Abstract
This study aimed to evaluate the activity of essential oils (EOs) against Streptococcus mutans biofilm by chemically characterizing their fractions responsible for biological and antiproliferative activity. Twenty EO were obtained by hydrodistillation and submitted to the antimicrobial assay (minimum inhibitory (MIC) and bactericidal (MBC) concentrations) against S. mutans UA159. Thin-layer chromatography and gas chromatography/mass spectrometry were used for phytochemical analyses. EOs were selected according to predetermined criteria and fractionated using dry column; the resulting fractions were assessed by MIC and MBC, selected as active fractions, and evaluated against S. mutans biofilm. Biofilms formed were examined using scanning electron microscopy. Selected EOs and their selected active fractions were evaluated for their antiproliferative activity against keratinocytes and seven human tumor cell lines. MIC and MBC values obtained for EO and their active fractions showed strong antimicrobial activity. Chemical analyses mainly showed the presence of terpenes. The selected active fractions inhibited S. mutans biofilm formation (P < 0.05) did not affect glycolytic pH drop and were inactive against keratinocytes, normal cell line. In conclusion, EO showed activity at low concentrations, and their selected active fractions were also effective against biofilm formed by S. mutans and human tumor cell lines.
1. Introduction
Despite the implementation of measures to control and treat dental caries with fluoride, they remain the most prevalent dental disease in many countries [1]. Caries are a multifactorial infectious disease caused by accumulation of biofilm on tooth surface [2]. Manifestations of the disease occur when there is an imbalance between the biofilm and the host due to changes in biofilm matrix pH caused by diet, microorganisms, or salivary flow and their components [3, 4].
Streptococcus mutans is considered the most cariogenic of all oral streptococci [5]. S. mutans is able to colonize the tooth surface and to produce large amounts of extra and intra-cellular polysaccharides. This microorganism is also highly acidogenic and aciduric, and it metabolizes several salivary glycoproteins, thus being responsible for the initial stage of oral biofilm formation and caries lesions [6].
Several products have been used to control dental caries, such as fluoride, chlorhexidine, and their associations [7]. However, natural products have contributed significantly to the discovery of chemical structures to create new medicaments to be used as innovative therapeutic agents against this prevalent disease [8, 9].
Essential oils (EOs) are important for their detected antimicrobial activity [10–12] including that against S. mutans [13]. They are complex, volatile, natural compounds formed by aromatic plants as secondary metabolites [14]. They are known for their bactericidal, virucidal, fungicidal, sedative, anti-inflammatory, analgesic, spasmolytic, and locally anesthetic properties [14]. The presence of complex chemical structures constituted of several groups, such as terpenes and terpenoids, aromatic and aliphatic constituents, all characterized by low molecular weight, may explain their successful bacteriostatic and bactericidal action [14].
Additionally, it was attested that the antimicrobial activity of a natural product, such as EO, is important to evaluate its effects on human normal cell lines and also against human tumor cell lines in order to evidence potential toxicity on human healthy and tumor cell lines [15]. For this reason, it is important that extensive studies involving EO as well as other sources of natural medicines are carried out.
The aim of this study was to evaluate the activity of EO and fractions against planktonic cells of S. mutans and also the selected active fractions of EO were chemically characterized and evaluated against mutans biofilm and antiproliferative activity on human cells.
2. Materials and Methods
2.1. Medicinal Plants
We studied 20 medicinal and aromatic plants (Table 1), which were obtained from the germoplasm bank of the Collection of Medicinal and Aromatic Plants (CPMA) of the Research Center for Chemistry, Biology and Agriculture (CPQBA), University of Campinas (UNICAMP), São Paulo, Brazil (http://www.cpqba.unicamp.br/), and identified by Glyn M. Figueira, curator of CPMA.
Table 1.
Medicinal and aromatic plants from the germplasm bank of the CPMA/CPQBA/UNICAMP selected for this study with their yield, MIC and MBC values, and MBC : MIC ratio.
| Medicinal species | Family | Popular name | Source | CPMA number | Voucher number1 | Yield (%) | MIC (μg/mL) | MBC (μg/mL) | MBC : MIC ratio2 | Popular use |
|---|---|---|---|---|---|---|---|---|---|---|
| Aloysia gratissima (Gillies & Hook) | Verbenaceae | Brazilian lavender | Leaf | 714 | UEC 121.393 | 1.1 | 125–250 | 250–500 | 2 : 1 | Digestive; antispasmodic |
| Aloysia triphylla (L'Hér.) Britton | Verbenaceae | Aloisia | Leaf | 274/700 | UEC 121.412 | 0.3 | 125–250 | 125–250 | 1 : 1 | Sedative; antispasmodic |
| Alpinia speciosa (Pers.) Burtt & Smith | Zingiberaceae | Colony | Root | 447 | UEC 145.185 | 0.2 | 125–250 | 250–500 | 2 : 1 | Antimicrobial |
| Baccharis dracunculifolia DC | Asteraceae | Broom weed | Leaf | 1841 | — | 0.8 | 62.5–125 | 250–500 | 4 : 1 | Tonic; eupeptic, antipyretic |
| Cinnamomum zeylanicum Blume | Lauraceae | Cinnamon | Leaf | 455 | IAC 19624 | 0.2 | 250–500 | 500–1000 | 2 : 1 | Carminative; antispasmodic |
| Coriandrum sativum L. | Apiaceae | Coriander | Leaf | 664 | — | 0.3 | 31.2–62.5 | 62.5–125 | 2 : 1 | Antimicrobial; antifungal |
| Cymbopogon citratus (DC) Stapf. | Poaceae | Lemon grass | Leaf | 503 | UEC 85.210 | 1.1 | 125–250 | 250–500 | 2 : 1 | Sedative; analgesic; anticough |
| Cymbopogon martini (Roxb.) J. F. Watson | Poaceae | Palmarosa | Leaf | 354 | UEC 127.115 | 0.6 | 125–250 | 250–500 | 2 : 1 | Antiseptic; antifungal |
| Cymbopogon winterianus Jowitt | Poaceae | Lemon verbena | Leaf | 712 | UEC 121.414 | 1.5 | 125–250 | 250–500 | 2 : 1 | Repellent, insecticide |
| Cyperus articulatus Vahl | Cyperaceae | Priprioca | Bulbs | 222 | UEC 121.396 | 0.5 | 125–250 | 250–500 | 2 : 1 | Anti-inflammatory |
| Elyonurus muticus Spreng | Poaceae | Agripalma | Leaf | 1701 | UEC 20.580 | 0.6 | 125–250 | 125–250 | 1 : 1 | Antibacterial |
| Eugenia florida DC. | Myrtaceae | Guamirim-cereja | Leaf | 1685 | IAC 49207 | 0.3 | 125–250 | 500–1000 | 4 : 1 | Anti-inflammatory |
| Eugenia uniflora L. | Myrtaceae | Pitanga | Leaf | 1816 | — | 0.7 | 125–250 | 250–500 | 2 : 1 | Antihypertensive; diuretic |
| Lippia alba (Mill.) N.E. Brown | Verbenaceae | False lemon balm | Leaf | 467/509 | UEC 121.413 | 0.3 | 125–250 | 250–500 | 2 : 1 | Treatment of migraines |
| Lippia sidoides Cham. | Verbenaceae | Rosemary | Leaf | 398/399 | — | 4.7 | 62.5–125 | 125–250 | 2 : 1 | Bactericide; fungicide |
| Mentha piperita L. | Lamiaceae | Mint | Leaf | 560 | UEC 127.110 | 2.2 | 250–500 | 250–500 | 1 : 1 | Antifungal; antibacterial |
| Mikania glomerata Spreng. | Asteraceae | Guaco | Leaf | 766 | UEC 102.047 | 0.4 | 62.5–125 | 125–250 | 2 : 1 | Anti-inflammatory; bronchodilator |
| Siparuna guianenses Aubl. | Monimiaceae | Wild lemon | Leaf | 2025 | — | 0.3 | 62.5–125 | 125–250 | 2 : 1 | Tranquilizer; diuretic |
| Syzygium aromaticum (L.) Merr. & L. M. Perry | Myrtaceae | Cloves | Leaf | 455 | IAC 19624 | 0.5 | 62.5–125 | 250–500 | 4 : 1 | Seasoning; antibacterial |
| Ziziphus joazeiro Mart. | Rhamnaceae | Joazeiro fruit | Leaf | 2119 | — | 0.5 | 250–500 | 500–1000 | 2 : 1 | Astringent; anti-inflammatory |
1A voucher herbarium specimen is a pressed plant sample deposited for future reference. Vouchers deposited at UEC herbarium (http://www.ib.unicamp.br/herbario/) at Biology Institute (IB) of UNICAMP, SP, Brazil. (—) Species with no voucher number registered. 2The EOs were considered bactericidal when the MBC : MIC ratio was between 1 : 1 to 2 : 1, and bacteriostatic if this ratio was higher than 2 : 1.
The plants were collected from November 2009 to January 2011, during the morning, after the dew point has been reached. The vouchers of each species were deposited in the herbarium of the Institute of Biology, at UNICAMP-UEC, and also registered in the herbarium of CPQBA, receiving identification numbers (CPMA number).
2.2. Essential Oil Extraction
EOs were obtained from 100 g of aerial fresh plant parts by hydrodistillation using a Clevenger-type system, for 3 hours. The aqueous phase was extracted with 50 mL of dichloromethane. Then, the organic layer was separated, dried over anhydrous sodium sulphate (Na2SO4), and filtered; the solvent was removed by vacuum evaporation at room temperature, resulting in EO. Oil samples were stored at −25°C in sealed glass vials [11].
2.3. Fractionation of Essential Oils
In order to select the EO that should be fractionated, we predetermined some criteria: best antimicrobial activity (MIC < 250 μg/mL), extract yield (>0.5%, except for Coriandrum sativum EO), commercial availability, presence of the EO in aerial parts of plants, and easy cultivation. The resulting fractions were also submitted to the antimicrobial assay.
Fractionation was performed using dry column chromatography (cellulose 2 cm × 20 cm) with Si gel 60 (Merck, Darmstadt, Germany) as the stationary phase and dichloromethane as the mobile phase, previously chosen by thin-layer chromatography (TLC), visualized under UV 254 nm, followed by anisaldehyde solution application and drying at 105°C for 5 min. After elution, columns were cut into different parts for each EO, according to polarity and extraction, using dichloromethane. The fractions so obtained were analyzed using TLC and gas chromatography coupled to mass spectrometry (GC-MS) and then bioguided using the antimicrobial assays [16]. All chemical wastes generated during this study were treated according to the Environmental Ethics Committee of UNICAMP (324/2009).
2.4. Analyses of the Selected Active Fractions using GC-MS
The chemical composition of each selected active fraction was evaluated using a Hewlett-Packard 6890 gas chromatograph equipped with an HP-5975 mass selective detector and HP-5 capillary column (30 m × 0.25 mm × 0.25 μm). GC-MS was performed using split injection with the injector set at 220°C, the column set at 60°C with a heating ramp of 3°C/min and a final temperature of 240°C, and the MS detector set at 250°C. Helium was used as a carrier gas at 1 mL/min. The GC-MS electron ionization system was set at 70 eV. The quantitative analyses were performed using a Hewlett-Packard 5890 gas chromatograph equipped with a flame ionization detector under the same conditions previously described. A sample of each EO or its selected active fraction was solubilized in ethyl acetate (15 mg/mL) for the analysis. Retention indices (RIs) were determined using injection of hydrocarbon standards and EO samples under the same conditions described above. The oil components were identified by comparison with data described in the literature and the profiles in the NIST 05 mass spectral library [11, 17].
2.5. Microorganisms
For the development of this study, Streptococcus mutans UA159 was used.
2.6. Antimicrobial Assay
We tested 20 EOs using the antimicrobial assay and selected them according to pre-determined criteria (item 2.3) before being fractionated and continuing the bioguided study.
MIC test was carried out using tissue culture microplates (96 wells) containing 100 μL/well BHI (Brain Heart Infusion, Difco, Franklin Lakes, NJ, USA) medium [18]. The stock solutions of EO and fractions from selected EO (item 2.3) were diluted with propylene glycol (4 mg/mL), transferred to the first well, and serial dilutions were performed to obtain concentrations ranging from 7.81 to 1000 μg/mL. We used 0.12% chlorhexidine (Sigma-Aldrich, St. Louis, MO, USA) as positive control and propylene glycol 6.25% as negative control. The bacterial inoculum (1 × 106 UFC/mL) was added to all wells, and the plates were incubated at 37°C and 5% CO2 for 24 hours. MIC was defined as the lowest concentration of EO or fraction from selected EO that inhibited microorganism visible growth indicated by resazurin 0.01% (Sigma-Aldrich, St. Louis, MO, USA) [19].
To determine MBC, an aliquot of each incubated well with concentrations higher than MIC was subcultured on BHI medium supplemented with 5% defibrinated sheep blood using a Whitley Automatic Spiral Plater (Don Whitley Scientific Limited, Shipley, West Yorkshire, UK). MBC was defined as the lowest concentration of EO or fraction that allowed no visible growth on the test medium.
To determine the nature of antibacterial effect of EO and fractions, the MBC : MIC ratio for bacteria was used [20]. When MBC : MIC ratio for S. mutans was between 1 : 1 and 2 : 1, the EO or fraction from selected EO was considered bactericidal against this microorganism [20], and when the ratio was higher than 2 : 1, it was considered bacteriostatic.
2.7. Action of Selected Active Fractions from Selected EO against S. mutans Biofilm
We tested 20 EOs, and those that fulfilled the pre-determined criteria (item 2.3) were selected to be chemically fractionated. The resulting fractions were also tested using the antimicrobial assay and selected according to MIC and MBC results and yields. The selected active fractions were then assessed regarding their action against S. mutans biofilm.
2.7.1. Inhibition of S. mutans Biofilm Growth
In order to evaluate the antimicrobial activity of EO selected active fractions against the formation of S. mutans biofilm, the samples were placed, at different concentrations (7.81–1000 μg/mL), in the wells of sterile polystyrene U-bottom microtiter plates, previously treated with saliva (the use of human saliva in this study was approved by the Research Ethics Committee of the Piracicaba Dental School, State University of Campinas (UNICAMP) (Approval 087/2011)) [21]. S. mutans cells (1.0 × 107 cells/mL in BHI medium) were added to wells containing BHI medium with 2% sucrose and the samples were incubated at 37°C for 18 hours. Biofilm growth was revealed and quantified using the crystal violet staining method and measuring absorbance at 575 nm [11, 22].
After 18 hours of incubation, the spent medium was aspirated, nonadhered cells were removed, the wells were washed three times with sterile distilled water, and the plates were dried for 45 min before carrying out biofilm quantification [22].
2.7.2. Glycolytic pH-Drop Assay
The effect of EO selected active fractions against S. mutans biofilm was measured using the standard glycolytic pH-drop assay [23]. Biofilm growth was carried out as previously described (item 2.7.1), in sterile polystyrene U-bottom microtiter plates without fractions. The biofilms so obtained were washed twice with 0.9% NaCl solution and salt solution (50 mM KCl + 1.0 mM MgCl2), containing EO selected active fractions at different concentrations (1000, 500, and 250 μg/mL), and vehicle (25% propylene glycol, v/v) was added. The pH was adjusted to 7.2 with 0.1 M KOH solution, and glucose was added to a final concentration of 1%, and pH-drop was assessed using Orion pH glass electrode attached to Orion 290 A+ pHmeter (Orion Scientific, Houston, TX, USA) for 90 min.
2.8. Scanning Electron Microscopy (SEM)
In order to evaluate S. mutans integrity using SEM, biofilms were first developed in Lab-Tek chambered coverglass (Nunc, Naperville, IL, USA), as described previously (item 2.7.1), were treated with vehicle (6.12% propylene glycol) or had their active fractions selected at concentrations able to inhibit more than 90% of S. mutans biofilm formation. Samples were fixed in 4% glutaraldehyde (v/v) in phosphate-buffered saline (PBS) at room temperature for 12–24 hours. After this procedure, the biofilms were dehydrated through a graded series of ethanol (50% to 100%), dried to a critical point, coated with gold, and observed using a scanning electron microscope JEOL JSM5600LV (JEOL Ltd., Tokyo, Japan) [11, 24].
2.9. Antiproliferative Assay
The in vitro antiproliferative assay [25] was performed in the present study using a human keratinocyte (HaCat) cell line, kindly donated by Dr. Ricardo Della Coletta (FOP, UNICAMP, Brazil), and seven human tumor cell lines (U251 (glioma), MCF-7 (breast), NCI-ADR/RES (ovarian expressing phenotype multiple drugs resistance), 786-0 (renal), NCI-H460 (lung, nonsmall cells), PC-3 (prostate), and OVCAR-03 (ovarian), kindly provided by M. A. Frederick (National Cancer Institute, USA). Stock and experimental cultures were grown in medium containing 5 mL RPMI-1640 (Gibco-BRL, Grand Island, NY, USA) supplemented with 5% fetal bovine serum (Gibco-BRL, Grand Island, NY, USA). A penicilline-streptomicine mixture (1000 U/mL : 1000 mg/mL, 1 mL/L RPMI) was added to experimental cultures. Cells in 96-well plates (100 μL cells/well) were exposed to each EO and selected active fractions in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) (0.25, 2.5, 25, and 250 μg/mL) at 37°C and 5% CO2 for 48 hours. Final DMSO concentration did not affect cell viability. Before (T0 plate) and after sample addition (T1 plates), cells were fixed with 50% trichloroacetic acid and cell proliferation was determined by spectrophotometric quantification (540 nm) of cellular protein content using sulforhodamine B assay. Using the concentration-response curve for each cell line, the total growth inhibition (TGI) was determined by nonlinear regression analysis using the software Origin 8.0 (OriginLab Corporation, Northampton, MA, USA) [26, 27].
2.10. Statistical Analysis
An exploratory data analysis was performed to determine the most appropriate statistical test. Inhibition of biofilm growth, and glycolytic pH-drop data were compared using the nonparametric Kruskal-Wallis test. P value < 0.05 was considered statistically significant. Triplicates from at least three separated experiments were conducted in each assay.
3. Results
3.1. Essential Oils and Fraction Yields
The EO yields, expressed in relation to dry weight of plant material (%, w/w), are shown in Table 1.
According to pre-determined criteria (item 2.3), four EOs were selected to be fractionated using dry column as follows: A. gratissima, B. dracunculifolia, C. sativum, and L. sidoides.
The yields of the fractions from selected EO were expressed as a function of the respective EO yield (%, w/w) and are shown in Table 2. The yields of A. gratissima fractions ranged from 14.4% to 29%, B. dracunculifolia from 20.1% to 30.6%, C. sativum from 4.9% to 30.9%, and L. sidoides from 1.7% to 33.3%.
Table 2.
Selected EO and their fractions with yield results, MIC and MBC values, and MBC : MIC ratio.
| Essential oil | Fraction | ||||||
|---|---|---|---|---|---|---|---|
| Identification | MIC (μg/mL) | MBC (μg/mL) | Identification | Yield (%) | MIC (μg/mL) | MBC (μg/mL) | MBC : MIC ratio1 |
| Aloysia gratissima (Ag) | 125–250 | 250–500 | Ag1 | 28.9 | 250–500 | 500–1000 | 2 : 1 |
| Ag2 | 17.9 | 250–500 | 500–1000 | 2 : 1 | |||
| Ag3 | 20.1 | 62.5–125 | 500–1000 | 8 : 1 | |||
| Ag4 2 | 14.4 | 31.2–62.5 | 62.5–125 | 2 : 1 | |||
| Baccharis dracunculifolia (Bd) | 62.5–125 | 250–500 | Bd1 | 30.5 | 250–500 | 500–1000 | 2 : 1 |
| Bd2 | 22.1 | 15.6–31.2 | 125–250 | 8 : 1 | |||
| Coriandrum sativum (Cs) | 31.2–62.5 | 62.5–125 | Cs1 | 6.6 | 125–250 | 500–1000 | 4 : 1 |
| Cs2 | 4.9 | 125–250 | 250–500 | 2 : 1 | |||
| Cs3 | 12.7 | 15.6–31.2 | 31.2–62.5 | 2 : 1 | |||
| Cs4 | 30.9 | 15.6–31.2 | 31.2–62.5 | 1 : 1 | |||
| Lippia sidoides (Ls) | 62.5–125 | 125–250 | Ls1 | 13.6 | 250–500 | 500–1000 | 2 : 1 |
| Ls2 | 33.3 | 62.5–125 | 250–500 | 4 : 1 | |||
| Ls3 | 26 | 62.5–125 | 125–250 | 2 : 1 | |||
| Ls4 | 6.1 | 62.5–125 | 125–250 | 2 : 1 | |||
| Ls5 | 1.7 | 62.5–125 | 125–250 | 2 : 1 | |||
1The fractions from selected EO were considered bactericidal when the MBC : MIC ratio was between 1 : 1 to 2 : 1, and bacteriostatic if this ratio was higher than 2 : 1. 2The fractions in bold font were selected as active fractions and evaluated against S. mutans biofilm and for their antiproliferative action. The subscript numbers of the fractions represent the numbers of parts obtained using the dry column fractionation.
3.2. Antimicrobial Activity
MIC and MBC values for all tested EO are shown in Table 1. MIC values ranged from 31.2 to 500 μg/mL, and MBC values ranged from 62.5 to 1000 μg/mL. The highest activities were observed for A. gratissima and A. triphylla (125–250 μg/mL), B. dracunculifolia, L. sidoides, M. glomerata, S. guianenses, S. aromaticum (62.5–125 μg/mL), and C. sativum (31.2–62.5 μg/mL).
Based on pre-determined criteria (item 2.3), four EOs (A. gratissima, B. dracunculifolia, C. sativum, and L. sidoides) were selected to be fractionated. MIC and MBC values of fractions from selected EO are shown in Table 2. MIC values obtained for all fractions ranged from 15.6 to 500 μg/mL, and MBC values ranged from 31.2 to 1000 μg/mL. The highest activities were observed for the fractions Ag4 (31.2–62.5 μg/mL), Bd2 (15.6–31.2 μg/mL), Cs4 (15.6–31.2 μg/mL), and Ls3 (62.5–125 μg/mL).
The MBC : MIC ratio (Table 1) showed that most EOs are bactericidal, except for B. dracunculifolia, E. florida, and S. aromaticum, which are considered bacteriostatic against S. mutans. Among the selected EO chosen to be fractionated, only that obtained from B. dracunculifolia was bacteriostatic. Most fractions from selected EO were bactericidal, except for Ag4, Cs1, Ls2, and Bd2, considered bacteriostatic against S. mutans (Table 2). Based on yield and antimicrobial activity, Ag4, Bd2, Cs4, and Ls3 fractions were selected for further evaluations.
3.3. Selected Active Fractions Activity against S mutans Biofilm
Figure 1 shows the development of S. mutans biofilm inhibiton after treatment with selected active fractions. Their growth was measured by optic density at 575 nm. The result showed that the selected active fractions tested at different concentrations were significantly different (P < 0.05) from the vehicle. Moreover, Cs4 and Bd2 fractions presented a better performance since they inhibited more than 90% of biofilm formation at lower concentrations (31.2 μg/mL).
Figure 1.
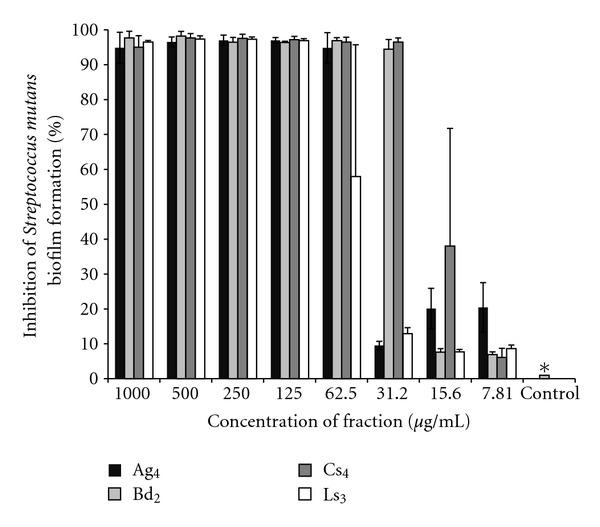
Influence of selected active fractions Ag4, Bd2, Cs4, and Ls3 from selected essential oils at different concentrations against Streptococcus mutans biofilm formation. All fractions tested were significantly different from the vehicle at all concentrations tested. Kruskal-Wallis test (P < 0.05).
3.4. pH-Drop Assay
The influence of selected active fractions from EO on glycolytic pH-drop of S. mutans biofilm formation in the presence of excess glucose was not significant (P > 0.05) for all fractions tested (Ag4, Bd2, Cs4, and Ls3).
3.5. Chemical Characterization of Fractions Constituents
The chemical composition of the selected EO and the selected active fractions is shown in Table 3.
Table 3.
Major compounds of the selected active fractions from essential oils with their retention time (Rt), retention index (RI), and relative percentage.
| Rt (min) | RI | Compound | Relative percentage1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ag EO | Ag4 | Bd EO | Bd2 | Cs EO | Cs4 | Ls EO | Ls3 | |||
| 4.02 | 899 | Cyclohexanone | — | — | — | — | — | — | 6.5 | — |
| 4.22 | 850 | 3-hexen-1-ol | — | — | — | 0.8 | 3.6 | 5.1 | — | — |
| 5.87 | 977 | Beta-pinene | 12.0 | — | — | — | — | — | — | — |
| 7.2 | 1024 | p-cymene | — | — | — | — | — | 17.3 | — | |
| 13.08 | 1140 | Trans-pinocarveol | — | 4.9 | — | — | — | — | — | — |
| 14.09 | 1165 | Trans-pinocamphone | 16.0 | 36.7 | — | — | — | — | — | — |
| 14.61 | 1177 | Cis-pinocamphone | 6.0 | 17.0 | — | — | — | — | — | |
| 16.7 | 1274 | 2-decen-1-ol <E> | — | — | — | — | 23.6 | 26.9 | — | — |
| 16.86 | 1277 | 1-decanol | — | — | — | — | 33.9 | 35.4 | — | — |
| 17.76 | 1299 | Trans-pinocarvyl acetate | 8.2 | — | — | — | — | — | — | — |
| 19.74 | 1300 | Thymol | — | — | — | — | — | 65.8 | 97.8 | |
| 19.95 | 1303 | Carvacrol | — | — | — | — | — | — | — | 0.6 |
| 21.84 | 1349 | Ethyl ester benzenepropanoic | — | — | — | 11.7 | — | — | — | — |
| 22.57 | 1416 | Trans-caryophyllene | 7.2 | — | 10.7 | — | — | — | 10.5 | — |
| 24.86 | 1473 | 2-dodecen-1-ol | — | — | — | — | 13.1 | 14.5 | — | — |
| 25.04 | 14.78 | Germacrene D | — | — | 4.9 | — | — | — | — | — |
| 25.66 | 1493 | Bicyclogermacrene | 4.2 | — | 6.8 | — | — | — | — | — |
| 27.97 | 1553 | M2 = 204 | 6.4 | — | — | — | — | — | — | |
| 30.59 | 1566 | Trans-nerolidol | — | — | 31.7 | 52.2 | — | — | — | — |
| 31.05 | 1578 | Spathulenol | — | — | 13.6 | 11.5 | — | — | — | — |
| 31.23 | 1582 | Caryophyllene oxide | 6.4 | 7.0 | — | 6.3 | — | — | — | 0.7 |
| 31.9 | 1600 | Guaiol | 8.5 | 12.7 | — | — | — | — | — | — |
| 33.44 | 1641 | Epi alpha cadinol | — | — | — | 3.1 | — | — | — | — |
| 32.47 | 1674 | 2-tetradecen-1-ol <E> | — | — | — | — | 5.5 | 5.2 | — | — |
| 34.40 | 1668 | Bulnesol | — | 3.5 | — | — | — | — | — | — |
1The selected active fractions Ag4, Bd2, Cs4, and Ls3 had their actions against S. mutans biofilm and their antiproliferative activity evaluated. Ag EO, Bd EO, Cs EO, and Ls EO correspond to the following essential oils: Aloysia gratissima, Baccharis dracunculifolia, Coriandrum sativum, and Lippia sidoides, respectively. Only the compounds with relative percentage above 3% are listed. 2 M: molecular weight of a nonidentified compound.
The analyses of EO and fractions indicated the presence of volatile compounds, mainly mono- and sesquiterpenes.
We identified 28 compounds in the EO of A. gratissima, representing 92.73% of the EO, 25 compounds in the EO of B. dracunculifolia, representing 93.45% of the EO, 15 compounds in the EO of C. sativum, representing 91.93% of the EO, and four compounds in the EO of L. sidoides, representing 100% of the EO. We also identified 19 compounds in fraction Ag4, representing 94.6% of the fraction, 10 compounds in fraction Bd2, representing 83.06% of the fraction, nine compounds in fraction Cs4, representing 89.71% of the fraction, and five compounds in fraction Ls3, representing 99.7% of the fraction.
The major compounds identified in each selected EO were: trans- and cis-pinocamphone, beta-pinene, and guaiol in A. gratissima; trans-nerolidol and spathulenol in B. dracunculifolia; 2-decen-1-ol and 1-decanol in C. sativum; and thymol in L. sidoides. The major compounds identified in each selected fractions were trans- and cis-pinocamphone and guaiol in Ag3; trans-nerolidol, spathulenol, and ethyl ester benzenepropanoic in Bd2; 2-decen-1-ol and 1-decanol in Cs4; thymol in Ls3.
3.6. Scanning Electron Microscopy (SEM)
The effect of selected active fractions against S. mutans biofilm formation was evaluated by SEM. Figure 2 shows a reduction in biofilm formation. Biofilms were first developed as described previously (Section 2.7.1), were treated with vehicle, or had their active fractions selected at concentrations able to inhibit more than 90% of S. mutans biofilm formation (Ag4 at 62.5 μg/mL, Bd2 and Cs4 at 31.2 μg/mL, and Ls3 at 125 μg/mL).
Figure 2.
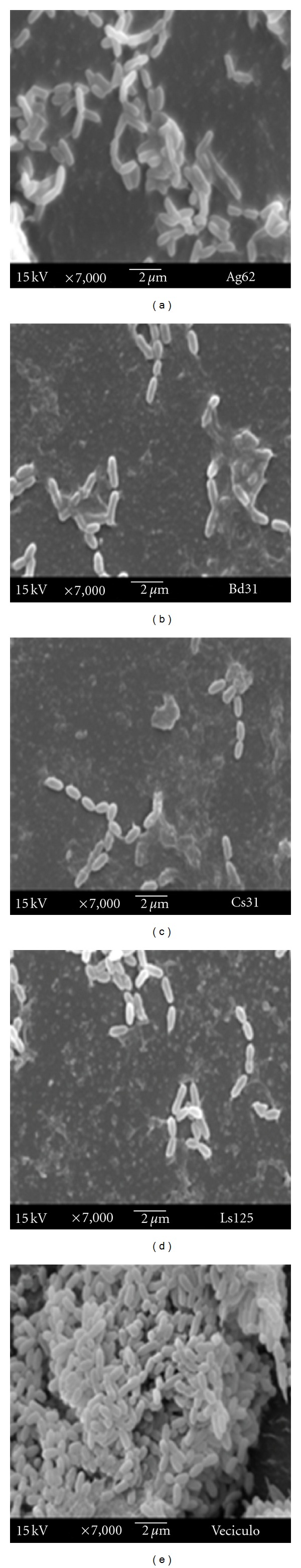
Scanning electron microscopy of Streptococcus mutans biofilms treated with the selected active fractions from selected essential oils and the vehicle. Images a, b, c, and d show the reduction of biofilm formation after treatment with Ag4, Bd2, Cs4, and Ls3 fractions, respectively, compared with the treatment with the vehicle (image (e)) (magnification of 7000x).
3.7. Antiproliferative Assay
Most EOs and their selected active fractions did not present activity against the human normal cell line evaluated in this study or presented high concentrations to totally inhibit its growth. TGI values are shown in Table 4.
Table 4.
Total growth inhibition (TGI) of selected essential oils and their selected active fractions tested against normal human cell and tumor cell lines.
| Cell line | TGI (μg/mL)1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ag EO | Ag4 | Bd EO | Bd2 | Cs EO | Cs4 | Ls EO | Ls3 | Dox | |
| Glioma (U251) | >250 | 55.6 | 38.2 | 51.4 | 8.3 | 61.5 | >250 | 94.9 | 0.92 |
| Breast (MCF-7) | >250 | 45.2 | 46.0 | 67.7 | 13.6 | 111.6 | >250 | 56.6 | 3.3 |
| Ovarian (NCI-ADR/RES) | >250 | 50.6 | 59.2 | 10.5 | 90.0 | 13.1 | >250 | 112.3 | 1.6 |
| Kidney (786-0) | >250 | 5.9 | 49.5 | 47.1 | 29.8 | 72.1 | >250 | 26.7 | >250 |
| Lung (NCI-H460) | >250 | 42.7 | 87.6 | 76.8 | 105.0 | 110.3 | >250 | 79.8 | 4.9 |
| Prostate (PC-3) | 99.9 | >250 | >250 | >250 | 118.1 | 141.9 | 26.7 | >250 | 11.7 |
| Ovarian (OVCAR-3) | <0.25 | 47.6 | <0.25 | 58.0 | <0.25 | 73.7 | <0.25 | 60.4 | 7.6 |
| Keratinocytes (HaCaT) | >250 | >250 | 92.3 | 95.7 | 129.4 | 145.6 | >250 | >250 | 2.3 |
1Data result from three replicates per treatment in two independent tests at 25°C for 48 hours. Ag EO, Bd EO, Cs EO, and Ls EO correspond to the following essential oils: Aloysia gratissima, Baccharis dracunculifolia, Coriandrum sativum, and Lippia sidoides, respectively. Ag4, Bd2, Cs4, and Ls3 are the selected active fractions evaluated. Dox: doxorubicin (positive control).
Among the EO evaluated, B. dracunculifolia and C. sativum were the most active inhibitors of human tumor cell lines growth, presenting selectivity for U251 (TGI = 38.2 μg/mL and TGI = 8.3 μg/mL, resp.) and OVCAR-3 (TGI < 0.25 μg/mL for both). On the other hand, A. gratissima and L. sidoides displayed the lowest activity, both presenting selectivity for OVCAR-3 (TGI < 0.25 μg/mL for both) and L. sidoides for PC-3 (TGI = 26.7 μg/mL). The reference compound, doxorubicin, presented antiproliferative activity against all cell lines, except for kidney (Table 4).
Table 4 also shows the activity of selected active fractions. Ag4 and Ls3 fractions presented better results than A. gratissima and L. sidoides EO, respectively, since these fractions were not active against human normal cell lines (TGI > 250 μg/mL) and showed lower TGI values, being selective for 786-0 (TGI = 5.9 μg/mL and TGI = 26.7 μg/mL, resp.). Cs4 fraction had better results than C. sativum EO only against NCI-ADR/RES (TGI = 13.1 μg/mL and TGI = 90 μg/mL, resp.). Bd2 displayed a better performance than B. dracunculifolia EO against NCI-ADR/RES (TGI = 10.5 μg/mL and TGI = 59.2 μg/mL, resp.), 786-0 (TGI = 47.1 μg/mL and TGI = 49.5 μg/mL, resp.), and NCI-H460 (TGI = 76.8 μg/mL and TGI = 87.6 μg/mL, resp.).
4. Discussion
The activity of natural products, especially EO, against microorganisms has been recently confirmed by several studies focusing on antimicrobial activity of EO against planktonic cells. However, bacteria growing in biofilms exhibit a specific phenotype and are often, but not always, more resistant to antimicrobial agents than their planktonic counterparts [10, 11]. Thus, it is important to search for natural products that have antibiofilm properties and antimicrobial activity against oral pathogens [28].
This study aimed to evaluate the activity of EO and their fractions against planktonic cells of S. mutans, and the active fractions were evaluated against biofilm formed by S. mutans. Also, EO and their active fractions were chemically characterized and their activity against human normal and tumor cell lines proliferation were determined.
The antimicrobial assay revealed low MIC values for almost all 20 EOs and 15 fractions from the selected EO tested. EO and the selected active fractions presented strong activity against S. mutans, since natural products are considered strong inhibitors of microbial activity, when MIC values are lower than 500 μg/mL [29].
These results demonstrate that the EO studied and especially those selected (A. gratissima, B. dracunculifolia, C. sativum, and L. sidoides) have potential for bioprospection of new active biomolecules. The fractionation process adopted showed good results, since the fractions obtained were more active than the original EO (Table 2). This bioguided study is a model for bioprospecting new drugs [30], and it can be considered successful since we found active fractions presenting higher activity than their respective EO.
Most EO and fractions studied showed MBC : MIC ratio that enables them to be classified as bactericidal compounds. This could be explained by their hydrophobicity, an important characteristic that exists in EO and their fractions [31] and may allow them to partition the lipids of the bacterial cell membrane, turning them more permeable and leading to leakage of ions and other cell constituents [32, 33]. On the other hand, B. dracunculifolia EO and its selected active fraction (Bd2) present compounds that could be capable of infiltrating the cell and interact with cellular metabolic mechanisms [34], demonstrating their bacteriostatic effect. Nevertheless, despite presenting bactericidal or bacteriostatic effect, the selected EO proved to be active against both S. mutans planktonic cells and biofilm, demonstrating the effectiveness of the substances present in these EO, since it is difficult to disrupt S. mutans biofilm [35].
The selected active fractions were also tested against S. mutans biofilm, and they were able to disrupt its formation at all tested concentrations. This disruption was observed using SEM, which showed the change the selected active fractions caused in the structure of S. mutans biofilm.
At the concentrations tested, it was possible to observe huge failures in S. mutans biofilm surface treated with the active fractions when compared with the treatment with the vehicle, which presented a more homogeneous biofilm surface. These changes were also observed in another study that tested the action of C. sativum and its bioactive fraction against Candida albicans [11]. Moreover, the simple conformational change in biofilm, caused by the action of the selected active fractions, could make it more susceptible and less virulent [4].
However, when the selected active fractions were tested in order to evaluate their ability to reduce S. mutans acid production, no significant results were observed (P > 0.05). Therefore, the selected active fractions could not act on this important virulence factor of S. mutans, different from the findings of another work with B. dracunculifolia extracts, which showed significant reduction in production of acid by this microorganism [36]. The difference between B. dracunculifolia EO and the active extracts from this plant may be attributed to the extraction method, which results in different compound mixtures with different mechanisms of action [37].
It is known that EOs are composed of numerous different chemical compounds, and their antimicrobial activity might be attributed to several different mechanisms, which could explain the variations in their mode of action [38].
The present data suggest the occurrence of a separation during the fractionation process of the selected EO in such a way that the selected active fractions presented higher amounts of bioactive compounds than their respective EO. The main biologically active compounds found in the selected active fractions were thymol, carvacrol, 2-decen-1-ol, trans-nerolidol, spathulenol, ethyl ester benzenepropanoic, trans-pinocamphone, cis-pinocamphone, and guaiol. These compounds have been extensively described in the literature for their effect on microorganisms [39, 40].
Both forms of trans- and cis-pinocamphone are major constituents of Ag4 fraction and were also found in Hyssopus officinalis L. EO [41]. These compounds are responsible for the antibacterial, antifungal, and antioxidant activities of H. officinalis EO, demonstrating that they pass through the cell wall and the plasma membrane, disrupting their structure [41]. The bactericidal activity of Ag4 fraction observed in the present study may be a consequence of this mode of action.
Trans-nerolidol and spathulenol, two compounds present in Bd2 fraction, have been considered active against unknown Gram-positive and Gram-negative bacteria [13]. Although spathulenol shows activity against S. mutans, its mechanism of action still remains unknown [13].
Other studies showed that certain alcohols, such as 2-decen-1-ol, have higher antimicrobial activity than aldehydes against Candida ssp. [11, 16]. These alcohols were found in Cs4 fraction and may be responsible for the action against S. mutans biofilm. Furthermore, considering the mode of action of C. sativum EO, it seems to result in bacterial cell permeabilization, leading to the impairment of other cell functions, such as membrane potential, respiratory activity, or efflux pump activity [42].
Thymol is an optic isomer of carvacrol, and both substances seem to make bacterial membrane more permeable [43]. In our study, both were found in Ls3 fraction as its major components. Previous studies have shown that these compounds present antimicrobial activity against fungi and bacteria [44], including species of the genus Streptococcus [12].
After determining the antimicrobial activity of a natural product, it is important to verify if it also exhibits antiproliferative activity, mainly after its fractionation, a procedure that may concentrate toxic compounds in the fractions that present biological activity.
Based on TGI values, the selected EO and selected active fractions could be classified as inactive (TGI > 50 μg/mL), weakly active (15 μg/mL < TGI < 50 μg/mL), moderately active (6.25 μg/mL < TGI < 15 μg/mL), and strongly active (TGI < 6.25 μg/mL) [45]. The absence of activity was clearly observed in this study since all selected EO and selected active fractions were inactive against the human normal cell line tested.
All EOs tested were selective against the ovarian tumor cell line, showing potent activity. Ag4 showed potent activity against the kidney tumor cell line, and Bd2 and Cs4 fractions showed only moderate activity against the ovarian tumor cell line. These results show the specificity of these EO and their fractions against some tumor cell lines, an important and desired characteristic for potential new chemotherapic drugs [15].
It is known that EO compounds, such as monoterpenes, have shown effects on mevalonate metabolism, linked to the maintenance of cell membrane, which could contribute to terpene tumor suppressive action [46]. Thereby, the presence of monoterpenes in the selected active fractions of our study may explain their antiproliferative actions against some tumor cell lines [47]; however, more studies are required to find the compounds of EO responsible for their anticancer activity, since little is known about essential oils and their antiproliferative activity.
5. Conclusion
The results of the present study indicate that all EO and fractions tested showed good antimicrobial activity, but only those showing activity at low concentrations were taken into consideration and fractionated for bioprospection of new agents against S. mutans. Among these fractions, the selected active fractions were able to disrupt S. mutans biofilm formation, did not inhibit normal cell line growth, and were more specific against human tumor cell lines. These features enable them to be tested in further studies and help the discovery of new bioactive molecules.
Acknowledgment
The authors thank São Paulo Research Foundation (FAPESP, no. 2009/12353-0 and no. 2011/14757-0) and the National Council for Scientific and Technological Development (CNPq, no. 308644/2011-5) for the financial support.
References
- 1. Brasil, Ministério da Saúde, Departamento de Atenção Básica, Coordenação Nacional de Saúde Bucal, Projeto SB Brasil 2010—Pesquisa Nacional de Saúde Bucal, Primeiros resultados, Brasília, Brazil, 2011.
- 2.Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149(2):279–294. doi: 10.1099/mic.0.26082-0. [DOI] [PubMed] [Google Scholar]
- 3.Bowen WH, Amsbaugh SM, Monell-Torrens S, Brunelle J, Kuzmiak-Jones H, Cole MF. A method to assess cariogenic potential of foodstuffs. The Journal of the American Dental Association. 1980;100(5):677–681. doi: 10.14219/jada.archive.1980.0211. [DOI] [PubMed] [Google Scholar]
- 4.Kajfasz JK, Rivera-Ramos I, Abranches J, et al. Two Spx proteins modulate stress tolerance, survival, and virulence in Streptococcus mutans . Journal of Bacteriology. 2010;192(10):2546–2556. doi: 10.1128/JB.00028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajdić D, McShan WM, McLaughlin RE, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(22):14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves TMS, Silva CA, Silva NB, Medeiros EB, Valença AMG. Atividade antimicrobiana de produtos fluoretados sobre bactérias formadoras do biofilme dentario: estudo in vitro . Pesquisa Brasileira em Odontopediatria e Clínica Integrada. 2010;10(2):209–216. [Google Scholar]
- 7.Bader JD, Shugars DA, Bonito AJ. Systematic reviews of selected dental caries diagnostic and management methods. Journal of Dental Education. 2001;65(10):960–968. [PubMed] [Google Scholar]
- 8.Clardy J, Walsh C. Lessons from natural molecules. Nature. 2004;432(7019):829–837. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- 9.Jeon JG, Rosalen PL, Falsetta ML, Koo H. Natural products in caries research: current (limited) knowledge, challenges and future perspective. Caries Research. 2011;45(3):243–263. doi: 10.1159/000327250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simões M. Antimicrobial strategies effective against infectious bacterial biofilms. Current Medicinal Chemistry. 2011;18(14):2129–2145. doi: 10.2174/092986711795656216. [DOI] [PubMed] [Google Scholar]
- 11.Furletti VF, Teixeira IP, Obando-Pereda G, et al. Action of Coriandrum sativum L. essential oil upon oral Candida albicans biofilm formation. Evidence-Based Complementary and Alternative Medicine. 2011;2011:9 pages. doi: 10.1155/2011/985832. Article ID 985832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botelho MA, Nogueira NAP, Bastos GM, et al. Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Brazilian Journal of Medical and Biological Research. 2007;40(3):349–356. doi: 10.1590/s0100-879x2007000300010. [DOI] [PubMed] [Google Scholar]
- 13.Silva F. Efeito antimicrobiano in vitro dos compostos isolados da Mikania glomerada sobre os patógenos orais. [Senior Research Project], Faculdade de Odontologia de Piracicaba, UNICAMP, Piracicaba, Brazil, 2005.
- 14.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—a review. Food and Chemical Toxicology. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho JE. Atividade antiulcerogênica e anticâncer de produtos naturais e de síntese. Construindo a História dos Produtos Naturais. 2006;7:1–18. [Google Scholar]
- 16.Begnami AF, Duarte MCT, Furletti V, Rehder VLG. Antimicrobial potential of Coriandrum sativum L. against different Candida species in vitro . Food Chemistry. 2010;118(1):74–77. [Google Scholar]
- 17.Adams RP. Identification of Essential Oils Components by Gas Chromatography/Mass Spectrometry. Carol Stream, Ill, USA: Allured; 2007. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard. 7th edition. 26, no. 2. Fort Wayne, Ind, USA: CLSI document M07-A7; 2006. [Google Scholar]
- 19.Soares SP, Vinholis AHC, Casemiro LA, Silva MLA, Cunha WR, Martins CHG. Atividade antibacteriana do extrato hidroalcoólico bruto de Stryphnodendron adstringens sobre microorganismos da cárie dental. Revista Odonto Ciência. 2008;23(2):141–144. [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 8th edition. 29, no. 2. Wayne, Pa, USA: CLSI document M07-A8; 2009. [Google Scholar]
- 21.Koo H, Hayacibara MF, Schobel BD, et al. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. Journal of Antimicrobial Chemotherapy. 2003;52(5):782–789. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- 22.Mattos-Graner RO, Jin S, King WF, Chen T, Smith DJ, Duncan MJ. Cloning of the Streptococcus mutans gene encoding glucan binding protein B and analysis of genetic diversity and protein production in clinical isolates. Infection and Immunity. 2001;69(11):6931–6941. doi: 10.1128/IAI.69.11.6931-6941.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duarte S, Rosalen PL, Hayacibara MF, et al. The influence of a novel propolis on mutans streptococci biofilms and caries development in rats. Archives of Oral Biology. 2006;51(1):15–22. doi: 10.1016/j.archoralbio.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Hawser SP, Douglas LJ. Biofilm formation by Candida species on the surface of catheter materials in vitro . Infection and Immunity. 1994;62(3):915–921. doi: 10.1128/iai.62.3.915-921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monks A, Scudiero D, Skehan P, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. Journal of the National Cancer Institute. 1991;83(11):757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 26.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nature Reviews Cancer. 2006;6(10):813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 27.Denny C, Zacharias ME, Ruiz ALTG, et al. Antiproliferative properties of polyketides isolated from Virola sebifera leaves. Phytotherapy Research. 2008;22(1):127–130. doi: 10.1002/ptr.2251. [DOI] [PubMed] [Google Scholar]
- 28.Koo H, Gomes BPFA, Rosalen PL, Ambrosano GMB, Park YK, Cury JA. In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Archives of Oral Biology. 2000;45(2):141–148. doi: 10.1016/s0003-9969(99)00117-x. [DOI] [PubMed] [Google Scholar]
- 29.Duarte MCT, Leme EE, Delarmelina C, Soares AA, Figueira GM, Sartoratto A. Activity of essential oils from Brazilian medicinal plants on Escherichia coli . Journal of Ethnopharmacology. 2007;111(2):197–201. doi: 10.1016/j.jep.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 30.Simões LMC, Gregório LE, Silva Filho AA, et al. Effect of Brazilian green propolis on the production of reactive oxygen species by stimulated neutrophils. Journal of Ethnopharmacology. 2004;94(1):59–65. doi: 10.1016/j.jep.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. International Journal of Food Microbiology. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Knobloch K, Weigand H, Weis N, Schwarm HM, Vigenschow H. Action of terpenoids on energy metabolism. In: Oils EJB, editor. In: Proceedings of the Progress in essential oil research: 16th International Symposium on Essential; 1986; De Gruyter; pp. 429–445. [Google Scholar]
- 33.Ultee A, Bennik MHJ, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus . Applied and Environmental Microbiology. 2002;68(4):1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marino M, Bersani C, Comi G. Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae . International Journal of Food Microbiology. 2001;67(3):187–195. doi: 10.1016/s0168-1605(01)00447-0. [DOI] [PubMed] [Google Scholar]
- 35.Marsh PD. Dental plaque: biological significance of a biofilm and community life-style. Journal of Clinical Periodontology. 2005;32(6):7–15. doi: 10.1111/j.1600-051X.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 36.Leitão DPS, Silva Filho AA, Polizello ACM, Bastos JK, Spadaro ACC. Comparative evaluation of in-vitro effects of Brazilian green propolis and Baccharis dracunculifolia extracts on cariogenic factors of Streptococcus mutans . Biological and Pharmaceutical Bulletin. 2004;27(11):1834–1839. doi: 10.1248/bpb.27.1834. [DOI] [PubMed] [Google Scholar]
- 37.Eloff JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants? Journal of Ethnopharmacology. 1998;60(1):1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 38.Calsamiglia S, Busquet M, Cardozo PW, Castillejos L, Ferret A. Invited review: essential oils as modifiers of rumen microbial fermentation. Journal of Dairy Science. 2007;90(6):2580–2595. doi: 10.3168/jds.2006-644. [DOI] [PubMed] [Google Scholar]
- 39.Bassole IHN, Nebie R, Savadogo A, Ouattara CT, Barro N, Traore SA. Composition and antimicrobial activities of the leaf and flower essential oils of Lippia chevalieri and Ocimum canum from Burkina Faso. African Journal of Biotechnology. 2005;4(10):1156–1160. [Google Scholar]
- 40.Parreira NA, Magalhães LG, Morais DR, et al. Antiprotozoal, schistosomicidal, and antimicrobial activities of the essential oil from the leaves of baccharis dracunculifolia . Chemistry and Biodiversity. 2010;7(4):993–1001. doi: 10.1002/cbdv.200900292. [DOI] [PubMed] [Google Scholar]
- 41.Kizil S, Haşimi N, Tolan V, Kilinç E, Karataş H. Chemical composition, antimicrobial and antioxidant activities of hyssop (Hyssopus officinalis L.) essential oil. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010;38(3):99–103. [Google Scholar]
- 42.Silva F, Ferreira S, Queiroz JA, Domingues FC. Coriander (Coriandrum sativum L.) essential oil: its antibacterial activity and mode of action evaluated by flow cytometry. Journal of Medical Microbiology. 2011;60(10):1479–1486. doi: 10.1099/jmm.0.034157-0. [DOI] [PubMed] [Google Scholar]
- 43.Lambert RJW, Skandamis PN, Coote PJ, Nychas GJE. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. Journal of Applied Microbiology. 2001;91(3):453–462. doi: 10.1046/j.1365-2672.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- 44.Lacoste E, Chaumont JP, Mandin D, Plumel MM, Matos FJ. Antiseptic properties of the essential oil of Lippia sidoides Cham: application to the cutaneous microflora. Annales Pharmaceutiques Francaises. 1996;54(5):228–230. [PubMed] [Google Scholar]
- 45.Fouche G, Cragg GM, Pillay P, Kolesnikova N, Maharaj VJ, Senabe J. In vitro anticancer screening of South African plants. Journal of Ethnopharmacology. 2008;119(3):455–461. doi: 10.1016/j.jep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Swanson KM, Hohl RJ. Anti-cancer therapy: targeting the mevalonate pathway. Current Cancer Drug Targets. 2006;6(1):15–37. doi: 10.2174/156800906775471743. [DOI] [PubMed] [Google Scholar]
- 47.Edris AE. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytotherapy Research. 2007;21(4):308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]


