Abstract
The author group has previously established an in vivo subchronic cigarette smoke (CS) exposure rat model, in which the systemic oxidative burden as well as the modulation of local anti-oxidative enzymes in the lung has been demonstrated. Oxidative stress has been shown to induce pro-inflammatory cytokine release, including interleukin (IL)-6 in the airways. In this study, we aimed to investigate the changes in IL-6 production, as well as the oxidative/anti-oxidative responses in the cerebral cortex using the same in vivo model. IL-6 was determined by RT-PCR and western-blot analysis. Local oxidative and anti-oxidative responses were determined by measuring cerebral cortical malondialdehyde (MDA) and advanced oxidation protein product (AOPP) levels, superoxide dismutase (SOD) and catalase activities, and the reduced to oxidized glutathione (GSH/GSSG) ratio. Nitrite level was measured by fluorescent spectrophotometry. Our results demonstrated a significant increase in both IL-6 mRNA and protein levels. Reductions of SOD activity and manganese (Mn)SOD protein level were observed together with the increased level of superoxide measured by chemiluminescent signal, after 56 days of CS exposure. There were no significant changes in the cerebral cortical levels of MDA, AOPP, catalase activity, and the GSH/GSSG ratio. Nitrite level was significantly reduced, together with the decreased protein level of nNOS in the cerebral cortex, after 56 days of CS exposure. Our results suggest that exposure to CS induces IL-6 expression in the cerebral cortex, which is not mediated by the oxidative/anti-oxidative imbalance.
Keywords: Neuroinflammation, Nitric oxide, Reactive oxygen species, Smoking
Interleukin (IL)-6 is a cytokine that mediates immune responses and inflammation processes. In the 1980s, IL-6 was first discovered to be produced by lymphocytes that mediated differentiation of B-cells (Hirano et al. 1986; Van Damme et al. 1987). Molecular pathway of IL-6 is initiated by the binding of IL-6 to its cell surface receptor, which triggers the recruitment of the signal transducer subunit gp130 (Heinrich et al. 2003), and induces the second messenger cascade. The inflammatory roles of IL-6 are contradictory. IL-6 has been reported to be involved in both pro-, and anti-inflammatory processes (Spooren et al. 2011). As an inflammatory cytokine, IL-6 plays a particularly important role in neuronal defensive mechanisms. It has been reported that the overexpression of IL-6 promotes astrogliosis and microgliosis in different in vivo models (Fattori et al. 1995; Tilgner et al. 2001). On the other hand, IL-6 is suggested to have an anti-inflammatory role, contributing toward the maintenance of the blood–brain barrier (BBB) integrity under neuroinflammatory conditions (Milner and Campbell 2006). Other than being an inflammatory cytokine, IL-6 is also considered as a neurotrophic factor. It has been shown to enhance neuronal differentiation in different cell types (Cao et al. 2006; Sterneck et al. 1996; Zhang et al. 2007) and promote neurogenesis (Islam et al. 2009). The exact role of IL-6 in neurological disorders is yet to be fully understood; however, elevated IL-6 level has been associated with many neurological diseases, including multiple sclerosis (Maimone et al. 1991), dementia (Zuliani et al. 2007), Parkinson’s disease (Nagatsu et al. 2000), autism, and schizophrenia (Patterson 2009). An in vivo study demonstrated that maternal immune activation—a popular hypothesis regarding the pathogenesis of neurodevelopmental disorders—failed to induce the behavior changes associated with autism and schizophrenia in IL-6−/− mice offspring model compared to that of the wild-type strain (Smith et al. 2007), indicating the role of IL-6.
Cigarette smoking is not only a major risk factor for airway diseases, but also for neurodegenerative disorders, including Alzheimer’s disease (AD, Ronnemaa et al. 2011; Barnes and Yaffe 2011). Cigarette smoke (CS) induces oxidative damage in different ways. Free radicals generated by CS induce lipid peroxidation, which can be measured by its byproducts, 8-isoprostane and malondialdehyde (MDA, Armstrong and Browne 1994). On the other hand, oxidant-mediated protein damage can be determined by the level of advanced oxidation protein products (AOPP, Witko-Sarsat et al. 1996). The oxidative/anti-oxidative imbalance that is triggered by long-term exposure to the abundant reactive oxygen species (ROS) in CS is known to induce pro-inflammatory cytokines, including IL-6 in the lung (Crapo 2003). It is unclear, however, whether exposure to CS would lead to an elevated level of IL-6 in the cerebral cortex secondary to oxidative/anti-oxidative imbalance.
We have previously found that serum cotinine, an alkaloid found in tobacco that can be used as an indicator for CS exposure and 8-isoprostane were significantly increased; lung superoxide dismutase (SOD) and catalase activity were also elevated as a self-defensive response after 56 days of CS exposure in our established subchronic CS exposure rat model (Chan et al. 2009). SOD is responsible for catalyzing the conversion of superoxide anions into hydrogen peroxide (Zelko et al. 2002) which is further decomposed into water and oxygen by catalase (Chelikani et al. 2004). On the other hand, hydrogen peroxide is also removed by the reduced glutathione (GSH) and oxidized glutathione (GSSG) system (Sies 1999). Based on our previous findings in the lung, we hypothesized that rat cerebral cortical levels of IL-6 would be increased after CS exposure for 56 days through oxidative pathway. We aimed to determine the cerebral cortical levels of IL-6 and changes in local oxidative/anti-oxidative markers using our established subchronic CS exposure rat model.
The subchronic CS exposure rat model was previously established in our group, and the protocol was published elsewhere (Chan et al. 2009). This approach was attempted to mimic the situation of secondary smoke exposure in healthy subjects (Chow et al. 1996), from adolescent-to-early adulthood in our study. In brief, 22 male Sprague–Dawley (SD) rats (150–200 g, around 5–6 weeks old) were purchased from the Laboratory Animal Unit (LAU) of The University of Hong Kong and were randomly divided into two groups. Cigarette smoking (CS) group was exposed with 4% (v/v, smoke/air) CS (11 mg Tar, 0.8 mg Nicotine; Camel; filter, R.J. Reynolds, Winston-Salem, NC, USA) with the mouthpiece filter removed (by cutting the wrapping paper circumferentially at the point where the glass-fiber filter meets the tobacco leaves) for 1 h daily for 56 consecutive days, while SA control group was exposed to fresh air (0%, v/v, smoke/air), simultaneously. After 56 days of exposure, the rats were euthanized by administering overdose of pentobarbitone. The cortex was dissected and stored in −80°C until further analysis. This protocol was approved by the Committee on the Use of Live Animals in Teaching and Research (CULATR) of The University of Hong Kong. From our previous findings, the mean value of serum cotinine in CS exposure group was 7.19 ± 0.80 ng/ml (mean ± SEM), cotinine was not detected in serum of SA control group (Chan et al. 2009).
IL-6 mRNA expression was determined using semi-quantitative RT-PCR method. Total RNA was extracted from cerebral cortical tissues using TRI reagent (Molecular Research Center, Inc., Cincinnati, OH, USA). cDNA was generated by reverse-transcription as follows: total RNA (1 μg) was added to a mixture of 5× RT buffer (USB Corporation, Cleveland, OH, USA), dNTP (20 mM), Oligo dt (1 μg, Invitrogen), RNaseOUT inhibitor (40 U, Invitrogen), and M-MLV reverse transcriptase (400 U, USB Corporation, Cleveland, OH, USA), topped up to 40 μl with DEPC water. The reaction mixture was incubated at 37°C for 30 min, and then at 75°C for 10 min. cDNA was stored at 4°C until further analysis. Gene transcript level was assessed by PCR. Guanine nucleotide-binding protein β-polypeptide 2-like 1 (GNB2L1) mRNA expression served as an internal control. The primers were generated by Invitrogen (for IL-6, forward primer: 5′-CCTATTGAAAATCTGCTCTGGTCTTCTGG-3′, backward primer: 5′-CTTCAAGTGCTTTCAAGATGAGTTGGATGG-3′; for GNB2L1, forward primer: 5′-GAGTGTGGCCTTCTCCTCT-3′, backward primer: 5′-GCTTGCAGTTAGCCAGGTT-3′). For quantitative analysis, band intensity was measured by software ImageJ (NIH, Bethesda, ML, USA). Results are expressed as target gene to GNB2L1 ratio.
Cerebral cortical tissues were lysed in ice-cold lysis buffer containing phenylmethylsulfonyl fluoride (1 mM) and protease inhibitor cocktail (Sigma-Aldrich). Protein concentration was determined by DC protein assay kit (Bio-Rad, Richmond, CA, USA). Protein extracts were separated in 8–15% SDS-PAGE gel and then transferred onto a PVDF membrane. The membrane was blocked by 5% skim milk in Tris-buffered saline (pH 7.4) containing 0.1% Tween-20. Subsequently, blocked membrane was incubated with diluted rabbit-anti-IL-6 (Abcam, 1:1,000), goat-anti-ionized calcium binding adaptor molecule-1 (Iba-1) (Abcam, 1:1,000), mouse-anti-glial fibrillary acidic protein (GFAP) (Sigma, 1:1,000), rabbit-anti-gp130 (Santa Cruz, 1:100), rabbit-anti-NOS1 (Santa Cruz, 1:200) or rabbit-anti-SOD2 (Santa Cruz, 1:200) primary antibody at 4°C overnight. The membrane was incubated with horseradish peroxidase (HRP)-conjugated goat-anti-rabbit, goat-anti-mouse, or rabbit-anti-goat secondary antibody (Dako, 1:2,000) for 1 h at room temperature. Bands were visualized on a Fuji X-ray film (Fujifilm, Tokyo, Japan) using an enhanced chemiluminescence (ECL) kit. After developed the target proteins, membranes were stripped and re-probed for corresponding internal control. Quantitative analysis of the chemiluminescent signal was done by software Image J. The protein of interest-to-internal control ratio was calculated. Results are expressed as fold of control.
Cortical MDA levels were measured by thiobarbituric acid reactive substances (TBARS) assay kit (Cayman Chemical) according to manufacturer’s instruction. The change in absorbance was measured at wavelength 540 nm. The concentration of MDA corrected with protein amount is expressed as fold of control.
Cortical AOPP levels were measured by spectrophotometric detection method. 200 μl of cerebral cortical homogenates or chloramin T (0–160 μM) were incubated with 1.16 M potassium iodide (10 μl) for 5 min at room temperature. The reaction was stopped by acetic acid (20 μl). The change in absorbance was measured at wavelength 340 nm. The concentration of AOPP corrected with protein amount is expressed as fold of control.
The SOD activity in cerebral cortical homogenates was measured with reference to the rate of cytochrome c reduction. The protocol has previously been reported by Chan et al. (2009). The rate of the change in absorbance was measured at wavelength 550 nm. SOD activity corrected with protein amount is expressed as fold of control.
The catalase activity in cerebral cortical homogenates was determined based on their reaction with hydrogen peroxide. The protocol has previously been reported by Chan et al. (2009). Catalase activity corrected with protein amount is expressed as fold of control.
Total GSH and GSSG concentrations were measured based on a protocol established previously (Rahman et al. 2006). For GSSG measurement, diluted cerebral cortical homogenates (25 μl in sodium phosphate buffer and 5% sulfosalicylic acid) were incubated with vinylpyridine (2 μl) at room temperature for 1 h. The mixture was then incubated with a master mix containing sodium phosphate buffer (143 mM), EDTA (6.3 mM), nicotinamide dinucleotide phosphate (NADPH, 2.39 mM), glutathione reductase, and 5,5-dithiobis-2-nitrobenzoic acid (DTNB, 10 mM) (Sigma-Aldrich) in dark at room temperature for 30 min. The absorbance was measured at wavelength of 405 nm. The procedure for measuring total GSH was the same as mentioned above, except that no incubation with 2-vinylpyridine was performed. Reduced GSH was calculated by subtracting the amount of GSSG from total GSH. The ratio of the reduced GSH/GSSG was calculated, and the results are expressed as fold of control.
The measurement for nitrite levels was performed as follows: Cerebral cortical homogenates were incubated with diluted 2,3-diaminonaphthalene (DAN) in the dark at room temperature for 10 min. The reaction was stopped by sodium hydroxide (1.4 M). Fluorometric signal was detected by excitation and emission wavelengths at 380 and 460 nm, respectively. The nitrite levels corrected with protein amount are expressed as fold of control.
The measurements for superoxide levels were performed as follows: Cerebral cortical homogenates (20 μg) were incubated with lucigenin (0.5 mM, Santa Cruz, CA, USA) in PBS. The chemiluminescent signal was measured immediately after the addition of lucigenin using a microplate reader (FLUOstar Optima, BMG LABTECH, Victoria, Australia). The chemiluminescent signal is expressed as relative luminescence unit (RLU) per microgram protein.
Numerical data are expressed as mean ± standard error of the mean (SEM). Differences in parameters between groups were analyzed by non-parametric Mann–Whitney U test, with the use of the Statistical Program for the Social Sciences (SPSS, edition 18.0). A p value less than 0.05 would be regarded as statistically significant.
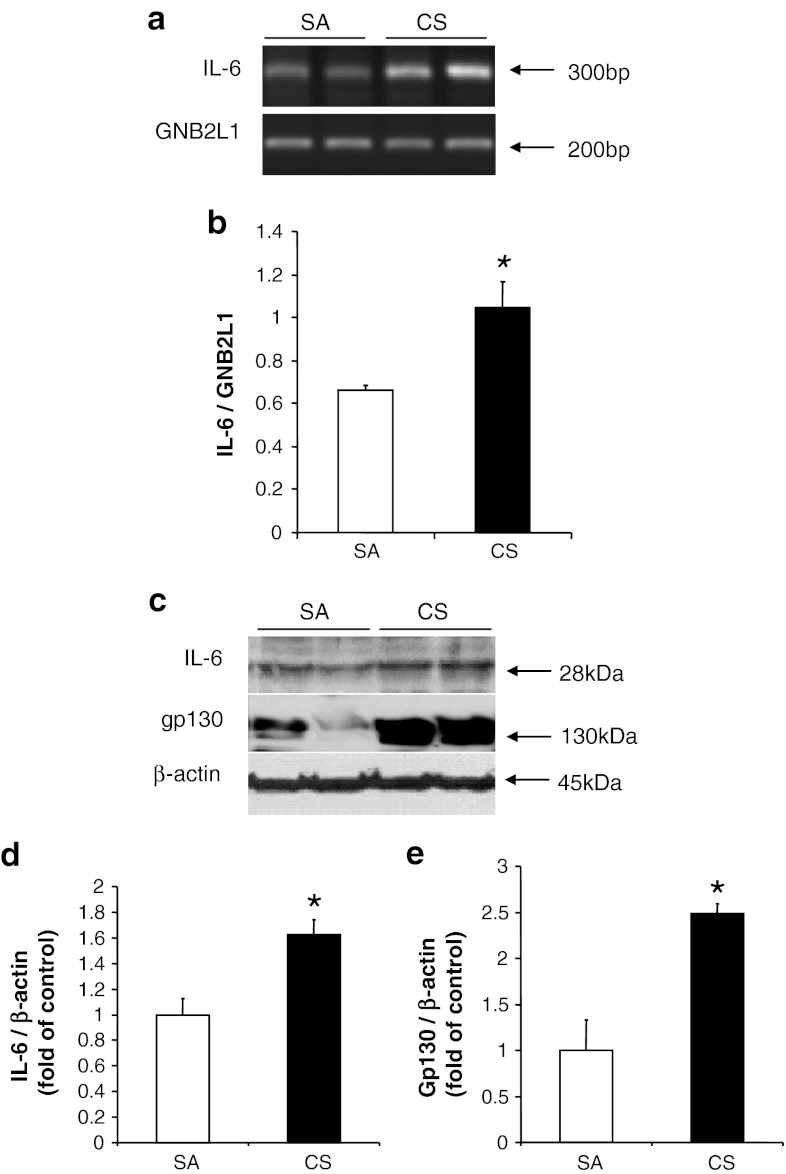
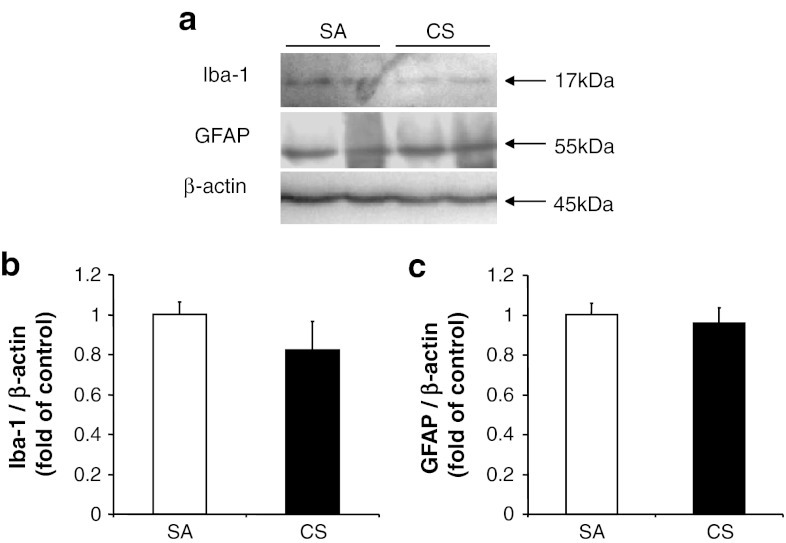
Our results demonstrated a significant increase of both IL-6 mRNA and protein levels in line with the protein level of gp130 in the rat cerebral cortex after 56 days of CS exposure (Fig. 1). The elevated protein level of gp130 indicated an increased binding of IL-6 to its receptor. Activations of microglia and astrocytes have been associated with the brain inflammation and injury, which can be induced by IL-6 (Balasingam et al. 1994). In our model, we observed no significant changes in the expression of Iba-1 or GFAP in cortex after CS exposure, which is consistent with the earlier literature (Fig. 2, Fuller et al. 2010). These findings suggest that the inflammatory pathway had not yet been activated even when IL-6 levels were elevated in our model.
Fig. 1.
Induction of IL-6 in cortex after 56 days of CS exposure. a Expression of IL-6 mRNA, with GNB2L1 serving as an internal control. b Quantitative analysis of luminescent band intensity of IL-6. Results are expressed as IL-6 to GNB2L1 ratio. c Protein levels of IL-6 and gp130, with β-actin serving as an internal control. d, e Quantitative analysis of band intensity by densitometry. Values represent the mean ± SEM. *p < 0.05, when comparing between SA and 56 days of CS exposure groups (n ≥ 4)
Fig. 2.
Expression of glial and astrocyte marker. a Protein levels of Iba-1 and GFAP, with β-actin serving as an internal control. b, c Quantitative analysis of band intensity from the blots. Results are expressed as fold of control. Values represent the mean ± SEM. No significant differences were obtained between the groups (n ≥ 4)
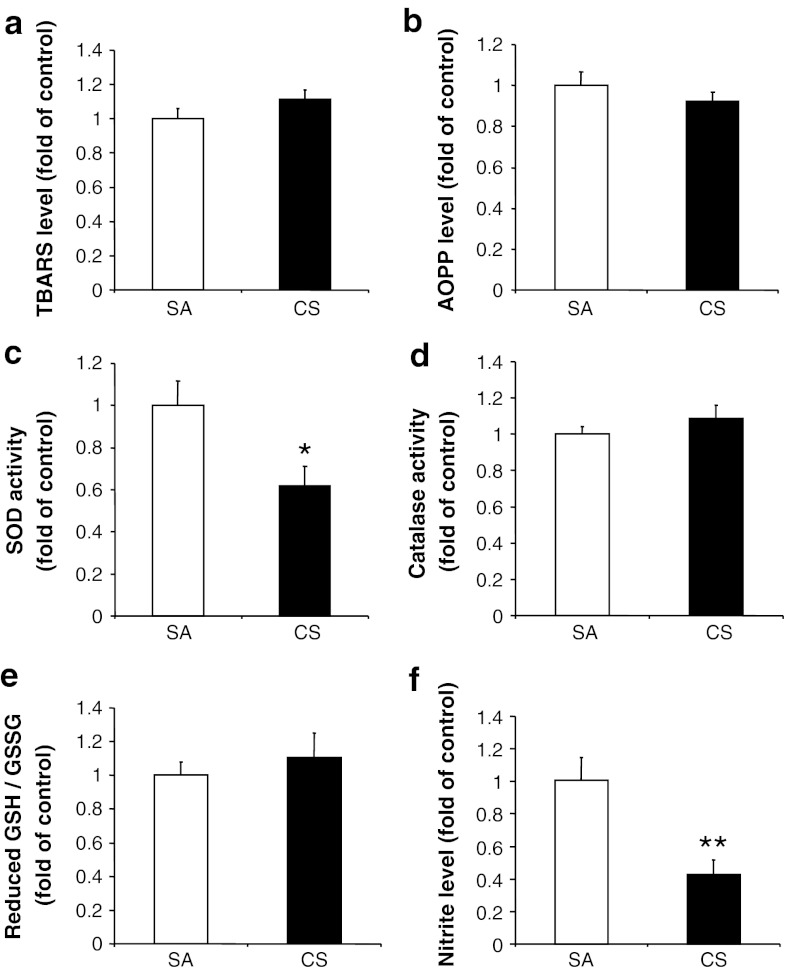
To determine the local oxidative response in the cortex, the MDA and AOPP levels were measured. Our results showed no significant changes in the cortical MDA (6.5 ± 1.1 nmol/mg protein for control values) or AOPP (47.7 ± 12.6 nmol/mg protein for control values) levels after 56 days of CS exposure (Fig. 3a, b), indicating no oxidative burden.
Fig. 3.
Changes in oxidative and anti-oxidative markers after 56 days of CS exposure. a MDA level, b AOPP level, c SOD activity, d catalase activity, e the GSH/GSSG ratio, and f nitrite level measured in cerebral cortex. Values represent the mean ± SEM. *p < 0.05, **p < 0.01, when comparing between SA and 56 days of CS exposure groups (n ≥ 10)
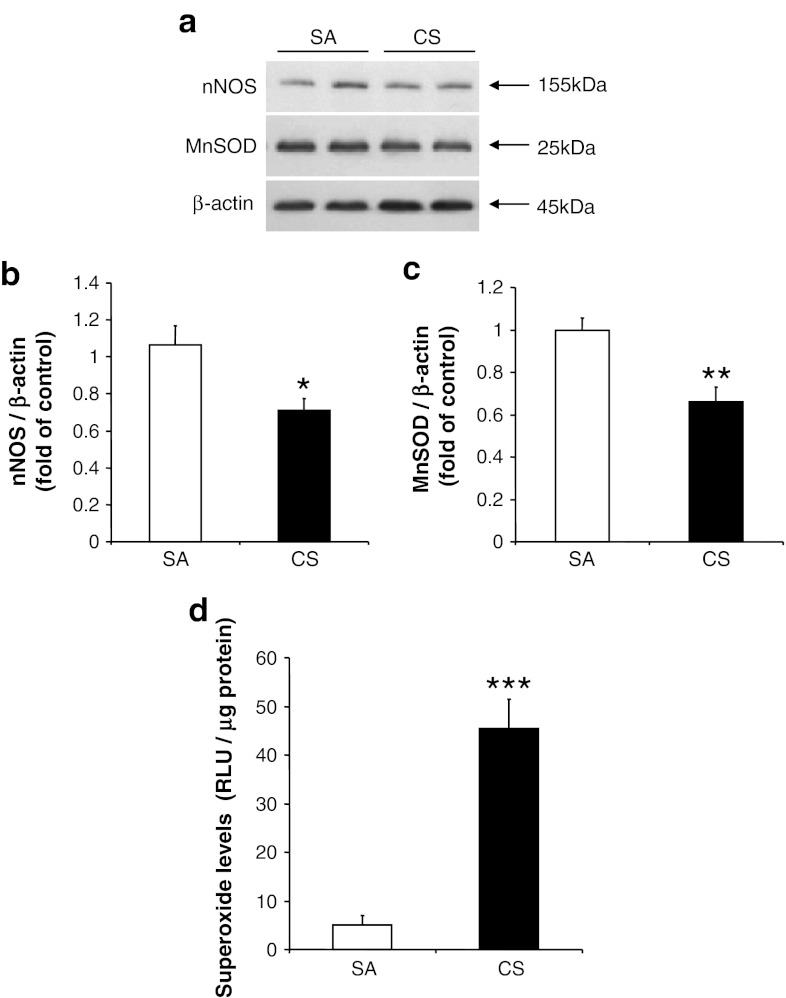
We further investigated the changes in different anti-oxidative markers in the cortex. We found that there was a significant reduction of total SOD activity (201.5 ± 82.7 mU/mg protein for control values) after CS exposure, in agreement with the earlier literature (Luchese et al. 2009), but not catalase activity (4.9 ± 0.6 U/mg protein for control values). Furthermore, no significant changes in GSH/GSSG ratio were observed after 56 days CS exposure (Fig. 3c–e). For further investigation of whether the decreased SOD activity would be due to reduction of its protein level, the protein level of manganese (Mn)SOD was determined after CS exposure. Our result demonstrated that CS reduced the protein level of MnSOD (Fig. 4a, c). SOD is the first line of anti-oxidative enzyme that is responsible for the defensive mechanism against ROS and other superoxide anion-free radicals (Zelko et al. 2002). The reduction of SOD activity might lead to the accumulation of superoxide anions in the brain. To confirm this, we examined the superoxide levels in the cerebral cortex after CS exposure by chemiluminescence assay. We found that the superoxide levels were significantly higher in the cortex (5.1 ± 1.9 RLU/μg protein versus 45.4 ± 6.0 RLU/μg protein) after CS exposure (Fig. 4d). The increased superoxide anions level can react with nitrogen species to form peroxynitrite which is a more potent oxidant involved in the nitration process of SOD. Post-translational modifications, including phosphorylation and nitration of SOD, have been associated with the reduced activity of SOD (Yamakura and Kawasaki 2010). The question as to whether these mechanisms are involved in the reduction of SOD activity in our model requires further investigation.
Fig. 4.
Changes in nNOS, MnSOD protein levels, and superoxide levels after 56 days of CS exposure. a Protein levels of nNOS and MnSOD, with β-actin serving as an internal control. b, c Quantitative analysis of band intensity from the blots. Results are expressed as fold of control. d Superoxide level measured in cerebral cortex. Values represent the mean ± SEM. *p < 0.05, **p < 0.01, *** p < 0.001, when comparing between SA and 56 days of CS exposure groups (n ≥ 7)
Nitrite levels are associated with oxidative stress in the rat brain (Vatassery et al. 2004). Intrahippocampal injection of IL-6 has been shown to induce nitrite levels (Ma and Zhu 2000). In contrast, we observed a decreased nitrite level in the cortex (74.5 ± 17.7 pmol/mg protein for control values, Fig. 3f) even with the increased IL-6 levels after 56 days of CS exposure. The reduced nitrite level may be explained by the decreased protein level of nNOS after CS exposure (Fig. 4a, b). On the other hand, inactive astrocytes and microglia may also explain the reduced level of nitrite after CS exposure, as active astrocytes and microglia are known to be responsible for the increased production of nitrite during inflammation (Storer et al. 2005).
CS has been shown to induce pro-inflammatory cytokines via nicotinic and oxidative pathways in dendritic cells (Vassallo et al. 2008). It is unclear as to whether the increased IL-6 levels in our in vivo model are mediated by the nicotinic pathways, which requires further investigation. Unlike what we found previously in the lung, our data appear to reject the hypothesis that CS-induced IL-6 is mediated by the oxidative/anti-oxidative imbalance in the cortex of our subchronic CS exposure in vivo model; however, it is unclear as to whether sustained exposure to CS in a chronic manner would trigger oxidative burden in the brain. Furthermore, our interpretations are limited by the age of the rats, since oxidative/antioxidative imbalance is more severe in an aged brain (Venkateshappa et al. 2011); hence, studies involving different age groups of rats would be required for better understanding on the dynamic oxidative changes at different ages. It is also noteworthy that, since all measurements were carried out in protein samples extracted from the whole cerebral cortex, the changes in IL-6 levels and oxidative/anti-oxidative markers in different cortical regions could not be assessed.
In conclusion, our data demonstrated that CS exposure elevated IL-6 in the rat cortex; however, in contrary to data obtained from our pulmonary studies, “oxidative/anti-oxidative imbalance” is not the mechanism involved in the cortex of our in vivo rat model in early adulthood. The increased IL-6 caused by CS, together with the reduction of nitrite levels, may alter brain function. Furthermore, reduced SOD activity after exposure to CS could play a role in neurodegeneration. More studies are warranted to elucidate the underlying mechanisms on CS-mediated IL-6 induction and the effect of elevated IL-6 in the brain.
Conflict of interest
The authors declare that they have no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Med Biol. 1994;366:43–58. doi: 10.1007/978-1-4615-1833-4_4. [DOI] [PubMed] [Google Scholar]
- Balasingam V, Tejada-Berges T, Wright E, Bouckova R, Yong VW. Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J Neurosci. 1994;14(2):846–856. doi: 10.1523/JNEUROSCI.14-02-00846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Gao Y, Bryson JB, Hou J, Chaudhry N, Siddiq M, Martinez J, Spencer T, Carmel J, Hart RB, Filbin MT. The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J Neurosci. 2006;26(20):5565–5573. doi: 10.1523/JNEUROSCI.0815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KH, Ho SP, Yeung SC, So WH, Cho CH, Koo MW, Lam WK, Ip MS, Man RY, Mak JC. Chinese green tea ameliorates lung injury in cigarette smoke-exposed rats. Respir Med. 2009;103(11):1746–1754. doi: 10.1016/j.rmed.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61(2):192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow JY, Ma L, Cho CH. An experimental model for studying passive cigarette smoking effects on gastric ulceration. Life Sci. 1996;58(26):2415–2422. doi: 10.1016/0024-3205(96)00245-7. [DOI] [PubMed] [Google Scholar]
- Crapo JD. Oxidative stress as an initiator of cytokine release and cell damage. Eur Respir J Suppl. 2003;44:4s–6s. doi: 10.1183/09031936.03.00000203a. [DOI] [PubMed] [Google Scholar]
- Fattori E, Lazzaro D, Musiani P, Modesti A, Alonzi T, Ciliberto G. IL-6 expression in neurons of transgenic mice causes reactive astrocytosis and increase in ramified microglial cells but no neuronal damage. Eur J Neurosci. 1995;7(12):2441–2449. doi: 10.1111/j.1460-9568.1995.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Fuller BF, Gold MS, Wang KK, Ottens AK. Effects of environmental tobacco smoke on adult rat brain biochemistry. J Mol Neurosci. 2010;41(1):165–171. doi: 10.1007/s12031-009-9316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(Pt 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Islam O, Gong X, Rose-John S, Heese K. Interleukin-6 and neural stem cells: more than gliogenesis. Mol Biol Cell. 2009;20(1):188–199. doi: 10.1091/mbc.E08-05-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchese C, Pinton S, Nogueira CW. Brain and lungs of rats are differently affected by cigarette smoke exposure: antioxidant effect of an organoselenium compound. Pharmacol Res. 2009;59(3):194–201. doi: 10.1016/j.phrs.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Ma TC, Zhu XZ. Effects of intrahippocampal infusion of interleukin-6 on passive avoidance and nitrite and prostaglandin levels in the hippocampus in rats. Arzneimittelforschung. 2000;50(3):227–231. doi: 10.1055/s-0031-1300190. [DOI] [PubMed] [Google Scholar]
- Maimone D, Gregory S, Arnason BG, Reder AT. Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. J Neuroimmunol. 1991;32(1):67–74. doi: 10.1016/0165-5728(91)90073-G. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. Increased expression of the beta4 and alpha5 integrin subunits in cerebral blood vessels of transgenic mice chronically producing the pro-inflammatory cytokines IL-6 or IFN-alpha in the central nervous system. Mol Cell Neurosci. 2006;33(4):429–440. doi: 10.1016/j.mcn.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson’s disease. J Neural Transm Suppl. 2000;58:143–151. [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204(2):313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1(6):3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- Ronnemaa E, Zethelius B, Lannfelt L, Kilander L. Vascular risk factors and dementia: 40-year follow-up of a population-based cohort. Dement Geriatr Cogn Disord. 2011;31(6):460–466. doi: 10.1159/000330020. [DOI] [PubMed] [Google Scholar]
- Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27(9–10):916–921. doi: 10.1016/S0891-5849(99)00177-X. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Haegeman G, Gerlo S. Interleukin-6, a mental cytokine. Brain Res Rev. 2011;67(1–2):157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Sterneck E, Kaplan DR, Johnson PF. Interleukin-6 induces expression of peripherin and cooperates with Trk receptor signaling to promote neuronal differentiation in PC12 cells. J Neurochem. 1996;67(4):1365–1374. doi: 10.1046/j.1471-4159.1996.67041365.x. [DOI] [PubMed] [Google Scholar]
- Storer PD, Xu J, Chavis JA, Drew PD. Cyclopentenone prostaglandins PGA2 and 15-deoxy-delta12, 14 PGJ2 suppress activation of murine microglia and astrocytes: implications for multiple sclerosis. J Neurosci Res. 2005;80(1):66–74. doi: 10.1002/jnr.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilgner J, Volk B, Kaltschmidt C. Continuous interleukin-6 application in vivo via macroencapsulation of interleukin-6-expressing COS-7 cells induces massive gliosis. Glia. 2001;35(3):234–245. doi: 10.1002/glia.1088. [DOI] [PubMed] [Google Scholar]
- Van Damme J, Opdenakker G, Simpson RJ, Rubira MR, Cayphas S, Vink A, Billiau A, Van Snick J. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J Exp Med. 1987;165(3):914–919. doi: 10.1084/jem.165.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo R, Kroening PR, Parambil J, Kita H. Nicotine and oxidative cigarette smoke constituents induce immune-modulatory and pro-inflammatory dendritic cell responses. Mol Immunol. 2008;45(12):3321–3329. doi: 10.1016/j.molimm.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatassery GT, SantaCruz KS, DeMaster EG, Quach HT, Smith WE. Oxidative stress and inhibition of oxidative phosphorylation induced by peroxynitrite and nitrite in rat brain subcellular fractions. Neurochem Int. 2004;45(7):963–970. doi: 10.1016/j.neuint.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Venkateshappa C, Harish G, Mythri RB, Mahadevan A, Srinivas Bharath MM, Shankar SK (2011) Increased oxidative damage and decreased antioxidant function in aging human substantia nigra compared to striatum: implications for Parkinson’s disease. Neurochem Res [Epub ahead of print]. doi:10.1007/s11064-011-0619-7 [DOI] [PubMed]
- Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49(5):1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- Yamakura F, Kawasaki H. Post-translational modifications of superoxide dismutase. Biochim Biophys Acta. 2010;1804(2):318–325. doi: 10.1016/j.bbapap.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33(3):337–349. doi: 10.1016/S0891-5849(02)00905-X. [DOI] [PubMed] [Google Scholar]
- Zhang PL, Levy AM, Ben-Simchon L, Haggiag S, Chebath J, Revel M. Induction of neuronal and myelin-related gene expression by IL-6-receptor/IL-6: a study on embryonic dorsal root ganglia cells and isolated Schwann cells. Exp Neurol. 2007;208(2):285–296. doi: 10.1016/j.expneurol.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Guerra G, Ranzini M, Rossi L, Munari MR, Zurlo A, Ble A, Volpato S, Atti AR, Fellin R. High interleukin-6 plasma levels are associated with functional impairment in older patients with vascular dementia. Int J Geriatr Psychiatry. 2007;22(4):305–311. doi: 10.1002/gps.1674. [DOI] [PubMed] [Google Scholar]