Abstract
Background and Objective. Various venom immunotherapy (VIT) protocols are available for Hymenoptera allergy. Although adverse reactions (ADRs) to VIT are widely reported, controlled trials are still needed. We conducted a randomized prospective study to evaluate ADRs and the efficacy of three VIT regimens. Methods. 76 patients with Hymenoptera allergy, aged 16–76 years, were randomized to receive an ultrarush protocol (group A: 27 patients), a rush protocol (group B: 25), or a slow protocol (group C: 24). Aqueous venom extract was used in incremental phase and an adsorbed depot in maintenance phase. ADRs and accidental Hymenoptera stings during VIT were used to evaluate efficacy. Results. During incremental treatment, ADRs occurred in 1.99%, 3.7%, and 3.9% of patients in groups A, B, and C, and in 0.99%, 1.46%, and 2.7%, respectively, during maintenance. ADRs were significantly fewer in group A (incremental + maintenance phase) than in group C (1.29% versus 3.2%; P = 0.013). Reactions to accidental Hymenoptera stings did not differ among groups (1.1%, 1.2%, and 1.1%). Conclusion. Ultrarush was as effective as the rush and slow protocols and was associated with a low incidence of reactions to stings. This study indicates that ultrarush VIT is a valid therapeutic option for Hymenoptera allergy.
1. Introduction
Reactions to stings by Hymenoptera species (Apis mellifera and Vespula Species) are classified as normal local reactions, large local reactions (LLRs), systemic anaphylactic reactions (SARs), systemic toxic reactions, and unusual reactions [1].All patients who have had an SAR to Hymenoptera stings should avoid insects that sting; they should also carry epinephrine for emergency self-administration, undergo examination for IgE antibodies specific for insect venom, and be considered candidates for venom immunotherapy (VIT) [1, 2]. Immunotherapy with purified Hymenoptera venom reduces the risk of anaphylactic reactions in most patients [2]. The primary goal of VIT is to prevent life-threatening reactions. A secondary benefit is that it helps relieve anxiety about insect stings and improves quality of life [3, 4]. The chance of a subsequent sting causing a more severe reaction than previous sting-induced reactions may be just 1% [5, 6]. Furthermore, a too short interval between stings increases the risk of a systemic reaction [7].
In recent decades, a number of VIT protocols have been proposed with the aim of reducing the number of injections, visits, and risk of SAR [2, 4, 8–10]. Among the various strategies devised to desensitize patients with SAR, an ultrarush protocol has been proposed as an alternative to rush and slow protocols in children, adolescents, and adults [11–18]. One advantage of rapid complete desensitization could be to reduce the risk of relapse of anaphylaxis if the patient is stung before the induction phase is completed. Rush therapy should be indicated for patients with moderate and frequent SAR at a high risk of frequent stings [14] and for patients with a single episode of SAR, but high psychological involvement [1, 13, 19, 20]. Although VIT-associated ADRs have been widely reported, there is a need for controlled, randomized trials that directly compare different regimens, that is, weekly, rush and ultrarush protocols, to determine whether the ultrarush VIT protocol is well tolerated and effective in patients with Hymenoptera sting-induced SAR.
The aim of this study was to evaluate, in a randomized controlled trial, the risk of adverse reactions (ADRs) and the efficacy of an ultrarush protocol compared to rush and weekly regimens in patients allergic to the Hymenoptera species, Apis mellifera or Vespula Species, commonly known as honey bee (HB) and yellow jacket (YJ).
2. Methods
2.1. Study Design
Patients with an indication for VIT were treated with commercially available preparations of Hymenoptera venom extracts and monitored for at least 24 months to record side effects and efficacy. According to three different treatment schedules (Table 1), the patients received injections of an aqueous extract of Hymenoptera venom (Aquagen SQ, ALK-Abelló, Hørsholm, Denmark) during the incremental stage of treatment and a purified aluminium-adsorbed depot extract of Hymenoptera venom (Alutard SQ, ALK-Abelló) during the maintenance stage. Both preparations were certified and prepared from the same source by the supplier (ALK-Abelló). The venom was purified by a Sephadex-gel filtration process by which the protein fractions are separated according to molecular weight. Venom extracts do not contain vasoactive amines. In addition, the extract undergoes filtration to reduce the presence of small peptides like apamin, kinins, and mast cell degranulating peptides. For depot VIT, the raw venom undergoes the same purification procedure that results in the recovery of a fraction containing only allergen, which is subsequently adsorbed onto aluminum hydroxide. All preparations contain the same amount of allergens, that is, 100 μg/mL, as specified by the manufacturer (ALK-Abelló). This ensured homogeneous immunogenicity. Injections were carried out according to EAACI Position Papers [2, 21].
Table 1.
Protocol of incremental treatment for ultrarush*, rush§, and slow conventional therapy*.
| Group A | Group B | Group C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| μg/dose | Cumulative μg/dose | Minute | μg/dose | Cumulative μg/dose | Day | Hour | μg/dose | Cumulative μg/dose | Week |
| 0.001 | 0.001 | 0 | 0.01 | 0.01 | 1 | 0 | 0.02 | 0.02 | 1 |
| 0.01 | 0.011 | 15 | 0.1 | 0.11 | 2 | 0.04 | 0.06 | 2 | |
| 0.04 | 0.051 | 30 | 1 | 1.11 | 4 | 0.08 | 0.14 | 3 | |
| 0.05 | 0.11 | 45 | 2 | 3.11 | 6 | 0.2 | 0.34 | 4 | |
| 0.1 | 0.21 | 60 | 3 | 5.11 | 2 | 0 | 0.4 | 0.74 | 5 |
| 0.4 | 0.61 | 75 | 3.5 | 9.61 | 2 | 0.8 | 1.54 | 6 | |
| 0.5 | 1.11 | 90 | 3.5 | 13.11 | 4 | 2 | 3.54 | 7 | |
| 1 | 2.11 | 105 | 10 | 23.11 | 3 | 0 | 4 | 7.54 | 8 |
| 4 | 6.11 | 120 | 15 | 38.11 | 2 | 8 | 15.54 | 9 | |
| 5 | 11.11 | 135 | 15 | 53.11 | 4 | 10 | 25.54 | 10 | |
| 10 | 21.11 | 150 | 20 | 73.11 | 4 | 0 | 20 | 45.54 | 11 |
| 40 | 61.11 | 165 | 25 | 98.11 | 2 | 40 | 85.54 | 12 | |
| 50 | 111.101 | 180 | 25 | 123.11 | 4 | 60 | 145.54 | 13 | |
| 30 | 153.11 | 5 | 0 | 80 | 225.54 | 14 | |||
| 35 | 188.11 | 2 | 100 | 325.54 | 15 | ||||
| 35 | 223.11 | 4 | |||||||
2.2. Cohort and Randomization of Patients
Seventy six patients (51 males, 25 females; aged 16–76 years) with history of SAR to HB or YJ venom were randomly assigned to different treatment for VIT as reported in Table 2. Patients were randomly assigned to group A, B, or C using a computer-generated random list and numbered envelopes. The envelopes were opened immediately before the start of venom immunotherapy. Patients of groups A (n = 27), B (n = 25), and C (n = 24) were treated with an ultrarush, rush, and weekly protocol, respectively. Groups A and B were admitted to hospital for VIT injections. Adverse reactions were graded according to Müller's classification [1, 9].
Table 2.
Demographic and clinical data of the 76 patients enrolled in the study.
| Group | N = | Treatment | Vespula/Apis | Sex (M/F) | Age (range) | Age (mean) | Local large reactions* | Systemic allergic reactions§ | Grade§
(I) (II)(III)(IV) |
|---|---|---|---|---|---|---|---|---|---|
| A | 27 | Ultrarush | 18/9 | 19/8 | 16–76 | 39.1 | 1 | 26 | (3) (3) (12) (8) |
| B | 25 | Rush | 16/9 | 16/9 | 18–68 | 40.3 | 1 | 24 | (5) (4) (13) (2) |
| C | 24 | Slow Conventional | 16/8 | 16/8 | 19–69 | 38.6 | 2 | 22 | (2) (6) (10) (4) |
|
| |||||||||
| Total | 76 | — | 50/26 | 51/25 | 16–76 | 39.3 | 4 | 72 | (10)(13)(35)(14) |
*It is defined as a swelling exceeding a diameter of 10 cm which lasts longer than 24 h.
§Classified according to Müller [9]: grade I: urticaria, pruritus, and malaise; grade II: angioedema, chest tightness, nausea, vomiting, abdominal pain, and dizziness; grade III: dyspnoea, wheeze, stridor, dysphagia, and hoarseness; grade IV: hypotension, collapse, loss of consciousness, incontinence, and cyanosis.
2.3. Incremental and Maintenance Phases
Group A patients were treated with a three-hour ultrarush protocol (Table 1a) [22]. Patients underwent continuous measurements of oxygen saturation and repeated measurements of blood pressure. The cumulative dose of Hymenoptera venom (HB or YJ) at the end of the incremental phase was 111.101 μg. Thirty minutes after the last injection, patients were discharged to home. Subsequently, on day 15, and once a month thereafter, the patients of each group received a subcutaneous injection of 50 μg adsorbed preparation on each arm, according to the parameter reported by the manufacturer (ALK Abelló, Milan, Italy): 100 μg of aqueous preparation = 100 000 SQ units of aluminum-adsorbed depot preparation. Group B patients received the aqueous preparation according to a daily rush schedule as reported in Table 1 (Group B) [23]. Group C was treated weekly with the slow conventional protocol shown in Table 1 (Group C) [22]. The cumulative doses of venom extract during the incremental phase in each group exceeded 100 mcg. No patient enrolled in this study received premedication before VIT.
2.4. Skin Prick Test
A skin test was done in all patients at least three weeks after the last sting [1, 24–26]. Sensitization was detected by skin prick tests with concentrations of 1, 10, and 100 μg/mL of Hymenoptera venom. If the prick test was negative, an intradermal test was done with 0.02 mL of venom concentrations from 0.001 to 1 μg/mL, injected into the volar surface of the forearm. The concentration was increased in 10-fold increments until there was a positive response or up to a maximum concentration of 1 μg/mL. Skin response was assessed after approximately 15–20 minutes. Physiological saline and histamine dihydrochloride 0.1% served as negative and positive controls, respectively.
2.5. IgE Assay
Total and specific IgE were measured in the patients' serum at diagnosis and 6, 12, 18, and 24 months after reaching the maintenance dose, using the Immulite 2000 Allergy system, according to the manufacturer's instructions (Diagnostic Products Corporation). The linear range of the assay was 0.2–100 kU/L for the Immulite 2000 sIgE method [27, 28].
2.6. Evaluation of Tolerance and Efficacy
Tolerance to VIT was evaluated on the basis of the ADRs recorded during the immunotherapy. The adverse reactions were classified as a large local reaction when there was swelling with a diameter more than 10 cm at the injection site that lasted for more than 24 hours (i.e., an LLR), or as a systemic anaphylactic reaction of different grades, as reported in Müller's classification [9]. The efficacy of VIT was evaluated on the basis of the outcome when a patient was accidentally restung by a Hymenoptera species. Patients were asked to report any of such stings during and after the VIT, and the type of reaction, according to Müller's classification [9]. Each patient had followup visits at the start of VIT and at 6, 12, 18, and 24 months thereafter from start of maintenance phase.
2.7. Statistical Analysis
Results are expressed as means ± SEM. Treatment groups (A, B, and C) and shift of preparations (from aqueous to depot) were compared. Categorical variables were compared by the chi-square test. Trends within a patient group were quantified by the Wilcoxon signed rank test. When the data were subjected to linear correlation analysis, correlations were calculated using the Spearman rank coefficient (rs) [29]. The level of statistical significance was P < 0.05.
3. Results
3.1. Tolerance of Each Venom Immunotherapy Regimen
During the incremental phase of venom immunotherapy, the numbers of LLR (defined as a swelling of more than 10 cm in diameter, lasting longer than 24 h) [9] were 7 out of 351 injections in group A (1.99%); 12/375 in group B (3.2%); 13/356 in group C (3.6%) (Figure 1). During the incremental phase, no SAR occurred in patients in group A (0/351 injections); two SARs occurred in group B (2/375; 0.9% of injections); one in group C (1/356; 0.56% of injections) (Figure 2). The rate of ADRs did not differ among the three groups: group A 7/351 (1.99%); group B 14/375 (3.7%); group C 14/356 (3.9%); (P = 0.27). During the incremental phase, two patients of group C withdrew from the study (at the fourth and seventh weeks). Adverse reactions were not the reasons for withdrawal. No patients in groups A and B withdrew from the trial.
Figure 1.
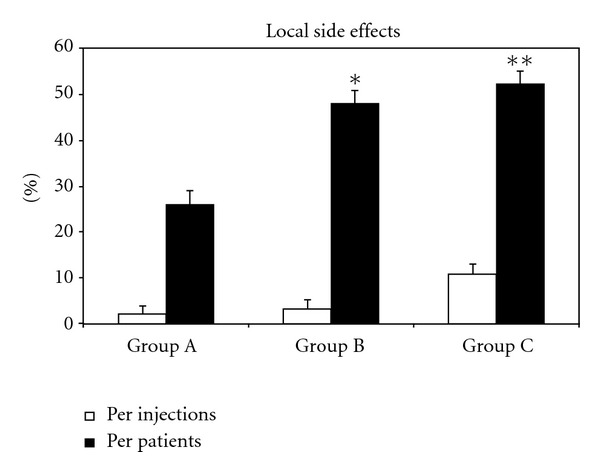
Frequency of local side effects in three groups of patients treated according to different venom immunotherapy (VIT) protocols (see Methods for treatments). Results are shown per injections (□) and per patients (▪). Each bar represents the mean ± SEM. *P < 0.05 compared with the corresponding group A versus group B. **P < 0.001 compared with the corresponding group A versus group C.
Figure 2.
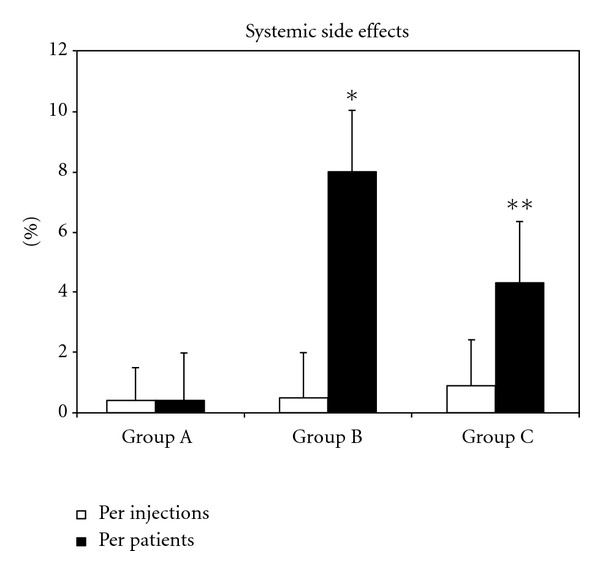
Frequency of systemic side effects in three groups of patients treated according to different venom immunotherapy (VIT) protocols. Results are shown per injections (□) or per patients (▪). Each bar represents the mean ± SEM. *P < 0.001 compared with the corresponding group A versus group B. **P < 0.05 compared with the corresponding group A versus group C.
In agreement with previous reports [30, 31], SARs occurred more frequently during the maintenance phase in patients with the HB venom preparation than in those receiving the YJ preparation (Table 3). In the maintenance phase, the ADRs were as follows: group A 8/810 (ADR/injections) (0.99%); group B 11/750 (1.46%); group C 12/440 (2.7%); P = 0.035; A versus C, P = 0.02; B versus C, (P = 0.03).
Table 3.
Side effects of patients treated with maintenance dose.
| Group A | Group B | Group C | Total | |
|---|---|---|---|---|
| Patients | 1 | 7 | 6 | 14 |
| maintenance dose of Apis m. | 1 | 6 | 5 | 12 |
| maintenance dose of Vespula spp. | 1 | 1 | 2 | |
| Local large reaction* (%) | — | 2(33.3) | 4(66.6) | 6 |
| Systemic anaphylactic reaction§ (%) | 1(12.5) | 5(62.5) | 2(25.0) | 8 |
| Grade I | 1 | 4 | 1 | 6 |
| Grade II | — | 1 | 1 | 2 |
During the whole study (incremental phase + maintenance phase), the ultrarush protocol (group A) resulted in less ADRs than the slow protocol (group C) (1.29% versus 3.2%; (P = 0.013)).
3.2. Shifting from the Incremental to the Maintenance Phase
No severe ADRs were observed when patients in groups A, B, and C were shifted from the aqueous preparations used during the incremental phase to adsorbed preparations for maintenance, which is in accordance with a previous report [32]. Rates of reactions were similar in each group: group A 1/27 (gastrointestinal symptoms 1), group B 2/25 (LLR 1; gastrointestinal symptoms 1), and group C 1/24 (headache 1).
3.3. Total and Specific IgE Levels and Evaluation of Serum s-IgE/Total IgE Ratio with SAR
Total and specific IgE was monitored before starting VIT, at the end of VIT, and during the study in the three groups (Figures 3 and 4). The serum s-IgE/total IgE ratio has been reported to predict the clinical response to allergen-specific immunotherapy [33]. We compared the serum s-IgE/total IgE ratio in our three groups of patients using the number of SARs before VIT and after hymenoptera sting during the maintenance phase. There was a similar highly significant direct correlation between the sIgE/tIgE post/preultrarush VIT delta and the SAR pre/postultrarush VIT delta in the three groups considered: group A (rho = 0.79; P = 0.034, Spearman rank correlation test), group B (rho = 0.83; P = 0.039, Spearman rank correlation test), and group C (rho = 0.77; P = 0.041, Spearman rank correlation test).
Figure 3.
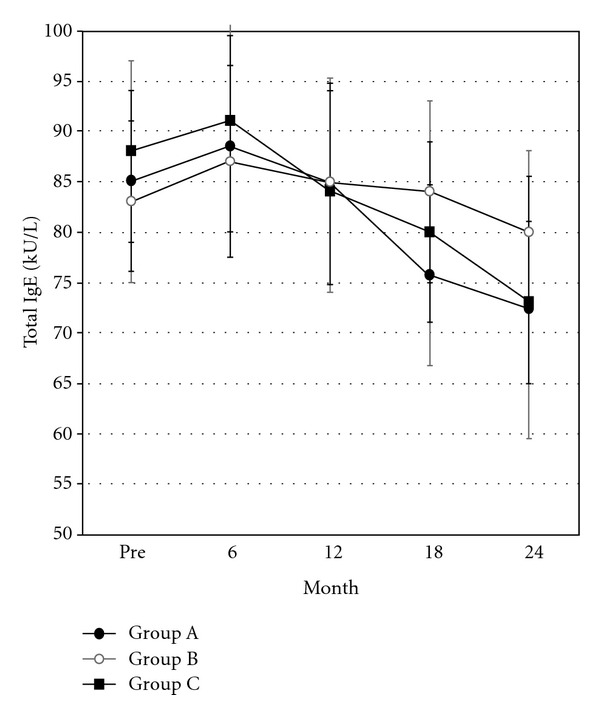
Total IgE during the incremental and maintenance phases in group A (Vespidae 18; Apidae 9; n = 27), group B (Vespidae 16; Apidae 9; n = 25), and group C (Vespidae 16; Apidae 8; n = 24). Vertical bars indicate the mean ± SEM.
Figure 4.
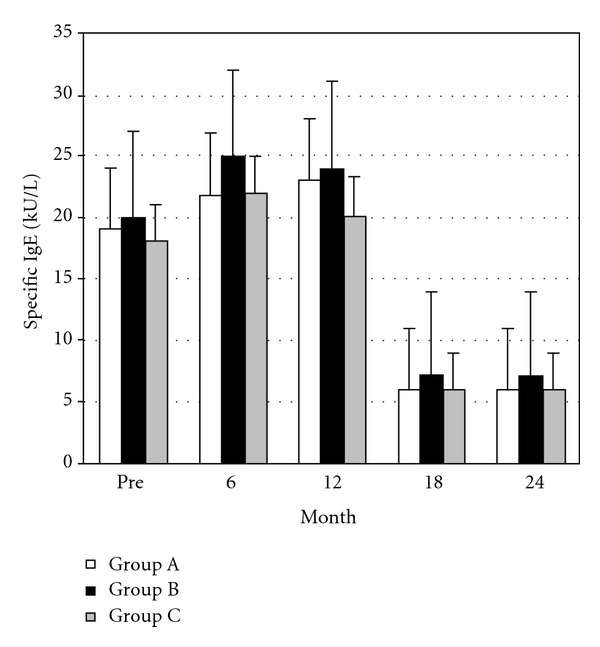
Specific IgE during the incremental and maintenance phases in group A (□) (Vespidae 18; Apidae: 9; n = 27), group B (▪) (Vespidae 16; Apidae 9 n = 25), and group C (▪) (Vespidae 16; Apidae 8; n = 24). Bars indicate the mean ± SEM.
3.4. Efficacy of Venom Immunotherapy
Accidental Hymenoptera stings during VIT were used to evaluate the efficacy of each of the three desensitization protocols. Of the 76 patients in the maintenance phase, 34 were restung accidentally, no allergic reactions were reported in 23 patients of these 34 patients (group A: 8; group B: 7; group C: 8) (Table 4). In the remaining 11 patients, there were three episodes of LLR and one of SAR (group A), three episodes of LLR and one of SAR (group B), and C two episodes of LLR and one of SAR (group C) (Table 4).
Table 4.
Allergic reactions to a field sting.
| Group A | Group B | Group C | Total | |
|---|---|---|---|---|
| Patients restung | 13 | 10 | 11 | 34 |
| maintenance dose of Apis m. | 9 | 6 | 7 | 22 |
| maintenance dose of Vespula spp. | 4 | 4 | 4 | 12 |
| No local and systemic effects | 8 | 7 | 8 | 23 |
| Local large reaction* | 3 | 3 | 2 | 8 |
| Systemic anaphylactic reaction§ | 1 | 1 | 1 | 3 |
| Grade I | — | 1 | 1 | |
| Grade II | 1 | — | 1 | 2 |
| Grade III | — | — | — | — |
| Grade IV | — | — | — | — |
*A large local reaction is defined as a swelling at the site of more than 10 cm lasting for more than 24 hours.
§A systemic anaphylactic reaction (SAR) was classified with modified classification of Müller [9].
4. Discussion
Various protocols have been proposed to obtain rapid desensitization of patients allergic to Hymenoptera venom [11, 12], but how to increase rapidly the doses is still debated. Some studies reported a high risk of ADR with rush protocols [31, 34, 35], while other studies showed that they were safe [13–15, 36–40]. In contrast with a previous study in which systemic reactions were found during the incremental phase of an ultrarush VIT protocol [12], no systemic reaction occurred in our group A patients, whereas they occurred in both groups B and C patients. However, in the maintenance phase, there was a case of SAR in group A, 5 in group B, and 2 in group C. We are unable to explain these findings; notwithstanding, no pretreatment, in terms of antihistamines and corticosteroids, was administered in our patients, unlike several previous studies [40, 41].
A wide range of systemic reactions have been reported during the incremental phase of VIT. Birnbaum et al. used an ultrarush VIT protocol similar to ours to treat 258 Hymenoptera venom-allergic patients with a cumulative dose of 101.1 μg, administered over a period of 3.5 hours. In 325 ultrarush immunotherapies performed, 33 (12.79%) patients experienced a systemic reaction during dose increment, namely, localized urticaria and/or angioedema and/or erythema in 24 patients and hypotension in 9 patients [14]. Bernstein et al. reported mild systemic reactions in only 5.2% of 77 patients; however, all patients received a cumulative total dose of only 58.55 μg on one day followed by an accelerated build-up over three weeks [38]. In our study, the frequency of reactions was comparable with the low number of ADRs observed in previous study, where the side effects during VIT were determined by percent of injections during the incremental and the maintenance phases [34, 42].
In our study, during the maintenance phase, in agreement with previous reports [30], ADRs occurred more frequently in our patients treated with the HB venom preparation than in those receiving the YJ preparation. We previously found that ultrarush VIT rapidly decreased ICAM-1 levels in patients with Hymenoptera allergy [43]. It is likely that the known ability of VIT to correct the imbalance in T lymphocyte subpopulations and in the associated production of cytokines may account for the different response to HB versus YJ venom [43]. In fact, these cytokines include IL-4 and TNF-alpha, which upregulate adhesion molecules [44]. In particular, a shift in cytokine responses from a Th2 to a Th1 pattern was demonstrated during rush VIT using both HB [45] and YJ venom [46]. Regarding T-reg, a recent study found an elevated IL-10 production by CD3(+) T cells few hours after rush VIT [47]. In our study, patients were treated with aqueous extracts of Hymenoptera venom during the incremental phase and well tolerated the shift to the maintenance dose with aluminum hydroxide-adsorbed extracts, confirming previous data [32, 48]. During VIT, the incidence of ADRs due to restings was similar in all three groups. The efficacy of ultrarush therapy is therefore comparable with that of rush and slow conventional protocols. After the incremental phase and also during maintenance treatment, specific IgE levels changed to the same extent in the three treatment groups, in agreement with other studies [49, 50]. The ultrarush protocol significantly reduced total and specific IgE levels as the rush and conventional protocol does. In all patients, there was a highly significant direct correlation between the sIgE/tIgE post/preultrarush VIT delta and the SAR pre/postultrarush VIT delta, which confirms the effectiveness of the three protocols.
Given our observation that the ultrarush VIT protocol is well tolerated and effective together with the fact that patients could be vaccinated before the Hymenoptera season starts [51], the fast protocol (3 hours) means patients have more time available for social activities and work. The working environment of some patients (beekeepers, farmers, etc.) with Hymenoptera allergy is an adjunctive risk factor for insect sting compared with the general population. Effective VIT frees these patients and their families from the worry of stings. Because of its short duration, ultrarush VIT is more easily accepted by the patients and has the additional advantages of rapid protection with a low cost. VIT improves the quality of life in all patients allergic to Hymenoptera venom, particularly those in the Müller classes III and IV and in the people who have been restung during VIT [3, 19, 52].
In conclusion, ultrarush was as effective as the rush and slow conventional protocols and was associated with a low incidence of reactions to Hymenoptera stings. This study indicates that ultrarush VIT is a valid therapeutic option for patients with Hymenoptera allergy.
Acknowledgments
The authors thank Silvana Pizza and her colleagues Rosa Vigorito and Massimo Caso for their excellent technical assistance in the Biological Analysis Laboratory, General Hospital of Agropoli, ASL, Salerno, Italy. They thank Jean Ann Gilder (Scientific Communication Srl) for revising and editing the paper.
Abbreviations
- ADR:
Adverse reaction
- HVA:
Hymenoptera venom allergy
- LLR:
Large local reaction
- SAR:
Systemic anaphylactic reaction
- VIT:
Venom immunotherapy.
References
- 1.Bilò BM, Rueff F, Mosbech H, Oude-Elberink JNG, EAACI Interest Group on Insect Venom Hypersensitivity Diagnosis of hymenoptera venom allergy. Allergy. 2005;60:1339–1349. doi: 10.1111/j.1398-9995.2005.00963.x. [DOI] [PubMed] [Google Scholar]
- 2.Bonifazi F, Jutel M, Bilò BM, Birnbaum J, Muller U. Prevention and treatment of hymenoptera venom allergy: guidelines for clinical practice. Allergy. 2005;60:1459–1470. doi: 10.1111/j.1398-9995.2005.00960.x. [DOI] [PubMed] [Google Scholar]
- 3.Golden DB, Moffitt J, Nicklas RA, et al. Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma & Immunology (AAAAI); American College of Allergy, Asthma & Immunology (ACAAI); Joint Council of Allergy, Asthma and Immunology. Journal of Allergy and Clinical Immunology. 2011;127:852–854. [Google Scholar]
- 4.Bilò BM, Bonifazi F. Hymenoptera venom immunotherapy. Immunotherapy. 2011;3(2):229–246. doi: 10.2217/imt.10.88. [DOI] [PubMed] [Google Scholar]
- 5.Van der Linden PWG, Hack CE, Struyvenberg A, Van der Zwan JK. Insect-sting challenge in 324 subjects with a previous anaphylactic reaction: current criteria for insect-venom hypersensitivity do not predict the occurrence and the severity of anaphylaxis. Journal of Allergy and Clinical Immunology. 1994;94(2):151–159. doi: 10.1016/0091-6749(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 6.Golden DBK, Marsh DG, Freidhoff LR, et al. Natural history of Hymenoptera venom sensitivity in adults. Journal of Allergy and Clinical Immunology. 1997;100(6 I):760–766. doi: 10.1016/s0091-6749(97)70270-7. [DOI] [PubMed] [Google Scholar]
- 7.Pucci S, Antonicelli L, Bilo MB, Garritani MS, Bonifazi F. Shortness of interval between two stings as risk factor for developing Hymenoptera venom allergy. Allergy. 1994;49(10):894–896. doi: 10.1111/j.1398-9995.1994.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 8.Hunt KJ, Valentine MD, Sobotka AK, Benton AW, Amodio FJ, Lichtenstein LM. A controlled trial of immunotherapy in insect hypersensitivity. The New England Journal of Medicine. 1978;299:157–161. doi: 10.1056/NEJM197807272990401. [DOI] [PubMed] [Google Scholar]
- 9.Müller UR. Insect Sting Allergy. New York, NY, USA: Gustav Fischer; 1990. [Google Scholar]
- 10.Johansson SGO, Hourihane JOB, Bousquet J, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56(9):813–824. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- 11.Birnbaum J, Charpin D, Vervloet D. Rapid hymenoptera venom immunotherapy: comparative safety of three protocols. Clinical and Experimental Allergy. 1993;23(3):226–230. doi: 10.1111/j.1365-2222.1993.tb00886.x. [DOI] [PubMed] [Google Scholar]
- 12.Brehler R, Wolf H, Kütting B, Schnitker J, Luger T. Safety of a two-day ultrarush insect venom immunotherapy protocol in comparison with protocols of longer duration and involving a larger number of injections. Journal of Allergy and Clinical Immunology. 2000;105(6):1231–1235. doi: 10.1067/mai.2000.105708. [DOI] [PubMed] [Google Scholar]
- 13.Ruëff F, Przybilla B, Brehler R, Luger T, Kütting B. Ultrarush immunotherapy in patients with hymenoptera venom allergy. Journal of Allergy and Clinical Immunology. 2001;107(5):928–929. doi: 10.1067/mai.2001.115138. [DOI] [PubMed] [Google Scholar]
- 14.Birnbaum J, Ramadour M, Magnan A, Vervloet D. Hymenoptera ultra-rush venom immunotherapy (210 min): a safety study and risk factors. Clinical and Experimental Allergy. 2003;33(1):58–64. doi: 10.1046/j.1365-2222.2003.01564.x. [DOI] [PubMed] [Google Scholar]
- 15.Schiavino D, Nucera E, Pollastrini E, et al. Specific ultrarush desensitization in Hymenoptera venom-allergic patients. Annals of Allergy, Asthma and Immunology. 2004;92(4):409–413. doi: 10.1016/S1081-1206(10)61775-9. [DOI] [PubMed] [Google Scholar]
- 16.Sturm G, Kränke B, Rudolph C, Aberer W. Rush Hymenoptera venom immunotherapy: a safe and practical protocol for high-risk patients. Journal of Allergy and Clinical Immunology. 2002;110(6):928–933. doi: 10.1067/mai.2002.129124. [DOI] [PubMed] [Google Scholar]
- 17.Köhli-Wiesner A, Stahlberger L, Bieli C, Stricker T, Lauener R. Induction of specific immunotherapy with hymenoptera venoms using ultrarush regimen in children: safety and tolerance. Journal of Allergy. 2012;2012:5 pages. doi: 10.1155/2012/790910. Article ID 790910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oren E, Chegini S, Hamilos DL. Ultrarush venom desensitization after systemic reactions during conventional venom immunotherapy. Annals of Allergy, Asthma and Immunology. 2006;97(5):606–610. doi: 10.1016/S1081-1206(10)61088-5. [DOI] [PubMed] [Google Scholar]
- 19.Confino-Cohen R, Melamed S, Goldberg A. Debilitating beliefs, emotional distress and quality of life in patients given immunotherapy for insect sting allergy. Clinical and Experimental Allergy. 1999;29(12):1626–1631. doi: 10.1046/j.1365-2222.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- 20.Oude Elberink JNG, De Monchy JGR, Van Der Heide S, Guyatt GH, Dubois AEJ. Venom immunotherapy improves health-related quality of life in patients allergic to yellow jacket venom. Journal of Allergy and Clinical Immunology. 2002;110(1):174–182. doi: 10.1067/mai.2002.125827. [DOI] [PubMed] [Google Scholar]
- 21.Müller U, Mosbech H. Position paper: immunotherapy with hymenoptera venoms. EAACI Allergy. 1993;48:36–46. [PubMed] [Google Scholar]
- 22.Patella V, Florio G, Spadaro G. Hymenoptera venom allergy: a new ultra-rush immunotherapy. In: Holgate ST, Marone G, Ring J, editors. Cellular and Molecular Targets in Allergy and Clinical Immunology. Hogrefe and Huber; 2008. p. 354. [Google Scholar]
- 23.Bilò BM, Cinti B, Brianzoni MF, Braschi MC, Bonifazi M, Antonicelli L. Honeybee venom immunotherapy: a comparative study using purified and non purified aqueous extracts in patients with normal basal serum tryptase concentrations. Journal of Allergy. 2012;2012:6 pages. doi: 10.1155/2012/869243. Article ID 869243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt KJ, Valentine MD, Sobotka AK, Lichtenstein LM. Diagnosis of allergy to stinging insects by skin testing with Hymenoptera venoms. Annals of Internal Medicine. 1976;85(1):56–59. doi: 10.7326/0003-4819-85-1-56. [DOI] [PubMed] [Google Scholar]
- 25.Cirillo A, Patella V, Ciccarelli A, Gallo L. Asthma in allergic patients to venom of Hymenoptera. In: Proceedings of the 17th International Congress of Allergology and Clinical Immunology; October 2000; Sydney, Australia. [Google Scholar]
- 26.Bjorkander J, Belin L. Diagnostic skin testing in Hymenoptera sensitivity. In: Oehling A, editor. Advances in Allergology and Applied Immunology. New York, NY, USA: Pergamon Press; 1980. p. 733. [Google Scholar]
- 27.Li TM, Chuang T, Tse S, Hovanec-Burns D, El Shami AS. Development and validation of a 3rd generation allergen-specific IgE assay on the continuous random access IMMULITE 2000 analyzer. Annals of Clinical and Laboratory Science. 2004;34(1):67–74. [PubMed] [Google Scholar]
- 28.Guilloux L, Hamberger C. Assessment of specific IgE assay using Immilite® 2000 DPC. Immuno-Analyse et Biologie Specialisee. 2004;19(1):71–80. [Google Scholar]
- 29.Snedecor GW, Cochram WG. Statistical Methods. Ames, Iowa, USA: The Iowa State University Press; 1980. [Google Scholar]
- 30.Charpin D, Birnbaum J, Vervloet D. Epidemiology of hymenoptera allergy. Clinical and Experimental Allergy. 1994;24(11):1010–1015. doi: 10.1111/j.1365-2222.1994.tb02736.x. [DOI] [PubMed] [Google Scholar]
- 31.Reisman RE. Natural history of insect sting allergy: relationship of severity of symptoms of initial sting anaphylaxis to re-sting reactions. Journal of Allergy and Clinical Immunology. 1992;90(3 I):335–339. doi: 10.1016/s0091-6749(05)80012-0. [DOI] [PubMed] [Google Scholar]
- 32.Cadario G, Marengo F, Ranghino E, et al. Higher frequency of early local side effects with aqueous versus depot immunotherapy for Hymenoptera venom allergy. Journal of Investigational Allergology and Clinical Immunology. 2004;14:127–133. [PubMed] [Google Scholar]
- 33.Di Lorenzo G, Mansueto P, Pacor ML, et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. Journal of Allergy and Clinical Immunology. 2009;123(5):1103–1110. doi: 10.1016/j.jaci.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Mosbech H, Muller U. Side-effects of insect venom immunotherapy: results from an EAACI multicenter study. Allergy. 2000;55(11):1005–1010. doi: 10.1034/j.1398-9995.2000.00587.x. [DOI] [PubMed] [Google Scholar]
- 35.Gillman SA, Cummins LH, Kozak PP, Hoffman DR. Venom immunotherapy: comparison of “rush” versus “conventional” schedules. Annals of Allergy. 1980;45(6):351–354. [PubMed] [Google Scholar]
- 36.Michils A, Baldassarre S, Ledent C, Mairesse M, Gossart B, Duchateau J. Early effect of ultrarush venom immunotherapy on the IgG antibody response. Allergy. 2000;55(5):455–462. doi: 10.1034/j.1398-9995.2000.00412.x. [DOI] [PubMed] [Google Scholar]
- 37.Van der Zwan JC, Flinterman J, Jankowski IG, Kerckhaert JAM. Hyposensitisation to wasp venom in six hours. British Medical Journal. 1983;287(6402):1329–1331. doi: 10.1136/bmj.287.6402.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernstein JA, Kagen SL, Bernstein DI, Bernstein IL. Rapid venom immunotherapy is safe for routine use in the treatment of patients with Hymenoptera anaphylaxis. Annals of Allergy. 1994;73(5):423–428. [PubMed] [Google Scholar]
- 39.Roll A, Hofbauer G, Ballmer-Weber BK, Schmid-Grendelmeier P. Safety of specific immunotherapy using a four-hour ultra-rush induction scheme in bee and wasp allergy. Journal of Investigational Allergology and Clinical Immunology. 2006;16(2):79–85. [PubMed] [Google Scholar]
- 40.Pasaoglu G, Sin BA, Misirligil Z. Rush hymenoptera venom immunotherapy is efficacious and safe. Journal of Investigational Allergology and Clinical Immunology. 2006;16(4):232–238. [PubMed] [Google Scholar]
- 41.Müller U, Hari Y, Berchtold E. Premedication with antihistamines may enhance efficacy of specific-allergen immunotherapy. Journal of Allergy and Clinical Immunology. 2001;107(1):81–86. doi: 10.1067/mai.2001.111852. [DOI] [PubMed] [Google Scholar]
- 42.Mosbech H, Muller U. Side-effects of insect venom immunotherapy: results from an EAACI multicenter study. Allergy. 2000;55(11):1005–1010. doi: 10.1034/j.1398-9995.2000.00587.x. [DOI] [PubMed] [Google Scholar]
- 43.Patella V, Incorvaia C, Ricciardi L, et al. The adhesion molecule ICAM-1 is overexpressed in patients with hymenoptera venom allergy and decreases after ultrarush venom immunotherapy. Journal of Biological Regulators & Homeostatic Agents. 2011;25:465–468. [PubMed] [Google Scholar]
- 44.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nature Reviews Immunology. 2006;6(10):761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 45.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. Journal of Allergy and Clinical Immunology. 2009;123(4):735–746. doi: 10.1016/j.jaci.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 46.McHugh SM, Deighton J, Stewart AG, Lachmann PJ, Ewan PW. Bee venom immunotherapy induces a shift in cytokine responses from a TH-2 to a TH-1 dominant pattern: comparison of rush and conventional immunotherapy. Clinical and Experimental Allergy. 1995;25(9):828–838. doi: 10.1111/j.1365-2222.1995.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 47.Schuerwegh AJ, De Clerck LS, Bridts CH, Stevens WJ. Wasp venom immunotherapy induces a shift from IL-4-producing towards interferon-gamma-producing CD4+ and CD8+ T lymphocytes. Clinical and Experimental Allergy. 2001;31(5):740–746. doi: 10.1046/j.1365-2222.2001.01066.x. [DOI] [PubMed] [Google Scholar]
- 48.Poli F, Longo G, Parmiani S. The safety and efficacy of immunotherapy with aluminum hydroxide-adsorbed venom extract of Vespula spp. An open, retrospective study. Allergologia et Immunopathologia. 2001;29(5):191–196. doi: 10.1016/s0301-0546(01)79054-4. [DOI] [PubMed] [Google Scholar]
- 49.Djurup R, Malling HJ, Sondergaard I, Weeke B. The IgE and IgG subclass antibody response in patients allergic to yellow jacket venom undergoing different regimens of venom immunotherapy. Journal of Allergy and Clinical Immunology. 1985;76(1):46–55. doi: 10.1016/0091-6749(85)90803-6. [DOI] [PubMed] [Google Scholar]
- 50.Jeep S, Meysel U, Kunkel G. IgE, IgG, IgG1 and IgG4 patterns in yellow jacket allergic patients during immunotherapy with a venom depot extract. Clinical and Experimental Allergy. 1992;22(2):297–302. doi: 10.1111/j.1365-2222.1992.tb03086.x. [DOI] [PubMed] [Google Scholar]
- 51.Bousquet J, Menardo JL, Velasquez G, Michel FB. Systemic reactions during maintenance immunotherapy with honey bee venom. Annals of Allergy. 1988;61(1):63–68. [PubMed] [Google Scholar]
- 52.Goldberg A, Confino-Cohen R. Rush venom immunotherapy in patients experiencing recurrent systemic reactions to conventional venom immunotherapy. Annals of Allergy, Asthma and Immunology. 2003;91(4):405–410. doi: 10.1016/S1081-1206(10)61689-4. [DOI] [PubMed] [Google Scholar]


