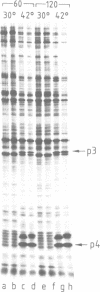
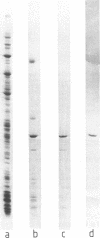
Abstract
By site-directed mutagenesis we have changed the serine residue 232 of the phi 29 terminal protein, involved in the covalent linkage to dAMP for the initiation of replication, into a threonine residue. The mutant terminal protein has been purified to homogeneity and shown to be inactive in the formation of the initiation complex; nevertheless, the mutant protein retains its ability to interact with the phi 29 DNA polymerase and with the DNA. The results obtained indicate a high specificity in the linking site of the terminal protein.
Full text
PDF



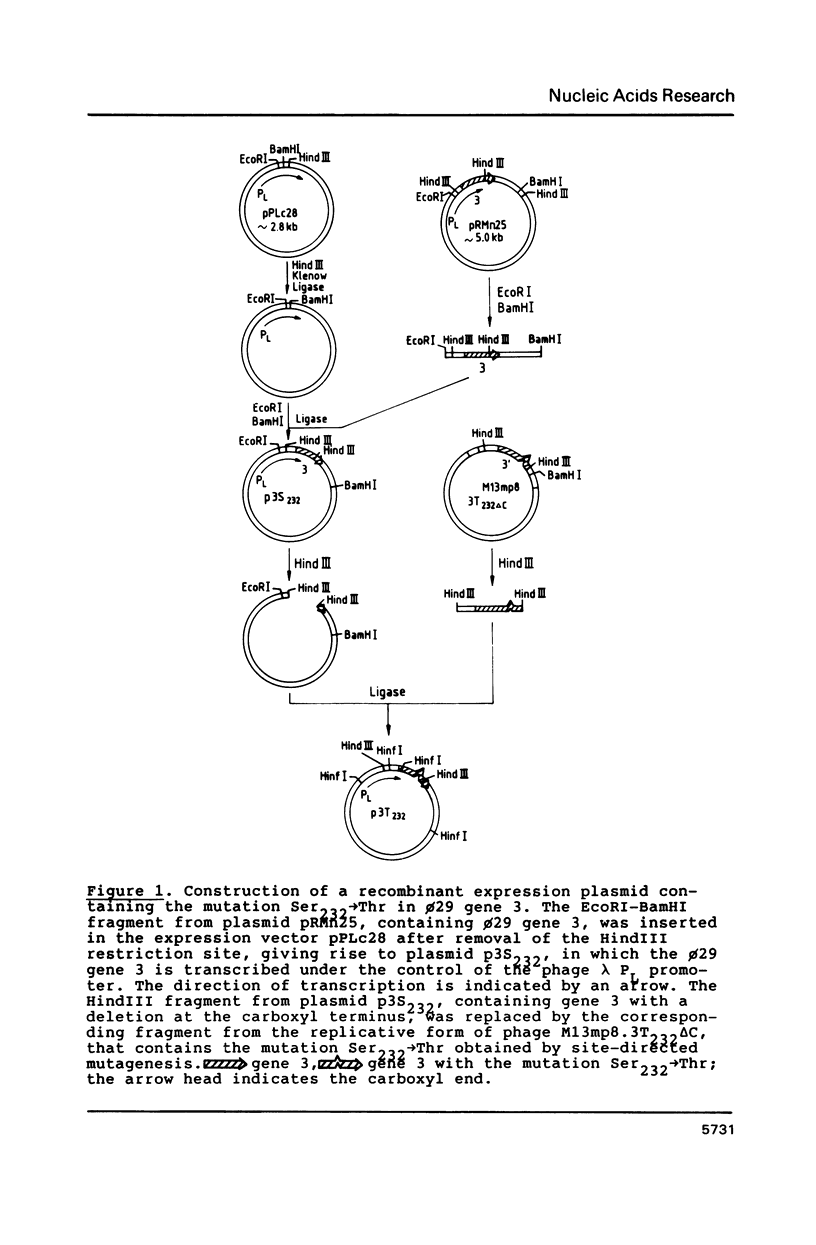



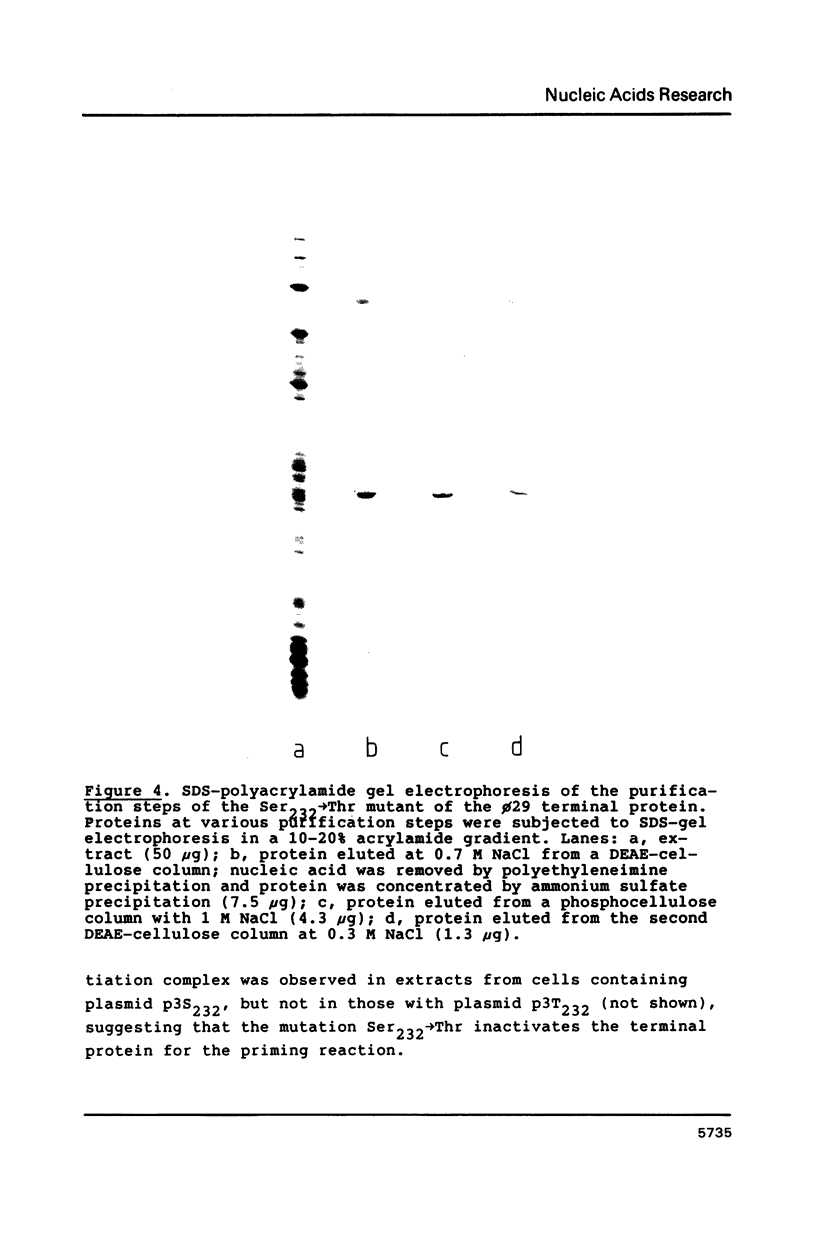

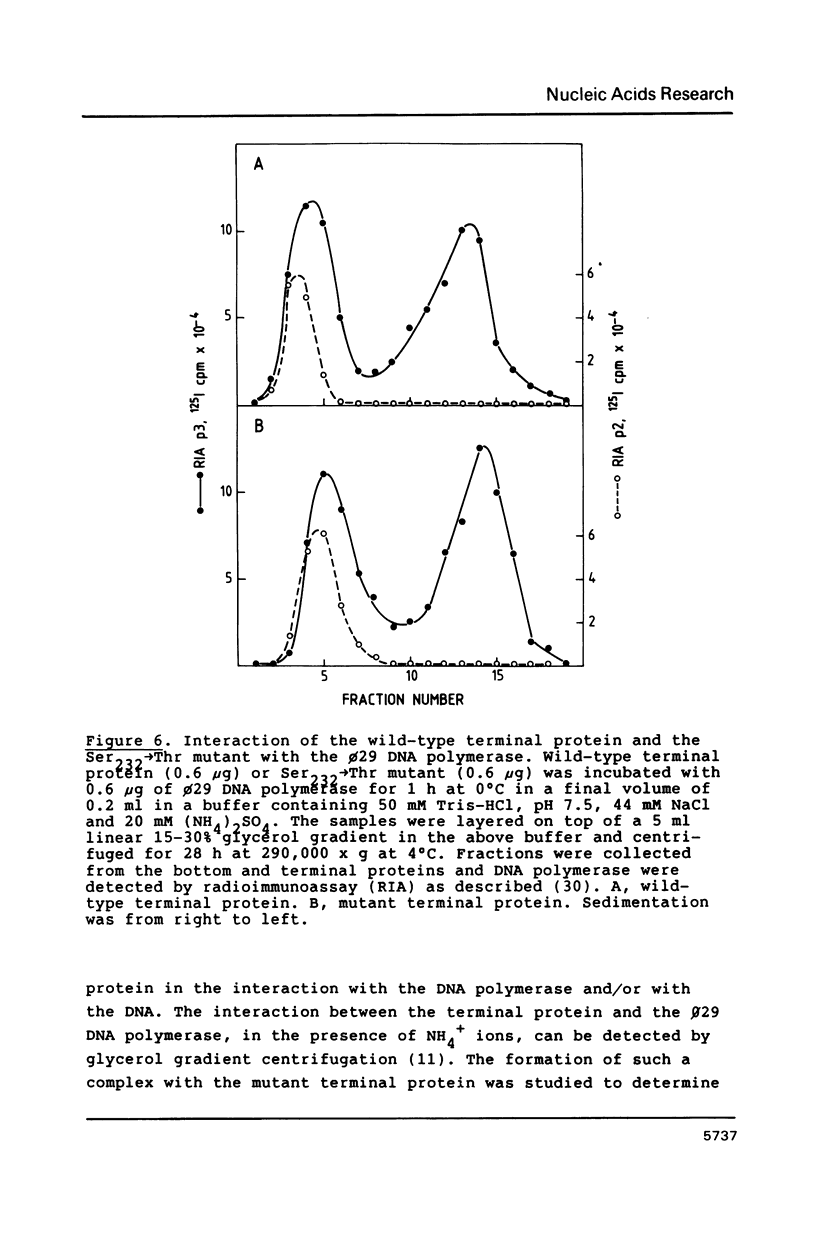
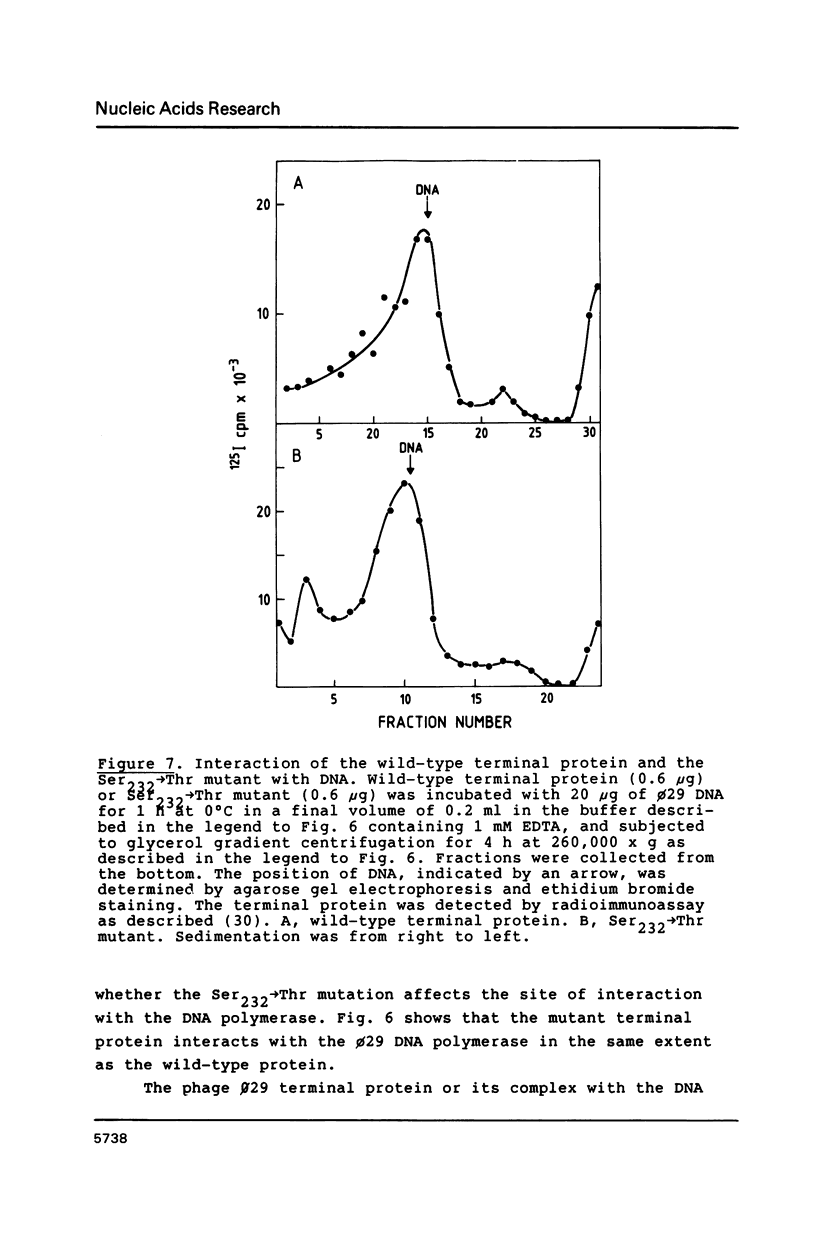


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard H. U., Remaut E., Hershfield M. V., Das H. K., Helinski D. R., Yanofsky C., Franklin N. Construction of plasmid cloning vehicles that promote gene expression from the bacteriophage lambda pL promoter. Gene. 1979 Jan;5(1):59–76. doi: 10.1016/0378-1119(79)90092-1. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Gutiérrez J., Lázaro J. M., Bernad A., Salas M. Replication of phage phi 29 DNA in vitro: role of the viral protein p6 in initiation and elongation. Nucleic Acids Res. 1986 Jun 25;14(12):4923–4937. doi: 10.1093/nar/14.12.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Prieto I., Gutiérrez J., Bernad A., Lázaro J. M., Hermoso J. M., Salas M. Effect of NH4+ ions on phi 29 DNA-protein p3 replication: formation of a complex between the terminal protein and the DNA polymerase. J Virol. 1987 Dec;61(12):3983–3991. doi: 10.1128/jvi.61.12.3983-3991.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Salas M. Characterization and purification of a phage phi 29-encoded DNA polymerase required for the initiation of replication. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5325–5329. doi: 10.1073/pnas.81.17.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Salas M. Replication of phage phi 29 DNA with purified terminal protein and DNA polymerase: synthesis of full-length phi 29 DNA. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6404–6408. doi: 10.1073/pnas.82.19.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García P., Hermoso J. M., García J. A., García E., López R., Salas M. Formation of a covalent complex between the terminal protein of pneumococcal bacteriophage Cp-1 and 5'-dAMP. J Virol. 1986 Apr;58(1):31–35. doi: 10.1128/jvi.58.1.31-35.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey K. J., Yoshikawa H., Ito J. The complete sequence of the Bacillus phage phi 29 right early region. Gene. 1985;40(2-3):301–309. doi: 10.1016/0378-1119(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J., García J. A., Blanco L., Salas M. Cloning and template activity of the origins of replication of phage phi 29 DNA. Gene. 1986;43(1-2):1–11. doi: 10.1016/0378-1119(86)90002-8. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J., Vinós J., Prieto I., Méndez E., Hermoso J. M., Salas M. Signals in the phi 29 DNA-terminal protein template for the initiation of phage phi 29 DNA replication. Virology. 1986 Dec;155(2):474–483. doi: 10.1016/0042-6822(86)90209-6. [DOI] [PubMed] [Google Scholar]
- Hermoso J. M., Méndez E., Soriano F., Salas M. Location of the serine residue involved in the linkage between the terminal protein and the DNA of phage phi 29. Nucleic Acids Res. 1985 Nov 11;13(21):7715–7728. doi: 10.1093/nar/13.21.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Mellado R. P., Salas M. High level synthesis in Escherichia coli of the Bacillus subtilis phage phi 29 proteins p3 and p4 under the control of phage lambda PL promoter. Nucleic Acids Res. 1982 Oct 11;10(19):5773–5784. doi: 10.1093/nar/10.19.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Pastrana R., Lázaro J. M., Blanco L., García J. A., Méndez E., Salas M. Overproduction and purification of protein P6 of Bacillus subtilis phage phi 29: role in the initiation of DNA replication. Nucleic Acids Res. 1985 May 10;13(9):3083–3100. doi: 10.1093/nar/13.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñalva M. A., Salas M. Initiation of phage phi 29 DNA replication in vitro: formation of a covalent complex between the terminal protein, p3, and 5'-dAMP. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5522–5526. doi: 10.1073/pnas.79.18.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto I., Lázaro J. M., García J. A., Hermoso J. M., Salas M. Purification in a functional form of the terminal protein of Bacillus subtilis phage phi 29. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1639–1643. doi: 10.1073/pnas.81.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Watabe K., Leusch M., Ito J. Replication of bacteriophage phi 29 DNA in vitro: the roles of terminal protein and DNA polymerase. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5374–5378. doi: 10.1073/pnas.81.17.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H., Ito J. Nucleotide sequence of the major early region of bacteriophage phi 29. Gene. 1982 Mar;17(3):323–335. doi: 10.1016/0378-1119(82)90149-4. [DOI] [PubMed] [Google Scholar]
- Zaballos A., Salas M., Mellado R. P. A set of expression plasmids for the synthesis of fused and unfused polypeptides in Escherichia coli. Gene. 1987;58(1):67–76. doi: 10.1016/0378-1119(87)90030-8. [DOI] [PubMed] [Google Scholar]
- Zaballos A., Salas M., Mellado R. P. Initiation of phage phi 29 DNA replication by mutants with deletions at the carboxyl end of the terminal protein. Gene. 1986;43(1-2):103–110. doi: 10.1016/0378-1119(86)90013-2. [DOI] [PubMed] [Google Scholar]