Abstract
Purpose
Curcumin has shown a variety of biological activity for various human diseases including cancer in preclinical setting. Its poor oral bioavailability poses significant pharmacological barriers to its clinical application. Here, we established a practical nano-emulsion curcumin (NEC) containing up to 20% curcumin (w/w) and conducted the pharmacokinetics of curcuminoids and curcumin metabolites in mice.
Methods
This high loading NEC was formulated based on the high solubility of curcumin in polyethylene glycols (PEGs) and the synergistic enhancement of curcumin absorption by PEGs and Cremophor EL. The pharmacokinetics of curcuminoids and curcumin metabolites was characterized in mice using a LC–MS/MS method, and the pharmacokinetic parameters were determined using WinNonlin computer software.
Results
A tenfold increase in the AUC0→24h and more than 40-fold increase in the Cmax in mice were observed after an oral dose of NEC compared with suspension curcumin in 1% methylcellulose. The plasma pharmacokinetics of its two natural congeners, demethoxycurcumin and bisdemethoxycurcumin, and three metabolites, tetrahydrocurcumin (THC), curcumin-O-glucuronide, and curcumin-O-sulfate, was characterized for the first time in mice after an oral dose of NEC.
Conclusion
This oral absorption enhanced NEC may provide a practical formulation to conduct the correlative study of the PK of curcuminoids and their pharmacodynamics, e.g., hypomethylation activity in vivo.
Keywords: Nano-emulsion curcumin (NEC), Pharmacokinetics, Curcuminoids, Curcumin metabolites, LC–MS/MS
Introduction
Curcumin, a naturally bioactive component in the Curcuma longa, has been shown to have a wide range of biological and pharmacological actions, including anti-tumor, anti-inflammatory, anti-virus, anti-oxidation, and anti-HIV activities in preclinical settings [1]. However, demonstration of these activities in clinical setting has been an unprecedented challenge because of its poor bioavailability despite curcumin is well tolerated at levels up to 12 g/day for 3–4 months in clinical settings [2]. The pharmacokinetic (PK) studies of curcumin in rodents and humans have consistently reported poor systemic bioavailability [3, 4]. Factors contributing to the low bioavailability of curcumin include its poor solubility and absorption, and its rapid metabolic elimination by reduction and conjugation [1]. No detectable curcumin in the urine, dose-independent low plasma level of curcumin (5 nM) and high levels of curcumin in the feces (>3 μmol/g) after dietary feeding of curcumin (0.1–0.5%) in rats suggest its poor absorption from the gut [5]. A maximal plasma level of 0.6 μM [3] curcumin has been reported in mice after oral administration of curcumin solution(s) or curcumin-fortified food up to the dose of 1 g/kg [3]. Similar to rodent studies, the oral systemic bioavailability of curcumin is poor in humans. In 25 patients with various precancerous lesions, the mean plasma levels were 0.19, 0.20, and 0.60 μg/ml (0.51, 0.54, and 1.6 μM) after taking 4, 6, and 8 g per day for 3 months, respectively [6]. However, only extremely low curcumin level (≤130 nM) in plasmas has been detected in several clinical trials after oral dosing of curcumin up to 12 g/day in healthy volunteers [4]. Therefore, the limited oral bioavailability of curcumin in humans poses a major obstacle to achieving adequate plasma levels with desirable pharmacological effects [1].
Natural curcumin is a mixture of three bis-α,β-unsaturated-γ-diketone hydrophobic curcuminoids: curcumin, demethoxycurcumin, and bisdemethoxycurcumin in a ratio of 77:17:3 [1]. Due to the pH-dependent tautomerization of ketone and enol isomers, curcuminoids exist as a predominant keto form in acidic and neutral solutions [3] and stable enol form in alkaline medium (Fig. 1). To improve the bioavailability of curcumin, several methods including adjuvant [7], phospholipid complexes [8], nano-particle formulation [8-21], microemulsion [22], and novel curcumin analogs [23] have been evaluated. Among these, nano-particle and microemulsion formulations have shown rather promising prospects for in vivo delivery of curcuminoids.
Fig. 1.
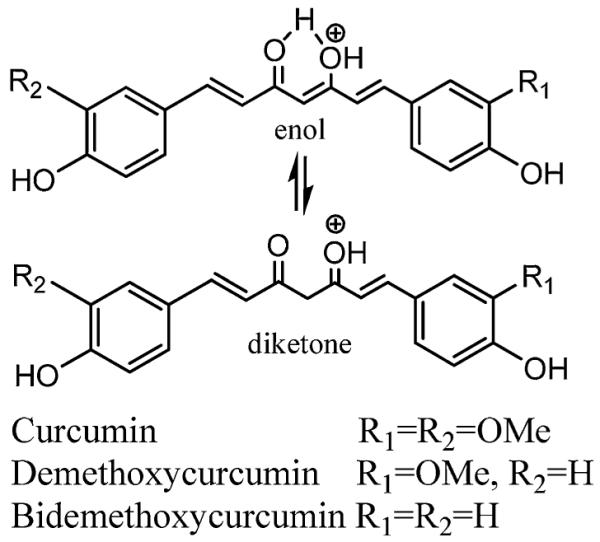
The tautomerized structures of three natural curcuminoids: curcumin, demethoxycurcumin, and bisdemethoxycurcumin
Recently, a self-microemulsion drug deliver system (SMEDDS) has been established to show significant enhanced absorption of curcumin [9]. However, no pharmacokinetics of curcumin has been reported for this system, which may be due to its low loading capacity of curcumin or the lack of a sensitive analytic method for curcumin. In this study, we first systematically evaluate the solubility of curcumin in several polyethylene glycols (PEGs) at different temperatures. Based on the finding of high solubility of curcumin in PEGs, a practical nano-emulsion curcumin was established containing up to 20% curcumin (w/w) in a mixture of PEG 600 and Cremophor EL. Then, the pharmacokinetics of curcumin, its two natural congeners (termed as curcuminoids collectively), and three in vivo metabolites: curcumin-O-glucuronide, curcumin-O-sulfate, and tetrahydrocurcumin (THC) was characterized in mice.
Materials and methods
Chemical and reagents
Curcumin as a mixture of curcumin, demethoxycurcumin, and bisdemethoxycurcumin (95%) was purchased from ACROS Organics (Belgium, USA), THC was prepared according to a published procedure [24], the internal standard (I.S.) hesperetin was obtained from National Cancer Institute (Bethesda, MD, USA), and analytical HPLC grade methanol, acetonitrile, ethyl acetate, and formic acid were purchased from Fisher Scientific (Pittsburgh, PA, USA). The heparin-treated mouse plasma was purchased from Harlan Bioproducts (Indianapolis, IN, USA). The phosphate buffer saline (PBS, pH 7.4), polyethylene glycol (PEG)-200, PEG-300, PEG-400, PEG-600, Cremophor EL, and Tween 80 were purchased from Sigma–Aldrich (St. Louis, MO, USA). All chemicals and reagents were used as received. An E-pure water purification system (Barnstead, Dubuque, IA) was used to obtain HPLC grade water (> 18 mΩ-cm).
Instrumentation
The LC–MS analyses used a Finnigan TSQ Quantum EMR Triple Quadrupole mass spectrometer (Thermo Fisher Scientific Corporation, San Jose, CA) coupled to a Shimadzu HPLC system (Shimadzu, Columbia, MD), which was equipped with a CBM-20A system controller, an LC-20 AD pump, a SIL-20AC auto-sampler, CTO-20A column oven, DGU-20A5 degasser, and FCV-11AL valve unit. The temperature of the auto-sampler was set at 4°C during operation. All operations were controlled by Finnigan Xcalibur software (Home Page Version 1.4 SR1) on a Windows XP operating system.
Liquid chromatographic–mass spectrometry
Curcumin, THC, and hesperetin were separated on a C8 column (2.1 9 50 mm, 5 lm, Thermo Hypersil–Keystone, Bellefonte, PA, USA) coupled with a c8 basic guard column (2.1 × 10 mm, 2 μm, Thermo Hypersil–Keystone, Bellefonte, PA, USA) at the flow rate of 0.20 ml/min with an isocratic eluent consisting of 50% mobile phase (MP) A (0.1% formic acid water) and 50% MP B (0.1% formic acid acetonitrile).
The mass spectrometer was operated in the positive ESI mode with a collision gas (Argon) pressure of 1.5 psi, a typical electro-spray needle voltage of 4,700 V, a sheath nitrogen gas flow of 25 (arbitrary unit), and a heated capillary temperature of 325°C. The samples and I.S. were analyzed by the multiple reaction monitor (MRM) mode using ion transitions at a proper collision energy (E) as follows: curcumin m/z 369.0 [m/z 177.0 (E = 30%), demethoxycurcumin 339.0 [m/z 177.0 (E = 30%), bisdemethoxycurcumin 309.0 [m/z 147.0 (E = 30%), tetrahydrocurcumin m/z 373.0 [m/z 137.0 (E = 30%), curcumin-O-glucuronide 545.0 [m/z 351.0 (E = 30%) and curcumin-O-sulfate 449.0 [m/z 351.0 (E = 30%), and I.S. hesperetin m/z 303.0 [177.0 m/z (E = 35%). The mass spectrometer was tuned to its optimal sensitivity by the direct infusion of curcumin and THC solution.
Extraction method
Curcumin, tetrahydrocurcumin, and the I.S. hesperetin were extracted from mouse plasma using ethyl acetate as follows: a 100 μl aliquot of blank mouse plasma spiked with various concentrations of curcumin, tetrahydrocurcumin, and a fixed concentration of hesperetin (1,000 ng/ml) were extracted with 1.0 ml ethyl acetate. The ethyl acetate layer was collected and dried under a mild stream of nitrogen. The residues were then reconstituted with 100 μl 50% acetonitrile/0.1% FA. The resulting solution was then analyzed by the LC–MS/MS method.
Solubility of curcumin in PEG 200, 300, 400, and 600
Curcumin (300 mg) was suspended in 1,100 mg PEG 200, 300, 400, and 600 in aluminum-foil-covered eppendoff tubes. These solutions stayed at room temperature or in a 60 or 100°C sand bath for 60 min with occasional vortex every 10 min. Then, the solution was stayed at room temperature overnight followed by centrifugation at 15,000×g for 3 min. An aliquot of 10 μl of the supernatant was diluted with 990 μl ethanol as solution A (20–250 μg/ml). Then, an aliquot of 100 μl of the supernatant was diluted with 900 μl ethanol as solution B. The absorption of solution B was measured on an UV–Vis spectrometer, and the concentration of curcumin was calculated based on the absorption at 425 nm against the calibration curves of curcumin in ethanol. The total amount of curcumin and the relative amount of curcumin, demethoxycurcumin, and bisdemethoxycurcumin were determined using the above-described LC–MS/MS method by serial dilution against the calibration curve of curcumin in mobile phase.
Preparation of nano-emulsion curcumin NEC1–NEC7
Curcumin (1.65 g) powder was added into 10 ml PEG 400 or PEG 600 (Sigma, St. Louis, MO. USA) in a glass tube covered by aluminum foil. After stirring, the tube was heated at 100°C in a heating block for 15 min till the curcumin powder was completely dissolved in PEG 400 or PEG 600. After cooling the tube at room temperature for about 10 min, various amounts of Cremophor EL and Tween 80 were added into the tube and the mixture was vortexed. The final concentration of curcumin of these seven formulations is 100 mg/ml—NEC1: one hundred mg/ml curcumin in PEG 400; NEC2–NEC5: 100 mg/ml curcumin in PEG 400 and Cremophor EL with a v/v of 4:1, 2:1; 4:3, and 1:1; NEC6: 100 mg/ml curcumin in PEG 400, Cremophor EL, and Tween 80 solution with a v/v/v ratio of 4:2:1; and NEC7: 100 mg/ml curcumin in PEG 600 and Cremophor EL with a v/v ratio of 2:1.
Stability of nano-emulsion curcumin (NEC7)
The stability of NEC7 was evaluated as follows: Formulations of curcumin (100 mg/ml) in 66.7% PEG 600 and 33.3% Cremophor® EL were stored at room temperature and at 37°C (in triplicates). Samples were taken at 0, 14, 30, and 60 days following storage to quantify the concentration of curcumin using UV–Vis spectrometer as detailed in the solubility section.
Particle size and morphology of NEC7
The particles of NEC7 were characterized for size and morphology using a JEOL-1210 transmission electron microscope (TEM) (JEOL, Tokyo, Japan) operating at 60 kV. For TEM measurements, 1 drop of nano-emulsion curcumin in water was carefully placed on 200-mesh formvar-coated copper TEM grid (grid size: 97 lm) (Ted Pella, Inc., Redding, CA, USA) followed by staining with 1% w/v of uranyl acetate solution for 5 min. The excess solution on the grid was removed using a piece of fine filter paper, and the samples were allowed to air dry for 10 h prior to imaging the particles under TEM.
Pharmacokinetics of curcuminoids and curcumin metabolites in mice after an oral administration of nano-emulsion curcumin and suspension curcumin
CD2F1 mice (~20 g) (Harlan, Indianapolis, IN) for formulation screening or CF-1 (Charles-river Laboratory, Wilmington, MA) for the full set of pharmacokinetics were used in this study. All animal procedures were performed according to a protocol in compliance with The Ohio State University Laboratory Animal Resources (ULAR) policies, which adhered to the guideline and “Principles of Laboratory Animal Care by National Institutes of Health.” For formulation optimization, gavage administration of approximately 250 μl of respective curcumin formulations results in a dose of 1,000 mg/kg. For complete pharmacokinetic analysis, approximately 450 or 250 μl (adjusted by body weight and doses) formulation NEC7 or SC in 1% methylcellulose was gavagely administered to mice resulting in a dose of 1,800 or 1,000 mg/kg curcumin. The blood was collected by cardiac puncture under CO2 anesthesia 1-h post-administration for formulation optimization, and the following time schedule of 0 (predose), 0.17, 0.33, 0.5, 1, 1.5, 2, 3, 4, 6, 8, and 24 h after dosing was collected for the full set of pharmacokinetics study. The blood samples in the heparinized tubes were centrifuged at 1,000×g for 5 min in a 4°C microcentrifuge, and the supernatant of each was collected and kept at −80°C until analysis. The curcumin levels in plasma were measured using the recently established LC–MS/MS assay. Plasma concentration–time data were analyzed by the WinNonlin computer software (Pharsight 5.0, Mountain View, CA) using appropriate pharmacokinetic models.
Analysis of curcumin in mouse plasma
An aliquot of 100 μl of PK plasma samples of NEC (10 × dilutions with blank mouse plasma) and SC formulations was mixed with 10 μl I.S. (10 μg/ml stock in 50% acetonitrile). The resulting solution was extracted and processed according to the protocol described in Extraction Method Section. An aliquot of 25 μl of the final solution was injected into the LC–MS system for LC-MS/MS analysis.
Statistical analyses
Results were subjected to ANOVA and linear regression analysis using Minitab (version 15) software packages. Plots of residuals were used to ensure that variances were homogeneous and that the residuals had a normal distribution. The Kruskal–Wallis ANOVA, followed by the Dunns’ multiple comparison test, was used to evaluate the curcumin levels at respective time points in the mice as a whole after an oral dosing of two curcumin formulations: NEC and SC. A two-tailed Mann–Whitney U test was used to compare plasma levels of curcumin obtained from NEC relative to plasma levels obtained from SC. Findings with P values ≤0.05 were considered significant.
Results
The solubility of curcumin in PEGs
It is well known that the poor oral bioavailability of curcumin relates at least in part from its poor solubility. Increased solubility may provide a practical way to increase its bioavailability. Recently, several research groups have demonstrated different practical methods to increase curcumin solubility including simply heating [25], formation of cyclodextrin complex [26], and non-covalent BSA complex [27]. Polyethylene glycols with different molecular weight have been used as co-solvents for many hydrophobic drugs. Here, the solubility of curcumin in several PEGs including PEG 200, 300, 400, and 600 at room temperature, 60, and 100°C was evaluated (Table 1). As shown in Table 1, the curcumin concentration in PEGs is dependent on the average molecular weight of PEGs at room temperature and 100°C, but independent at 60°C. Notably, a curcumin concentration in the homogenous PEG solutions can be achieved up to 250 mg/ml when the solution was heated up to 100°C, without formation of a precipitate upon cooling down to room temperature. Since precipitation was not observed when curcumin-saturated PEG solutions at 100°C cool to room temperature, we were interested in determining whether chemical structural alterations of curcumin occurred during the heating and cooling process. To understand this phenomenon, the exact concentrations of curcumin, demethoxycurcumin, and bisdemethoxycurcumin were evaluated using the LC–MS/MS method [28] and a comparable concentrations of these curcuminoids (250 mg/ml in PEG 600) in a ratio of 86:10:4 as determined by UV–Vis spectrometry. This result demonstrated that there was no significant decomposition of curcumin during this process since the concentration of curcumin determined by LC–MS/MS is the concentration of the free form of unmodified curcumin. This result is also consistent with a recent report on the increase of the solubility of curcumin in water by heating [25].
Table 1.
The solubility of curcumin in different PEGs and temperatures
| Co-solvent and emulsifier | 200 | 300 | 400 | 600 |
|---|---|---|---|---|
| Final conc. @ RT (mg/ml) | 83.2 ± 3.4 | 93.8 ± 6.4 | 128.3 ± 4.7 | 126.0 ± 9.2 |
| Final conc. 60°C (mg/ml) | 135.7 ± 5.3 | 136.3 ± 2.1 | 131.4 ± 9.0 | 136.0 ± 8.3 |
| Final conc. 100°C (mg/ml) | 193.1 ± 1.2 | 252.3 ± 7.9 | 195 ± 11.2 | 241.8 ± 7.8 |
Optimization of several nano-emulsion curcumin (NEC) formulations guided by 1-h post-dosing in mice
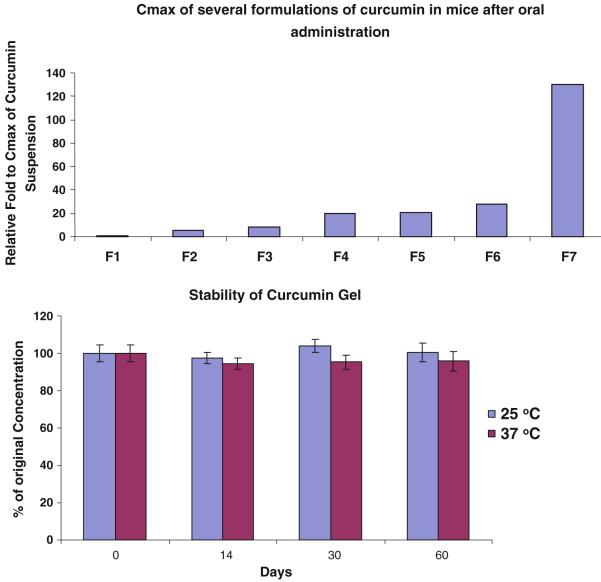
Nano-particle formulations of curcumin including self-microemulsified drug delivery system (SMEDDS), solid lipid nano-particle (SLN), and sustained-release curcumin formulations have shown promising results to enhance the absorption of curcumin. However, due to the limited loading capacity of these formulations, the in vivo plasma level of curcumin has not yet to reach the effective concentrations shown in in vitro cell studies. We evaluated whether the mixture of PEGs and some non-ionic emulsifiers including Cremophor EL and Tween 80 would yield a nano-emulsion curcumin to enhance gastrointestinal absorption of curcumin. As most pharmacokinetic studies of curcumin in murine models have shown peak plasma concentrations at 1–2 h, we evaluate 1-h plasma concentration of curcumin to test several formulations of curcumin in different PEGs and mixtures with Cremophor EL and Tween 80 at different ratios in mice. Seven formulations NEC1 to NEC7 prepared as described in method section were administered orally at 100 mg/ml in 0.25 ml resulting in a dose of 1,000 mg/kg curcumin in mice. As shown in Fig. 2a, the plasma level of curcumin in mice is the lowest after oral dose of NEC1 and the highest for NEC7 (see composition in their preparation section). The plasma level of curcumin achieved with NEC2–NEC5 which varied in the ratio of Cremophor EL to PEG 400 is higher than that of NEC1 in PEG 400 alone. No significant difference of the plasma level of curcumin between NEC5 (PEG 400 plus Cremophor EL) and NEC6 (PEG 400 plus Tween 80) was observed, indicating that Tween 80 and Cremophor EL serve similar functions. The significant difference in plasma curcumin concentrations after oral dosing with NEC6 (PEG 400 plus Cremophor EL) and NEC7 (PEG 600 plus Cremophor EL) suggests that PEG 600 can enhance its absorption compared to that of PEG 400. As shown in Fig. 2, the plasma level of curcumin after oral administration of formulation NEC7 is about sixfold higher than that of NEC6 in mice.
Fig. 2.
Plasma concentration 1-h post-oral administration of several NECs and SC after single dose administration (1,000 mg/kg; n = 2) a and the stability of formulation F7 for 60 days b
The stability and particle size of nano-emulsion curcumin
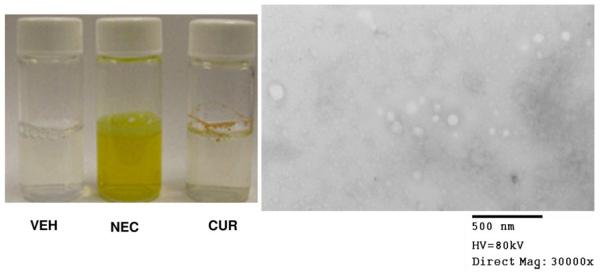
These screening results demonstrated that PEG 600 and Cremophor EL have synergistic effects to enhance the plasma level of curcumin in mice. The stability of this formulation was then evaluated as described above. It was found that there was no significant change in curcumin content in this formulation, and <5% of curcumin in this formulation decomposed in 60 days at r.t. (Fig. 2b). After dilution in water, the average particle size of NEC measured by TEM is 68.7 ± 26.1 nm (Fig. 3).
Fig. 3.
The solubility of VEH (vehicle), NEC and CUR in water and the NEC particle in water (0.26 mg/ml) measured by transmission electron microscope (TEM) image size
Pharmacokinetics analysis of orally administered SC and NEC
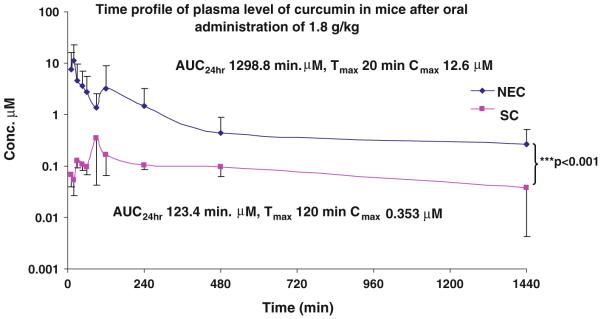
After optimization of NEC as 100 mg/ml curcumin formulated in volume ratio 2:1 of PEG 600 and Cremophor EL, the pharmacokinetics of this formulation and its suspension in 1% methylcellulose were characterized at the oral dose of 1,800 mg/kg in CD2F1 mice using a validated LC–MS/MS method [28]. The plasma concentration–time profiles of curcumin in mice following an oral administration of SC and NEC have been shown in Fig. 4, and pharmacokinetic parameters in plasma obtained from the pooled concentration–time data are summarized in Table 2. As shown in Fig. 4 and Table 2, the AUc0→24h of NEC was 1,299 lM min, while the AUc0→24h of SC was 123.4 μM min. This resulted in a 10.5-fold increased relative bioavailability (Fig. 4). These results also demonstrated cmax values of 12.6 μM for NEC, about 39.4 times higher as compared to 0.353 μM for SC, indicating that NEC can deliver an in vivo effective plasma level of curcumin for most of predefined molecules and a significant increase in its oral bioavailability after its oral administration in mice.
Fig. 4.
Plasma concentration–time profile following single oral administration of NEC and SC after single dose administration (1,800 mg/kg; n = 6), pink SC, and blue NEC
Table 2.
The pharmacokinetic parameters for nano-emulsion curcumin (NEC) and suspension curcumin (SC) in mice (n = 6)
| Parameter (units) | SC | NEC |
|---|---|---|
| λz (1/min) | 0.0009 | 0.0017 |
| HL(λz) (min) | 776.05 | 413.25 |
| Tmax (min) | 90.00 | 20.0 |
| Cmax (μM) | 0.353 | 12.60 |
| Clast (μM) | 0.037 | 0.480 |
| AUClast (min × μM) | 123.64 | 1,298.8 |
| Vz_F_obs (l/kg) | 33.91 | 1,243.13 |
| Cl_F_obs (l/min/kg) | 0.0303 | 2.085 |
| AUMClast (min × min × μM) | 60,397.9 | 718,777.9 |
Pharmacokinetic analysis of curcumin two natural congeners and metabolites of NEC
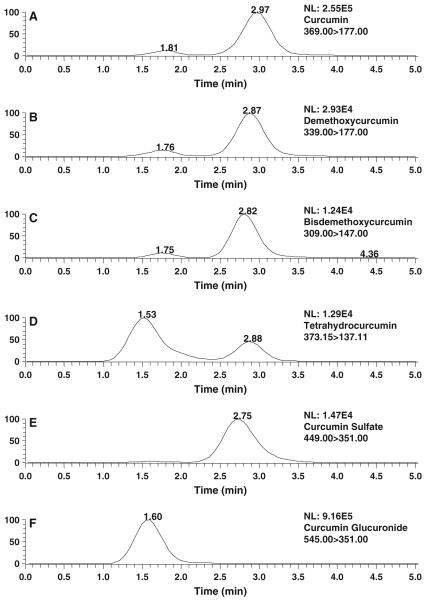
Natural curcumin is a mixture of three curcuminoids: curcumin, demethoxycurcumin, and bisdemethoxycurcumin. Despite the pharmacokinetics of curcumin has been extensively studied, the pharmacokinetics of demethoxycurcumin and bisdemethoxycurcumin remains largely unexplored. This could be due to their relative low abundance in the commercial curcumin [29]. In addition, curcumin has been shown to be metabolized extensively into two major conjugates, curcumin-O-glucuronide and curcumin-O-sulfate [29] with some pharmacokinetic data in human after an oral dose of 12 g curcumin [4]. Recently, we demonstrated that curcumin, demethoxycurcumin, and bisdemethoxycurcumin, and one of its reduced metabolites tetrahydrocurcumin have shown comparable inhibitory activity on M. SssI, a bacterial analog of human DNA methyltransferase 1 (DNMT1) as predicted by human DNMT1 molecular modeling [30]. Also the human DNMT1 model demonstrated that curcumin-O-glucuronide and curcumin-O-sulfate may possess similar inhibitory activity on M. SssI. The above pharmacokinetic study demonstrated that NEC can enhance the absorption of curcumin. Therefore, the pharmacokinetics of demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin, curcumin-O-glucuronide, and curcumin-O-sulfate was also characterized simultaneously in mice after an oral administration of 1 g/kg NEC. The following several putative ionic transitions of demethoxycurcumin m/z 339.0 [m/z 177.0, bisdemethoxycucumin m/z 309.0 [m/z 147.0, tetrahydrocurcumin m/z 373.0 [m/z 137.0, curcumin-O-glucuronide m/z 545.0 [m/z 351.0, and curcumin-O-sulfate m/z 449.0 [m/z 351 were used to monitor these species, and their concentrations were calculated against the calibration curve of curcumin. First, the evaluation of the total and relative amount of curcumin, demethoxycurcumin, and bisdemethoxycurcumin in NEC demonstrated that the concentrations of curcumin, demethoxycurcumin, and bisdemethoxycurcumin are 86, 10, and 4 mg/ml in NEC, respectively. As shown in Fig. 5, demethoxycurcumin (Fig. 5b), bisdemethoxycurcumin (Fig. 5c), curcumin-O-sulfate (Fig. 5d), curcumin-O-glucuronide (Fig. 5e), and tetrahydrocurcumin (Fig. 5f) can be detected in mouse plasma 30 min after an oral administration of 1 g/kg NEC. The occurrence of these species has previously been detected in mouse and rat plasma following oral administration of suspension curcumin [15]. Therefore, the results suggest that PEG 600 and Cremophor EL do not confound the metabolic profile of curcumin in vivo. Based on the plasma concentration–time profile of these species, curcumin-O-glucuronide is the predominant circulating species, followed by curcumin, demethoxycurcumin, tetrahydrocurcumin, bisdemethoxycurcumin, and curcumin-O-sulfate (Fig. 6), and the relative ratio of plasma concentrations of curcumin, demethoxycurcumin, and bisdemethoxycurcumin at the early time points (30 min) reflected their normal distribution in the NGC formulation. The calculated pharmacokinetic parameters (Table 3) reveal that [1] the terminal elimination half-life is in the following order: curcumin-O-glucuronide > curcumin > demethoxycurcumin > tetrahydrocurcumin > bisdemethoxycurcumin > curcumin-O-sulfate; [2] the time to reach to the peak concentration is from 20 to 30 min; [3] the AUCs/dose of curcumin, demethoxycurcumin, and bisdemethoxycurcumin during 24 h is in the order bisdemethoxycurcumin > demethoxycurcumin > curcumin [4]; and the AUC of curcumin-O-glucuronide, tetrahydrocurcumin, and curcumin-O-sulfate is about 5.08-, 0.085-, and 0.033-fold of that of curcumin, respectively.
Fig. 5.
Representative LC–MS/MS chromatograms of extracts of plasma from mouse, which had received NEC at 1,800 mg/kg by oral gavage, demonstrating multiple reaction monitoring transitions indicative of curcumin a, demethoxycurcumin b, bisdemethoxycurcumin c, tetrahydrocurcumin d, curcumin-O-monoglucuronide e, curcumin-O-monosulfate f, plasmas were obtained 20 min after gel curcumin administration. For details of administration and LC-MS analysis see “Materials and methods”
Fig. 6.
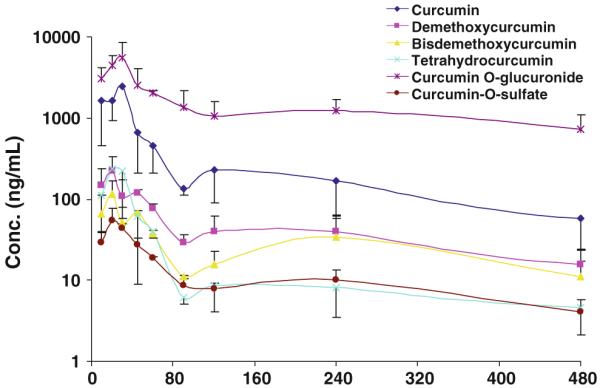
Plasma concentration-time profile of curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin, curcumin-O-glucuronide, and curcumin-O-sulfate following single oral administration of after a single dose administration of NEC containing 860 mg/kg curcumin, 100 mg/kg demethoxycurcumin, and 40 mg/kg bisdemethoxycurcumin (n = 3)
Table 3.
The pharmacokinetic parameters for curcumin (CUR), demethoxycurcumin (DMC), bisdemethoxycurcumin (BDM), tetrahydrocurcumin (THC), curcumin-O-sulfate (CURS), and curcumin-O-glucuronide (CURG) at the dose of 1 g/kg NEC in mice (n = 3)
| Parameters (unit) | BDM | CUR | DMC | THC | CURS | CURG |
|---|---|---|---|---|---|---|
| λz (1/min) | 0.0034 | 0.0039 | 0.0028 | 0.0018 | 0.0046 | 0.0014 |
| HL(λz) (min) | 203.8 | 177.0 | 245.6 | 386.3 | 149.1 | 503.4 |
| Tmax (min) | 20 | 30 | 20 | 20 | 20 | 30 |
| Cmax (μM) | 0.374 | 6.66 | 0.663 | 0.64 | 0.123 | 10.1 |
| Cmax/D (kg/l) | 0.0029 | 0.0029 | 0.0022 | NA* | NA | NA |
| AUClast (min × μM) | 43.2 | 385.6 | 63.7 | 29.4 | 11.9 | 1,209.7 |
| AUCINF_obs (min × μM) | 53.6 | 426.1 | 79.8 | 36.4 | 13.9 | 2,166.3 |
| AUCINF/Dobs (min × kg/ml) | 0.413 | 0.182 | 0.270 | NA | NA | NA |
| Vz_F_obs (l/kg) | 727.9 | 1,396 | 1,295 | NA | NA | NA |
| Cl_F_obs (l/min/kg) | 2.48 | 5.47 | 3.65 | NA | NA | NA |
| AUMClast (min × min × μM) | 7,901 | 42,311 | 9,906 | 2,560 | 1,857 | 213,187 |
| AUMCINF_obs (min × min × μM) | 15,970 | 72,049 | 23,346 | 9,820 | 3,212 | 1,367,217 |
| % Curcumin (AUC) | 12.6 | 100 | 18.7 | 8.54 | 3.26 | 508.4 |
| % Curcumin (NGC) | 6 | 100 | 12 | NA | NA | NA |
Not applicable
Discussion
To overcome the pharmacological barriers of curcumin in clinical setting application, several novel curcumin formulations such as liposomal curcumin [15], nano-particle curcumin [10, 11, 13, 16-21, 31], solid disperse (SD) curcumin [32], and self-microemulsified (SME) curcumin [22] have been established. Pharmacokinetic studies have demonstrated that liposomal curcumin, SD curcumin, and nano-particle curcumin can improve the absorption and oral bioavailability of curcumin with enhanced plasma concentrations and increased AUC in rats. However, the in vitro effective concentration of curcumin remains unachievable in vivo at all test doses. This may be due to their low loading capacity of curcumin. It is expecting that increasing the solubility of curcumin will increase its loading capacity and absorption. Here, we have found that the solubility of curcumin in several PEGs is high up to 250 mg/ml, thus we wished to develop a gel formulation with incorporation of well-established and widely used emulsifiers Cremophor EL and Tween 80. Notably, it was found that Cremophor EL and PEGs can synergistically enhance the plasma levels of curcumin in mice, and PEG 600 is a better co-solvent to enhance the plasma levels compared to that of PEG 400. Therefore, an improved NEC formulated in the mixture of PEG 600 and Cremophor EL has a tenfold loading capacity of 200 mg/ml compared to that of a reported SMEDDS 20 mg/ml with an average particle size of 68.7 nm [22].
Despite the pharmacokinetics of orally administrated curcumin as chow-feeding or some of these above-mentioned formulated curcumin has been characterized in rats and humans, the pharmacokinetics of orally administrated curcumin has rarely been characterized in mice. Lin et al. [3] first reported that the plasma level of curcumin can achieve 0.60 μM in plasma and then declined to under detection limit (5 ng/ml) within 6 h after an oral administration of 1.0 g/kg curcumin. Gescher [5] reported that the steady-state levels of curcumin in plasma was at levels near the limit of detection (5 pmol/ml) in C57Bl/6J after having received diet containing 0.1, 0.2, or 0.5% (150, 300, 750 mg/kg) curcumin for 1 week, and no products of metabolic conjugation or reduction of curcumin were detected. Recently, pharmacokinetics of curcumin in mice [33] demonstrated that no curcumin could be detected in plasma after an oral dosing of 75 mg/kg curcumin in 0.5 N NaOH, and the curcumin plasma level was 0.095 and 0.465 μM and THC plasma level was 0.025 and 0.115 μM in mice after 4-month feeding of 0.05% (75 mg/kg) and 0.2% curcumin (300 mg/kg) in chow, respectively.
Here, we are reporting for the first time that the plasma level of curcumin can achieve [10 μM in mice after an oral dose of 1,800 mg/kg NEC due to the enhanced oral bioavailability of NEC. The results were in accordance with the synergistically enhancement of curcumin absorption or other hydrophobic drug absorption by PEG 600 and Cremophor EL as an emulsion formulation [34-36]. The enhanced oral bioavailability may be arisen from the following three factors: first, PEG can enhance its solubility as a co-solvent to ensure the formation of a homogenous solution of curcumin as a gel, unlike a particle in curcumin suspension in 1% CMC. It is well accepted that the oral bioavailability of lipophilic compounds increases with the following orders: powder, suspension, and solutions. Second, the use of Cremophor EL and PEG for NEC may enhance the permeability of curcumin in the intestinal membrane or affinity between lipid particles and intestinal membrane, and bioadhesion to the GI tract wall. Last, the average particle size of nano-particles was maintained below 100 nm, and it helps bypassing the liver first-pass metabolism that has been reported to be the major site of curcumin degradation, which is consistent with the relative smaller ratio of curcumin-O-glucuronide to curcumin (<6:1) than the reported higher ratio >20:1 in human. Despite several metabolites of curcumin including curcumin-O-glucuronide, curcumin-O-sulfate have been detected as the major metabolites in human and rats after an oral administration of curcumin and detected in plasma after an i.p. administration of curcumin in mice [29], no conjugation of metabolites of curcumin has been detected in mice after an oral administration of curcumin. As mentioned above, tetrahydrocurcumin has been detected in mice after 4-month feeding of curcumin-containing diets. Then, metabolic profiling and semi-quantification of three putative hypomethylating curcumin metabolites demonstrated first that curcumin-O-glucuronide, the major metabolite, followed by tetrahydrocurcumin and curcumin-O-sulfate, two minor metabolites, can be detected in plasma after an oral dose of 1.8 g/kg NEC. This is the first time to detect two curcumin conjugates in mouse plasma. Due to its unavailability, the biological activity of curcumin-O-glucuronide remains largely unknown. Recently, we have found that the synthetic curcumin-O-glucuronide has shown differential biological activity on the epigenetic patterns from that of curcumin in several human cancer cells (Data not shown). Therefore, NEC will provide a unique formulation to evaluate the association of these predefined biological activities of curcumin in vivo with its plasma levels and exposure duration. With these defined biological activity of curcumin-O-glucuronide, NEC will also provide a unique resource to differentiate biological activities in vivo by different orally administrated doses in murine models.
Previously, the pharmacokinetics of demethoxycurcumin and bisdemethoxycurcumin, two natural congeners of curcumin in natural curcumin remains unexplored. One more thing to be mentioned is the finding that the ratio of AUC/dose of curcumin, demethoxycurcumin, and bisdemethoxycurcumin is in the following decreasing order: bisdemethoxycurcumin, demethoxycurcumin, and curcumin in mice after an oral dose of 1 g/kg NEC. This result implicated that the bioavailability of both demethoxycurcumin and bisdemethoxycurcumin is more than that of curcumin. Bisdemethoxycurcumin has the highest bioavailability. The success of the characterization of demethoxycurcumin and bisdemethoxycurcumin is ascribed to the enhanced oral bioavailability of NEC and the sensitive LC–MS/MS method for quantification of curcumin and its congeners up to 8 h. Interestingly, the increasing order of the oral bioavailability of curcumin, demethoxycurcumin, and bisdemethoxycurcumin is consistent with their tendency to be glucuronidated and the current scenario of predominant metabolite of curcuminoids as their glucuronides [4, 37-39]. The finding should have significant implication for the future research for curcumin since several comparative studies on the biological activities of curcumin, demethoxycurcumin, and bisdemethoxycurcumin have shown that demethoxycurcumin and bisdemethoxycurcumin possess similar biological activities to that of curcumin on several targets and their cytotoxicities on several human cancer cell lines [40]. With the favorable pharmacokinetic properties of demethoxycurcumin and bisdemethoxycurcumin, it is logic to shift the current research scenario of curcumin using commercial curcumin mixture as a mixture of curcumin, demethoxycurcumin, and bisdemethoxycurcumin to use single curcuminoids. Also our study has demonstrated that curcumin, demethoxycurcumin, and bisdemethoxycurcumin have shown comparable inhibitory activity on M. SssI [30]. Therefore, further characterization of the pharmacokinetics and pharmacodynamics of these respective hypomethylating curcumininoids and their metabolites in murine models is ongoing to advancing NEC to the clinical setting as a potential means of overcoming the pharmacological barrier of curcumin.
Acknowledgments
This work was supported by National Institute of Health (NIH) grants [R21 CA135478] (Zhongfa Liu) and Bio-medical Mass Spectrometric Laboratory (Kenneth K. Chan and Zhongfa Liu) at The Ohio State University.
Abbreviations
- PEG
Polyethylene glycol
- NEC
Nano-emulsion curcumin
- SC
Suspension curcumin
- PK
Pharmacokinetic
- MP
Mobile phase
- LC
Liquid chromatography
- MS/MS
Tandem mass spectrometric detection
- I.S.
Internal standard
- CV
Coefficient of variation
- ULAR
University laboratory animal resources
- XIC
Extract ion mass chromatograms
- SD
Solid disperse
Footnotes
Conflict of interest The contents of this manuscript have been submitted for a patent application.
Contributor Information
Liu Zhongfa, College of Pharmacy, The Ohio State University, Room 152, Riffe Building, 500 W. 12th Avenue, Columbus, OH 43210, USA, liu.550@osu.edu; Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
Ming Chiu, College of Pharmacy, The Ohio State University, Room 152, Riffe Building, 500 W. 12th Avenue, Columbus, OH 43210, USA, liu.550@osu.edu; Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
Jiang Wang, College of Pharmacy, The Ohio State University, Room 152, Riffe Building, 500 W. 12th Avenue, Columbus, OH 43210, USA, liu.550@osu.edu; Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
Wei Chen, College of Pharmacy, The Ohio State University, Room 152, Riffe Building, 500 W. 12th Avenue, Columbus, OH 43210, USA, liu.550@osu.edu; Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
Winston Yen, College of Pharmacy, The Ohio State University, Room 152, Riffe Building, 500 W. 12th Avenue, Columbus, OH 43210, USA, liu.550@osu.edu; Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA.
Patty Fan-Havard, School of Pharmacy and Pharmaceutical Sciences, The State University of New York, Amherst, NY 14260, USA.
Lisa D. Yee, The Department of Surgery, The Ohio State University, Columbus, OH 43210, USA
Kenneth K. Chan, College of Pharmacy, The Ohio State University, Room 152, Riffe Building, 500 W. 12th Avenue, Columbus, OH 43210, USA, liu.550@osu.edu; Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA;College of Pharmacy, The Ohio State University, Room 152, Riffe Building, 500 W. 12th Avenue, Columbus, OH 43210, USA, liu.550@osu.edu; Comprehensive Cancer Center, The Ohio State University, Columbus, OH 43210, USA; College of Medicine and Public Health, The Ohio State University, Columbus, OH 43210, USA
References
- 1.Lopez-Lazaro M. Anticancer and carcinogenic properties of curcumin: considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol Nutr Food Res. 2008;52(Suppl 1):S103–S127. doi: 10.1002/mnfr.200700238. [DOI] [PubMed] [Google Scholar]
- 2.Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471–480. doi: 10.1007/978-0-387-46401-5_21. [DOI] [PubMed] [Google Scholar]
- 3.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos Biol Fate Chem. 1999;27:486–494. [PubMed] [Google Scholar]
- 4.Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, Williams ML, Steward WP, Gescher AJ. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse: a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002;11:535–540. [PubMed] [Google Scholar]
- 6.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 7.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 8.Thangapazham RL, Puri A, Tele S, Blumenthal R, Maheshwari RK. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int J Oncol. 2008;32:1119–1123. [PMC free article] [PubMed] [Google Scholar]
- 9.Cui J, Yu B, Zhao Y, Zhu W, Li H, Lou H, Zhai G. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm. 2009;371:148–155. doi: 10.1016/j.ijpharm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Li Z, Sun M, Li H, Guo C, Cui J, Li A, Cao F, Xi Y, Lou H, et al. Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Dev Ind Pharm. 2010;36:1225–1234. doi: 10.3109/03639041003695139. [DOI] [PubMed] [Google Scholar]
- 11.Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem. 2010;58:2095–2099. doi: 10.1021/jf9024807. [DOI] [PubMed] [Google Scholar]
- 12.Ma Z, Shayeganpour A, Brocks DR, Lavasanifar A, Samuel J. High-performance liquid chromatography analysis of curcumin in rat plasma: application to pharmacokinetics of polymeric micellar formulation of curcumin. Biomed Chromatogr. 2007;21:546–552. doi: 10.1002/bmc.795. [DOI] [PubMed] [Google Scholar]
- 13.Mach CM, Chen JH, Mosley SA, Kurzrock R, Smith JA. Evaluation of liposomal curcumin cytochrome p450 metabolism. Anticancer Res. 2010;30:811–814. [PubMed] [Google Scholar]
- 14.Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats. Int J Pharm. 2007;330:155–163. doi: 10.1016/j.ijpharm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60:171–177. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 16.Mohanty C, Acharya S, Mohanty AK, Dilnawaz F, Sahoo SK. Curcumin-encapsulated MePEG/PCL diblock copolymeric micelles: a novel controlled delivery vehicle for cancer therapy. Nanomed (Lond, Engl) 2010;5:433–449. doi: 10.2217/nnm.10.9. [DOI] [PubMed] [Google Scholar]
- 17.Mohanty C, Sahoo SK. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials. 2010;31:6597–6611. doi: 10.1016/j.biomaterials.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 18.Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29:3867–3875. [PubMed] [Google Scholar]
- 19.Onoue S, Takahashi H, Kawabata Y, Seto Y, Hatanaka J, Timmermann B, Yamada S. Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J Pharm Sci. 2010;99:1871–1881. doi: 10.1002/jps.21964. [DOI] [PubMed] [Google Scholar]
- 20.Shahani K, Swaminathan SK, Freeman D, Blum A, Ma L, Panyam J. Injectable sustained release micro particles of curcumin: a new concept for cancer chemoprevention. Cancer Res. 2010;70:4443–4452. doi: 10.1158/0008-5472.CAN-09-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yallapu MM, Gupta BK, Jaggi M, Chauhan SC. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci. 2009;351:19–29. doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Cui J, Yu B, Zhao Y, Zhu W, Li H, Lou H, Zhai G. Enhancement of oral absorption of curcumin by self-microemulsifying drug delivery systems. Int J Pharm. 2009;371(1–2):148–155. doi: 10.1016/j.ijpharm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv Exp Med Biol. 2007;595:77–103. doi: 10.1007/978-0-387-46401-5_2. [DOI] [PubMed] [Google Scholar]
- 24.Ohtsu H, Xiao Z, Ishida J, Nagai M, Wang HK, Itokawa H, Su CY, Shih C, Chiang T, Chang E, et al. Antitumor agents. 217 Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J Med Chem. 2002;45:5037–5042. doi: 10.1021/jm020200g. [DOI] [PubMed] [Google Scholar]
- 25.Kurien BT, Singh A, Matsumoto H, Scofield RH. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev Technol. 2007;5:567–576. doi: 10.1089/adt.2007.064. [DOI] [PubMed] [Google Scholar]
- 26.Tomren MA, Masson M, Loftsson T, Tonnesen HH. Studies on curcumin and curcuminoids XXXI. Symmetric and asymmetric curcuminoids: stability, activity and complexation with cyclodextrin. Int J Pharm. 2007;338:27–34. doi: 10.1016/j.ijpharm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Barik A, Priyadarsini KI, Mohan H. Photophysical studies on binding of curcumin to bovine serum albumins. Photochem Photobiol. 2003;77:597–603. doi: 10.1562/0031-8655(2003)077<0597:psoboc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Vijaya Saradhi UV, Ling Y, Wang J, Chiu M, Schwartz EB, Fuchs JR, Chan KK, Liu Z. A liquid chromatographytandem mass spectrometric method for quantification of curcuminoids in cell medium and mouse plasma. J Chromatogr. 2010;878:3045–3051. doi: 10.1016/j.jchromb.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin JK, Pan MH, Lin-Shiau SY. Recent studies on the biofunctions and biotransformations of curcumin. Biofactors (Oxf, Engl) 2000;13:153–158. doi: 10.1002/biof.5520130125. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Xie Z, Jones W, Pavlovicz RE, Liu S, Yu J, Li PK, Lin J, Fuchs JR, Marcucci G, et al. Curcumin is a potent DNA hypomethylation agent. Bioorg Med Chem Lett. 2009;19:706–709. doi: 10.1016/j.bmcl.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 31.Bisht S, Feldmann G, Soni S, Ravi R, Karikar C, Maitra A, Maitra A. Polymeric nanoparticles-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnol. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu DH, Wang S, Mei XT, Luo XJ, Xu SB. Studies on solubility enhancement of curcumin by polyvinylpyrrolidione K30. Zhong Yao Cai = Zhong Yao Cai = J Chin Med Mater. 2008;31:438–442. [PubMed] [Google Scholar]
- 33.Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo JS, Song YK, Hong JY, Lim SJ, Kim CK. Reduced food-effect and enhanced bioavailability of a self-microemulsifying formulation of itraconazole in healthy volunteers. Eur J Pharm Sci. 2008;33:159–165. doi: 10.1016/j.ejps.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Patel D, Sawant KK. Oral bioavailability enhancement of acyclovir by self-microemulsifying drug delivery systems (SMEDDS) Drug Dev Ind Pharm. 2007;33:1318–1326. doi: 10.1080/03639040701385527. [DOI] [PubMed] [Google Scholar]
- 36.Wang DK, Shi ZH, Liu L, Wang XY, Zhang CX, Zhao P. Development of self-microemulsifying drug delivery systems for oral bioavailability enhancement of alpha-asarone in beagle dogs. PDA J Pharm Sci Technol/PDA. 2006;60:343–349. [PubMed] [Google Scholar]
- 37.Dempe JS, Pfeiffer E, Grimm AS, Metzler M. Metabolism of curcumin and induction of mitotic catastrophe in human cancer cells. Mol Nutr Food Res. 2008;52:1074–1081. doi: 10.1002/mnfr.200800029. [DOI] [PubMed] [Google Scholar]
- 38.Hoehle SI, Pfeiffer E, Metzler M. Glucuronidation of curcuminoids by human microsomal and recombinant UDP-glucuronosyltransferases. Mol Nutr Food Res. 2007;51:932–938. doi: 10.1002/mnfr.200600283. [DOI] [PubMed] [Google Scholar]
- 39.Pfeiffer E, Hoehle SI, Walch SG, Riess A, Solyom AM, Metzler M. Curcuminoids form reactive glucuronides in vitro. J Agric Food Chem. 2007;55:538–544. doi: 10.1021/jf0623283. [DOI] [PubMed] [Google Scholar]
- 40.Matsunaga T, Endo S, Soda M, Zhao HT, El-Kabbani O, Tajima K, Hara A. Potent and selective inhibition of the tumor marker AKR1B10 by bisdemethoxycurcumin: probing the active site of the enzyme with molecular modeling and site-directed mutagenesis. Biochem Biophys Research Commun. 2009;389:128–132. doi: 10.1016/j.bbrc.2009.08.107. [DOI] [PubMed] [Google Scholar]