Abstract
Benzodiazepines exert their anxiolytic, anticonvulsant, muscle-relaxant and sedative-hypnotic properties by allosterically enhancing the action of GABA at GABAA receptors via their benzodiazepine-binding site. Although these drugs have been used clinically since 1960, the molecular basis of this interaction is still not known. By using multiple homology models and an un biased docking protocol, we identified a binding hypothesis for the diazepam-bound structure of the benzodiazepine site, which was confirmed by experimental evidence. Moreover, two independent virtual screening approaches based on this structure identified known benzodiazepine-site ligands from different structural classes and predicted potential new ligands for this site. Receptor-binding assays and electrophysiological studies on recombinant receptors confirmed these predictions and thus identified new chemotypes for the benzodiazepine-binding site. Our results support the validity of the diazepam-bound structure of the benzodiazepine-binding pocket, demonstrate its suitability for drug discovery and pave the way for structure-based drug design.
GABAA receptors (GABAARs) are the major inhibitory transmitter receptors in the brain and the site of action of a variety of pharmacologically and clinically important drugs, such as benzodiazepines, barbiturates, neuroactive steroids, anesthetics and convulsants1. These receptors are chloride ion channels composed of five subunits that can belong to different subunit classes. A total of 19 GABAA receptor subunits (α1–6, β1–3, γ1–3, δ, ε, π, θ, ρ1–3) have been identified in mammalian brain. The majority of GABAARs are composed of one γ-, two β- and two α-subunits. The two GABA-binding sites of these receptors are located extra-cellularly at β-α interfaces (Fig. 1a). Classical benzodiazepines, such as diazepam, predominantly exert their action via GABAARs composed of α1βγ2, α2βγ2, α3βγ2 and α5βγ2 subunits and are known to bind at the extracellular α-γ interface2 (Fig. 1b). Owing to the lack of high-resolution structural data for this important drug-binding site, however, a consensus binding-mode hypothesis explaining all of the experimental data is still lacking.
Figure 1. Structure of diazepam-sensitive GABAARs and of benzodiazepine ligands.
(a) Top view of the extracellular domain of the pentameric α1β2γ2 GABAAR in ribbon mode. The benzodiazepine-binding site (BZ) and the two GABA-binding sites are indicated by arrows. Diazepam in the benzodiazepine-binding site is depicted in space-filling mode. (b) view from a perspective nearly parallel with the lipid bilayer of the α1γ2 extracellular interface in ribbon mode with a diazepam-bound pose of CBM I in space-filling mode. The dashed window indicates the section shown in Figures 2-5. Segments (loops) A, B, C, D, E, F and G are labeled, and the corresponding protein segment is rendered in a darker shade. In both panels the plus (+) and minus (−) sides of the subunits are indicated. (c) Chemical structures of compounds used in this study. 1-9 were used for docking and 10-14 were used for validation. The pharmacophoric features l1, l3, H1 and H2 (ref. 48) are superimposed on two-dimensional structures of diazepam and flumazenil (11). As an additional feature we introduce H1′ to denote the interaction with the second lone pair of the carbonyl oxygen. More detailed information on the compounds can be found in Supplementary Table 5.
GABAARs, together with nicotinic acetylcholine receptors (nAChRs), glycine receptors and serotonin type 3 receptors, are members of the cysteine-loop receptor superfamily. Although so far no crystal structure of a GABAAR is available, a variety of crystal structures from a functional and structural homolog of the ligand-binding domain of cysteine-loop receptors, the acetylcholine-binding protein (AChBP), and more recently from the nAChR and bacterial homologs thereof, is available3 and demonstrates a high structural conservation within this receptor superfamily. Some of these structures have served as templates for protein homology models and docking studies that have yielded controversial binding-mode hypotheses for various benzodiazepines4-8. However, because of their low sequence identity of <20%, different members of the superfamily have appreciable variability in local interface and pocket structure3. To overcome this problem, we constructed multiple homology models of the GABAAR from a variety of structural templates to broadly sample the benzodiazepine-pocket geometry. We then developed a workflow that, in contrast to automated ligand-supported modeling approaches9, did not rely on energetic scoring functions. This workflow allowed unbiased selection and refinement of those models best suited to describe a diazepam-bound state as well as evaluation of several thousand docking poses of diazepam and its close structural analogs. The procedure yielded a binding mode for diazepam and analogs that is convincingly supported by experimental evidence. Using this binding hypothesis in a virtual screening of a large compound library, we identified new allosteric modulators of GABAARs acting via the benzo diazepine site, thus confirming their suitability for drug discovery and structure-based drug design.
RESULTS
Computational modeling and docking
To account for the substantial variability in local interface and pocket structures10 of cysteine-loop receptor family members, we constructed a total of 37 homology models of the GABAAR derived from eight distinct structural templates (Supplementary Methods, Supplementary Table 1), multiple sequence alignments (Supplementary Fig. 1) and variations in degrees of protein flexibility (Supplementary Schemes 1 and 2). We then docked diazepam in its bioactive conformation (M conformation; Supplementary Fig. 2 and Supplementary Table 2) into all initial models (Supplementary Results, Supplementary Tables 3 and 4 and Supplementary Fig. 3) using the software package FlexX11. FlexX and other docking algorithms are able to explore the conformational space sufficiently well to generate correctly docked poses, but they often fail in ranking the correct poses on top. Furthermore, the binding-mode prediction accuracy of scoring functions is target dependent12. We therefore evaluated the 100 top-scoring poses (FlexX score), thus ensuring that correct poses were retained. We then eliminated homology models that predominantly provided poses with poor lipophilic interaction for ligand features L1 and L3 (Fig. 1c) of the diazepam structure or featured no simultaneous ligand contacts with α1 and γ2 subunits. For the remaining 14 models, we extended docking to multiple ligands (1, 3–5, 8, 9; in M conformation in Fig. 1c) and used the docking protocol FlexE13 to enable the flexibility of those amino acid side chains that have been shown to be important for ligand recognition2 and/or constitute the main steric determinants of the binding site. By applying the above-mentioned criteria for each ligand docked, we further reduced the number of homology models. The three best-performing models were based on Protein Data Bank (PDB) entries 2BYQ3 (Aplysia californica AChBP, epibatidine bound), 1UW6 (ref. 3) (Lymnea stagnalis AChBP, nicotine bound) and 2QC1 (ref. 3) (mouse nAChR, α1 subunit, α-bungarotoxin bound). To improve conformational sampling, we further increased the flexibility of these three models by considering additional rotamers of amino acid side chains in direct contact with pocket-defining residues, especially at the crowded subunit interface between loops A and B. This resulted in three additional models (Supplementary Scheme 2).
Subsequently, we docked ligands 1–9 (Fig. 1c) into the six final models (Supplementary Table 3) using the FlexE protocol. We then performed an energy minimization of all poses and calculated the L1 and L3 interaction strengths. Of the 4,997 energy-minimized poses, we retained 1,463 with L1 and L3 interaction strengths above the median value. These poses still sampled a wide variety of putative binding geometries for smaller ligands such as diazepam and flunitrazepam. Ligands with bulky substituents (Fig. 1c; 4–9) assumed more restricted poses. Thus, by using only lipophilic interaction strength as our selection criterion, we retrieved a set of poses in which all of the binding modes compatible with the sampled pocket topologies were still present.
Binding-mode search
To compare binding modes in different templates, we threedimensionally aligned the ligand-protein complexes on the basis of the conserved domains of the α- and γ-subunits of their benzodiazepine-binding pocket. Then, we identified similar binding modes through cluster analysis (Supplementary Scheme 3) after computing the r.m.s. deviation values of the ligands’ common atom positions14 between each pair of poses and storing them as a matrix. Cluster analysis then produced groups of poses with small differences in r.m.s. deviation. By allowing 2-Å scatter around each centroid pose, thirty clusters, each comprising a group of similar binding modes, emerged (Supplementary Figs. 4 and 5).
Common binding-mode screen
To define candidate geometries representing a common binding mode (CBM), we split the docked ligands (Fig. 1c and Supplementary Table 5) into mandatory ‘core ligands’ (Fig. 1c; 1–5), and ‘accessory ligands’ (Fig. 1c; 6–9). Core ligands have very similar chemical structures, affinities, efficacies and patterns in their response to mutation, suggesting tightly overlapping bound-state geometries. Although this assumption is not necessarily true in each case, it is the basis of all rational hit-optimization efforts and (quantitative) structure-activity studies. Additionally, we also considered compound 5 ‘core’, not as a ligand but as affinity chromatography bait (Supplementary Fig. 6). Accessory ligands should also bind in a similar binding mode as the core ligands, but they either do not share all pharmacophore features with diazepam or, because of steric requirements, may induce some-what different conformations of the binding site. Only three of the clusters contained all of the mandatory core ligands and were thus further considered as CBMs (Supplementary Scheme 3).
For these three clusters, we extended the measure of ‘binding-mode similarity’ to include not only the orientation of the ligand’s common substructure but also the pocket atoms interacting with this substructure. The resulting ‘bound-complex r.m.s. deviation’ value describes the similarity of ligand-pocket interaction patterns. By clustering the bound-complex r.m.s. deviation values, we finally extracted the most homogenous subsets of poses from each parent cluster, resulting in CBM candidates CBM I, CBM II and CBM III (Fig. 2 and Supplementary Tables 6–8).
Figure 2. CBM candidates.

view of the benzodiazepine-binding pockets of the CBM pose pools as depicted in the dashed window of Figure 1b. (a-f) In all panels, the l1-forming annealed benzene group is depicted in orange and the l3-forming pendant phenyl group in purple. The α-subunits are shown in yellow fine-line representation, and the γ-subunits are shown in blue. Color codes used for the seven-membered ring and the side chains: gray for carbon, blue for nitrogen and red for oxygen. All labels are color-matched to the interaction partner. All side chains providing major interactions (H1, H1′, H2, l1 and l3 as defined in Fig. 1c) in a given CBM are indicated in panels a-c to show the variability. The scatter of core ligand poses (Supplementary Table 9) is depicted for each CBM in panels d-f.
Evaluation of the CBM candidates
CBM I (Figs. 2 and 3 and Supplementary Fig. 7) contains the highest number of poses of core and accessory ligands and is more homogeneous in terms of ligand overlap (small average r.m.s. deviation distance to centroid) than the other putative binding modes (Fig. 2 and Supplementary Table 9).
Figure 3. Representation of a CBM I pose of diazepam.

The l1 hydrophobic feature (Fig. 1c) shows strong interactions with α1 Tyr159 and α1 Tyr209 (ref. 26). The l3 hydrophobic feature (Fig. 1c) shows strong interactions with α1 His101 and γ2 Phe77. The asterisk on γ2 Phe77 indicates the position of the hydroxyl group in the ligand-bound F77Y mutants24 (Supplementary Fig. 9a). The arrow indicates rotational freedom of the pendant phenyl ring. The imine nitrogen forms a hydrogen bond with α1 Ser204. The carboxyl oxygen of diazepam with its two lone pairs interacts with γ2 Thr142 and α1 Thr206. The α1 subunit is in yellow, and the γ2 subunit is in blue.
Analysis of all types of ligand interactions with pocket residues indicated that not all of the poses of a given CBM feature all interactions. Whereas in particular the highly rotamer-sensitive hydrogen bonds fluctuate considerably, major lipophilic interactions are present in most poses and are CBM defining. CBM I is the only pose pool in which both of the lipophilic L-features (Fig. 1c) and hydrogen bonds of all of the core ligands are matched. CBM II fails to provide a consensus H1 hydrogen bond (Fig. 1c), and CBM III has a very heterogeneous L-feature pattern (Supplementary Tables 6 and 9).
A comparison of the crystal structures of ligand-bound members of the cysteine-loop receptor family indicated that the positions of large side chains within the binding pocket seem to be well conserved. Notably, the positions of CBM I α1 Tyr159 and α1 Tyr209, the key residues defining its L1 pocket (Fig. 2), are similar to those of homologous residues of the ligand-bound complexes of both AChBP and nAChR, even though we kept all large side chains in the pocket flexible during docking (Supplementary Fig. 8).
Subjecting all poses from the five core ligands used for the CBM screen to two scoring functions15,16 indicated that 13 of the 50 top ten scoring poses were located in CBM I, none were in CBM II and one was in CBM III. The remaining top-scoring poses were distributed randomly across the other 27 clusters (Supplementary Table 9).
Validation of binding modes by experimental evidence
The correct binding mode must be supported by experimental evidence. When flunitrazepam was used as a photolabel for the benzodiazepine-binding pocket, covalent modification of α1 His101 (loop A) was observed17. Similarly, when covalent labeling of various cysteines engineered into the benzodiazepine pocket was investigated with compound 10 (Fig. 1c), which carries a cysteine-reactive isothio cyanate at a position equivalent to that of the nitro group in flunitrazepam, irreversible labeling and permanent modulation of receptors containing the α1 H101C mutation was reported, suggesting that the active state was captured18. CBM I (Fig. 4) and CBM II allow such covalent labeling, whereas CBM III does not (Table 1).
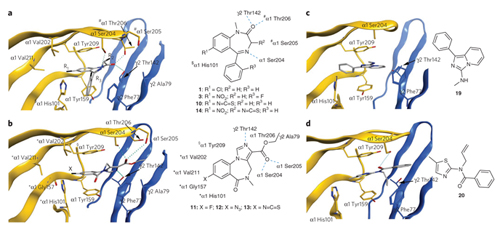
Figure 4. CBM I reference binding modes of diazepam and flumazenil compared to docking poses of virtual screening hits 19 and 20, selected by iFP scoring.
Representation shows the α-subunit (yellow) and γ-subunit (blue). ligand and key residues are rendered in stick representation, and hydrogen-bond interactions are indicated by cyan dotted lines. (a) Two-dimensional scheme shows diazepam 1 and analogs 3, 10 and 14 in CBM I. Position of covalent incorporation of 7-NCS18 is indicated by ($), and the sites of 3-NCS28 reactivity are indicated by (#). (b) Two-dimensional scheme shows flumazenil (11) and analogs 12 and 13 in CBM I. Residues whose respective cysteine mutants react with the imid 13 (ref. 23) are marked (*), and the α1 Tyr209 whose bovine homolog is photolabeled by 12 (ref. 22) is marked (§). The ester group is localized between loops C and D. (c,d) 19 (c) and 20 (d) place their phenyl and thiazol groups in the same tight aromatic binding pocket between α1 His101, α1 Tyr159 and α1 Tyr209 as that occupied by the L1 ring of 1 in a and 11 in b, and they stack with the same γ2 Phe77 as the reference ligands.
Table 1. CBM candidates in light of experimental data.
| Ligandsa | CBM I | CBM II | CBM III | ||||
|---|---|---|---|---|---|---|---|
| α1 His101 | |||||||
| (1) Flunitrazepam photolabeling17 | 3 | All | ++ | All | ++ | None | − |
| (2) 7-NCS covalent labeling18 | 10 | All | ++ | All | ++ | None | − |
| (3) α1 His101 interacts with L3 (ref. 20)b | 3 | 13/16c | ++ | None | − | 3/6c | + |
| α1 Tyr209 | |||||||
| (4) α1 Tyr209 interacts with L1 (ref. 21) | 1 | All | ++ | None | − | All | ++ |
| (5) Ro15-4513 photolabeling22 | 12 | All | ++ | None | − | All | ++ |
| α1 His101, α1 Gly157, α1 Val202 and α1 Val211 | |||||||
| (6) ‘Imid-NCS’ compound covalent labeling23 | 13 | All | ++ | None | − | None | − |
| γ2 Phe77 | |||||||
| (7) γ2 Phe77 interacts with L3 (ref. 24)b | 1-3 | All | ++ | 3/8c | + | 7/12c | + |
| α1 Thr206 | |||||||
| (8) α1 Thr206 hydrogen bonding24 | 1,3 | 34/37c | ++ | 3/7c | + | None | − |
| (9) α1 Thr206 contacts ester24 | 8 | All | ++ | 1/3c | + | None | − |
| α1 Ser205 and α1 Thr206 | |||||||
| (10) 3-NCS covalent labeling28 | 14 | All | ++ | All | ++ | None | − |
| γ2 Ala79 | |||||||
| (11) γ2 Ala79 near ester29 | 8 | All | ++ | All | ++ | None | − |
Only poses of the defined ligands (Fig. 1c) were used for evaluation.
Interactions were calculated using SCORING.SVL.
Number of poses fulfilling the respective criterion in relation to number of total poses in a CBM (Supplementary Table 7 lists the total number of poses per ligand within a CBM).
Receptors that contain the α1 H101R mutation have a drastically reduced affinity for classical benzodiazepines such as flunitrazepam. Compounds lacking the pendant phenyl ring, such as flumazenil, tolerate this mutation19, possibly suggesting an interaction of α1 His101 with the pendant phenyl ring. Alternatively, the loss of flunitrazepam binding in the α1 H101R mutant could reflect an unfavorable steric interaction with arginine7. However, having a cysteine in this position20 results in a loss of affinity for flunitrazepam by a factor of 200, whereas glutamine leads to a loss of affinity by a factor of only 15. These findings were interpreted as evidence for a strong aromatic or hydrophobic interaction between the pendant phenyl ring (L3) of flunitrazepam and α1 His101 (ref. 20). Such an interaction is present in CBM I (Figs. 2 and 3), in which α1 His101 is an essential part of the pocket accommodating L3, and to a lesser degree in CBM III but not in CBM II.
The loop C mutation α1 Y209A, though producing a near wild-type GABA response, leads to a decrease in binding affinity both for diazepam (by a factor of 40) and the imidazobenzodiazepine flumazenil (by a factor of 41) (Fig. 1c; 11)21. This suggests that α1 Tyr209, in contrast to α1 His101, provides an essential interaction with a structure common to diazepam and flumazenil. In CBM I, the L1 pharmacophoric feature common to both compounds forms a strong aromatic interaction with α1 Tyr209 (Fig. 4a,b). Notably, the imidazobenzodiazepine Ro15-4513 (Fig. 1c; 12), which is structurally similar to flumazenil, photolabels α1 Tyr209 (ref. 22) (Fig. 4b). More recently, a derivative of Ro15-4513, in which the azido group has been replaced by an isothiocyanate group (Fig. 1c; 13), has also been shown to react with several engineered cysteines in the α-subunit23. Only CBM I is fully consistent with the reaction patterns of both studies (Fig. 4b) and with the additional reaction of compound 13 with the α1 H101C mutant, which was interpreted as strong evidence for an overlapping binding mode of diazepam and flumazenil23. Together, these results suggest that only CBM I can provide a single structural hypothesis for all of these covalently incorporated derivatives of diazepam, flunitrazepam and flumazenil.
Compounds with a pendant phenyl ring suffer an affinity loss in the loop D γ2 F77Y mutant, which is relatively less pronounced (by a factor of 250) for diazepam but is much stronger in diazepam analogs with chlorine substituents (monochlorodiazepam, by a factor of 530; dichlorodiazepam (Fig. 1c), by a factor of 700 (ref. 24)) that decrease the flexibility of the pendant phenyl ring. Thus, γ2 Phe77 has been proposed to interact with the pendant phenyl ring of diazepam and its analogs24. CBM I features precisely this interaction and has a sufficiently narrow L3 pocket, defined mostly by γ2 Phe77 and α1 His101, to possibly cause steric problems when phenylalanine is replaced by tyrosine. Diazepam might be able to accommodate to the loss of space by its pendant phenyl ring adapting another rotational state (Fig. 3) that is probably not possible for its more rigid analogs. Neither CBM II nor CBM III offer interactions consistent with these findings.
Other mutations in γ2 Phe77 have subtle effects on the affinities of diazepam and flumazenil. The point mutation γ2 F77L is tolerated well by both ligands, whereas γ2 F77I leads to a loss of affinity for flumazenil by a factor of 2,000 but to a loss of affinity for diazepam25 or Ro15-8670 by a factor of only 4-5 (Supplementary Fig. 9a,b). Docking of flumazenil into the final models indicated that flumazenil, much like Ro15-8670, can be accommodated in all three CBM geometries, thus supporting the postulated CBM between diazepam and flumazenil23 by direct docking. The CBM I pose (Fig. 4b) features a strong hydrophobic interaction between γ2 Phe77 and flumazenil. After replacement of phenylalanine with leucine in our flumazenil-bound wild-type CBM I structures, both the hydrophobic interaction and local ligand burial of flumazenil are reduced, although the pocket structure tolerates the point mutation without detectable unfavorable interactions. When isoleucine replaces phenylalanine, the loss of hydrophobic interaction and ligand burial are much more pronounced. In addition, steric clashes are observed and thus suggest that this mutation would induce local rearrangements in the pocket involving both subunits. CBM I structures are thus fully compatible with experimental data on the F77X mutants.
Several other experimental findings, such as the importance of α1 Tyr159 (loop B) for the action of diazepam, flunitrazepam and flumazenil23,26 or of subtle effects of the α1 T206V substitution in loop C for the potency of diazepam and Ro15-8670 (ref. 24), together can only be explained by CBM I and suggest a close approximation between the ester group of Ro15-8670 (8) and α1 Thr206 (Table 1 and Supplementary Fig. 9a,c). This conclusion is supported by the effects of α1 S205N substitutions on compounds with or without this ester group27 as well as by covalent labeling of α1 S205C and α1 T206C mutants by a nitrazepam derivative carrying a reactive isothiocyanate in the seven-membered ring’s 3C position28 (Fig. 1c; 14; also Fig. 4b). Finally, other experiments indicated that the ester group of 3′-ester–substituted imidazobenzodiazepines must be located close to γ2 Ala79 (ref. 29) (Fig. 4b and Supplementary Fig. 9c). All of these results are consistent with CBM I poses of these ligands (Table 1) and thus again support an overlapping orientation of benzodiazepines and imidazobenzodiazepines within the benzodiazepine binding pocket.
Alternative workflow leads to the same binding mode
To avoid the assumption of a common binding mode, we also subjected the initial pool of 30 pose clusters to an alternative workflow (Supplementary Scheme 3) and evaluated all poses by geometric restraints (distance between a pocket residue and a ligand atom) derived from experiments 2, 3, 7, 8, 10 and 11 cited in Table 1. Only two clusters met all criteria at different cutoff levels for the selected distances. These two clusters are the parent clusters of CBM I and CBM II. When all poses were scored by two scoring functions as above, the group of poses containing CBM I once again emerged as a strong candidate.
Neither of the two approaches led to a single bound-state model. The bound-state hypotheses derived in this work represent groups of poses with some uncertainty associated with atomic positions and protein-ligand interactions. The pose groups derived from the CBM analysis are smaller and more homogeneous, as we used their interaction pattern in defining the binding mode (Supplementary Tables 6–8).
Retrospective validation of CBM I by virtual screening
If CBM I structures are indeed binding competent, they should be suitable for enriching other benzodiazepine-site ligands from different structural classes out of a library of decoy molecules. To investigate this possibility, we generated a ‘validation’ library in analogy to the directory of useful decoys (DUD) database30 (Supplementary Scheme 4) by extracting 41 benzodiazepine-binding-site ligands from different structural classes from the literature (Supplementary Table 10). We property-matched decoys on the basis of the five terms used to construct the DUD database, plus net formal charge31. Thus, for each of the 41 ligands, we generated all protonation states near pH 7.0, resulting in 48 unique ligand protomers. For each of them, we created 50 decoy molecules that are physically similar but topologically distinct and thus should not bind the benzodiazepine site, resulting in a library of 2,400 decoys. After seeding the 48 unique ligand protomers into the decoy library, the resulting validation library was used for virtual screening using structure-based pharmacophore models. These we generated using the software program LigandScout32 using bound-state complexes from all three CBM pools as our basis (Supplementary Fig. 10). We sorted the compounds in the validation library for those that best matched these pharmacophore models. In the sorted libraries, we computed33 the enrichment of known binders in the top 0.5%, 1%, 2%, 5% and 10% for each CBM. CBM I performed much better than CBM II and CBM III (Supplementary Table 9), resulting in an enrichment over random selection of 24.8 when the top 0.5% was analyzed. With the exception of purely two-dimensional similarity searching, this screen outperforms those conducted with a ligand-based pharmacophore model and shape-based similarity search (Supplementary Table 11). Such enrichment factors are comparable to the average enrichment factors obtained for the 40 crystal structures of the original DUD database30, clearly indicating that CBM I complexes are sufficiently binding competent to identify known benzodiazepine-site ligands from different structural classes.
CBM I binding hypotheses predict new ligands
We then investigated whether CBM I–derived pharmacophore models (Supplementary Fig. 10) can be used for the discovery of new ligands (Supplementary Scheme 5). As a screening library we used the large DUD databank30, which contains 95,357 compounds and covers a large chemical space. After the screening run, we grouped the top 0.5% of compounds in the DUD screen according to their chemical scaffolds. Some of the compound classes in the top 0.5% were found to have anticonvulsive activity in animal models but were never investigated for a possible interaction with GABAARs. Representatives from four of these compound classes were commercially available and were tested for displacement of [3H]flunitrazepam binding from mouse cerebellum membrane preparations (Table 2).
Table 2. Effects of top 0.5% compounds on [3H]flunitrazepam binding to cerebellar membranes and on GABA-stimulated currents in recombinant α1β3γ2 receptors.
In contrast to compounds 16–18, the 3-hydroxyoxindole 15 at a 10-μM concentration was able to inhibit [3H]flunitrazepam binding to 18 ± 7% of the control values, indicating that it interacts with the benzodiazepine-binding site of GABAARs. This compound at 1-μM and 10-μM concentrations also significantly (P < 0.001) stimulated GABA-induced currents in recombinant α1β3γ2 receptors expressed in Xenopus laevis oocytes (Table 2). Notably, the ligand-based screening approaches would have missed this new hit compound (Supplementary Table 12).
A subsequent similarity search revealed that other 3-hydroxyoxindoles were also present in the top 0.5%, four of which (15a–15d) were commercially available. To further increase the number of compounds for the follow-up study, we bought five additional analogs (15e–15i) not present in the DUD database. We then tested all nine additional 3-hydroxyoxindoles, as shown in Table 2. Displacement of [3H]flunitrazepam binding at 10-μM compound concentrations revealed that, in addition to compound 15, the compound 15b at 10 μM was able to inhibit [3H]flunitrazepam binding to about 34 ± 5% of control binding (P <0.001), whereas compounds 15a and 15c inhibited this binding to approximately 70% (P < 0.01). The other 3-hydroxyoxindoles investigated did not significantly (P < 0.5) inhibit [3H]flunitrazepam binding at this concentration. All four compounds able to displace [3H]flunitrazepam binding were also able to significantly (P < 0.001) enhance GABA-elicited currents in α1β3γ2 recombinant receptors expressed in X. laevis oocytes at 10-μM concentration (Table 2). We then characterized compounds 15b and 15, which had the highest potency for inhibition of [3H] flunitrazepam binding (half-maximum inhibitory concentration (IC50) values of 4.9 ± 1.5 μM and 3.8 ± 0.8 μM, respectively; Fig. 5 and Supplementary Fig. 11). Both compounds showed a dose-dependent stimulation of GABA-induced currents and shifted the GABA dose-response curve to the left. In addition, the effect of these compounds could be reduced by coapplication of 100 nM of the benzodiazepine-site antagonist flumazenil, indicating that the effect was at least partially generated by interaction with the benzodiazepine-binding site of these receptors. Flumazenil inhibited the effects of 15b more strongly than those of 15. The additional effect of these compounds in the presence of flumazenil might have been caused by their interaction with additional binding sites at these receptors34.
Figure 5. 3-hydroxyoxindoles as a new class of benzodiazepine-binding-site ligands of GABAARs.
(a) The CBM I–derived pharmacophore model (Supplementary Fig. 10a) based on the diazepam-bound structure and its match with 15b is depicted. Both dark blue aromatic features match, as do the red hydrogen bond and the yellow lipophilic feature. The steric exclusion volumes are depicted in an overlay with a ribbon structure to provide orientation in the model. (b) Two-dimensional structure of 15b. (c) Inhibition of 2 nM [3H]flunitrazepam binding to mouse cerebellar membranes by 15b. Data represent means ± s.d. from three experiments performed in triplicate. nH, Hill coefficient. (d) Electrophysiological traces and dose-response curve of 15b on GABA EC3 (3% of maximum GABA current) currents in the absence (squares) or presence of 100 nM flumazenil (asterisks), obtained from X. laevis oocytes expressing recombinant GABAARs composed of α1β3γ2 subunits. Data represent means ± s.d. from three separate experiments performed in different oocytes from two different batches. (e) GABA dose-response curves in α1β3γ2 receptors expressed in X. laevis oocytes with (open squares) and without (filled squares) 30 μM 15b. Data represent means ± s.d. from three separate experiments performed in different oocytes from two different batches. In c, d and e, x-axis scale is logarithmic.
These data clearly indicate that 3-hydroxyoxindoles are unique modulators of GABAARs via the benzodiazepine-binding site. The structural novelty of this compound class is also reflected by the fact that the most similar known benzodiazepine-site ligand (Supplementary Table 12) has a Tanimoto similarity of 0.56 (based on molecular access system (MACCS) keys).
Results obtained for this small data set indicate that an aromatic ring at R3 in combination with an unsubstituted nitrogen atom seems to be beneficial for biological activity. This idea is also supported by the orientation of compound 15b in the pharmacophore model, which shows a considerable overlap of substituent R3 with the pendant phenyl ring of diazepam and a hydrogen bond of the NH group to the backbone carbonyl of α1 Tyr159 (Fig. 5). However, systematic studies have to be performed to get deeper insights into the structure-activity relationship and binding mode of this unique ligand class. Finally, whether this activity accounts for some of the previously reported anticonvulsive activity of this compound class35 has to be clarified in future experiments.
In an alternative approach, we performed docking-based virtual screening studies (Supplementary Scheme 5) against the diazepam-bound (Fig. 4a) and flumazenil-bound (Fig. 4b) GABAAR models (CBM I) used for generating the protein-based pharmacophore model (Supplementary Fig. 10)36. To assess the robustness of the benzodiazepine-binding-site models, we used a docking method (protein-ligand ANT system (PLANTS)37) different from that used to derive ligand binding modes (FlexX) for virtual screening studies. To consider protein flexibility, we performed two independent docking simulations for each of the two benzodiazepine-pocket models: a ‘rigid’ docking run considering no side chain flexibility and a ‘flexible’ docking run allowing flexibility of the side chains of γ2 Thr142 (ref. 38) and α1 Thr206 (ref. 24) (important hydrogen bond–donating residues involved in ligand binding and activation). We used the original ligand poses of diazepam and flumazenil in the respective benzodiazepine-binding-site models to define reference interaction fingerprints (IFPs) for scoring the docking poses of an in-house library of 1,010 chemically diverse fragment-like molecules, as described previously39. The IFP scoring method determines ligand binding mode similarity to experimentally supported ligand binding poses (Fig. 4a,b). IFPs have been used as an efficient alternative postprocessing method of docking poses36,39 to overcome target-dependent scoring problems12. We used seven different interaction types (negatively charged, positively charged, hydrogen bond–accepting, hydrogen bond–donating, aromatic face-to-edge, aromatic-face-to-face and hydrophobic) to define the IFP (Supplementary Table 13). We used the Tanimoto coefficient measuring IFP similarity with the reference ligand pose in the benzodiazepine-binding-site receptor model to score the docking poses of known actives and decoys. Normalized IFP scores (Z values) of the docking poses in the diazepam and flumazenil binding pocket receptor models were merged and ranked with respect to the normalized IFP score. We successfully used this docking-based virtual screening procedure to identify two new benzodiazepine-binding-site ligands from the 1,010 chemically diverse fragment-like molecules (Supplementary Scheme 5)40. From the top-ranked 30 fragment-like compounds, we selected 10 fragments, chemically dissimilar from known benzodiazepine-binding-site ligands, for radioligand binding assays. Two of these in silico hits, 19 and 20, inhibited [3H]flunitrazepam binding (IC50 values of 25.6 ± 9.6 μM and 34.7 ± 2.6 μM, respectively; Table 2). Remarkably, the new ligands are proposed to make the same interactions with the benzodiazepine-binding-site as diazepam and flumazenil 11, but most of the functional groups (hydrogen bond acceptors and aromatic ring systems) mediating these conserved interactions are located at positions different than those of the corresponding functional groups in the reference ligands (Fig. 4). The newly identified chemical scaffolds (the MACCS Tanimoto similarity of 0.58 was the closest to any of the 41 benzodiazepine-binding-site ligands; Supplementary Table 12) illustrate the suitability of this molecular IFP approach for scaffold-hopping purposes41.
CBM I models are similar to the GluCl structure
After submission of this study, a nematode glutamate-gated chloride channel (GluCl) structure was reported (PDB code 3RIF)42. This cysteine-loop receptor features 28%, 35% and 29% sequence identity in the extracellular domain with α1, β2 and γ2 subunits, respectively, and thus it is clearly the template of choice for future studies. Careful analysis of this structure and homology models derived from it (Supplementary Table 4) indicated that our CBM I models indeed are more similar to the structure with PDB code 3RIF than the AChBP structures used in modeling and, more importantly, much more so than the models that we rejected through our workflow. This again highlights the validity of our approach.
DISCUSSION
In the absence of a crystal structure of the ligand-bound benzodiazepine-binding-site of GABAARs, protein homology modeling and computational ligand dockings are the only bases on which structure-based hypotheses for the interaction of benzodiazepines with their binding site can be formed. However, accurate prediction of membrane-protein structures and ligand interactions remains a challenge43 and requires accurate modeling of structurally divergent regions and extensive use of experimental evidence, as shown recently in a community-wide G protein–coupled receptor structure-prediction assessment. Here we compensated for the local uncertainty resulting from structurally divergent regions of related cysteine-loop receptor family members by constructing a variety of homology models from different templates. We then exploited the given structure of diazepam and its derivatives to select those models best accommodating these compounds. To do so, we developed a workflow that allowed handling of multiple models and several thousand diazepam docking poses. The only selection criteria used were a reasonable lipophilic interaction with the hydrophobic parts of the diazepam structure as well as simultaneous contacts of diazepam with the α1 and the γ2 subunit of GABAARs. With the assumption that diazepam and its close structural analogs have a CBM within the benzodiazepine-binding pocket and by clustering of ligand poses according to a similar orientation of their common structure and according to similar interactions with pocket residues, we were finally led to three CBM geometries, CBMs I-III.
CBM I binding geometry and interactions are supported by a large variety of structural, computational and experimental evidence. Furthermore, CBM I can provide a single structural hypothesis for seemingly discrepant results obtained with various covalently incorporated compounds and supports the hypothesis that diazepam and the imidazobenzodiazepines Ro15-4513 or fluma zenil show a similar binding mode within the benzodiazepine pocket. Finally, CBM I can also explain subtle changes in affinity of various ligands for various amino acid substitutions. Taken together, this abundance of evidence indicates that CBM I is the correct binding mode of diazepam in the benzodiazepine-binding pocket.
Previous studies also featured docking poses of diazepam or its analogs. Overall, however, the conclusions remained contradictory. In one study5, a flunitrazepam orientation similar to the CBM III poses was reported. Another study6 identified a diazepam pose vaguely similar to the CBM I pose, but apparently it had a very different interaction pattern owing to a different pocket topology. A pharmacophore-derived diazepam pose4 is in a position intermediate between that of CBM II and CBM I. Most recently, binding modes vaguely resembling our CBM II were proposed based on modeling structures from a single template and covalently incorporated ligands7. In a study more concerned with zolpidem8, flunitrazepam poses featured the P conformation, which has been demonstrated to be inactive at the benzodiazepine-binding site (Supplementary Table 2).
A correct structure, however, should also be able to accommodate other ligands of this site from different structural classes. Thus, by using structure-based pharmacophore models derived from CBM I, we identified 5 out of 41 benzodiazepine-site ligands from different structural classes within the top 0.5% of hits of a benzodiazepine-focused set of decoy molecules (validation library), and we found five additional compounds within the top 2% and eight more in the top 10% of compounds (Supplementary Tables 9 and 10). Such enrichment factors, as well as the remaining false negatives (binders that are not ranked in the top range) are comparable to those obtained from docking into ligand-bound crystal structures30.
Virtual screening with the DUD database30 identified not only additional known benzodiazepine-site ligands present in this database but also a variety of other anticonvulsive compounds that so far have not been associated with GABAARs. By investigating a commercially available subset for a possible interaction with the benzodiazepine-binding site of GABAARs, we identified several 3-hydroxyoxindole derivatives that were able to modulate GABA-induced currents in recombinant receptors via the benzodiazepine-binding site. Furthermore, in a complementary approach, docking-based virtual screening studies using a molecular protein-ligand IFP scoring method identified two additional new chemical scaffolds, which were proven active in [3H]flunitrazepam displacement assays. These data further support the validity of our diazepam-bound structure of the benzodiazepine-binding site and demonstrate its suitability for structure-based drug discovery.
Further exploration of our in silico screening results presumably will identify additional new ligand classes for the benzodiazepine-binding site. Information from docking studies with other benzodiazepine-site ligands as well as molecular dynamics studies will identify the flexible and rigid parts of the pocket and define their ligand-pocket interactions as well as the mechanism of allosteric modulation of positive, negative and neutral interactors. The present structural models can also be used for modeling of the benzodiazepine-binding sites of other GABAAR subtypes, and docking of unselective and subtype-selective ligands into their binding sites will ultimately lead to appropriate structural hypotheses that allow lead optimization and fragment-based drug design. Finally, our structures can be used for the modeling of similar extracellular pockets of GABAARs34, again enabling lead optimization and drug discovery.
METHODS
Template characterization and preparation
Six AChBP structures (PDB codes 1I9B, 1UW6, 2BYN, 2BYQ, 2BYR and 2BYS)3 and two nAChR structures (PDB codes 2BG9 and 2QC1)3 were used as GABAA modeling templates (Supplementary Table 1) and structurally aligned with ProFit (http://www.bioinf.org.uk/software/profit/). Only the extracellular domains of the structures with PDB codes 2BG9 and 2QC1 were considered, and both complete pentamers and individual subunits, reassembled into multitemplates, were used.
Alignment and model building
A multiple-sequence alignment with all templates’ extracellular domains and the GABAAR α1, γ2 and β2 extracellular domains’ sequences was constructed with clustalX44 (Supplementary Scheme 1 and Supplementary Fig. 1). All templates were structurally aligned with secondary-structure matching10. Individual pairwise sequence-to-structure alignments between the GABAAR subunits and all template subunits were obtained from the Fugue server45. From these data, alignment variants of variable segments were constructed46, and homology models were built using Modeller. For the two subunits that contribute to the benzodiazepine-binding site, multiple template combinations were used to sample the structural variations in the templates.
Creation of input ligands
Seven 1,4-benzodiazepines and three 1,4-imidazobenzodiazepines were built in Molecular Operating Environment (MOE) version 2007.09 (http://www.chemcomp.com), using the M conformation of the seven-membered ring that is supported by experimental studies (Supplementary Table 2 and Supplementary Fig. 2).
FlexX docking
Diazepam was docked into all models using FlexX v2.0.3 (ref. 11). RING_MODE was set to 0 (constraining the ring conformation), and all other parameters were left in their default values. The pose output limit was set to 100 for each run for extensive conformational sampling.
FlexE docking
FlexE13 allows protein flexibility through an ensemble of superposed protein structures where similar parts of the structures are merged and dissimilar areas are treated as separate alternatives. To prepare such ensembles, we explored the rotameric states of α1 His101, α1 Tyr159, α1 Val202, α1 Ser204, α1 Ser205, α1 Thr206, α1 Tyr209, γ2 Tyr58, γ2 Phe77 and γ2 Thr142 with the MOE tool Rotamer Explorer. In cases in which more than ten rotamers were obtained for a certain side chain, the ten most diverse rotamers were retained. Each benzodiazepine-binding-site ligand was docked into the ensemble structures of the corresponding homology models, generating 100 poses for each ligand. Finally, each ligand-receptor complex of the final pose pool was refined using the MOE tool LigX energy minimize. Lipophilic interactions of L1 and L3 both in docked and energy-minimized poses were calculated with the Scientific Vector Language (SVL)-exchange tool SCORING.SVL (http://svl.chemcomp.com).
CBM candidate selection
We defined the CBM of a molecular scaffold to require common orientation of the scaffold and common binding-site topology surrounding the common scaffold. Poses were superposed on the backbone atoms of the conserved protein segments using MOE to determine ligands’ common scaffold r.m.s. deviation. The SVL-exchange script MOL_RMSD.SVL (http://svl.chemcomp.com) was used for the r.m.s. deviation computation of the common ligand scaffolds. Then, the Microsoft Excel add-in XLSTAT (http://www.xlstat.com) was used for hierarchical clustering of the r.m.s. deviation dissimilarity matrix using the WARD method.
All protein heavy atoms within 4 Å of any heavy atom of the molecular scaffold in more than 90% of all poses of the cluster were considered part of the binding site of the common scaffold. The identified atoms and the common scaffold were used for the calculation of the bound-complex r.m.s. deviation, which was used to cluster the poses to candidate binding modes within their parent cluster.
Evaluating covalent incorporation and mutagenesis data
Poses were considered to fit experimental data if the smallest distance between the atoms in any of the following combinations was <6 Å: (i) any atom of the nitro group (flunitrazepam) and any atom of α1 His101 (ref. 17), (ii) the photoreactive nitrogen of the arylazido group (Ro15-4513) and α1 Tyr209 (ref. 22), (iii) any atom of the ester moiety (Ro15-8670) and side chain atoms of α1 Thr206 or the Cβ of γ2 Ala79 (ref. 29) or (iv) the carbon of isothiocyanate (NCS)-substituted ligands and the sulfur of the respective cysteine mutant.
Generation and validation of pharmacophore models
Two structure-based pharmacophore models were created for each CBM, one derived from the top-scoring diazepam pose (pharmacophore D) and the other from the flumazenil pose, which showed the lowest bound-complex r.m.s. deviation from the diazepam-selected pose (pharmacophore F). Pharmacophore models were created using LigandScout 3.0 (ref. 32) (Supplementary Methods).
Validation library
For each of the 41 known benzodiazepine-binding-site ligands (Supplementary Table 10), protonation states near pH 7.0 were generated, resulting in 48 unique ligand protomers. For each of them, we created 50 decoy molecules that are physically similar but topologically distinct and thus should not bind the benzodiazepine site, resulting in a library of 2,400 decoys (Supplementary Methods). A conformational database was created with OMEGA v2.3.3 (http://www.eyesopen.com/omega) and screened against pharmacophore D and pharmacophore F using LigandScout 3.0. Hits of each pharmacophore model were sorted according to scoring value, and enrichment factors33 were determined for the top 0.5%, 1%, 2%, 5% and 10% for each CBM.
Discovery library
To test the ability of the CBM I–derived pharmacophore model to identify new ligands, we generated three-dimensional conformations for the DUD30 databank (using OMEGA v2.4.1 (http://www.eyesopen.com/omega) and screened the resulting database of 93,597 compounds against pharmacophore D and pharmacophore F using LigandScout 3.0 (ref. 32). Compounds 15, 15a–15i and 18 were bought from Ambinter, and compounds 16 and 17 were bought from Asinex.
PLANTS docking and IFP scoring
PLANTS37 speedup settings were used twice to generate 15 poses for each compound in two independent docking runs: one run considering no side chain flexibility and another run allowing flexibility of the γ2 Thr142 and α1 Thr206 side chains. The original ligand poses in the respective GABAA models were used to define reference IFPs, as described previously36,39. The cavity used for the IFP analysis consisted of all of the residues within 5 Å of the reference ligand. Standard IFP scoring parameters36 and a Tanimoto coefficient measuring IFP similarity with the reference ligand pose were used to rank the docking poses.
Experimental section
Recombinant GABAARs were expressed in X. laevis oocytes, and HEK cells and compound modulatory effects were investigated using the two-electrode voltage-clamp technique at a GABA concentration eliciting 3% of the maximum current34. Binding of compounds was investigated by [3H]flunitrazepam displacement studies47 in mouse cerebellar membrane preparations.
Supplementary Material
Acknowledgments
Financial support from the Doctoral Fellowship Programme of the Austrian Academy of Science (L.R.), the Austrian Science Fund grant P19653 (M.E.) and the European Commission Seventh Framework Programme grant HEALTH-F4-2008-202088 (W.S. and I.J.P.d.E.) is gratefully acknowledged. We also thank Inte:Ligand for providing us a free license for LigandScout 3.0 and Open Eye for an Omega license. We thank M. Mysinger and J. Irwin from the Shoichet laboratory for generating the focused ‘benzodiazepine’ decoy set. In addition we thank E. Sigel for helpful suggestions and discussions during generation of this work; M. Stojanovic for technical assistance in the binding assays; A. Chalikiopoulos and M. Verheij for assistance in fragment docking studies; and E. Urban, S. Haselmaier, J. König, H. Custers and A. van de Stolpe for providing HRMS and 1H- and 13C-NMR spectral data.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
Additional information
Supplementary information and chemical compound information are available online at http://www.nature.com/naturechemicalbiology/. Reprints and permissions information is available online at http://www.nature.com/reprints/index.html. Correspondence and requests for materials should be addressed to M.E.
Accession codes. PDB: the previously determined crystal structures for A. californica AChBP, L. stagnalis AChBP, mouse nAChR, a nematode glutamategated chloride channel, an nAChR structure and four AChBP structures are deposited under accession codes 2BYQ, 1UW6, 2QC1, 3RIF, 2BG9, 1I9B, 2BYN, 2BYR and 2BYS, respectively.
references
- 1.Sieghart W. Structure and pharmacology of γ-aminobutyric acid A receptor subtypes. Pharmacol. Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 2.Sigel E. Mapping of the benzodiazepine recognition site on GABAA receptors. Curr. Top. Med. Chem. 2002;2:833–839. doi: 10.2174/1568026023393444. [DOI] [PubMed] [Google Scholar]
- 3.Miller PS, Smart TG. Binding, activation and modulation of Cys-loop receptors. Trends Pharmacol. Sci. 2010;31:161–174. doi: 10.1016/j.tips.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Clayton T, et al. An updated unified pharmacophore model of the benzodiazepine binding site on γ-aminobutyric acidA receptors: correlation with comparative models. Curr. Med. Chem. 2007;14:2755–2775. doi: 10.2174/092986707782360097. [DOI] [PubMed] [Google Scholar]
- 5.Mokrab Y, et al. Exploring ligand recognition and ion flow in comparative models of the human GABA type A receptor. J. Mol. Graph. Model. 2007;26:760–774. doi: 10.1016/j.jmgm.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Ci S, Ren T, Su Z. Investigating the putative binding-mode of GABA and diazepam within GABAA receptor using molecular modeling. Protein J. 2008;27:71–78. doi: 10.1007/s10930-007-9109-9. [DOI] [PubMed] [Google Scholar]
- 7.Berezhnoy D, Gibbs TT, Farb DH. Docking of 1,4-benzodiazepines in the α1/γ2 GABAA receptor modulator site. Mol. Pharmacol. 2009;76:440–450. doi: 10.1124/mol.109.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sancar F, Ericksen SS, Kucken AM, Teissére JA, Czajkowski C. Structural determinants for high-affinity zolpidem binding to GABAA receptors. Mol. Pharmacol. 2007;71:38–46. doi: 10.1124/mol.106.029595. [DOI] [PubMed] [Google Scholar]
- 9.Evers A, Gohlke H, Klebe G. Ligand-supported homology modelling of protein binding-sites using knowledge-based potentials. J. Mol. Biol. 2003;334:327–345. doi: 10.1016/j.jmb.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Krissinel E, Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 11.Rarey M, Kramer B, Lengauer T, Klebe G. A fast flexible docking method using an incremental construction algorithm. J. Mol. Biol. 1996;261:470–489. doi: 10.1006/jmbi.1996.0477. [DOI] [PubMed] [Google Scholar]
- 12.Moitessier N, Englebienne P, Lee D, Lawandi J, Corbeil CR. Towards the development of universal, fast and highly accurate docking/scoring methods: a long way to go. Br. J. Pharmacol. 2008;153(suppl 1):S7–S26. doi: 10.1038/sj.bjp.0707515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Claussen H, Buning C, Rarey M, Lengauer T. FlexE: efficient molecular docking considering protein structure variations. J. Mol. Biol. 2001;308:377–395. doi: 10.1006/jmbi.2001.4551. [DOI] [PubMed] [Google Scholar]
- 14.Chema D, Eren D, Yayon A, Goldblum A, Zaliani A. Identifying the binding mode of a molecular scaffold. J. Comput. Aided Mol. Des. 2004;18:23–40. doi: 10.1023/b:jcam.0000022561.76694.5b. [DOI] [PubMed] [Google Scholar]
- 15.Wang R, Lai L, Wang S. Further development and validation of empirical scoring functions for structure-based binding affinity prediction. J. Comput. Aided Mol. Des. 2002;16:11–26. doi: 10.1023/a:1016357811882. [DOI] [PubMed] [Google Scholar]
- 16.Labute P. The generalized Born/volume integral implicit solvent model: estimation of the free energy of hydration using London dispersion instead of atomic surface area. J. Comput. Chem. 2008;29:1693–1698. doi: 10.1002/jcc.20933. [DOI] [PubMed] [Google Scholar]
- 17.Duncalfe LL, Carpenter MR, Smillie LB, Martin IL, Dunn SM. The major site of photoaffinity labeling of the γ-aminobutyric acid type A receptor by [3H]flunitrazepam is histidine 102 of the α subunit. J. Biol. Chem. 1996;271:9209–9214. doi: 10.1074/jbc.271.16.9209. [DOI] [PubMed] [Google Scholar]
- 18.Berezhnoy D, et al. On the benzodiazepine binding pocket in GABAA receptors. J. Biol. Chem. 2004;279:3160–3168. doi: 10.1074/jbc.M311371200. [DOI] [PubMed] [Google Scholar]
- 19.Wieland HA, Lüddens H, Seeburg PH. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J. Biol. Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]
- 20.Davies M, Bateson AN, Dunn SM. Structural requirements for ligand interactions at the benzodiazepine recognition site of the GABAA receptor. J. Neurochem. 1998;70:2188–2194. doi: 10.1046/j.1471-4159.1998.70052188.x. [DOI] [PubMed] [Google Scholar]
- 21.Buhr A, Schaerer MT, Baur R, Sigel E. Residues at positions 206 and 209 of the α1 subunit of γ-aminobutyric acidA receptors influence affinities for benzodiazepine binding site ligands. Mol. Pharmacol. 1997;52:676–682. doi: 10.1124/mol.52.4.676. [DOI] [PubMed] [Google Scholar]
- 22.Sawyer GW, Chiara DC, Olsen RW, Cohen JB. Identification of the bovine γ-aminobutyric acid type A receptor α subunit residues photolabeled by the imidazobenzodiazepine [3H]Ro15-4513. J. Biol. Chem. 2002;277:50036–50045. doi: 10.1074/jbc.M209281200. [DOI] [PubMed] [Google Scholar]
- 23.Tan KR, et al. Proximity-accelerated chemical coupling reaction in the benzodiazepine-binding site of γ-aminobutyric acid type A receptors: superposition of different allosteric modulators. J. Biol. Chem. 2007;282:26316–26325. doi: 10.1074/jbc.M702153200. [DOI] [PubMed] [Google Scholar]
- 24.Sigel E, Schaerer MT, Buhr A, Baur R. The benzodiazepine binding pocket of recombinant α1β2γ2 γ-aminobutyric acidA receptors: relative orientation of ligands and amino acid side chains. Mol. Pharmacol. 1998;54:1097–1105. doi: 10.1124/mol.54.6.1097. [DOI] [PubMed] [Google Scholar]
- 25.Buhr A, Baur R, Sigel E. Subtle changes in residue 77 of the γ subunit of α1β2γ2 GABAA receptors drastically alter the affinity for ligands of the benzodiazepine binding site. J. Biol. Chem. 1997;272:11799–11804. doi: 10.1074/jbc.272.18.11799. [DOI] [PubMed] [Google Scholar]
- 26.Amin J, Brooks-Kayal A, Weiss DS. Two tyrosine residues on the α subunit are crucial for benzodiazepine binding and allosteric modulation of γ-aminobutyric acidA receptors. Mol. Pharmacol. 1997;51:833–841. doi: 10.1124/mol.51.5.833. [DOI] [PubMed] [Google Scholar]
- 27.Derry JMC, Dunn SMJ, Davies M. Identification of a residue in the γ-aminobutyric acid type A receptor α subunit that differentially affects diazepam-sensitive and -insensitive benzodiazepine site binding. J. Neurochem. 2004;88:1431–1438. doi: 10.1046/j.1471-4159.2003.02264.x. [DOI] [PubMed] [Google Scholar]
- 28.Tan KR, Baur R, Charon S, Goeldner M, Sigel E. Relative positioning of diazepam in the benzodiazepine-binding-pocket of GABAA receptors. J. Neurochem. 2009;111:1264–1273. doi: 10.1111/j.1471-4159.2009.06419.x. [DOI] [PubMed] [Google Scholar]
- 29.Kucken AM, Teissére JA, Seffinga-Clark J, Wagner DA, Czajkowski C. Structural requirements for imidazobenzodiazepine binding to GABAA receptors. Mol. Pharmacol. 2003;63:289–296. doi: 10.1124/mol.63.2.289. [DOI] [PubMed] [Google Scholar]
- 30.Huang N, Shoichet BK, Irwin JJ. Benchmarking sets for molecular docking. J. Med. Chem. 2006;49:6789–6801. doi: 10.1021/jm0608356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mysinger MM, Shoichet BK. Rapid context-dependent ligand desolvation in molecular docking. J. Chem. Inf. Model. 2010;50:1561–1573. doi: 10.1021/ci100214a. [DOI] [PubMed] [Google Scholar]
- 32.Wolber G, Langer T. LigandScout: 3-D pharmacophores derived from protein-bound ligands and their use as virtual screening filters. J. Chem. Inf. Model. 2005;45:160–169. doi: 10.1021/ci049885e. [DOI] [PubMed] [Google Scholar]
- 33.Wei BQ, Baase WA, Weaver LH, Matthews BW, Shoichet BK. A model binding site for testing scoring functions in molecular docking. J. Mol. Biol. 2002;322:339–355. doi: 10.1016/s0022-2836(02)00777-5. [DOI] [PubMed] [Google Scholar]
- 34.Ramerstorfer J, et al. The GABAA receptor α+β-interface: a novel target for subtype selective drugs. J. Neurosci. 2011;31:870–877. doi: 10.1523/JNEUROSCI.5012-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popp FD, Parson R, Donigan BE. Synthesis of potential anticonvulsants: condensation of isatins with acetone and related ketones. J. Pharm. Sci. 1980;69:1235–1237. doi: 10.1002/jps.2600691035. [DOI] [PubMed] [Google Scholar]
- 36.Marcou G, Rognan D. Optimizing fragment and scaffold docking by use of molecular interaction fingerprints. J. Chem. Inf. Model. 2007;47:195–207. doi: 10.1021/ci600342e. [DOI] [PubMed] [Google Scholar]
- 37.Korb O, Stutzle T, Exner TE. Empirical scoring functions for advanced protein-ligand docking with PLANTS. J. Chem. Inf. Model. 2009;49:84–96. doi: 10.1021/ci800298z. [DOI] [PubMed] [Google Scholar]
- 38.Mihic SJ, Whiting PJ, Klein RL, Wafford KA, Harris RA. A single amino acid of the human γ-aminobutyric acid type A receptor γ2 subunit determines benzodiazepine efficacy. J. Biol. Chem. 1994;269:32768–32773. [PubMed] [Google Scholar]
- 39.de Graaf C, Rognan D. Selective structure-based virtual screening for full and partial agonists of the β2 adrenergic receptor. J. Med. Chem. 2008;51:4978–4985. doi: 10.1021/jm800710x. [DOI] [PubMed] [Google Scholar]
- 40.Verheij MH, et al. Fragment library screening reveals remarkable similarities between the G protein-coupled receptor histamine H and the ion channel serotonin 5-HTA. Bioorg. Med. Chem. Lett. 2011;21:5460–5464. doi: 10.1016/j.bmcl.2011.06.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venhorst J, Nunez S, Terpstra JW, Kruse CG. Assessment of scaffold hopping efficiency by use of molecular interaction fingerprints. J. Med. Chem. 2008;51:3222–3229. doi: 10.1021/jm8001058. [DOI] [PubMed] [Google Scholar]
- 42.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michino M, et al. Community-wide assessment of GPCR structure modelling and ligand docking: GPCR Dock 2008. Nat. Rev. Drug Discov. 2009;8:455–463. doi: 10.1038/nrd2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 45.Shi J, Blundell TL, Mizuguchi K. FUGUE: sequence-structure homology recognition using environment-specific substitution tables and structure-dependent gap penalties. J. Mol. Biol. 2001;310:243–257. doi: 10.1006/jmbi.2001.4762. [DOI] [PubMed] [Google Scholar]
- 46.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 47.Sieghart W, Schuster A. Affinity of various ligands for benzodiazepine receptors in rat cerebellum and hippocampus. Biochem. Pharmacol. 1984;33:4033–4038. doi: 10.1016/0006-2952(84)90017-0. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W, Koehler KF, Zhang P, Cook JM. Development of a comprehensive pharmacophore model for the benzodiazepine receptor. Drug Des. Discov. 1995;12:193–248. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.