Abstract
Although mitochondria are key determinants of myocardial injury during ischemia–reperfusion (I/R), their interaction with critical cytoprotective signaling systems is not fully understood. Sphingosine-1-phosphate (S1P) produced by sphingosine kinase-1 protects the heart from I/R damage. Recently a new role for mitochondrial S1P produced by a second isoform of sphingosine kinase, SphK2, was described to regulate complex IV assembly and respiration via interaction with mitochondrial prohibitin-2. Here we investigated the role of SphK2 in cardioprotection by preconditioning. Littermate (WT) and sphk2−/− mice underwent 45 min of in vivo ischemia and 24 h reperfusion. Mice received no intervention (I/R) or preconditioning (PC) via 5 min I/R before the index ischemia. Despite the activation of PC-cytoprotective signaling pathways in both groups, infarct size in sphk2−/− mice was not reduced by PC (42 ± 3% PC vs. 43 ± 4% I/R, p = ns) versus WT (24 ± 3% PC vs. 43 ± 3% I/R, p < 0.05). sphk2−/−mitochondria exhibited decreased oxidative phosphorylation and increased susceptibility to permeability transition (PTP). Unlike WT, PC did not prevent ischemic damage to electron transport or the increased susceptibility to PTP. To evaluate the direct contribution to the resistance of mitochondria to cytoprotection, SphK2, PHB2 or cytochrome oxidase subunit IV was depleted in cardiomyoblasts. PC protection was abolished by each knockdown concomitant with decreased PTP resistance. These results point to a new action of S1P in cardioprotection and suggest that the mitochondrial S1P produced by SphK2 is required for the downstream protective modulation of PTP as an effector of preconditioning protection.
Keywords: Mitochondria, Permeability transition pore, Sphingosine kinase-2, Mitochondrial S1P, Oxidative phosphorylation, Ischemic preconditioning
Introduction
Coronary heart disease is the leading cause of death in Western countries. Ischemic preconditioning (PC) reduces infarct size after a prolonged ischemia–reperfusion [47], including in humans [66]. In most species, repeated short episodes of I/R activate intrinsic prosurvival signaling kinases via the reperfusion injury salvage kinases (RISK) pathway, that includes PI3K/Akt and ERK1/2, and the alternative survivor activating factor enhancement (SAFE) pathway [7] which activates the cytokine tumor necrosis factor alpha (TNFα) [42] and the transcription factor signal transducer and activator of transcription-3 (STAT-3) [40, 57] at reperfusion.
These two pathways converge on mitochondria and are proposed to result in inhibition of mitochondrial permeability transition pore (PTP) opening, by distal components of the cascades which include NO and inhibition of glycogen synthase kinase 3β (GSK3β) [21, 23, 37] and likely hexokinase II (HKII) [5, 19]. Mitochondria are centrally involved in the signal transduction of PC [30] which leads to a partial uncoupling of mitochondrial respiration, resulting in decreased mitochondrial injury following subsequent sustained ischemia [9, 10]. Opening of the PTP is a major determinant of cardiomyocyte death and inhibition of the PTP is a key mechanism of protection by ischemic post- and preconditioning [3, 15, 29, 31, 34, 48], including in humans [51]. Opening of the PTP is characterized by a sudden change of inner mitochondrial membrane permeability. However, the mechanism whereby cytoprotective kinase pathways modulate PTP opening remains an area of active investigation.
Previous studies have demonstrated a role of sphingosine-1-phosphate (S1P) in regulation of cardioprotection [38, 53, 61]. Indeed, activation of sphingosine kinase 1 (SphK1) and production of S1P is an important mediator of PC as shown by the use of a SphK inhibitor [35]. It has been suggested that this protection is due to the “inside-out” effect of S1P [61] whereby the S1P formed inside cells by SphK1 is released and activates plasma membrane S1P receptors, either on the same cell or on nearby cells. S1P receptor ligation then triggers signaling cascades that suppress apoptosis and leads to cardioprotection [59, 61, 67]. As a G protein-coupled receptor (GPCR) ligand, S1P produced by SphK1 activates sarcolemmal receptors, which in turn activate the RISK and SAFE pro-survival kinases.
Although there is no doubt regarding the extracellular function of S1P produced by SphK1 during ischemia–reperfusion [36, 59, 60], much less is known regarding the S1P produced by SphK2. Recent studies have elucidated two new intracellular roles of SphK2. First, SphK2 localized in the nucleus can produce S1P, which binds to and inhibits histone deacetylases 1 and 2, causing an increase in acetylation of specific histone lysines and an increase of specific gene transcription [25]. Second, our group has recently shown that S1P produced by SphK2 in the mitochondria interacts with prohibitin 2 (PHB2) to control the assembly and function of cytochrome oxidase (COX) in the electron transport chain [56]. SphK2 null mitochondria present a reduced mitochondrial S1P content in the presence of a normal cytosolic S1P content, consistent with the fact that SphK1 activity was unchanged in the SphK2 deficient mice [56]. Furthermore, the absence of SphK2 and thus the decreased mitochondrial S1P content led to a dysfunction in mitochondrial respiration through cytochrome oxidase at baseline in these mice [56].
A recent study on STAT3-deficient mice that contain defects in mitochondrial respiration at baseline showed that these knockout mice were not more sensitive to ischemia–reperfusion but refractory to cardioprotection [6].
Since mitochondria contribute a critical role in determining whether or not the heart recovers during reperfusion [28], we investigated the cardiac role of mitochondrial S1P produced by SphK2 on modulation of PTP opening during ischemia–reperfusion. The present work is the first in vivo study to demonstrate that, due to a preexisting defect in mitochondrial respiration and enhanced susceptibility to PTP opening, SphK2 deficient mice cannot be protected by preconditioning and sustain an increase in myocardial injury in vivo despite activation of PC dependent cytoprotective kinase pathways. The PHB2/COX mechanism of SphK2 action in mitochondria is supported by the attenuation of cardioprotection by the acute downregulation of PHB2 and COX subunit IV. Mitochondrial S1P generated by SphK2 acts at the mitochondrial level via modulation of PTP opening and the regulation of oxidative phosphorylation.
Methods
Protocols and studies involving animals were performed in accordance with the Virginia Commonwealth University Institutional Animal Care and Use Committee guidelines. SphK2 knockout mice were obtained from Dr. Richard Proia (NIH).
Murine left anterior descending coronary artery occlusion model
WT littermates and transgenic male mice (8–10 weeks old) were anesthetized by intraperitoneal injection of pentobarbital sodium (90 mg/kg) and buprenorphine (0.01 mg/ kg SC). Animals were orally intubated and ventilated by a rodent ventilator (minivent, Harvard Apparatus).
A left thoracotomy was performed in the fourth intercostal space. A small curved needle was passed around the left anterior descending coronary artery to induce ischemia reperfusion. Mice were subjected to 45 min of regional myocardial ischemia followed by either 10 min (for isolation of mitochondria, see below) or 24 h of reperfusion. Myocardial infarct size was then determined by triphenyltetrazolium staining [20–22, 64]. Mice were randomly assigned to receive no additional treatment (ischemia–reperfusion, I/R) or ischemic preconditioning (PC), induced by one cycle of 5 min ischemia and 5 min of reperfusion before the index ischemia.
Isolation of a mixed population of murine cardiac mitochondria
Heart mitochondria were isolated at 10 min reperfusion. Myocardial area at risk was removed and placed into buffer A (100 mM KCl, 50 mM 3-(N-morpholino) propanesulfonic acid, 1 mM EGTA, 5 mM MgSO4, and 1 mM ATP, pH 7.4 at 4°C) containing 0.2% bovine serum albumin. Cardiac tissue was homogenized with a polytron tissue processor (Kinematica GmbH, Switzerland) for 2.5 s at a rheostat setting of 10,000 rpm. The homogenate was centrifuged at 3,000g to collect the crude cytosolic fraction. The pellet was resuspended in buffer A and incubated with 5 mg/g trypsin for 15 min at 4°C. After homogenization with a Teflon pestle at 600 rpm, the nuclear fraction was pelleted at 500g. The remaining supernatant was then centrifuged at 3,000g to pellet the mitochondria. After washing, the mitochondria were resuspended in 100 mM KCl, 50 mM MOPS, and 0.5 mM EGTA, pH 7.4. Protein content was measured by Lowry determination. Mitochondria were kept on ice and used within 4 h.
Mitochondrial oxidative phosphorylation
Oxygen consumption in mitochondria was measured using a Clark-type oxygen electrode at 30°C. Mitochondria were incubated in buffer containing 80 mM KCl, 50 mM MOPS, pH 7.4, 1 mM EGTA, 5 mM KH2PO4, and 1 mg/ml BSA. Glutamate/malate (complex I substrate, 20/5 mM) and the complex IV substrate TMPD (1 mM)-ascorbate (10 mM) plus rotenone (7.5 μM) were used as electron donors. Maximal rate of state 3 respiration (2 mM ADP) was measured as previously described [13]. The net reactive oxygen species (ROS) production was measured as net H2O2 production (pmol/30 min/mg protein).
Calcium retention capacity (CRC)
CRC is defined as the amount of Ca2+ required to trigger a massive Ca2+ release by isolated cardiac mitochondria. It is used as an indicator of the PTP sensitivity to Ca2+ and expressed as nmol CaCl2/mg mitochondrial protein [21, 32]. CRC was evaluated in medium containing 150 mM sucrose, 50 mM KCl, 2 mM KH2PO4, 5 mM succinic acid in 20 mM Tris/HCl, pH 7.4 by gradual addition to fresh mitochondria (125 μg/ml at 25°C) of a known amount of calcium (5 nmol). Extramitochondrial Ca2+ concentration was recorded with 0.5 μM Calcium Green-5N and fluorescence monitored with excitation and emission wavelengths set at 500 and 530 nm, respectively. Assessment of the CRC was performed in each experimental group (n = 4/group).
Analysis of ERK1/2, Akt, GSK3β and STAT3 phosphorylation by western blot
After the preconditioning stimulus (5 min ischemia followed by 5 min reperfusion), the area at risk was removed and homogenized in buffer A supplemented with protease and phosphatase inhibitors (Roche Diagnostics, Meylan, France). A total of 50 μg of each sample was separated by SDS-PAGE on 10% gels. The phosphorylation state and the total protein of ERK1/2, Akt, STAT3 and GSK3β were determined by immunoblotting with antibodies from Cell Signaling Technology (Danvers, MA) (n = 4/group). Relative levels were determined by densitometry using ImageJ (NIH, USA; http://rsb.info.nih.gov/ij/).
Cell culture and transfection
H9c2 cardiomyoblasts were issued to Centre National de la Recherche Scientifique (CNRS) (C. Kieda, patent 99-16169, France). All cell culture reagents were obtained from Invitrogen (Cergy Pontoise, France). Cells were cultured under 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) containing 4.5 mM glucose and supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin. Cells were plated at a density of 15,000 cells/cm2 and passaged when they were 70–80% confluent. Specific siRNAs targeted to SphK2, PHB2 or COX IV were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Cells were grown to 80% confluence and transfected with 100 nM each siRNA using DharmaFECT 1 siRNA transfection reagent (Fisher-Bio-block, Illkirch, France). 24 h later, transfection mixtures were replaced with complete regular medium antibiotic-free. 48 h after transfection, cells were lysed and proteins analyzed by western blotting.
Cellular model of hypoxia–reoxygenation (H/R)
H9c2 cardiomyoblasts at 37°C were subjected to 180 min hypoxia followed by 60 min reoxygenation. siRNA transfected cells were randomized to receive no further intervention (H/R) or preconditioning (PC) performed by 20 min hypoxia followed by 20 min reoxygenation before the long period of hypoxia. During hypoxia, the cell culture medium was replaced with an acidic medium containing (in mM): 118 NaCl, 2.6 KCl, 14.5 NaHCO3, 1.2 MgSO4, 1.2 KH2PO4 at pH 6.2, and cardiomyoblasts were exposed to hypoxia in a controlled hypoxic chamber (Adelbio®, Clermont-Ferrand, France) by 95% nitrogen and 5% CO2 gas mixture flushing up to partial O2 pressure of 1–2%. Reoxygenation was conducted in a normoxic incubator at 37°C, by replacing the acidic medium for 1 h with a pH 7.4 Krebs–Henseleit buffer containing 11 mM glucose and 2% BSA.
Measurement of H9c2 cell death after H/R
To determine cell death, cells were loaded with 5 μM propidium iodide (PI) which only permeates damaged cells. Cardiomyoblasts were imaged with an Olympus IX-50 (Japan) inverted microscope equipped with a 100 W mercury lamp (HBO) as a source of epifluorescence illumination and with a 12-bit cooled digital CCD camera (ORCA R2, Hamamatsu). Propidium iodide (PI) fluorescence was excited at 520–550 nm and recorded at 580 nm. Images (4 fields of 250–300 cells each) were acquired after an illumination time of 20 ms per image using digital epifluorescence imaging software (ImageJ, NIH, USA; http://rsb.info.nih.gov/ij). The cell death percentage after the reoxygenation period was calculated from the number of cells stained with PI divided by the total number of cells.
Measurement of PTP opening in cells
Direct assessment of PTP opening in H9c2 cells was performed with calcein acetoxymethyl ester (calcein-AM) loading and CoCl2, resulting in mitochondrial localization of calcein fluorescence [39, 50]. Cells were loaded with 1 μM calcein-AM for 20 min at 37°C in 1 ml, pH 7.4, reoxygenation buffer supplemented with 1 mM CoCl2. They were then washed free of calcein and CoCl2 and incubated in reoxygenation medium. PTP opening was determined from the reduction in mitochondrial calcein signal (expressed as the percentage of the baseline value) after injection of H2O2 (100 μM). Fluorescence was measured in cells every 10 s after an illumination time of 15 ms per image (emitting from 460 to 490 nm and detecting at 515 nm) using digital epifluorescence imaging software (ImageJ, NIH, USA; http://rsb.info.nih.gov/ij).
Immunoblotting studies of H9c2 cells
After transfection, cells were lysed in buffer containing 20 mM Tris pH 8.0, 138 mM NaCl, 1% NP40, 1 mM DTT, 2.7 mM KCl, 1 mM MgCl, 2.5% glycerol, 5 mM EDTA supplemented with a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Cells were then centrifuged to remove insoluble material, and the protein concentration was determined using the Bradford assay. Equal amounts of protein (50 μg) were separated by SDS-PAGE, and immunopositive bands (antibodies from Santa Cruz Biotechnology, Santa Cruz, CA, USA) were visualized by enhanced chemiluminescence as previously described [25, 56].
Statistical analysis
Data are expressed as the means ± SEM. Differences in the relationship between infarct size and area at risk were evaluated by analysis of covariance (ANCOVA) and post hoc Tukey’s test, with infarct size as the dependent variable and area at risk as the covariant. Statistical calculations were performed using Statview® 5.0 Power PC version (SAS Institute Inc., Cary, NC). For other analyses, differences between groups were compared by one-way analysis of variance (ANOVA). When a significant F value was obtained, means were compared using a Tukey’s test. Statistical significance was defined as a value of p < 0.05.
Results
Infarct size is not reduced by ischemic preconditioning in sphk2−/− mice
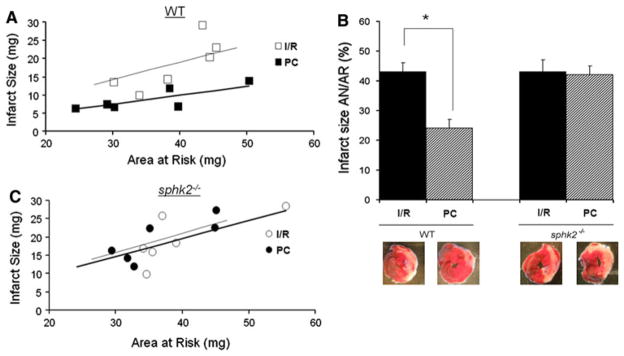
A genetic approach combined with an in vivo model of ischemia–reperfusion was used to assess the involvement of decreases in mitochondrial S1P in myocardial reperfusion injury. Areas at risk (AAR) were comparable among groups, ranging from 41 ± 1 to 43 ± 2% of the left ventricle (p = ns among groups). When infarct size was plotted versus its major determinant in this mouse model, AAR, wild type mice exhibited a significant linear relationship between infarct size and AAR in the untreated I/R group (Fig. 1a) with an infarct size averaging 43 ± 3% of AAR (Fig. 1b). As expected [24, 47], most data points for preconditioned hearts were below the regression line for the untreated I/R group (Fig. 1a), indicating that for any size of AAR, PC hearts developed significantly smaller infarcts, averaging 24 ± 3% of AAR (Fig. 1b). Ischemic sphk2−/− mice developed similar infarct size compared to WT averaging 43 ± 4% of AAR (p = ns, Fig. 1b). In contrast, PC failed to induce protection in sphk2−/− mice, with an infarct size averaging 42 ± 3% of AAR (Fig. 1b) and a comparable slope of the regression line compared to ischemic sphk2−/− group (p = ns, Fig. 1c). These data suggest that SphK2, although not contributing to infarct size in our model, is required for effective protection by ischemic preconditioning.
Fig. 1.
sphk2−/− mice are not protected by preconditioning. Scatterplots of relation between infarct size (IS) and area at risk (AAR) in ischemic (I/R, n = 6) versus preconditioned (PC, n = 6) groups of WT (a) or sphk2−/− mice (b). There is a close linear correlation between IS and AAR in all four groups. In WT but not in sphk2−/−mice, the slope of the regression line is significantly lower in the PC compared to I/R group. c Infarct size expressed as a % of the area at risk in WT or sphk2−/− mice. *p < 0.05
Deletion of SphK2 does not affect preconditioning cytoprotective pathways
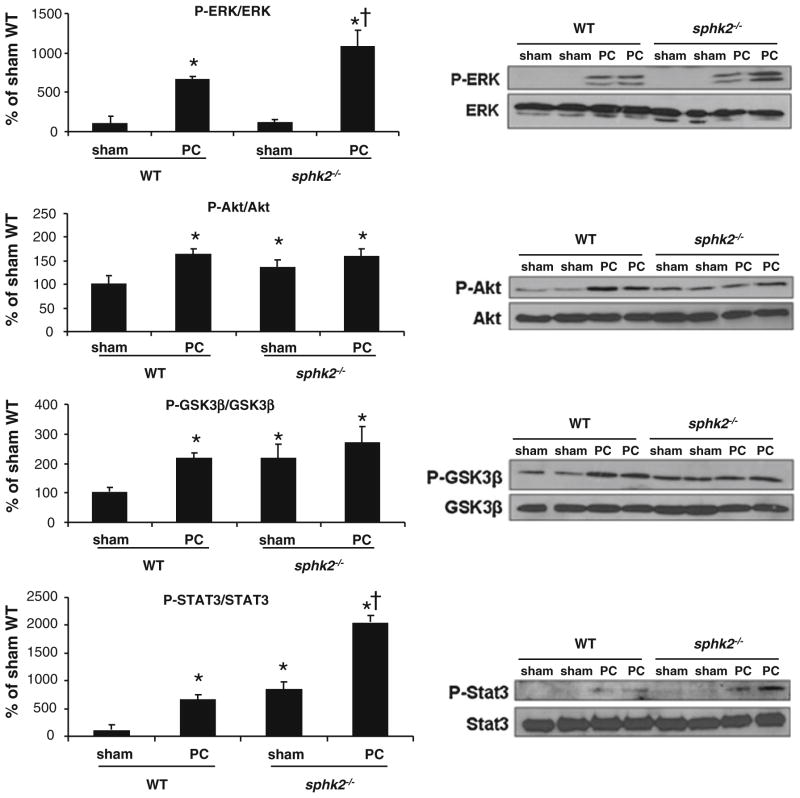
In order to address the mechanism of a resistant phenotype to cardioprotection in sphk2−/− mice, signaling pathways that mediate cytoprotection were assessed. Following the PC stimulus, phosphorylated forms of ERK1/2, Akt, STAT3 and GSK3β were significantly increased in both WT and sphk2−/− groups compared to sham-WT (p < 0.05, Fig. 2). Surprisingly, although there is no reduction of infarct size in sphk2−/− mice, protective signaling pathways are activated. In the basal condition (sham), phosphorylated forms of Akt, GSK3β and STAT3 were slightly increased in sphk2−/− compared to the WT group (p < 0.05, Fig. 2). Thus, in the absence of SphK2, cardioprotection is ineffective despite the activation of cytoprotective signaling pathways, including those known to be activated by SphK1 [35].
Fig. 2.
Cardioprotective kinase signaling pathways are preserved in sphk2−/− mice. Right panel Typical immunoblots are shown. Left panel Analysis of ERK1/2, Akt, GSK3β and STAT3 phosphorylation in sham and preconditioned (PC) WT and sphk2−/− mice. Results are expressed as percentage of sham-WT. *p < 0.05 versus sham-WT. <p < 0.05 versus sham-sphk2−/− (n = 4/group)
Preconditioning failed to protect mitochondrial function in sphk2−/− mice
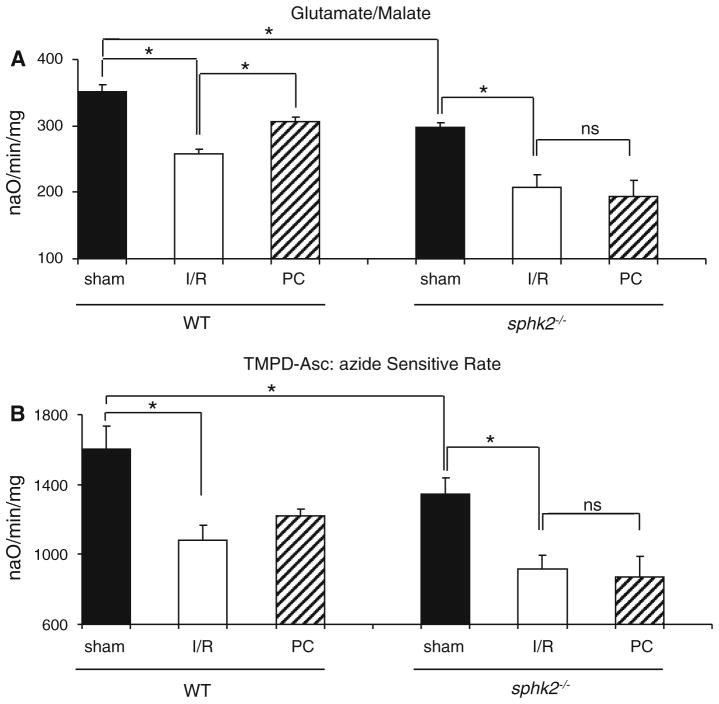
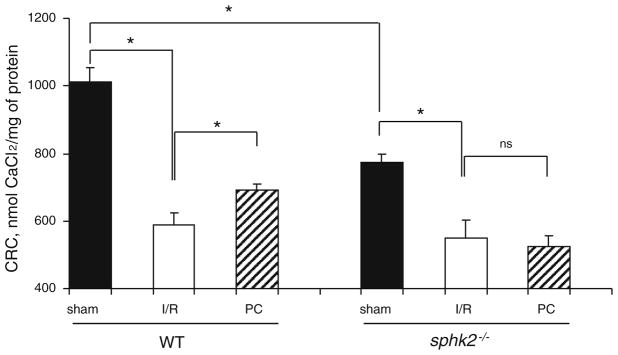
Since cytoprotective signaling pathways were activated by PC, we next focused on the effector of protection, the mitochondria. In the baseline state (sham groups), respiration measured with substrate donors to complex I was lower in mitochondria isolated from sphk2−/− compared to WT mice (Fig. 3a, [56]). A similar decrease was observed with an electron donor to COX (Fig. 3b), localizing the defect to respiration through this complex [56]. One consequence of these basal defects is that sphk2−/− mitochondria exhibit an increased capacity for maximal production of ROS with glutamate/malate as complex I substrates averaging 62 ± 8 pmol/30 min/mg protein versus 37 ± 7 pmol/30 min/mg WT group (p < 0.05). Sham mitochondria isolated from sphk2−/− hearts showed a significant decrease in CRC compared to WT (Fig. 4). In concert with our previous study [56], these results indicate that deletion of SphK2 altered mitochondrial function in the baseline state, underlined by a sensitization of the PTP opening and a lower respiratory capacity.
Fig. 3.
Preconditioning failed to rescue OXPHOS in sphk2−/−isolated mitochondria. Oxidative phosphorylation was measured in isolated mitochondria (after 45 min index ischemia followed by 10 min reperfusion) with glutamate/malate as complex I substrate (a) and TMPD-ascorbate as substrate of complex IV via cytochrome c (b). Maximal state 3 rates were expressed in naO/min/mg (n = 4–5/group). Preconditioning failed to rescue oxidative phosphorylation dependent upon complex I substrates in mitochondria isolated from sphk2−/− hearts
Fig. 4.
Isolated cardiac mitochondria from sphk2−/−mice have increased susceptibility to PTP despite ischemia preconditioning. Calcium retention capacity (CRC) in baseline (sham), ischemic (I/R) and preconditioned (PC) WT and transgenic mitochondria (n = 4–5/group). The reduction of CRC induced by I/R was reversed by preconditioning in WT but not in sphk2−/− mice
Next, the impact of these mitochondrial alterations was assessed during I/R. In WT animals, I/R resulted in a significant reduction in CRC that averaged 588 ± 36 nmol CaCl2/mg protein (p < 0.05 vs. sham-WT, Fig. 4), concomitant with a decrease in respiration with electron donors to complex I as well as to COX (Fig. 3a, b). As expected, PC significantly improved CRC when compared to I/R alone (Fig. 4) and preserved oxidative phosphorylation measured with complex I and IV substrates in the WT group (Fig. 3a, b). In contrast to WT mice, although I/R exhibited a similar alteration in CRC that averaged 550 ± 54 nmol CaCl2/mg protein (p = ns vs. I/R-WT, Fig. 4), PC failed to improve CRC in sphk2−/− mitochondria (Fig. 4). Moreover, PC also failed to protect mitochondrial respiration in sphk2−/− mitochondria (Fig. 3a, b).
Inhibition of the mS1P–PHB2–COX complex prevents preconditioning protection
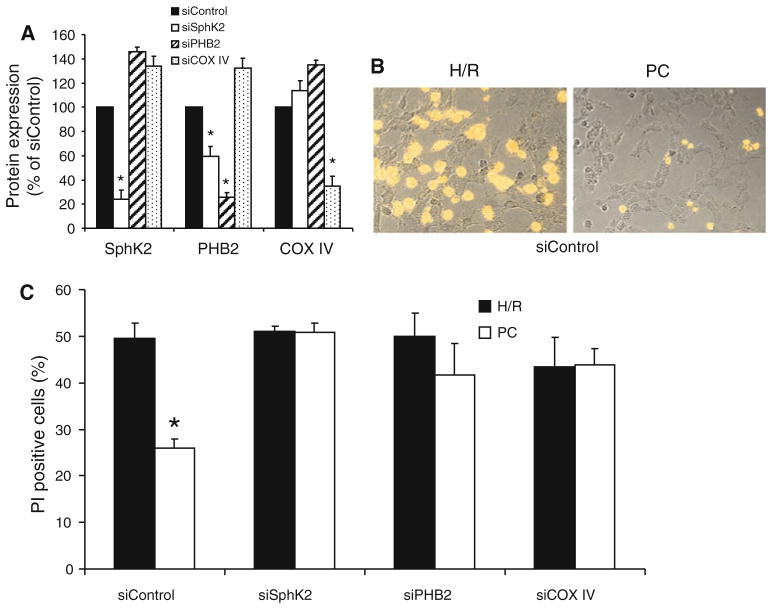
We previously demonstrated that deficiency in mitochondrial S1P in sphk2−/− mice led to a PHB2-dependent aberrant assembly of the COX complex [56]. In order to examine whether PHB2 and possibly COX is involved in the ischemia-tolerant phenotype, we investigated the extent of cell death observed in cardiomyoblasts submitted to hypoxia–reoxygenation following depletion of these proteins. Specific siRNAs targeted to SphK2, PHB2 or the nuclear-encoded COX subunit IV markedly decreased the respective protein expression in cells (Fig. 5a). In line with the previous study performed on HeLa cells [56], depletion of PHB2 induced a decrease of SphK2 expression on H9c2 cells (Fig. 5a).
Fig. 5.
Down-regulation of SphK2, PHB2 or COX IV inhibits preconditioning protection. a–c H9c2 cells were transfected with siControl, siSphK2, siPHB2 or siCOX IV, as indicated. a Protein expression was determined and quantified by immunoblotting of cell lysates. b Example of a typical cell image obtained after hypoxia–reoxygenation (H/R) or preconditioning (PC) in the siControl group. c Preconditioning significantly reduced cell death in the siControl group (p < 0.05). In contrast, preconditioning failed to reduce cell death in transfected cells treated with siRNA targeted to SphK2, PHB2 and COX IV
In the siControl group, 180 min hypoxia followed by 60 min reoxygenation induced cell death averaging 49 ± 3% (Fig. 5b, c). Preconditioning significantly reduced cell death by approximately half, averaging 26 ± 2% (p < 0.05 vs. H/R group, Fig. 5b, c). Cell death after hypoxia–reoxygenation was similar to the siControl group for each knockdown. However, in contrast to the siControl group, preconditioning failed to reduce cell death when SphK2, PHB2, or COX IV was down regulated (Fig. 5c). Taken together, these data suggest that mS1P–PHB2–COX complex [56] contributes to the protection elicited by preconditioning.
Down-regulation of SphK2, PHB2 or COX IV sensitizes PTP opening
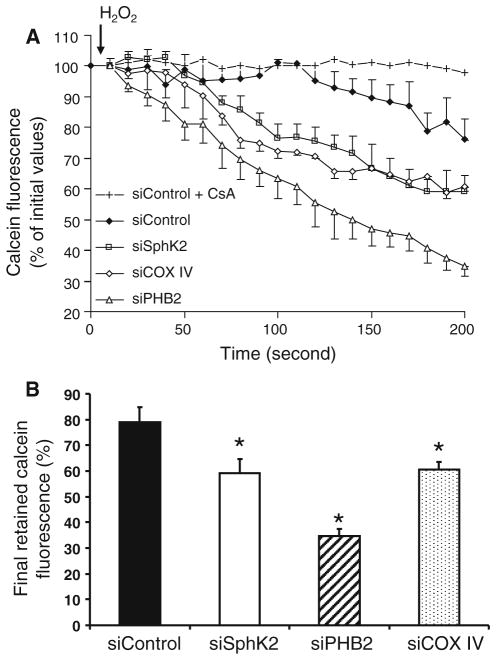
Since PTP resistance represents a key role in preconditioning protection [27], in order to further delineate the mechanism involved in the preconditioning-resistant phenotype, the effect of the knockdown of SphK2, PHB2, and COX IV on PTP resistance was studied. H2O2 stress of calcein-loaded siControl cardiomyoblasts resulted in a reduction in mitochondrial calcein fluorescence up to 82 ± 6% of the initial value (Fig. 6a). This effect was abolished by cyclosporine A treatment (1 μM), a potent PTP inhibitor, confirming that the reduction in mitochondrial calcein fluorescence was due to PTP opening (siControl + CsA, Fig. 6a). Interestingly, PTP opening was sensitized when SphK2, PHB2 or COX IV were down regulated, characterized by a significant reduction of mitochondrial calcein fluorescence compared to siControl (Fig. 6b). Thus, the deletion of SphK2, PHB2 or COX subunit IV lowers the resistance to PTP opening.
Fig. 6.
Depletion of SphK2, PHB2 or COX IV altered PTP opening in cells. Measurement of calcein fluorescence in H9c2 cells. a Residual mitochondrial calcein fluorescence (expressed as percentage of baseline fluorescence) after activation of PTP opening induced by 100 μM H2O2. b Threshold of PTP opening was reduced when the expression of SphK2, PHB2 or COX IV was downregulated. *p < 0.05 versus siControl
Discussion
The potent lipid mediator S1P is recognized as a critical cardioprotective molecule [38]. S1P produced inside the cell is released and binds to specific G protein-coupled cell surface receptors that activate a signaling cascade that leads to cardioprotection [59, 63, 68]. The majority of research to date has focused on the activation of these receptors, but there is now evidence that S1P exerts intracellular functions independently of cell surface receptors, notably in the mitochondria [56]. Utilizing genetic in vivo and in vitro models, the present study demonstrates that due to a preexisting defect in mitochondrial respiration, SphK2 deficient mice cannot be protected by preconditioning (PC). This work suggests that SphK2 is downstream of the SphK1-cytoprotective pathway and, that presence of SphK2 is required to maintain a functional COX and normal mitochondrial respiration to modulate PTP opening during preconditioning.
Indeed, even if cytosolic signaling kinase pathways are activated in sphk2−/− mice, shown by the phosphorylation of ERK1/2, Akt, STAT3 and GSK3β, preconditioning fails to afford protection in vivo. The recent finding of Vessey et al. [62] in the isolated perfused heart and several previous reports suggest a crucial role for SphK2 in ischemic protection. Wacker et al. [65] reported that the expression and activity level of the SphK2 isoform in the cerebral micro-vasculature is increased after preconditioning, whereas SphK1 is unchanged. These authors further demonstrated that dimethylsphingosine treatment, an inhibitor of SphK2, completely abolished the reduction of infarct volume provided by the cerebral hypoxic preconditioning stimulus [65]. In the current model, activity of SphK2 and consequently the mitochondrial S1P content were decreased in transgenic mice despite a normal activity of SphK1 and a normal cytosolic S1P content. Taken together, these results suggest first, that mitochondrial S1P constitutes a distinct pool of S1P which acts independently of the cytosolic S1P pool generated via SphK1; and second, that mitochondrial S1P produced by mitochondrial SphK2 was responsible for inducing an ischemia-tolerant phenotype. In this way, it is known that preconditioning is not effective in the aged heart and a recent study demonstrated that the activity of SphK2 is reduced with aging [60], supporting the relevance of SphK2 activity in cardioprotection.
The current study subsequently focused on the mitochondrial-based mechanism since the sphk2−/− mitochondrial phenotype is refractory to protection via the activation of upstream mediators including GSK3β. Preconditioning failed to attenuate the ischemia-induced defects in mitochondrial respiration in sphk2−/− mice. However, since the mitochondrial respiratory function is already altered in the baseline state, we hypothesized that the preexisting mitochondrial defect was responsible for the inability to protect the heart by preconditioning. Indeed, the sole known role of mitochondrial S1P involves its interaction with the mitochondrial chaperone PHB2 that is required for the proper assembly of complex IV, resulting in a decrease in mitochondrial respiration [56]. In the present study, in vitro results clearly demonstrated that reduction of mitochondrial respiration at baseline, even by indirect (SphK2 or PHB2) or direct (COX subunit IV) knockdown, prevented the protective effect of preconditioning, consistent with the phenomenon of aging where cardioprotection from preconditioning is ineffective [1] and perhaps in line with the presence of a cytochrome oxidase defect in the aged heart [17, 49]. Future work is required to understand the relative contributions of defects in cytoprotective kinase cascades [58] in relation to the relative refractoriness of the mitochondria to undergo protective modulation following the activation of cytoprotective networks [46].
Since sphk2−/− mitochondria presented an increase in PTP sensitivity at baseline, we propose that SphK2 could modulate the PTP through an interaction between mitochondrial S1P–PHB2–COX complex and components of the PTP. Generally, myocardial cardioprotection studies correlated the reduction of infarct size with the resistance of PTP opening [2, 4, 16, 18, 26, 43]. Here we investigated whether basal altered PTP sensitivity may cause the observed failure of preconditioning. We tested the effect of the acute knockdown of each constituent in the interaction (SphK2, PHB2 and COX IV) on PTP sensitivity and hypoxia–reoxygenation stress. Interestingly, knockdown of each potential component led to a failure of preconditioning and to a clear decrease in the resistance to PTP opening in response to cell stress. In contrast, the extent of cell death in the absence of preconditioning was unaltered. Hence, our results support the notion that mitochondrial S1P (produced by SphK2) is required for preconditioning by regulating the mitochondrial respiratory chain and that the respiratory chain function can modulate the PTP. The enhanced calcium sensitivity to PTP seems to be the mitochondrial mechanism responsible for the mitochondrial phenotype that is resistant to cardioprotection, perhaps related to PHB2 interactions or alternatively via the COX defect. It is interesting to note that blockade of the electron transport chain at proximal sites protects mitochondrial function [10] and attenuates the susceptibility to PTP [11], whereas blockade at COX does not protect [14]. Thus, sphk2−/− mitochondria with a defect in COX (and an increased net ROS production from the electron transport chain) may offer a relative resistance to cardioprotection due to the distal site of the defect in the chain. Defects in electron transport, especially distal in the chain at COX [12, 41], favor increased ROS production.
It has been proposed that SphK1 mediates prosurvival effects whereas SphK2 is proapoptotic [33, 44, 45, 52]. In the current study, SphK2 deletion did not induce protection following I/R, inconsistent with a proapoptotic role of SphK2 in the heart during the stress of ischemia and reperfusion. The observed difference could be explained by the fact that the proapoptotic effect of SphK2 was only demonstrated following overexpression in different models [44, 45, 52].
Although SphK2 deficient mice present mitochondrial defects at baseline, we were surprised to find that there was no difference in infarct size between sphk2−/− and WT mice. However, this finding is consistent with the fact that our SphK2 knockout mice exhibit no evident cardiac phenotype, with no differences in baseline cardiac function including left ventricle mass index, development of fibrosis or impairment of diastolic function (supplemental data Figure S and Table 1). In the recent study in a non blood perfused system, Vessey et al. [62] observed an increased infarct size after ischemia–reperfusion in their SphK2 transgenic mice accompanied by an increase in phosphorylated p38. The discrepancy with our study could be explained by the different transgenic model and also by the fact that our in vivo model is a more complex integrative setting. Thus, it appears that the modest increase in susceptibility to ischemic injury, evident in the isolated perfused organ, is not so severe as to emerge in the setting of the more complex, in vivo model of cardiac injury. Furthermore, our observations are confirmed by the in vitro analysis in H9c2 cells, where knockdown of SphK2 did not affect cell death following hypoxia-reoxygenation. Thus, baseline mitochondrial defects appear to contribute to the effectiveness of cytoprotective intervention and less so to the extent of ischemic injury in the unprotected state (since infarct sizes were comparable between ischemic WT and sphk2−/− mice). This concept has been previously observed in other transgenic models including the STAT3-KO mice and the connexin 43-deficient mice, where cardioprotection is lost but without increased infarct size after ischemia–reperfusion compared to the WT [8, 55]. Indeed, although these mice displayed mitochondrial defects at baseline with decreased respiration and resistance of PTP, they did not show greater ischemic insults [8, 55].
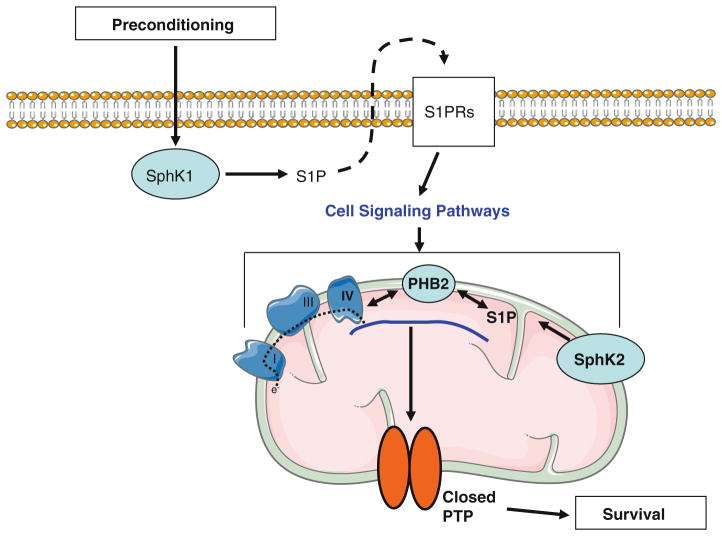
In summary, the current study in concert with previous work [62] points to a new role for mitochondrial S1P generated by mitochondrial SphK2 in cardioprotection. Based on these studies and the previous study of mitochondrial function [56], we present a working hypothesis regarding the involvement of the different subcellular S1P pools in cardioprotection (Fig. 7): despite a normal SphK1 activity, the absence of SphK2 induces a reduction of mitochondrial S1P content which in turn alters mitochondrial respiration and enhances PTP susceptibility. This mitochondrial phenotype is refractory to the protection of PC. Thus, the COX defect and the S1P–PHB2–COX interaction seem to contribute to PTP sensitivity; its regulation suggests that in addition to regulation by GSK3β [37] and HKII [5], PTP is responsive to peptide modulators including STAT-3 [8], connexin 43 [54] and likely PHB2. To conclude, SphK2 is required downstream of the SphK1-activated signaling pathways for PTP-dependent preconditioning protection. In other words, preconditioning first activates SphK1 which recruits the cytosolic S1P pool to trigger the cytoprotective signaling pathways by the inside-out effect; second, the mitochondrial pool of S1P is activated to regulate oxidative phosphorylation (through mS1P–PHB2–COX complex) and thus the ROS production, leading to an inhibition of PTP opening. Deficiencies in SphK2 or PHB2 may contribute to mitochondrial phenotypes relatively refractory to preconditioning in the presence of co-morbid cardiac conditions including aging and diabetes.
Fig. 7.
Proposed involvement of different subcellular S1P pools in cardioprotection. Preconditioning activates SphK1 which increases the cytosolic pool of S1P leading to cellular release and the activation of S1P receptors on the cell surface. Receptor activation activates cytoprotective kinase cascades that converge on mitochondria as the effector of protection. The mitochondrial pool of S1P regulates oxidative phosphorylation (via the S1P–PHB2–COX complex) and ROS production, leading to an inhibition of PTP opening and cell survival. A deficiency in SphK2 contributes to a reduction of mitochondrial S1P content leading to a mismatch of the respiratory chain responsible for a defect in mitochondrial respiration and dysfunctional PTP regulation
Supplementary Material
Acknowledgments
The authors thank Drs. Anindita Das and Robert Paillard for their advice and technical help. We thank Dr. Fadi N. Salloum from the Division of Cardiology, Department of Internal Medicine, Virginia Commonwealth University for echocardiographic measurements. And we thank Dr. Claudine Kieda from the Centre Biophysique Moléculaire (CNRS, France) for the gift of H9c2 cells. This work was supported by U.S. National Institutes of Health (NIH) grants 2PO1AG15885 (E.J.L.) and R37GM043880 (S.S), Medical Research Service, Department of Veterans Affairs Merit Review Award to E.J.L. and the Pauley Heart Center, Virginia Commonwealth University (E.J.L., Q.C., L.G., M.P.). L.G was supported by SERVIER grant “jeune chercheur” (France) and M.P. was supported by French grant EXPLORA’DOC 2009 (Rhône-Alpes, France).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00395-011-0223-7) contains supplementary material, which is available to authorized users.
Contributor Information
Ludovic Gomez, Department of Medicine, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. INSERM U886, Université Claude Bernard Lyon I, 69373 Lyon, France.
Melanie Paillard, Department of Medicine, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. INSERM U886, Université Claude Bernard Lyon I, 69373 Lyon, France.
Megan Price, Department of Biochemistry and Molecular Biology, Virginia Commonwealth University School of Medicine, Richmond, VA 23298, USA.
Qun Chen, McGuire Veterans Affairs Medical Center, Richmond, VA 23249, USA.
Geoffrey Teixeira, INSERM U886, Université Claude Bernard Lyon I, 69373 Lyon, France.
Sarah Spiegel, Department of Biochemistry and Molecular Biology, Virginia Commonwealth University School of Medicine, Richmond, VA 23298, USA.
Edward J. Lesnefsky, Email: ejlesnefsky@vcu.edu, Department of Medicine, Virginia Commonwealth University, School of Medicine, Richmond, VA 23298, USA. Department of Biochemistry and Molecular Biology, Virginia Commonwealth University School of Medicine, Richmond, VA 23298, USA. McGuire Veterans Affairs Medical Center, Richmond, VA 23249, USA
References
- 1.Abete P, Ferrara N, Cioppa A, Ferrara P, Bianco S, Calabrese C, Cacciatore F, Longobardi G, Rengo F. Preconditioning does not prevent postischemic dysfunction in aging heart. J Am Coll Cardiol. 1996;27:1777–1786. doi: 10.1016/0735-1097(96)00070-8. [DOI] [PubMed] [Google Scholar]
- 2.Argaud L, Gateau-Roesch O, Augeul L, Couture-Lepetit E, Loufouat J, Gomez L, Robert D, Ovize M. Increased mitochondrial calcium coexists with decreased reperfusion injury in postconditioned (but not preconditioned) hearts. Am J Physiol Heart Circ Physiol. 2008;294:H386–H391. doi: 10.1152/ajpheart. 01035.2007. [DOI] [PubMed] [Google Scholar]
- 3.Argaud L, Gateau-Roesch O, Chalabreysse L, Gomez L, Loufouat J, Thivolet-Bejui F, Robert D, Ovize M. Preconditioning delays Ca2+-induced mitochondrial permeability transition. Cardiovasc Res. 2004;61:115–122. doi: 10.1016/j.cardiores. 2003.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, Ovize M. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. Journal of Molecular and Cellular Cardiology. 2005;38:367–374. doi: 10.1016/j.yjmcc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Beutner G, Ruck A, Riede B, Brdiczka D. Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim Biophys Acta. 1998;1368:7–18. doi: 10.1016/S0005-2736(97)00175-2. [DOI] [PubMed] [Google Scholar]
- 6.Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, Schulz R. Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res. 2008;102:131–135. doi: 10.1161/CIRCRESAHA.107.164699. [DOI] [PubMed] [Google Scholar]
- 7.Boengler K, Hilfiker-Kleiner D, Drexler H, Heusch G, Schulz R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol Ther. 2008;120:172–185. doi: 10.1016/j.pharmthera. 2008.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–C147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Hoppel CL, Lesnefsky EJ. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther. 2006;316:200–207. doi: 10.1124/jpet.105.091702. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Lesnefsky EJ. Blockade of electron transport during ischemia preserves bcl-2 and inhibits opening of the mitochondrial permeability transition pore. FEBS Lett. 2011;585:921–926. doi: 10.1016/j.febslet.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294:C460–C466. doi: 10.1152/ajpcell. 00211.2007. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther. 2006;319:1405–1412. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, Yin G, Stewart S, Hu Y, Lesnefsky EJ. Isolating the segment of the mitochondrial electron transport chain responsible for mitochondrial damage during cardiac ischemia. Biochem Biophys Res Commun. 2010;397:656–660. doi: 10.1016/j.bbrc. 2010.05.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen MV, Yang XM, Downey JM. Acidosis, oxygen, and interference with mitochondrial permeability transition pore formation in the early minutes of reperfusion are critical to postconditioning’s success. Basic Res Cardiol. 2008;103:464–471. doi: 10.1007/s00395-008-0737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson SM, Hausenloy D, Duchen MR, Yellon DM. Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol. 2006;38:414–419. doi: 10.1016/j.biocel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- 18.Gateau-Roesch O, Argaud L, Ovize M. Mitochondrial permeability transition pore and postconditioning. Cardiovasc Res. 2006;70:264–273. doi: 10.1016/j.cardiores.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Gimenez-Cassina A, Lim F, Cerrato T, Palomo GM, Diaz-Nido J. Mitochondrial hexokinase II promotes neuronal survival and acts downstream of glycogen synthase kinase-3. J Biol Chem. 2009;284:3001–3011. doi: 10.1074/jbc.M808698200. [DOI] [PubMed] [Google Scholar]
- 20.Gomez L, Chavanis N, Argaud L, Chalabreysse L, Gateau-Roesch O, Ninet J, Ovize M. Fas-independent mitochondrial damage triggers cardiomyocyte death after ischemia–reperfusion. Am J Physiol Heart Circ Physiol. 2005;289:H2153–H2158. doi: 10.1152/ajpheart.00165.2005. [DOI] [PubMed] [Google Scholar]
- 21.Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- 22.Gomez L, Thibault H, Gharib A, Dumont JM, Vuagniaux G, Scalfaro P, Derumeaux G, Ovize M. Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2007;293:H1654–H1661. doi: 10.1152/ajpheart.01378.2006. [DOI] [PubMed] [Google Scholar]
- 23.Gross ER, Hsu AK, Gross GJ. The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3beta. Am J Physiol Heart Circ Physiol. 2006;291:H827–H834. doi: 10.1152/ajpheart.00003.2006. [DOI] [PubMed] [Google Scholar]
- 24.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275:H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/ science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 27.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 28.Hausenloy DJ, Baxter G, Bell R, Botker HE, Davidson SM, Downey J, Heusch G, Kitakaze M, Lecour S, Mentzer R, Mocanu MM, Ovize M, Schulz R, Shannon R, Walker M, Walkinshaw G, Yellon DM. Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Res Cardiol. 2010;105:677–686. doi: 10.1007/s00395-010-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/S0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 30.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 31.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol. 2010;105:151–154. doi: 10.1007/ s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 32.Ichas F, Jouaville LS, Sidash SS, Mazat JP, Holmuhamedov EL. Mitochondrial calcium spiking: a transduction mechanism based on calcium-induced permeability transition involved in cell calcium signalling. FEBS Lett. 1994;348:211–215. doi: 10.1016/0014-5793(94)00615-6. [DOI] [PubMed] [Google Scholar]
- 33.Igarashi N, Okada T, Hayashi S, Fujita T, Jahangeer S, Nakamura S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278:46832–46839. doi: 10.1074/jbc. M30657720. [DOI] [PubMed] [Google Scholar]
- 34.Javadov SA. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J Physiol (Lond) 2003;549:513–524. doi: 10.1113/jphysiol. 2003.03423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin ZQ, Goetzl EJ, Karliner JS. Sphingosine kinase activation mediates ischemic preconditioning in murine heart. Circulation. 2004;110:1980–1989. doi: 10.1161/01.CIR.0000143632. 06471.93. [DOI] [PubMed] [Google Scholar]
- 36.Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, Goetzl EJ, Karliner JS, Gray MO. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1970–H1977. doi: 10.1152/ajpheart. 01029.2001. [DOI] [PubMed] [Google Scholar]
- 37.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karliner JS. Sphingosine kinase and sphingosine 1-phosphate in cardioprotection. J Cardiovasc Pharmacol. 2009;53:189–197. doi: 10.1097/FJC.0b013e3181926706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh H, Nishigaki N, Hayashi H. Diazoxide opens the mitochondrial permeability transition pore and alters Ca2+ transients in rat ventricular myocytes. Circulation. 2002;105:2666–2671. doi: 10.1161/01.CIR.0000016831.41648.04. [DOI] [PubMed] [Google Scholar]
- 40.Kelly RF, Lamont KT, Somers S, Hacking D, Lacerda L, Thomas P, Opie LH, Lecour S. Ethanolamine is a novel STAT-3 dependent cardioprotective agent. Basic Res Cardiol. 2010;105:763–770. doi: 10.1007/s00395-010-0125-0. [DOI] [PubMed] [Google Scholar]
- 41.Korge P, Ping P, Weiss JN. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ Res. 2008;103:873–880. doi: 10.1161/CIRCRESAHA.108.180869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lacerda L, McCarthy J, Mungly SF, Lynn EG, Sack MN, Opie LH, Lecour S. TNFalpha protects cardiac mitochondria independently of its cell surface receptors. Basic Res Cardiol. 2010;105:751–762. doi: 10.1007/s00395-010-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007;75:530–535. doi: 10.1016/j.cardiores.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278:40330–40336. doi: 10.1074/jbc.M30445520. [DOI] [PubMed] [Google Scholar]
- 45.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH, Jr, Milstien S, Spiegel S. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 46.Miura T, Tanno M, Sato T. Mitochondrial kinase signalling pathways in myocardial protection from ischaemia/reperfusion-induced necrosis. Cardiovasc Res. 2010;88:7–15. doi: 10.1093/ cvr/cvq206. [DOI] [PubMed] [Google Scholar]
- 47.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 48.Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–423. doi: 10.1093/cvr/ cvq129. [DOI] [PubMed] [Google Scholar]
- 49.Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Age-dependent decline in the cytochrome c oxidase activity in rat heart mitochondria: role of cardiolipin. FEBS Lett. 1997;406:136–138. doi: 10.1016/S0014-5793(97)00264-0. [DOI] [PubMed] [Google Scholar]
- 50.Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, Di Lisa F. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J. 1999;76:725–734. doi: 10.1016/S0006-3495 (99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 52.Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, Wattenberg BW, Vadas MA. Phosphorylation-dependent translocation of sphingosine kinase to the plasma membrane drives its oncogenic signalling. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sattler KJ, Elbasan S, Keul P, Elter-Schulz M, Bode C, Graler MH, Brocker-Preuss M, Budde T, Erbel R, Heusch G, Levkau B. Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res Cardiol. 2010;105:821–832. doi: 10.1007/s00395-010-0112-5. [DOI] [PubMed] [Google Scholar]
- 54.Schulz R, Boengler K, Totzeck A, Luo Y, Garcia-Dorado D, Heusch G. Connexin 43 in ischemic pre- and postconditioning. Heart Fail Rev. 2007;12:261–266. doi: 10.1007/s10741-007-9032-3. [DOI] [PubMed] [Google Scholar]
- 55.Schwanke U, Konietzka I, Duschin A, Li X, Schulz R, Heusch G. No ischemic preconditioning in heterozygous connexin43-deficient mice. Am J Physiol Heart Circ Physiol. 2002;283:H1740–H1742. doi: 10.1152/ajpheart.00442.2002. [DOI] [PubMed] [Google Scholar]
- 56.Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, Kordula T, Milstien S, Lesnefsky EJ, Spiegel S. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. Faseb J. 2011;25(2):600–612. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suleman N, Somers S, Smith R, Opie LH, Lecour SC. Dual activation of STAT-3 and Akt is required during the trigger phase of ischaemic preconditioning. Cardiovasc Res. 2008;79:127–133. doi: 10.1093/cvr/cvn067. [DOI] [PubMed] [Google Scholar]
- 58.Tani M, Honma Y, Hasegawa H, Tamaki K. Direct activation of mitochondrial K(ATP) channels mimics preconditioning but protein kinase C activation is less effective in middle-aged rat hearts. Cardiovasc Res. 2001;49:56–68. doi: 10.1016/S0008-6363 (00)00240-6. [DOI] [PubMed] [Google Scholar]
- 59.Theilmeier G, Schmidt C, Herrmann J, Keul P, Schafers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, von Wnuck Lipinski K, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/ CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 60.Vessey DA, Kelley M, Li L, Huang Y. Sphingosine protects aging hearts from ischemia/reperfusion injury: superiority to sphingosine 1-phosphate and ischemic pre- and post-conditioning. Oxid Med Cell Longev. 2009;2:146–151. doi: 10.4161/oxim.2.3.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vessey DA, Kelley M, Li L, Huang Y, Zhou HZ, Zhu BQ, Karliner JS. Role of sphingosine kinase activity in protection of heart against ischemia reperfusion injury. Med Sci Monit. 2006;12:BR 318–BR 324. [PubMed] [Google Scholar]
- 62.Vessey DA, Li L, Jin ZQ, Kelley M, Honbo N, Zhang J, Karliner JS. A sphingosine kinase form 2 knockout sensitizes mouse myocardium to ischemia/reoxygenation injury and diminishes responsiveness to ischemic preconditioning. Oxid Med Cell Longev. 2011;2011:8. doi: 10.1155/2011/961059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vessey DA, Li L, Kelley M, Karliner JS. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem Biophys Res Commun. 2008;375:425–429. doi: 10.1016/j.bbrc.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vivaldi MT, Kloner RA, Schoen FJ. Triphenyltetrazolium staining of irreversible ischemic injury following coronary artery occlusion in rats. Am J Pathol. 1985;121:522–530. [PMC free article] [PubMed] [Google Scholar]
- 65.Wacker BK, Park TS, Gidday JM. Hypoxic preconditioning-induced cerebral ischemic tolerance: role of microvascular sphingosine kinase 2. Stroke. 2009;40:3342–3348. doi: 10.1161/STROKEAHA.109.560714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yellon DM, Alkhulaifi AM, Pugsley WB. Preconditioning the human myocardium. Lancet. 1993;342:276–277. doi: 10.1016/0140-6736(93)91819-8. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H3150–H3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 68.Zhao ZQ, Vinten-Johansen J. Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res. 2006;70:200–211. doi: 10.1016/j.cardiores.2006.01.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.