marker and predictor of vaso-occlusive complications of SCD, including stroke, vaso-occlusive pain episodes, acute chest syndrome, pulmonary hypertension, and progressive lung dysfunction.5,34–38 Not only do patients with SCD have decreased oxygen carrying capacity due to chronic anemia, they have elevated COHb and MetHb, which are unable to transport oxygen. In SCD, as in other hemolytic diseases, a by-product of hemoglobin catabolism is carbon monoxide which binds to hemoglobin, forming COHb.10–12 Methemoglobin is also elevated in sickle erythrocytes which have impaired ability to return methemoglobin to its reduced (ferrous) state due to reduced NADH content and abnormal membrane associated ferric iron.13,14 Since COHb and MetHb are hemoglobin based derivatives, measurements of dissolved oxygen in plasma will not detect dyshemoglobins, and the PaO2 may be normal, as in our subjects (Table 1), despite a decrease in oxygen carrying capacity.
Accurate measurements of blood oxygen levels have important clinical implications in hypoxemic SCD patients. Basing treatment decisions on conventional two-wavelength pulse oximetry data alone in children with SCD may be inappropriate since the assessment of oxygen status in SCD patients with elevated dyshemoglobins requires multi-wavelength co-oximetry. We have shown in children with SCD, as in other patients with elevated amounts of COHb and MetHb, SpO2 over estimates SaO2 and FaO2Hb (Table 1).21,22 Despite the normal PaO2 and SpO2 of our subjects, the mean FaO2Hb was 93±3.5%. Among subjects with arterial blood measurements, we found the presence of COHb and MetHb explained 55% of the variability in FaO2Hb (Figure 6) and among all subjects, the dyshemoglobins explained 17% of the variability in SpO2 (Figure 7). The remaining variability may have been due to other important variables, such as: ventilation/perfusion mismatch, shunt, diffusion defects, and/or the rightward shift of the oxyhemoglobin dissociation curve. For every 1% increase in the amount of dyshemoglobins measured (COHb + MetHb) there was a 2.7% decline in FaO2Hb measured by co-oximetry, and a 1.2% decline in SpO2 measured by pulse oximetry.
When calculating arterial blood oxygen content in SCD patients, FaO2Hb is the best index of oxyhemoglobin saturation.23,24,39–41 However, without knowing the amounts of COHb and MetHb, FaO2Hb cannot be determined.42,43 Co-oximetry is reliable for analyzing oxyHb, deoxyHb, COHb, and MetHb, and there is no difference in the spectral absorbance of blood from SCD-SS patients compared to individuals with normal hemoglobin genotype (AA).25,26,44 Unfortunately, measuring dyshemoglobin concentrations via blood sampling is invasive, painful, and subject to significant reporting delays. Additionally, blood co-oximeters are not available in many medical facilities. The new pulse co-oximeter used in this study utilizes the principles of spectrophotometry and plethysmography to non-invasively perform rapid estimates of COHb and MetHb percentages.
In this methods comparison study, the use of a pulse co-oximeter to non-invasively measure COHb and MetHb was evaluated for accuracy and reliability by comparison with an accepted standard invasive method, whole blood co-oximetry. The Bland and Altman technique was used to analyze the level of agreement between non-invasive and invasive measurements by calculating bias and precision, rigorous measures of agreement between two clinical measurement techniques, each with their own inherent limitations and errors.32,33 Bland and Altman analysis was chosen over correlation analysis because, as Nickerson, et al. emphasized in their comparison of pulse oximeters and co-oximeters, two instruments can have a perfect correlation and yet quantitatively disagree with each other.41 Our Bland and Altman analysis in children with SCD showed SpCO and COHb values within the average range of 0.4 – 6.1%, and SpMet and MetHb values within the average range of 0.1 – 1.1%, values similar to those reported in other studies analyzing dyshemoglobin percentages in patients with SCD.8,45 Normal COHb and MetHb levels in nonsmokers without SCD are less than 1.5% and less than 0.6%, respectively.46
There was no significant difference in the COHb values of the 16 subjects (32%) who had venous blood sampled compared to the 34 subjects (68%) who had arterial blood sampled (Figure 3). There was a small, but statistically significant difference (p=0.002) in the MetHb levels measured from arterial and venous blood samples. These small differences in MetHb levels between arterial and venous blood should not significantly impact the measurement of FaO2Hb when using blood co-oximetry. The pulse co-oximeter values, SpCO and SpMet, were not statistically different in subjects who had arterial or venous blood sampled.
We found SpCO, with a bias of 0.1%, and SpMet, with a bias of −0.22%, to be closely comparable to invasive laboratory measurements. The precision of the pulse co-oximeter measurements, ±2.1% for SpCO and ±0.33% for SpMet, was within an acceptable clinical range of accuracy, and within published uncertainties for the pulse co-oximeter (SpCO: ±3% and SpMet: ±1%).30 Similar uncertainties of SpCO and SpMet using the pulse co-oximeter in healthy adults have been reported.20 While the clinical agreement of the two measurement techniques was good, Lin’s concordance correlations were low for COHb and MetHb measurements. This may be because the range of COHb and MetHb in the subjects analyzed was only slightly broader than the published accepted uncertainty of the pulse co-oximeter for COHb (±3%) and MetHb (±1%). This does not detract from the clinical usefulness of the non-invasive measurement technique, whose bias and precision for measuring the dyshemoglobins were within the acceptable published range of the pulse co-oximeter.
Although our SCD subjects had elevated COHb and MetHb levels, this study was limited to clinically stable subjects who were at a steady disease state. Thus, further studies will be necessary to assess the performance of the pulse co-oximeter during episodes of acute chest syndrome or vaso-occlusive pain when COHb and MetHb may be further elevated.
Patients with SCD can have elevated levels of dyshemoglobins, and in combination with chronic anemia, have decreased arterial oxygen content. To better understand why a patient with SCD is having OHD, it is necessary to accurately measure the oxyhemoglobin saturation; knowing COHb and MetHb concentrations is essential. Non-invasive measurements of SpCO and SpMet with a pulse co-oximeter are beneficial in helping clinicians more accurately detect hypoxemia in children with SCD. Although the non-invasive technique slightly overestimated the invasive COHb measurements and slightly underestimated the MetHb measurements, there was close agreement between the two methods.
Acknowledgments
Financial Support
This work was supported by the National Heart, Lung, and Blood Institute, USA [Grant R01-HL79911] and the National Center for Research Resources, USA [Grant UL1RR024134]. CM has received support from Philips Respironics for investigator-initiated research, unrelated to the current study.
Footnotes
Financial/Nonfinancial Disclosures
No potential conflicts of interest exist between any of the authors and any companies/organizations whose products or services were discussed in this article. The authors have no competing interests.
References
- 1.Fowler NO, Smith O, Greenfield JC. Arterial blood oxygenation in sickle cell anemia. Am J Med Sci. 1957;234:449–458. doi: 10.1097/00000441-195710000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Jensen WN, Rucknagel D, Taylor WL. Arterial oxygen unsaturation and possible mechanisms of its production in sickle cell anemia. J Clin Invest. 1957;36:905–911. [Google Scholar]
- 3.Rodman T, Close HP, Cathcart R, Purcell MK. The oxyhemoglobin dissociation curve in the common hemoglobinopathies. Am J Med. 1959;27:558–566. doi: 10.1016/0002-9343(59)90041-5. [DOI] [PubMed] [Google Scholar]
- 4.Needleman JP, Franco ME, Varlotta L, Reber-Brodecki D, Bauer N, Dampier C, Allen JL. Mechanisms of nocturnal oxyhemoglobin desaturation in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28:418–422. doi: 10.1002/(sici)1099-0496(199912)28:6<418::aid-ppul6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Quinn CT, Ahmad N. Clinical correlates of steady-state oxyhaemoglobin desaturation in children who have sickle cell disease. Br J Haematol. 2005;131(1):129–134. doi: 10.1111/j.1365-2141.2005.05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Homi J, Levee L, Higgs D, Thomas P, Serjeant G. Pulse oximetry in a cohort study of sickle cell disease. Clin Lab Haematol. 1997;19(1):17–22. doi: 10.1046/j.1365-2257.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- 7.Rackoff WR, Kunkel N, Silber JH, Asakura T, Ohene-Frempong K. Pulse oximetry and factors associated with hemoglobin oxygen desaturation in children with sickle cell disease. Blood. 1993;81(12):3422–3427. [PubMed] [Google Scholar]
- 8.Needleman JP, Setty BN, Varlotta L, Dampier C, Allen JL. Measurement of hemoglobin saturation by oxygen in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28:423–428. doi: 10.1002/(sici)1099-0496(199912)28:6<423::aid-ppul7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Bromberg PA, Jensen WN. Blood oxygen dissociation curves in sickle cell disease. J Lab Clin Med. 1967;70:480–488. [PubMed] [Google Scholar]
- 10.Sears DA, Udden MM, Thomas LJ. Carboxyhemoglobin levels in patients with sickle-cell anemia: relationship to hemolytic and vasoocclusive severity. Am J Med Sci. 2001;322:345–348. doi: 10.1097/00000441-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Uetani Y, Nakamura H, Okamoto O, Yamazaki T, Vreman HJ, Stevenson DK. Carboxyhemoglobin measurements in the diagnosis of ABO hemolytic disease. Acta Paediatr Jpn. 1989;31:171–176. doi: 10.1111/j.1442-200x.1989.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 12.Engel RR, Rodkey FL, Krill CE., Jr Carboxyhemoglobin levels as an index of hemolysis. Pediatrics. 1971;47(4):723–730. [PubMed] [Google Scholar]
- 13.Shalev O, Hebbel RP. Catalysis of soluble hemoglobin oxidation by free iron on sickle red cell membranes. Blood. 1996;87:3948–3952. [PubMed] [Google Scholar]
- 14.Zerez CR, Lachant NA, Tanaka KR. Impaired erythrocyte methemoglobin reduction in sickle cell disease: dependence of methemoglobin reduction on reduced nicotinamide adenine dinucleotide content. Blood. 1990;76:1008–1014. [PubMed] [Google Scholar]
- 15.Fitzgerald RK, Johnson A. Pulse oximetry in sickle cell anemia. Crit Care Med. 2001;29:1803–1806. doi: 10.1097/00003246-200109000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Pianosi P, Charge TD, Esseltine DW, Coates AL. Pulse oximetry in sickle cell disease. Arch Dis Child. 1993;68(6):735–738. doi: 10.1136/adc.68.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comber JT, Lopez BL. Evaluation of pulse oximetry in sickle cell anemia patients presenting to the emergency department in acute vasoocclusive crisis. Am J Emerg Med. 1996;14:16–18. doi: 10.1016/S0735-6757(96)90005-4. [DOI] [PubMed] [Google Scholar]
- 18.Blaisdell CJ, Goodman S, Clark K, Casella JF, Loughlin GM. Pulse oximetry is a poor predictor of hypoxemia in stable children with sickle cell disease. Arch Pediatr Adolesc Med. 2000;154:900–903. doi: 10.1001/archpedi.154.9.900. [DOI] [PubMed] [Google Scholar]
- 19.Zijlstra WG, Buursma A, Meeuwsen-van der Roest WP. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin Chem. 1991;37(9):1633–1638. [PubMed] [Google Scholar]
- 20.Barker SJ, Curry J, Redford D, Morgan S. Measurement of carboxyhemoglobin and methemoglobin by pulse oximetry: a human volunteer study. Anesthesiology. 2006;105:892–897. doi: 10.1097/00000542-200611000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Barker SJ, Tremper KK. The effect of carbon monoxide inhalation on pulse oximetry and transcutaneous PO2. Anesthesiology. 1987;66(5):677–679. doi: 10.1097/00000542-198705000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Barker SJ, Tremper KK, Hyatt J. Effects of methemoglobinemia on pulse oximetry and mixed venous oximetry. Anesthesiology. 1989;70(1):112–117. doi: 10.1097/00000542-198901000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Craft JA, Alessandrini E, Kenney LB, Klein B, Bray G, Luban NL, Meek R, Nadkarni VM. Comparison of oxygenation measurements in pediatric patients during sickle cell crises. J Pediatr. 1994;124(1):93–95. doi: 10.1016/s0022-3476(94)70260-8. [DOI] [PubMed] [Google Scholar]
- 24.Kress JP, Pohlman AS, Hall JB. Determination of hemoglobin saturation in patients with acute sickle chest syndrome: a comparison of arterial blood gases and pulse oximetry. Chest. 1999;115(5):1316–1320. doi: 10.1378/chest.115.5.1316. [DOI] [PubMed] [Google Scholar]
- 25.DeYoung A, Noble RW. Oxygen binding to sickle cell hemoglobin. Methods Enzymol. 1981;76:792–803. doi: 10.1016/0076-6879(81)76158-5. [DOI] [PubMed] [Google Scholar]
- 26.Sunshine HR, Hofrichter J, Ferrone FA, Eaton WA. Oxygen binding by sickle cell hemoglobin polymers. J Mol Biol. 1982;158(2):251–273. doi: 10.1016/0022-2836(82)90432-6. [DOI] [PubMed] [Google Scholar]
- 27.Suner S, Partridge R, Sucov A, et al. Non-invasive pulse co-oximetry screening in the emergency department identifies occult carbon monoxide toxicity. J Emerg Med. 2008;34(4):441–450. doi: 10.1016/j.jemermed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Plante T, Harris D, Savitt J, Akhlaghi F, Monti J, Jay GD. Carboxyhemoglobin monitored by bedside continuous CO-oximetry. J Trauma. 2007;63(5):1187–1190. doi: 10.1097/01.ta.0000242769.04246.5d. [DOI] [PubMed] [Google Scholar]
- 29.Schembre D, Ayub K, Gibbons E, Simmons S, Hampson N. Noninvasive monitoring for methemoglobin after topical application of benzocaine during upper endoscopy and trans-esophageal echocardiography [abstract] Gastrointest Endosc. 2007;65:AB324. [Google Scholar]
- 30.Masimo Corporation. Radical-7 signal extraction pulse CO-Oximeter operator’s manual. 2007. [Google Scholar]
- 31.Siemens Medical Solutions Diagnostics. Rapidlab 1200 systems operator’s manual. 2007. [Google Scholar]
- 32.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 33.Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. Statistician. 1983;32:307–317. [Google Scholar]
- 34.Hargrave DR, Wade A, Evans JP, Hewes DK, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101:846–848. doi: 10.1182/blood-2002-05-1392. [DOI] [PubMed] [Google Scholar]
- 35.Kirkham FJ, Hewes DK, Prengler M, Wade A, Lane R, Evans JP. Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. Lancet. 2001;357:1656–1659. doi: 10.1016/s0140-6736(00)04821-2. [DOI] [PubMed] [Google Scholar]
- 36.Quinn CT, Sargent JW. Daytime steady-state haemoglobin desaturation is a risk factor for overt stroke in children with sickle cell anaemia. Br J Haematol. 2008;140(3):336–339. doi: 10.1111/j.1365-2141.2007.06927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Setty BN, Stuart MJ, Dampier C, Brodecki D, Allen JL. Hypoxaemia in sickle cell disease: biomarker modulation and relevance to pathophysiology. Lancet. 2003;362:1450–1455. doi: 10.1016/S0140-6736(03)14689-2. [DOI] [PubMed] [Google Scholar]
- 38.Minniti CP, Sable C, Campbell A, et al. Elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease: association with hemolysis and hemoglobin oxygen desaturation. Haematologica. 2009;94(3):340–347. doi: 10.3324/haematol.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed S, Siddiqui AK, Sison CP, Shahid RK, Mattana J. Hemoglobin oxygen saturation discrepancy using various methods in patients with sickle cell vaso-occlusive painful crisis. Eur J Haematol. 2005;74(4):309–314. doi: 10.1111/j.1600-0609.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 40.Ortiz FO, Aldrich TK, Nagel RL, Benjamin LJ. Accuracy of pulse oximetry in sickle cell disease. Am J Respir Crit Care Med. 1999;159:447–451. doi: 10.1164/ajrccm.159.2.9806108. [DOI] [PubMed] [Google Scholar]
- 41.Nickerson BG, Sarkisian C, Tremper K. Bias and precision of pulse oximeters and arterial oximeters. Chest. 1988;93(3):515–517. doi: 10.1378/chest.93.3.515. [DOI] [PubMed] [Google Scholar]
- 42.Forte VA, Jr, Malconian MK, Burse RL, et al. Operation Everest II: comparison of four instruments for measuring blood O2 saturation. J Appl Physiol. 1989;67(5):2135–2140. doi: 10.1152/jappl.1989.67.5.2135. [DOI] [PubMed] [Google Scholar]
- 43.Haymond S, Cariappa R, Eby CS, Scott MG. Laboratory assessment of oxygenation in methemoglobinemia. Clin Chem. 2005;51(2):434–444. doi: 10.1373/clinchem.2004.035154. [DOI] [PubMed] [Google Scholar]
- 44.Nahavandi M, Nichols JP, Hassan M, Gandjbakhche A, Kato GJ. Near-infrared spectra absorbance of blood from sickle cell patients and normal individuals. Hematology. 2009;14(1):46–48. doi: 10.1179/102453309X385133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nahavandi M, Millis RM, Tavakkoli F, et al. Arterialization of peripheral venous blood in sickle cell disease. J Natl Med Assoc. 2002;94(5):320–326. [PMC free article] [PubMed] [Google Scholar]
- 46.Crapo RO, Jensen RL, Hegewald M, Tashkin DP. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Respir Crit Care Med. 1999;160:1525–1531. doi: 10.1164/ajrccm.160.5.9806006. [DOI] [PubMed] [Google Scholar]