Abstract
The potent lipid mediator sphingosine-1-phosphate (S1P) is produced inside cells by two closely related kinases, sphingosine kinase 1 (SPHK1) and SPHK2, and has emerged as a crucial regulator of immunity. Many of the actions of S1P in innate and adaptive immunity are mediated by its binding to five G protein-coupled receptors, designated S1PR1–5, but recent findings have also identified important roles for S1P as a second messenger during inflammation. In this Review, we discuss recent advances in our understanding of the roles of S1P receptors and describe the newly identified intracellular targets of S1P that are crucial for immune responses. Finally, we discuss the therapeutic potential of new drugs that target S1P signalling and functions.
Sphingosine-1-phosphate (S1P) is a potent bioactive sphingolipid metabolite that regulates diverse cellular processes that are important for inflammation and immune responses. These processes include cell growth, survival, differentiation, lymphocyte trafficking, vascular integrity and cytokine and chemokine production. Interest in S1P as a signalling molecule increased dramatically two decades ago with the discoveries that it regulates cell growth1 and is formed and signals in response to external stimuli2. Since then, there has been an explosion of reports on the importance of S1P in physiology and pathophysiology. We now realize why this simple lipid has such pleiotropic actions: S1P is not only an agonist of five specific G protein-coupled S1P receptors (S1PR1–5) that activate diverse downstream signalling pathways, it also has important intracellular (second messenger) actions.
S1P is formed by phosphorylation of sphingosine, a backbone component of all sphingolipids, in a reaction catalysed by two isoforms of sphingosine kinase, SPHK1 and SPHK2, which have distinct and overlapping functions3. SPHK1 is activated by numerous stimuli, including pro-inflammatory cytokines, and promotes the formation of S1P. S1P, in turn, can be exported by specific transporters to activate its own receptors in autocrine and/or paracrine manners (FIG. 1a). This process, known as S1P ‘inside-out signalling’4, is important for many of the immune cell functions known to be regulated by S1P, and the diversity of these functions is explained by the repertoire of S1PR expression on various immune cells.
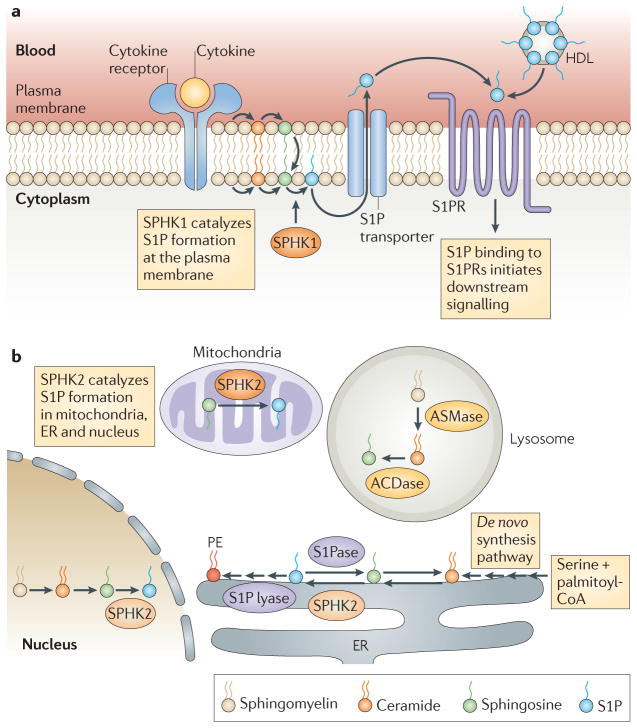
Figure 1. A simplified scheme of S1P synthesis and metabolism and inside-out signalling.
Sphingosine-1-phosphate (S1P) is synthesized by phosphorylation of sphingosine in a reaction that is catalysed by sphingosine kinase 1 (SPHK1) at the plasma membrane (a) and by SPHK2 at the endoplasmic reticulum (ER), mitochondria and nucleus (b). At the ER, S1P is irreversibly degraded by S1P lyase or dephosphorylated to sphingosine by an S1P phosphatase (S1Pase). S1P produced at the plasma membrane in response to stimuli is released by specific transporters and regulates immune functions by binding to specific S1P receptors (S1PRs) and initiating downstream signalling pathways (inside-out signalling). S1P produced in the mitochondria and nucleus by SPHK2 has direct intracellular targets, and S1P generated by SPHK1 at the plasma membrane can also function intracellularly. In the blood, S1P is produced mainly by erythrocytes, is bound to albumin and high-density lipoprotein (HDL) and can activate S1PRs. ACDase, acid ceramidase; ASMase, acid sphingomyelinase.
As with any signalling molecule, S1P levels are tightly controlled by its rapid degradation. S1P can be dephosphorylated by specific and nonspecific phosphatases back to sphingosine, which can then be reused for ceramide and sphingolipid biosynthesis. Alternatively, S1P can be irreversibly cleaved by S1P lyase in the final degradative step of sphingolipid metabolism (FIG. 1b). Constitutive levels of S1P in most tissues are low, and this is probably due to the activity of S1P lyase5. Erythrocytes and platelets are exceptions, as they do not have S1P lyase or S1P phosphatase activity and they produce and contain high levels of S1P. Erythrocytes are the main source of the high levels of S1P in plasma6, where S1P circulates bound to albumin and high density lipoproteins, whereas in the lymph, high levels of S1P are produced by the lymphatic endothelium7. Interestingly, in vivo studies in knockout mice indicate a homeostatic role for SPHK1 and SPHK2 in supplying blood and lymph with large amounts of S1P6,7. This sharp gradient between the levels of S1P in the circulation and those in the tissues and the tightly regulated spatial and temporal expression of S1PRs are crucial for the trafficking of immune cells. As this has been the subject of several comprehensive reviews8–11, we focus in this Review on the recent advances in this area. In addition, we outline other new developments, such as the recently identified intracellular targets of S1P, that have improved our understanding of the roles of S1P receptors in immune responses and in disorders of the immune system. Finally, we describe new therapeutics designed to alter S1P signalling and function in immunity.
S1P receptors in immune cell trafficking
S1P and its receptor S1PR1 have long been known to be crucial regulators of cell movement12. It is now recognized that in both homeostatic and disease settings, the S1P–S1PR1 axis controls the trafficking and migration of numerous types of immune cells, including T and B lymphocytes, natural killer T (NKT) cells, dendritic cells (DCs), macrophages, neutrophils, haematopoietic progenitors, mast cells and osteoclasts8,13–19. Some recent studies using pharmacological and genetic approaches combined with sophisticated intravital staining have increased our understanding of precisely how S1PR1 regulates the egress of newly formed T cells from the thymus and the exit of mature T cells and B cells from secondary lymphoid organs13,20–22.
Regulation of thymocyte egress
After entering the thymus, early thymic progenitor cells (TPCs) differentiate into T cell receptor (TCR)-expressing CD4+CD8+ double-positive thymocytes in the cortex and then mature into single-positive thymocytes in the medulla. As explained below, expression of CD69 on immature thymocytes prevents their expression of S1PR1, and this ensures proper selection and maturation13. After surviving negative selection, immature thymocytes upregulate expression of the transcription factor Krüppel-like factor 2 (KLF2) and its target gene S1pr1 (REF. 23). This enables naive T cells to exit the thymus in response to S1P produced by neural crest-derived perivascular cells that surround the thymus. The T cells subsequently encounter the endothelium13 and respond to the high levels of S1P in the blood6 (FIG. 2a).
Figure 2. Regulation of T cell egress by S1PR1.
When mature single-positive (SP) thymocytes are ready to exit the thymus (a), they upregulate Krüppel-like factor 2 (KLF2) and its target sphingosine-1-phosphate receptor 1 (S1PR1). The re-expression of S1PR1 enables thymocytes to exit the thymus in response to S1P locally supplied by perivascular cells before encountering the endothelium and then the high levels of S1P in blood vessels. S1PR1 on circulating T cells is internalized owing to high levels of S1P and reappears when the T cells enter non-inflamed lymphoid tissues (b) that contain low levels of S1P. However, in inflamed lymphoid tissues (c), CD69 is expressed on lymphocytes and causes internalization and degradation of S1PR1 to delay exit. After undergoing several rounds of division, the newly generated effector T cells upregulate S1PR1, lose CC-chemokine receptor 7 (CCR7) expression and exit into the circulation. TCR, T cell receptor.
It was proposed that the microenvironment in perivascular channels protects S1P from degradation. The study by Zachariah and Cyster13 explained why plasma S1P appeared to be insufficient to promote thymic egress6 and demonstrated that mature T cells exit the thymus at the corticomedullary junction via blood vessels rather than lymphatic vessels. It is still not clear how the local gradient of S1P is maintained and regulated, and it is also uncertain whether production of S1P is a general property of pericytes and occurs in other vascular beds. Answers to these questions have important clinical implications, as changes in S1P concentrations at tissue-specific sites and in the blood have been noted in many diseases, such as asthma, rheumatoid arthritis and coronary artery disease. Hopefully, the development of more sensitive mass spectrometry methods to measure S1P will make it possible to measure changes in the levels of S1P in different microenvironments. Surprisingly, however, it has been suggested that S1P produced in the blood might be a major source of S1P in lymphoid tissues24.
The thymus must import TPCs from the blood to sustain T cell production. Importation occurs in waves over several weeks and is thought to be a gated process. A recent study showed that the adhesion molecule P-selectin and the CC-chemokine receptor 9 (CCR9) ligand CC-chemokine ligand 25 (CCL25) are periodically expressed by the thymic endothelium, correlating with the periodic filling and emptying of thymic niches, and are essential for the gate-keeping mechanism25. This study also identified S1P in blood as another feedback signal that mediates the effects of the peripheral lymphocyte pool size on TPC receptivity. Changes in S1P levels in the blood seemed to positively correlate with changes in thymic P-selectin expression, and disruption of S1PR1 function or the S1P gradient between the thymus and the blood resulted in reduced P-selectin expression and inhibited the entry of TPCs25. This study raises several perplexing questions. How does peripheral lymphocyte depletion cause an increase in plasma S1P? How can small changes in the high circulating levels of S1P in mouse plasma lead to such profound changes in thymic endothelium P-selectin expression? Could the known effects of S1P on the regulation of vascular permeability have a role in this process?
Regulation of mature T cell trafficking
After entering the circulation, lymphocytes internalize S1PR1 in response to high S1P levels in the blood, but they then re-express S1PR1 during transit through non-inflamed secondary lymphoid organs8 (FIG. 2b). Indeed, surface expression of S1PR1 is a primary determinant of lymphocyte egress kinetics26. Competing chemotactic signalling between the egress-promoting S1P–S1PR1 axis and the retention signals delivered by the CCL21–CCR7 chemokine receptor axis determines lymphocyte transit time27.
A multistep model of lymph node egress was proposed by Cyster and colleagues20,28 in which cortical sinus ‘probing’ by T cells is followed by S1PR1-dependent entry of cells into the sinus. The cells then flow into medullary sinuses and the efferent lymph, which contains high levels of S1P produced by lymphatic endothelial cells7. Following exposure to inflammatory mediators, lymphocyte egress from lymphoid organs is transiently shut down to intensify the local immune response. Thus, in an inflamed lymphoid organ, exposure to type I interferons (IFNs) increases lymphocyte expression of the activation antigen CD69, which binds to S1PR1 and induces its internalization and degradation29 (FIG. 2c). Shortly after the activation of TCRs, the expression of S1PR1 decreases. However, once antigen-engaged T cells undergo 3–4 cell divisions, the newly generated effector cells upregulate S1PR1 and are no longer retained in the lymphoid organ by CCR7-mediated signalling, enabling them to rapidly exit into the circulation and travel to sites of inflammation27 (FIG. 2c). The S1P–S1PR1 axis also has a regulatory role in the egress of mature T cells from the bone marrow30.
Control of marginal zone B cell positioning
In addition to regulating systemic lymphocyte circulation, the S1P–S1PR1 axis is also important for the positioning of marginal zone B cells in the spleen10,14. These are a distinct lineage of B cells that capture blood-borne antigens and deliver them to follicular DCs, which then process and present these antigens to patrolling lymphocytes31. It has been proposed that exposure to high concentrations of S1P in the marginal zone rapidly downregulates the expression of S1PR1 and S1PR3 on marginal zone B cells and allows them to migrate in a CXC-chemokine receptor 5 (CXCR5)-dependent manner towards CXC-chemokine ligand 13 (CXCL13), which is produced by follicular DCs14. The low levels of S1P in the follicles permit re-expression of S1PR1 and S1PR3, enabling the B cells to return to the marginal zone. This proposed cyclical shuttling of B cells between the marginal zone and the follicle seems to provide an efficient mechanism that explains how antigens from the blood can reach the follicles in the spleen. However, more experiments are needed to fully delineate the molecular basis of marginal zone B cell shuttling.
In contrast to the confirmed link between KLF2 and S1PR1 expression and function in T cells23, such a link in B cells is still tenuous. Several recent studies have demonstrated that KLF2 has a crucial role in subset differentiation and function of mature B cells32–34. One study reported that KLF2 deficiency causes S1pr1 and Cxcr5 gene expression to be elevated in follicular B cells and reduced in marginal zone B cells32, allowing follicular B cells to encroach on the marginal zone and respond to marginal zone-associated antigens. By contrast, other investigations have provided more convincing evidence that KLF2 is not required to maintain surface expression of S1PR1 on B cells33,34. The differences in these studies may be partially due to the lack of S1PR1-specific antibodies to quantify its surface expression. Further studies are also needed to clarify why the immune system uses KLF2-dependent homing mechanisms that seem to hinder the clearance of blood-borne pathogens.
Control of mature B cell emigration
Similarly to T cell egress, the egress of B cells from lymphoid tissue via medullary sinuses and efferent lymphatics is dependent on S1PR1 (REF. 22), and immature B cells also require S1PR1 for efficient egress into the bone marrow vascular compartment and peripheral blood21,35. Furthermore, expression of CD69 on bone marrow B cells negatively regulates S1PR1 expression and inhibits the entry of these cells into the blood21. However, chemotaxis of B cells towards S1P is independent of S1PR1 and is largely mediated by S1PR3, despite the fact that B cell emigration from the bone marrow and transit through lymph nodes are normal in S1PR3-deficient mice21,22. Nevertheless, S1PR3 is required for normal B cell development and is involved in positioning transitional B cells within bone marrow sinusoids during tolerance induction and maturation36. Therefore, it seems unlikely that directed chemotaxis to higher concentrations of S1P in blood and lymph explains why B cell egress is dependent on S1PR1. Rather, it is tempting to speculate that S1PR1 plays a crucial role in the reverse transmigration of lymphocytes by regulating the small GTPase RAC37 and the potent actin nucleation factor DIAPH1 (protein diaphanous homologue 1; also known as mDIA1)38, which are required for the reorganization of the actin cytoskeleton. In agreement with this role for RAC as a downstream effector of S1PR1-mediated migration39, it was recently shown that RAC is required for T cell transmigration through the endothelium and for S1P-induced lymph node egress37.
S1PRs and control of DC migration
Both S1PR1 and S1PR3 have been implicated in the migration of DCs. The intrasplenic positioning of immature DCs to marginal zone bridging channels seems to depend on S1PR1 but not S1PR3 (REF. 16). By contrast, relocation of activated DCs to the T cell zone is independent of S1PR1 and S1PR3.
After sensing pathogens through Toll-like receptors (TLRs), DCs mature and upregulate MHC and co-stimulatory molecules, increase production of inflammatory cytokines and remodel their chemokine and S1P receptor profiles. In immature DCs, expression of CCR7 is low and CCL19 is unable to activate RAC, which is required for their migration (FIG. 3a). Moreover, signalling through S1PR2 leads to RHO activation (which is known to inhibit migration) and also causes translocation of the transcriptional co-activator FHL2 (four-and-a-half LIM domains protein 2) to the nucleus, where it represses the transcription of S1pr1 to further decrease the migratory ability of the immature DC17. Maturation of DCs results in upregulation of CCR7 and downregulation of S1PR2, thereby decreasing RHO activation and the nuclear translocation of FHL2. This leads to increased S1P signalling via S1PR1, which promotes RAC activation and DC migration17 (FIG. 3b).
Figure 3. FHL2-mediated repression of S1pr1 expression and regulation of DC migration.
Ligation of sphingosine-1-phosphate receptor 2 (S1PR2) on immature dendritic cells (DCs) (a) activates the small GTPase RHO, leading to the translocation of four and a half LIM domains protein 2 (FHL2) to the nucleus, where it represses S1pr1 expression. In addition, immature DCs express only low levels of CC-chemokine receptor 7 (CCR7) and this ensures that RAC activation is limited. Such repression of S1PR1 and RAC ensures that migratory responses of immature DCs are restricted. During maturation (b), DCs upregulate CCR7 and downregulate S1PR2; this results in increasedS1pr1 expression and RAC activation, leading to enhanced migration of mature DCs. CCL19, CC-chemokine ligand 19.
Regulatory roles of S1PRs in innate leukocyte migration
Although S1PR2 inhibits the migratory responses induced by S1PR1 and S1PR3 in a variety of cell types40, S1PR4 and S1PR5 have recently been shown to have limited and specialized roles in immune cell trafficking. Egress of NKT cells from lymph nodes and bone marrow requires S1PR5 (REF. 41), and NKT cell recruitment to inflamed lymphoid organs was shown to be aberrant in S1PR5-deficient mice41. The transcription factor T-bet regulates the expression of S1PR5 in NK cells15 but, unlike S1PR1, S1PR5 is not inhibited by CD69, and this property may facilitate trafficking of activated NKT cells to inflamed sites15.
It is well established that disruption of the S1P gradient by inhibition or deletion of S1P lyase causes a remarkable increase in S1P levels in lymphoid tissues and prevents lymphocyte egress5,42. S1P lyase-deficient mice also have elevated levels of pro-inflammatory cytokines and impaired neutrophil trafficking43. Deletion of S1pr4 partially decreases neutrophilia and inflammation in S1P lyase-deficient mice, implicating S1PR4 in neutrophil migration from blood into tissues. It was also suggested that the reduced entry of neutrophils into tissues in these mice is caused by decreased expression of adhesion molecules on the neutrophils, thereby disrupting the IL-23–IL-17–G-CSF (granulocyte colony-stimulating factor) cytokine-controlled loop that regulates granulopoiesis43.
Inside-out signalling by S1P in inflammation
In addition to their functions in controlling leukocyte migration, S1P and its receptors have important roles in regulating allergic responses, lymphocyte differentiation (reviewed in REF. 11), endothelial barrier integrity44–46 and cytokine and adhesion molecule expression (reviewed in REFS 47–49). Many of these functions depend on inside-out signalling by S1P, as discussed in the following section.
Regulation of mast cell function
Crosslinking of high-affinity IgE receptors on mast cells upregulates SPHK1 (and probably SPHK2), leading to S1P production18,50–52. S1P is then secreted by ATP-binding cassette (ABC) transporters53 or possibly by the newly identified S1P transporter spinster homologue 2 (SPNS2)54,55. S1P in turn can activate its own receptors in an autocrine and/or paracrine manner, promoting mast cell activation and degranulation. This inside-out signalling by S1P has important implications for the many functions of S1P and emphasizes the complexity of its actions in inflammation. Accordingly, inhalation of SPHK inhibitors improved disease severity in a mouse model of asthma56.
Although engagement of S1PR1 promotes the migration of mast cells, activation of S1PR2 halts their migration and contributes to mast cell degranulation18 and secretion of chemokines and cytokines57,58. Interestingly, functional variants of the S1PR1 gene were recently associated with asthma susceptibility and severity59. Consistent with a crucial role for mast cells in the initiation of anaphylaxis, IgE-mediated anaphylactic responses in mice were substantially attenuated by an S1PR2 antagonist and in S1PR2-deficient mice. By contrast, blocking the functions of S1PR2 had no effect in models of anaphylaxis induced by the administration of histamine or platelet-activating factor58, which are major mediators of anaphylaxis in mice and are released by activated mast cells.
Control of inflammation-induced vascular permeability
Anaphylactic shock, unlike other mast cell-dependent allergic responses, affects the function of multiple organ systems, including cutaneous, vascular and pulmonary systems. A recent study showed that S1P is not only involved in the mast cell-dependent onset of anaphylaxis, but also in the recovery from anaphylaxis in a mast cell-independent manner60. Histamine released from mast cells stimulates SPHK1 and enhances S1P production by both haematopoietic and non-haematopoietic sources, and this is crucial for the clearance of histamine60. The SPHK1–S1PR2 axis participates in this counter-regulatory feedback loop by controlling the drop in blood pressure that is characteristic of allergen-induced anaphylaxis. Surprisingly, in this non-lethal model of histamine-induced anaphylaxis there was only a modest involvement of S1PR1 and vascular permeability60.
However, an elegant study by Coughlin and colleagues that used mice engineered to lack circulating S1P and a more severe model of anaphylaxis provided convincing evidence that plasma S1P maintains vascular integrity by activating S1PR1, probably on endothelial cells46. Two models were proposed, which are not mutually exclusive, to explain how plasma S1P might communicate with endothelial cells. Either S1P continuously activates luminal endothelial S1PR1 to maintain tight cell–cell junctions or, following entry of S1P into the subendothelial space via ‘leaky’ endothelium, dynamic S1PR1 signalling activates abluminal surface S1PRs to close intercellular gaps46. S1P ligation of S1PR1 on endothelial cells induces RAC-dependent cytoskeletal rearrangements and promotes adherens junction assembly to enhance barrier integrity45 (FIG. 4). By contrast, S1PR2 and S1PR3 promote vascular permeability through activation of the RHO pathway and its downstream effectors RHO-associated protein kinase (ROCK) and phosphatase and tensin homologue (PTEN)48,61 (FIG. 4).
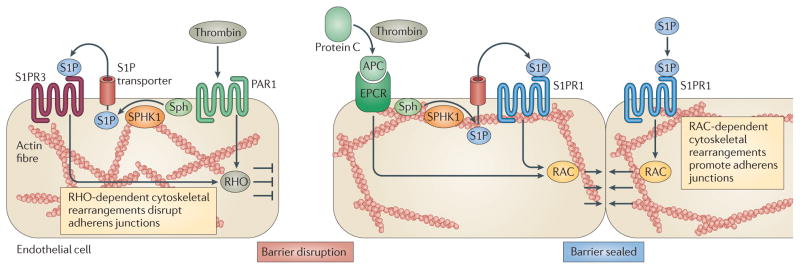
Figure 4. The dual role of PAR1 and inside-out signalling by S1P in the regulation of endothelial barrier function.
Under physiological conditions, plasma sphingosine-1-phosphate (S1P) is produced and released by erythrocytes to maintain barrier integrity. Protease-activated receptor 1 (PAR1) activation by thrombin (the levels of which are elevated during pathology) increases endothelial permeability; this process may also be dependent on sphingosine kinase 1 (SPHK1) and S1P receptor 3 (S1PR3)61. Permeability is gradually counteracted by thrombin-mediated cleavage of protein C to activated protein C (APC), which then binds to the endothelial protein C receptor (EPCR), stimulating SPHK1 expression and signalling through the S1P–S1PR1 axis that reseals the endothelial cell barrier. S1PR1 or S1PR3 induce RAC- or RHO-dependent cytoskeletal rearrangements to promote or disrupt adherens junctions, respectively. Sph, sphingosine.
During severe inflammatory responses, the coagulation protease thrombin increases vascular permeability by proteolytic cleavage and activation of protease- activated receptor 1 (PAR1) present on the endothelial cell surface. Thrombin–PAR1 signalling is disruptive to the endothelial barrier and recent studies suggest that this barrier disruption may also involve activation of SPHK1 and inside-out signalling through S1PR3 (REF. 61). The initial increase in vascular permeability induced by PAR1 is gradually counteracted by activated protein C, which is produced by thrombin-mediated cleavage of protein C. Binding of activated protein C to its receptor stimulates SPHK1 and S1P inside-out signalling through S1PR1 that reseals the endothelial cell barrier61,62.
Similarly, it has been suggested that upregulation of SPHK1, formation of S1P and the subsequent activation of S1PR1 is a negative feedback mechanism that limits the increase in endothelial permeability induced by lipopolysaccharide (LPS)62,63. By contrast, LPS-induced barrier disruption is mediated, at least in part, by ligation and activation of S1PR2 and S1PR3 (REF. 63). Thus, the concept is emerging that tonic signalling of endothelial cell S1PR1 maintains the homeostatic barrier property of the vascular system and, during infection and inflammation, the SPHK1–S1P–S1PR1 axis is involved in the restoration of normal vascular barrier integrity (FIG. 4). Understanding these barrier control mechanisms is important, as abnormal vascular permeability and increased fluid retention in the lung are serious complications in infectious diseases.
Regulation of TNFR signalling by intracellular S1P
S1P and SPHK1 have long been implicated in the actions of certain cytokines, such as tumour necrosis factor (TNF)64, a pleiotropic cytokine with important effects in a wide range of autoimmune disorders from rheumatoid arthritis and inflammatory bowel disease to asthma. Gene targeting of Sphk1 in animal models of these disorders has provided further support for this notion65–67. In response to TNF and other cytokines, SPHK1 is activated and translocates to the plasma membrane to catalyse the production of S1P68, which is then exported out of the cell by specific transporters to activate its receptors in an autocrine manner69. This inside-out signalling by S1P is thought to promote certain TNF functions (FIG. 5), including activation of nitric oxide synthase in endothelial cells70 and enhancement of microvascular tone and reduction of blood flow in the cochlea71. Nevertheless, it has long been thought that S1P that is generated intra-cellularly in response to TNF might also function inside the cell independently of S1PRs72,73. Recently, it was discovered that S1P can activate the key inflammatory transcription factor nuclear factor-κB (NF-κB)74, and this was found to be independent of S1PRs75.
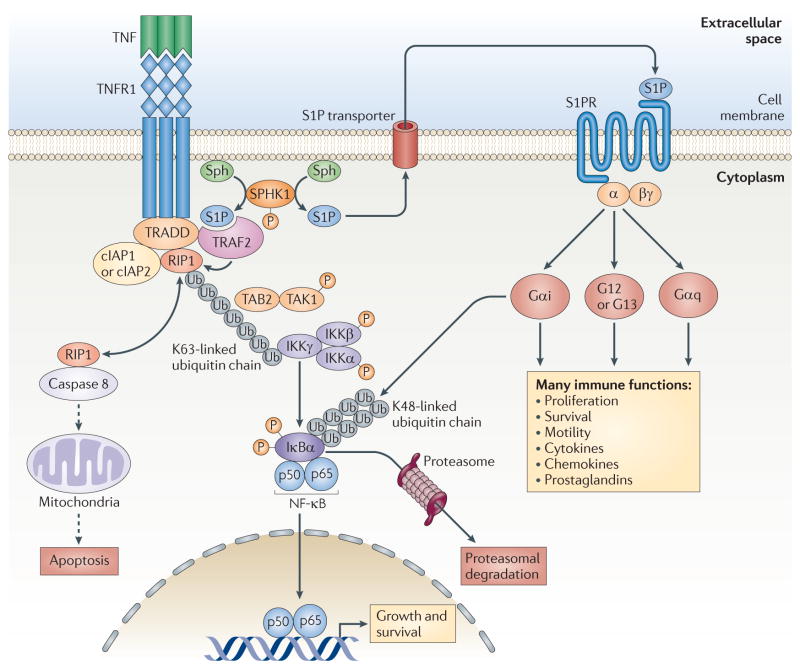
Figure 5. Roles of S1P produced by SPHK1 in TNFR signalling.
Engagement of TNF receptor 1 (TNFR1) by tumour necrosis factor (TNF) leads to the recruitment of a signalling complex containing TNFR1-associated death domain protein (TRADD), TNFR-associated factor 2 (TRAF2), receptor-interacting protein 1 (RIP1; also known as RIPK1), cellular inhibitor of apoptosis 1 (cIAP1) and cIAP2. Interaction of TRAF2 with sphingosine kinase 1 (SPHK1) stimulates it and brings it to the plasma membrane where its substrate, sphingosine, resides. Intracellular sphingosine-1-phosphate (S1P) is a required cofactor for the K63-linked polyubiquitylation of RIP1 by TRAF2, and the ubiquitin chain then acts as a scaffold to recruit and activate TGFβ-activated kinase 1 (TAK1; also known as MAP3K7) and IκB kinase (IKK) complexes. The IKK complex phosphorylates NF-κB inhibitor-α (IκBα), leading to the activation of nuclear factor-κB (NF-κB). Polyubiquitylation of RIP1 also prevents its interaction with pro-caspase 8, and this limits the processing of this caspase precursor and thus the initiation of apoptosis. Intracellular S1P can be exported out of cells and can then activate its cell surface receptors and downstream signalling pathways, culminating in proliferation, survival, motility and the production of cytokines, chemokines and prostaglandins. S1PR, S1P receptor; Sph, sphingosine; TAB2, TAK1-binding protein 2; Ub, ubiquitin.
Engagement of TNF receptor 1 (TNFR1) results in the assembly of multicomponent signalling complexes by adaptor proteins, including TNFR-associated factor 2 (TRAF2), leading to K63-linked polyubiquitylation (in which ubiquitin molecules are linked through lysine 63) of receptor-interacting protein 1 (RIP1; also known as RIPK1). The polyubiquitin scaffold then recruits and activates both TGFβ-activated kinase 1 (TAK1; also known as MAP3K7) and the IκB kinase (IKK) complex (which is composed of two kinase subunits, IKKα and IKKβ) by binding to their regulatory subunits — TAB2 and IKKγ (also known as NEMO), respectively. Phosphorylation of NF-κB inhibitor-α (IκBα) by the IKK complex leads to its K48-linked polyubiquitylation and subsequent proteasomal degradation. This liberates NF-κB (a dimeric transcription factor consisting of p65 and p50 subunits), which then enters the nucleus and regulates the transcription of its target genes76 (FIG. 5).
It had previously been demonstrated that the interaction of SPHK1 with TRAF2 activates SPHK1, which then participates in TNF signalling that leads to the activation of NF-κB73. However, the mechanism for this involvement of SPHK1 in TRAF2-mediated activation of the canonical NF-κB pathway had not been elucidated. Although genetic evidence indicated that TRAF2 is necessary for the polyubiquitylation of RIP1 (REF. 77), many studies failed to detect ubiquitin ligase activity of purified or recombinant TRAF2 (REF. 78), leading to the notion that TRAF2 was an adaptor protein for other E3 ubiquitin ligases79 rather than an E3 ubiquitin ligase itself. This conundrum was recently resolved by the discovery that S1P is a cofactor for TRAF2 that is necessary for TRAF2-mediated ubiquitylation of RIP1 (REF. 75). In the presence of S1P, TRAF2 was found to efficiently catalyse K63-linked polyubiquitylation of RIP1, providing the first evidence that TRAF2 has E3 ubiquitin ligase activity. This cofactor function of S1P is very specific to this molecule, as other structurally related lipids — including dihydro-S1P, which only lacks the double bond of S1P and is a ligand for all of the S1PRs — do not mimic its actions. It was also shown that S1P enhances the addition of both wild-type and K63-only ubiquitin (mutated ubiquitin with only one lysine residue, at position 63), but not K48-only ubiquitin, to RIP1. This finding is especially relevant because unlike K48-linked polyubiquitylation, which targets proteins for proteasomal degradation, K63-linked polyubiquitylation of RIP1 serves as a scaffold to recruit proteins containing specific ubiquitin-binding domains, and this leads to the activation of NF-κB76,80,81.
These results suggest a new paradigm linking SPHK1 and S1P to K63-linked polyubiquitylation of RIP1 and activation of NF-κB. Thus, in response to TNF, recruitment of TRAF2 to TNFR1 at the plasma membrane activates its binding partner SPHK1 (REF. 73), which produces S1P. S1P in turn is an essential cofactor for TRAF2-mediated RIP1 K63-linked polyubiquitylation, which is necessary for NF-κB activation and the anti-apoptotic programme initiated by TNF76. This suggests a feed-forward type of interdependent regulation in which TRAF2 and SPHK1 require and/or regulate the actions of each other (FIG. 5). Polyubiquitylation of RIP1 also prevents its interaction with pro-caspase 8, and this limits the processing of pro-caspase 8 to its effector form that initiates apoptosis (FIG. 5). This provides a mechanism for the numerous observations of the importance of SPHK1 in inflammatory, anti-apoptotic and immune responses and explains why only S1P suppresses apoptosis, even though dihydro-S1P is also a ligand for the S1PRs, as only S1P binds to and activates TRAF2.
It is still not clear how TNF stimulates SPHK1 through TRAF2. It has been suggested that phosphorylation of SPHK1 by ERK1 and ERK2 might be involved68. However, TNF only weakly activates ERK1 and ERK2, and TRAF2 itself is required for TNF-mediated induction of ERK1 and ERK2 (REF. 82). Phosphorylation of TRAF2 by protein kinase Cδ (PKCδ) mediates recruitment of the IKK complex and determines TRAF2-mediated K63-linked polyubiquitylation78. As PKCδ has also been shown to activate and phosphorylate SPHK1, leading to its translocation to the plasma membrane83, we speculate that PKCδ may be the potential link between TNFR1 and SPHK1 activation.
Many other questions remain unanswered. For example, what is the mechanism by which S1P affects the E3 ubiquitin ligase activity of TRAF2? Does it involve the recruitment of specific E2 ubiquitin-conjugating enzymes? Is S1P required for signalling downstream of other receptors that use TRAF2? Does S1P bind to and regulate the E3 ubiquitin ligase activity of other TRAFs or other RING domain-containing E3 ubiquitin ligases? Just answering the last question may be a daunting task, as bioinformatics analyses indicate that the human genome encodes 300 RING domain proteins and at least half possess intrinsic E3 ubiquitin ligase activity84. Although much more needs to be learnt about the in vivo significance of SPHK1 in TNF-mediated signalling, a recent study demonstrated that deletion of Sphk1 in a mouse model of TNF-induced arthritis markedly decreases synovial inflammation and joint erosions and diminishes cyclooxygenase 2 expression67.
The plot thickens: S1P and TLR4 in sepsis
Sepsis is an overwhelming systemic inflammatory response that results from bacterial infections and frequently leads to death. Cells of the innate immune system (primarily monocytes, macrophages and DCs) sense bacterial components through members of the TLR family, which depend on TRAFs to control NF-κB activation and inflammatory and immune responses, in a similar manner to TNFR1.
Both TLR2 and TLR4 — which recognize bacterial lipopeptides and LPS from Gram-negative bacteria, respectively — stimulate and upregulate SPHK1 (REFS 85,86). There is some evidence to support a role for inside-out signalling by S1P in their inflammatory responses. For example, it has been suggested that, in macrophages, the binding of S1P to S1PR3 or S1PR2 contributes respectively to endotoxin-induced production of CCL2 (also known as MCP1)87 and the production of the pro-inflammatory cytokines IL-1β and IL-18 (REF. 88). However, the role of S1PR1 in this system is still controversial87,89. In addition, LPS-induced production of IL-6 and IL-8 by human gingival epithelial cells was shown to be mediated by increased expression and activation of S1PR1 and S1PR3 (REF. 90). Furthermore, as discussed below, there are some indications that intracellular S1P generated by SPHK1 might also contribute to inflammatory responses to endotoxins.
Much has been learnt about the complex signalling pathways downstream of TLRs91. Briefly, the engagement of TLR4 and TLR2 causes the recruitment of receptor-specific adaptors, including TIR domain-containing adaptor protein (TIRAP) and myeloid differentiation primary response protein 88 (MYD88). MYD88 recruits TRAF6 and members of the IL-1R-associated kinase (IRAK) family, leading to the oligomerization and auto-ubiquitylation of TRAF6, which then recruits and activates TAK1. Activated TAK1 phosphorylates and activates the IKK complex, leading to the activation of NF-κB, p38 and JUN N-terminal kinase (JNK), and this culminates in pro-inflammatory cytokine production. TIR domain-containing adaptor protein inducing IFNβ (TRIF), which is only recruited to TLR4, interacts with TRAF3, TANK-binding kinase 1 (TBK1) and IKKε, which mediate the phosphorylation of IFN-regulatory factor 3 (IRF3). Phosphorylated IRF3 dimerizes and translocates to the nucleus, where it induces an IFN response (FIG. 6).
Figure 6. Roles of S1P produced by SPHK1 in TLR4 signalling.
Engagement of Toll-like receptor 4 (TLR4) by lipopolysaccharide (LPS) at the plasma membrane promotes the recruitment of the adaptor myeloid differentiation primary response protein 88 (MYD88) and the assembly of a signalling complex that includes another adaptor called TIR domain-containing adaptor protein (TIRAP), TNFR-associated factor 6 (TRAF6) and the protein kinase TGFβ-activated kinase 1 (TAK1; also known as MAP3K7).
Sphingosine-1-phosphate (S1P) generation by sphingosine kinase 1 (SPHK1) mediates the activation of protein kinase Cδ (PKCδ), which phosphorylates an unknown target to promote IκB kinase (IKK) and nuclear factor-κB (NF-κB) activation through an ill-defined mechanism. It is also possible that S1P enhances the autoubiquitylation of TRAF6, which then recruits and activates TAK1. TAK1 phosphorylates the IKK complex, leading to the activation of NF-κB and mitogen-activated protein kinases (MAPKs; namely, extracellular signal-regulated kinase (ERK), JUN N-terminal kinase (JNK) and p38). Dashed arrows indicate pathways in TLR4 signalling that could potentially involve S1P. AP1, activator protein 1; IκBα, NF-κB inhibitor-α; IRAK, IL-1R-associated kinase; IRF3, interferon-regulatory factor 3; MD2, myeloid differentiation factor 2 (also known as LY96); RIP1, receptor-interacting protein 1 (also known as RIPK1); Sph, sphingosine; TAB, TAK1-binding protein; TBK1, TANK-binding kinase 1; TRAM, TRIF-related adaptor molecule; TRIF, TIR domain-containing adaptor protein inducing IFNβ; Ub, ubiquitin.
Recently, two elegant studies with important clinical implications have enhanced our understanding of the participation of SPHK1 in the signalling pathways downstream of TLR2 and TLR4, and have provided compelling evidence for the involvement of S1P in the development of septic shock. The studies demonstrated that deletion or inhibition of SPHK1 prevents sepsis in mouse models of LPS challenge or caecal ligation and puncture49,86. However, although the conclusions of these two studies both highlighted the essential role of SPHK1 and S1P in sepsis, the proposed mechanisms diverged.
Ruf and colleagues49 suggested that DC activation induced by coagulation in the lymphatics, which promotes systemic inflammation and lethality during severe sepsis, depends on inside-out signalling by S1P via activation of S1PR3. They showed that the SPHK1–S1P–S1PR3 axis is a downstream component of PAR1 signalling in DCs and regulates late-phase amplification of inflammation during sepsis. On the other hand, Melendez and co-workers86 proposed a completely different mechanism for the involvement of SPHK1 and intracellular S1P in sepsis, focusing on the functions of macrophages. They showed that SPHK1 expression is upregulated in peritoneal macrophages isolated from patients with severe sepsis and that administration of a SPHK1 inhibitor to mice suppresses LPS-induced production of inflammatory cytokines86, in agreement with in vivo and in vitro studies of Sphk1 silencing86,92. However, Sphk1-null mice are not protected from LPS-induced death86,93; this may be due to adaptive functional redundancy that takes place during embryonic development owing to the essential functions of SPHK1.
Interestingly, TLR2 and TLR4 triggering enhances cytoplasmic production of S1P in phagocytes but does not increase its release, suggesting that intracellular rather than S1PR-mediated actions of S1P occur downstream of these TLRs86. An important observation is that PKCδ is associated with the IKK complex in LPS-treated macrophages and is required for NF-κB activation in response to LPS and bacterial lipoprotein. Intriguingly, it has been shown that SPHK1 is required for PKCδ activation by LPS and that S1P stimulates the enzymatic activity of recombinant PKCδ, suggesting that PKCδ is a direct intracellular target of S1P (FIG. 6).
It is still not clear how TLR4 signalling activates PKCδ or which particular targets PKCδ phosphorylates. Although it was suggested that PKCδ phosphorylates and activates the IKK complex86, this has not been confirmed. It is possible that PKCδ has a different substrate in the TLR4 signalling pathway and several intriguing candidates come to mind. TRAF6, a key E3 ubiquitin ligase that is involved in the activation of both the TLR2 and TLR4 signalling pathways, could be phosphorylated by PKCδ in a similar manner to that discussed above for TRAF2 (REF. 78). Alternatively, a PKCδ substrate yet to be identified could promote IKK and NF-κB activation through an unknown mechanism. Finally, PKCδ could phosphorylate and activate SPHK1, either directly83 or via activation of ERK1 and ERK2. If so, PKCδ would be upstream of SPHK1 and S1P formation. S1P might then enhance the E3 ubiquitin ligase activity of TRAF6, by analogy with its effect on TRAF2. Some support for this notion is provided by our own observations that S1P specifically binds to TRAF6, enhances its in vitro autoubiquitylation and interacts with its RING domain, although the affinity of this interaction is lower than that of S1P binding to the RING domain of TRAF2 (K. B. Harikumar, C. Luo, S.M. and S.S., unpublished observations).
Importantly, the administration of a specific SPHK1 inhibitor has been shown to protect mice against systemic inflammation and mortality induced by caecal ligation and puncture, even when the inhibitor is given 2 hours after induction of polymicrobial sepsis. As might be expected, the protective effect is lessened when the inhibitor is given at later time points86. However, combining a SPHK1 inhibitor with the broad spectrum antibiotic co-amoxiclav (which is currently used to treat patients with sepsis) markedly improves the efficacy of the antibiotic, extending the time window for treatment and resulting in better outcomes than either drug alone, although off-target effects of the SPHK1 inhibitor cannot be excluded. New treatments for sepsis are needed, as resistance to antibiotics is developing faster than new antibiotics become available, and these findings86 may pave the way for the exploration of SPHK1 inhibitors for the treatment of sepsis in humans.
Regulation of histone deacetylases by S1P
Compared with our understanding of the functions of SPHK1 in inflammation, much less is known about the role of SPHK2, although recent reports have begun to highlight the specific roles of this isoenzyme in immune cell activities24,51,52,94,95. Although many studies point to an important role for SPHK1 rather than SPHK2 in mast cell activation18, SPHK2 may also contribute to some mast cell functions51,52. Interestingly, downregulation of SPHK1 and SPHK2 in mice has opposing effects during collagen-induced arthritis, decreasing and increasing severity, respectively. Even before the development of the clinical symptoms of arthritis, depletion of SPHK2 enhances production of the pro-inflammatory cytokines IL-6, TNF and IFNγ 66. Similarly, T cells from SPHK2-deficient mice show enhanced proliferation and production of cytokines, and promote pathology in a model of inflammatory bowel disease94. Hence, it has been suggested that therapeutic enhancement of SPHK2 activity could be useful in chronic inflammatory diseases (including colitis) and, conversely, that SPHK2 inhibition could treat disorders associated with immunosuppression (such as chronic infections and cancers)94.
The increased expression of many pro-inflammatory genes that accompanies chronic inflammatory diseases is regulated by acetylation of core histones. Histone deacetylases (HDACs) remove acetyl groups from histones, thereby inducing chromatin condensation and transcriptional repression, and have emerged as key targets to reverse aberrant epigenetic changes associated with human diseases, such as cancer and inflammation96,97. HDACs can also deacetylate numerous non-histone proteins that regulate immune functions, including the transcription factors signal transducer and activator of transcription 1 (STAT1), STAT3, NF-κB and forkhead box P3 (FOXP3)98.
Although several HDAC inhibitors have been approved for the treatment of cancer, some HDAC inhibitors have important anti-inflammatory or immunosuppressive effects that might be of therapeutic benefit in inflammatory disorders, including rheumatoid arthritis, juvenile idiopathic arthritis and chronic obstructive pulmonary disease (COPD)97. For example, cell populations, inhibit HDAC inhibitors expand TReg TH17 cell differentiation and suppress the activation of macrophages and DCs98.
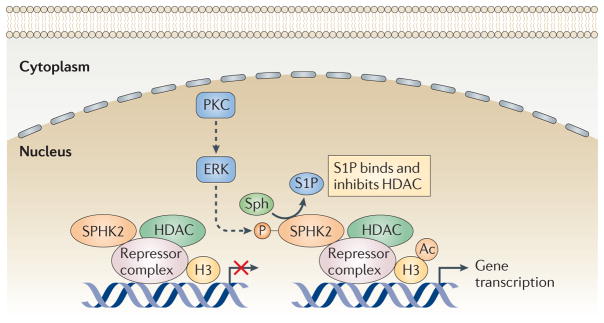
Despite the widespread interest in HDACs and their inhibitors, the factors that regulate HDAC activity remain largely unknown. Recent findings have revealed a connection between SPHK2, S1P and HDAC functions95 (FIG. 7). In contrast to SPHK1, SPHK2 is present in the nucleus of many types of cells where it has been shown to inhibit cell proliferation99, probably by upregulating expression of the cyclin-dependent kinase inhibitor p21 (encoded by CDKN1A) in a p53-independent manner100. Recent studies have shown that endogenous SPHK2 is associated with histone H3 and produces S1P that binds to and inhibits HDAC1 and HDAC2, which are classical class I HDACs that are primarily localized in the nucleus in multiprotein complexes with co-repressors. SPHK2 associates with HDAC1 and HDAC2 in repressor complexes and is selectively enriched at the promoters of CDKN1A and the transcriptional regulator FOS, where it enhances local acetylation of histone H3 and transcription95 (FIG. 7). Interestingly, phorbol 12-myristate 13-acetate (PMA), an activator of protein kinase C that enhances the phosphorylation and catalytic activity of SPHK2 (REF. 101), rapidly increases nuclear S1P and the colocalization of SPHK2 with HDAC1. However, thereafter, PMA induces the phosphorylation and nuclear export of SPHK2; this is probably mediated by protein kinase D102 and ensures that the inhibition of HDACs by S1P is only transient.
Figure 7. S1P produced by SPHK2 in the nucleus inhibits HDACs and regulates gene transcription.
Nuclear sphingosine kinase 2 (SPHK2) is associated with histone deacetylase 1 (HDAC1) and HDAC2 in repressor complexes at the promoters of specific genes (such as CDKN1A, which encodes p21). In response to external stimuli, protein kinase C (PKC) is activated (probably via extracellular signal-regulated kinase 1 (ERK1)) and phosphorylates and activates SPHK2, resulting in increased production of sphingosine-1-phosphate (S1P). S1P binds to and inhibits HDAC1 and HDAC2, leading to increased levels of histone acetylation and gene transcription. Dashed arrows indicate pathways that could potentially activate SPHK2. Ac, acetyl; H3, histone H3.
The finding that S1P and SPHK2 are part of a co-repressor complex that influences histone acetylation and gene expression95 suggests a fascinating scenario for sphingolipid signalling in the nucleus and for HDAC regulation. Future studies are needed to address several important questions. Does SPHK2, by regulating HDAC1 and HDAC2, control global or inflammation-specific programmes of gene expression? How is SPHK2 recruited to specific target genes? Does S1P bind to and regulate the deacetylase activity of other members of the HDAC family, in addition to HDAC1 and HDAC2? What are the inflammatory consequences of the inhibition? Deciphering the role of S1P in the regulation of histone acetylation is important, as HDAC inhibitors are a potential therapeutic approach for the treatment of chronic inflammation. A better understanding of how HDACs are regulated will boost the search for safer and more effective drugs that are capable of interfering with HDAC functions in a highly specific manner.
Therapeutic targeting of S1P signalling
Multiple sclerosis
Much attention has been focused recently on S1P and its receptors, especially S1PR1, following the elucidation of the mechanism of action of the immunosuppressive drug FTY720 (also known as fingolimod). This drug has been approved for the treatment of multiple sclerosis103, a common inflammatory disorder of the central nervous system. FTY720, a sphingosine analogue, is a prodrug that is phosphorylated in vivo by SPHK2 to generate a S1P mimetic that is a ligand for all of the S1PRs except S1PR2. However, in vivo, FTY720 has a complex mode of action. Phosphorylated FTY720 acts as a ‘functional antagonist’ of S1PR1 by inducing its prolonged internalization, downregulation and ubiquitin-dependent proteosomal degradation26,27. Surprisingly, it has been shown that signalling by phosphorylated FTY720 continues even after S1PR1 internalization, suggesting that persistent agonism contributes to the actions of FTY720 and might be important for enhanced endothelial barrier function104. The clinical efficacy of FTY720 has been attributed to its ability to promote the retention of naive T cells and central memory T cells (including autoreactive TH17 cells) in lymph nodes, preventing terminally differentiated effector T cells and effector memory T cells from entering the central nervous system and driving pathological responses105. On the other hand, FTY720 minimally affects peripheral effector memory T cells, which are important for protection against infection103.
FTY720 may also have direct neuroprotective effects within the central nervous system, as it is concentrated to high levels in the brain and its effectiveness in patients with multiple sclerosis does not correlate with the degree of lymphopaenia in these individuals. Indeed, a recent study demonstrated that the protective functions of FTY720 in experimental autoimmune encephalomyelitis (EAE), a mouse model for multiple sclerosis, depend on the expression of S1PR1 by astrocytes106. As S1P levels are increased in the spinal cords of mice during EAE106 and levels of S1P in cerebrospinal fluid correlate with increasing disability in patients with multiple sclerosis107, it is possible that targeting S1P generation might also be beneficial in neuroinflammatory disorders.
Rheumatoid arthritis
As discussed above, deletion of S1P lyase (the enzyme principally responsible for S1P degradation), or its inhibition by the food colourant 2-acetyl-4-tetrahydroxybutylimidazole, disrupts local S1P gradients and interferes with lymphocyte emigration5. LX2931, an oral S1P lyase inhibitor, has been developed as a potential therapeutic for autoimmune and inflammatory disorders108. LX2931 reduced joint inflammation and destruction in mouse and rat models of arthritis and is now in Phase II clinical trials for use in rheumatoid arthritis, a chronic systemic inflammatory disorder that severely affects synovial joints108.
In summary, these preclinical and clinical studies suggest that diverse pharmacological agents that target the functions of S1P and its receptors show therapeutic potential for treating a wide range of inflammatory and autoimmune disorders. Development of a second generation of drugs, with improved specificity and efficacy, will provide new treatment strategies for these inflammatory disorders and, moreover, will enhance our understanding of how this ‘simple’ sphingolipid metabolite functions both inside and outside of cells.
Acknowledgments
This work was supported by grants from the US National Institutes of Health (to S.S.).
Glossary
- S1P receptors
A family of five G protein-coupled receptors. The binding of sphingosine-1-phosphate (S1P) to these receptors activates heterotrimeric GTP-binding proteins, leading to the activation of downstream signalling
- Inside-out signalling
The process by which intracellular signalling mechanisms result in the activation of cell surface receptors. By contrast, outside-in signalling is the process by which ligation of a cell surface receptor activates signalling pathways inside the cell
- Transitional B cells
Transitional B cells are short-lived immature B cells that either die or are selected into the peripheral mature B cell repertoire. Transitional B cells can be subdivided into three subsets (T1, T2 and T3 cells) based on differential phenotypical and functional characteristics
- Reverse transmigration
Migration of cells across the endothelial basement membrane and, subsequently, across the endothelial barrier
- Marginal zone bridging channels
Structures in the spleen that are thought to allow the passage of lymphocytes from the red pulp to the white pulp
- Anaphylaxis
A severe whole body allergic reaction that is life threatening
- E3 ubiquitin ligases
Enzymes that attach the molecular tag ubiquitin to proteins. Depending on the number of ubiquitin molecules that are attached and the positioning of the links between them, the ubiquitin tag can target proteins for degradation by the proteasome, sort them to specific subcellular compartments or modify their biological activity
- Sepsis
A potentially serious medical condition that involves a whole-body inflammatory response to an infection
- FTY720
A sphingosine-like drug that is phosphorylated intracellularly by sphingosine kinase. Phosphorylated FTY720 is an agonist of all of the sphingo-sine-1-phosphate receptors (S1PRs) except S1PR2, but its immunosuppressive functions are due to prolonged downregulation and degradation of S1PR1
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Sarah Spiegel’s homepage: http://www.biochemistry.vcu.edu/directory/faculty/spiegel.html
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Zhang H, et al. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114:155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olivera A, Spiegel S. Sphingosine-1-phosphate as a second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. This study provided the first demonstration that S1P is formed and signals in response to external stimuli. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nature Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 5.Schwab SR, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 6.Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. This was the first demonstration that erythrocytes are the major source of plasma S1P. [DOI] [PubMed] [Google Scholar]
- 7.Pham TH, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nature Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 9.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nature Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira JP, Kelly LM, Cyster JG. Finding the right niche: B-cell migration in the early phases of T-dependent antibody responses. Int Immunol. 2010;22:413–419. doi: 10.1093/intimm/dxq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi H. Sphingosine-1-phosphate and immune regulation: trafficking and beyond. Trends Pharmacol Sci. 2011;32:16–24. doi: 10.1016/j.tips.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang F, et al. Sphingosine 1-phosphate stimulates cell migration through a Gi-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- 13.Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010;328:1129–1135. doi: 10.1126/science.1188222. This study identified the major thymic egress route and implicated pericytes in reverse transmigration of mature thymocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster J. G Follicular shuttling of marginal zone B cells facilitates antigen transport. Nature Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenne CN, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rathinasamy A, Czeloth N, Pabst O, Forster R, Bernhardt G. The origin and maturity of dendritic cells determine the pattern of sphingosine 1-phosphate receptors expressed and required for efficient migration. J Immunol. 2010;185:4072–4081. doi: 10.4049/jimmunol.1000568. [DOI] [PubMed] [Google Scholar]
- 17.Konig K, et al. Four-and-a-half LIM domain protein 2 is a novel regulator of sphingosine 1-phosphate receptor 1 in CCL19-induced dendritic cell migration. J Immunol. 2010;185:1466–1475. doi: 10.4049/jimmunol.0903449. [DOI] [PubMed] [Google Scholar]
- 18.Jolly PS, et al. Transactivation of sphingosine-1-phosphate receptors by FcεRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishii M, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grigorova IL, et al. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nature Immunol. 2009;10:58–65. doi: 10.1038/ni.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allende ML, et al. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J Exp Med. 2010;207:1113–1124. doi: 10.1084/jem.20092210. This study demonstrated that S1PR1 is required for transfer of newly generated immature B cells from the bone marrow to the blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha RK, Park C, Hwang IY, Davis MD, Kehrl JH. B lymphocytes exit lymph nodes through cortical lymphatic sinusoids by a mechanism independent of sphingosine-1-phosphate-mediated chemotaxis. Immunity. 2009;30:434–446. doi: 10.1016/j.immuni.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takada K, et al. Kruppel-like factor 2 is required for trafficking but not quiescence in postactivated T cells. J Immunol. 2011;186:775–783. doi: 10.4049/jimmunol.1000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sensken SC, et al. Redistribution of sphingosine 1-phosphate by sphingosine kinase 2 contributes to lymphopenia. J Immunol. 2010;184:4133–4142. doi: 10.4049/jimmunol.0903358. [DOI] [PubMed] [Google Scholar]
- 25.Gossens K, et al. Thymic progenitor homing and lymphocyte homeostasis are linked via S1P-controlled expression of thymic P-selectin/CCL25. J Exp Med. 2009;206:761–778. doi: 10.1084/jem.20082502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thangada S, et al. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J Exp Med. 2010;207:1475–1483. doi: 10.1084/jem.20091343. This study established that S1PR1 surface residency on T cells is the primary determinant of lymphocyte egress kinetics in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by Gαi-coupled receptors to promote T cell egress. Immunity. 2008;28:122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grigorova IL, Panteleev M, Cyster JG. Lymph node cortical sinus organization and relationship to lymphocyte egress dynamics and antigen exposure. Proc Natl Acad Sci USA. 2010;107:20447–20452. doi: 10.1073/pnas.1009968107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem. 2010;285:22328–22337. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda Y, Seki N, Sato N, Sugahara K, Chiba K. Sphingosine 1-phosphate receptor type 1 regulates egress of mature T cells from mouse bone marrow. Int Immunol. 2010;22:515–525. doi: 10.1093/intimm/dxq036. [DOI] [PubMed] [Google Scholar]
- 31.Cyster JG. B cell follicles and antigen encounters of the third kind. Nature Immunol. 2010;11:989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 32.Hoek KL, et al. Follicular B cell trafficking within the spleen actively restricts humoral immune responses. Immunity. 2010;33:254–265. doi: 10.1016/j.immuni.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hart GT, Wang X, Hogquist KA, Jameson SC. Kruppel-like factor 2 (KLF2) regulates B-cell reactivity, subset differentiation, and trafficking molecule expression. Proc Natl Acad Sci USA. 2011;108:716–721. doi: 10.1073/pnas.1013168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkelmann R, et al. B cell homeostasis and plasma cell homing controlled by Kruppel-like factor 2. Proc Natl Acad Sci USA. 2011;108:710–715. doi: 10.1073/pnas.1012858108. References 33 and 34 provided evidence that KLF2 is not required to maintain surface expression of S1PR1 on B cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira JP, Cyster JG, Xu Y. A role for S1P and S1P1 in immature-B cell egress from mouse bone marrow. PLoS ONE. 2010;5:e9277. doi: 10.1371/journal.pone.0009277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donovan EE, Pelanda R, Torres RM. S1P3 confers differential S1P-induced migration by autoreactive and non-autoreactive immature B cells and is required for normal B-cell development. Eur J Immunol. 2010;40:688–698. doi: 10.1002/eji.200939858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faroudi M, et al. Critical roles for Rac GTPases in T-cell migration to and within lymph nodes. Blood. 2010;116:5536–5547. doi: 10.1182/blood-2010-08-299438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakata D, et al. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med. 2007;204:2031–2038. doi: 10.1084/jem.20062647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. This was the first study on S1P receptor knockout mice and revealed the importance of S1PR1 in blood vessel formation and vascular maturation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang F, et al. Sphingosine-1-phosphate receptor-2 deficiency leads to inhibition of macrophage proinflammatory activities and atherosclerosis in apoE-deficient mice. J Clin Invest. 2010;120:3979–3995. doi: 10.1172/JCI42315. This study demonstrated an important role for S1PR2 in atherogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Walzer T, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nature Immunol. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 42.Vogel P, et al. Incomplete inhibition of sphingosine 1-phosphate lyase modulates immune system function yet prevents early lethality and non-lymphoid lesions. PLoS ONE. 2009;4:e4112. doi: 10.1371/journal.pone.0004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allende ML, et al. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J Biol Chem. 2010;286:7348–7358. doi: 10.1074/jbc.M110.171819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia JG, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dudek SM, et al. Abl tyrosine kinase phosphorylates nonmuscle myosin light chain kinase to regulate endothelial barrier function. Mol Biol Cell. 2010;21:4042–4056. doi: 10.1091/mbc.E09-10-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camerer E, et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. This elegant study demonstrated that plasma S1P maintains vascular integrity by activating S1PR1 on endothelial cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsolais D, Rosen H. Chemical modulators of sphingosine-1-phosphate receptors as barrier-oriented therapeutic molecules. Nature Rev Drug Discov. 2009;8:297–307. doi: 10.1038/nrd2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez T, et al. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 49.Niessen F, et al. Dendritic cell PAR1–S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. This study showed for the first time that the SPHK1–S1P–S1PR3 axis is a downstream component of PAR1 signalling in dendritic cells that regulates late phase amplification of inflammation during sepsis. [DOI] [PubMed] [Google Scholar]
- 50.Olivera A, et al. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J Biol Chem. 2006;281:2515–2525. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- 51.Olivera A, et al. The sphingosine kinase–sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 2007;26:287–297. doi: 10.1016/j.immuni.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Oskeritzian CA, et al. Distinct roles of sphingosine kinases 1 and 2 in human mast-cell functions. Blood. 2008;111:4193–4200. doi: 10.1182/blood-2007-09-115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitra P, et al. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci USA. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osborne N, et al. The spinster homolog, two of hearts, is required for sphingosine 1-phosphate signaling in zebrafish. Curr Biol. 2008;18:1882–1888. doi: 10.1016/j.cub.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawahara A, et al. The sphingolipid transporter Spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 56.Nishiuma T, et al. Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1085–L1093. doi: 10.1152/ajplung.00445.2007. [DOI] [PubMed] [Google Scholar]
- 57.Price MM, et al. Sphingosine-1-phosphate induces development of functionally mature chymase-expressing human mast cells from hematopoietic progenitors. FASEB J. 2009;23:3506–3515. doi: 10.1096/fj.08-128900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oskeritzian CA, et al. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J Exp Med. 2010;207:465–474. doi: 10.1084/jem.20091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun X, et al. Functional variants of the sphingosine-1-phosphate receptor 1 gene associate with asthma susceptibility. J Allergy Clin Immunol. 2010;126:241–249. doi: 10.1016/j.jaci.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olivera A, et al. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J Clin Invest. 2010;120:1429–1240. doi: 10.1172/JCI40659. This study showed that S1P produced by SPHK1 regulates blood pressure, histamine clearance and recovery from anaphylaxis in a S1PR2-dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Niessen F, et al. Endogenous EPCR/aPC-PAR1 signaling prevents inflammation-induced vascular leakage and lethality. Blood. 2009;113:2859–2866. doi: 10.1182/blood-2008-12-192385. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Tauseef M, et al. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res. 2008;103:1164–1172. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sammani S, et al. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol. 2010;43:394–402. doi: 10.1165/rcmb.2009-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia P, et al. Tumor necrosis factor-α induces adhesion molecule expression through the sphingosine kinase pathway. Proc Natl Acad Sci USA. 1998;95:14196–14201. doi: 10.1073/pnas.95.24.14196. This was the first paper to demonstrate a role for SPHK1 in signal transduction by TRAF2 that is important for the activation of NF-κB and for preventing apoptosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snider AJ, et al. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB J. 2008;23:143–152. doi: 10.1096/fj.08-118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lai WQ, et al. Distinct roles of sphingosine kinase 1 and 2 in murine collagen-induced arthritis. J Immunol. 2009;183:2097–2103. doi: 10.4049/jimmunol.0804376. [DOI] [PubMed] [Google Scholar]
- 67.Baker DA, Barth J, Chang R, Obeid LM, Gilkeson GS. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-α-induced arthritis. J Immunol. 2010;185:2570–2579. doi: 10.4049/jimmunol.1000644. This study demonstrated that SPHK1 plays a key role in TNF-induced inflammatory arthritis in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pitson SM, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Palma C, Meacci E, Perrotta C, Bruni P, Clementi E. Endothelial nitric oxide synthase activation by tumor necrosis factor α through neutral sphingomyelinase 2, sphingosine kinase 1, and sphingosine 1 phosphate receptors: a novel pathway relevant to the pathophysiology of endothelium. Arterioscler Thromb Vasc Biol. 2006;26:99–105. doi: 10.1161/01.ATV.0000194074.59584.42. [DOI] [PubMed] [Google Scholar]
- 71.Scherer EQ, et al. Tumor necrosis factor-α enhances microvascular tone and reduces blood flow in the cochlea via enhanced sphingosine-1-phosphate signaling. Stroke. 2010;41:2618–2624. doi: 10.1161/STROKEAHA.110.593327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vann LR, et al. Involvement of sphingosine kinase in TNF-α-stimulated tetrahydrobiopterin biosynthesis in C6 glioma cells. J Biol Chem. 2002;277:12649–12656. doi: 10.1074/jbc.M109111200. [DOI] [PubMed] [Google Scholar]
- 73.Xia P, et al. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-α signaling. J Biol Chem. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 74.Siehler S, Wang Y, Fan X, Windh RT, Manning DR. Sphingosine 1-phosphate activates nuclear factor-κB through Edg receptors. Activation through Edg-3 and Edg-5, but not Edg-1, in human embryonic kidney 293 cells. J Biol Chem. 2001;276:48733–48739. doi: 10.1074/jbc.M011072200. [DOI] [PubMed] [Google Scholar]
- 75.Alvarez SE, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. This study identified S1P as an essential cofactor for the E3 ligase activity of TRAF2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 77.Lee TH, Shank J, Cusson N, Kelliher MA. The kinase activity of Rip1 is not required for tumor necrosis factor-α-induced IκB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J Biol Chem. 2004;279:33185–33191. doi: 10.1074/jbc.M404206200. [DOI] [PubMed] [Google Scholar]
- 78.Li S, Wang L, Dorf ME. PKC phosphorylation of TRAF2 mediates IKKα/β recruitment and K63-linked polyubiquitination. Mol Cell. 2009;33:30–42. doi: 10.1016/j.molcel.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Varfolomeev E, et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor α(TNFα)-induced NF-κB activation. J Biol Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 81.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-κB activation [corrected] Nature Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 82.Devin A, Lin Y, Liu ZG. The role of the death-domain kinase RIP in tumour-necrosis-factor-induced activation of mitogen-activated protein kinases. EMBO Rep. 2003;4:623–627. doi: 10.1038/sj.embor.embor854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paugh BS, et al. EGF regulates plasminogen activator inhibitor-1 (PAI-1) by a pathway involving c-Src, PKCδ, and sphingosine kinase 1 in glioblastoma cells. FASEB J. 2008;22:455–465. doi: 10.1096/fj.07-8276com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 85.Wu W, Mosteller RD, Broek D. Sphingosine kinase protects lipopolysaccharide-activated macrophages from apoptosis. Mol Cell Biol. 2004;24:7359–7369. doi: 10.1128/MCB.24.17.7359-7369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Puneet P, et al. SphK1 regulates proinflammatory responses associated with endotoxin and polymicrobial sepsis. Science. 2010;328:1290–1294. doi: 10.1126/science.1188635. This study identified PKCδ as an intracellular target of S1P and demonstrated a crucial role for SPHK1 in sepsis. [DOI] [PubMed] [Google Scholar]
- 87.Keul P, et al. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- 88.Skoura A, et al. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:81–85. doi: 10.1161/ATVBAHA.110.213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hughes JE, et al. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eskan MA, et al. TLR4 and S1P receptors cooperate to enhance inflammatory cytokine production in human gingival epithelial cells. Eur J Immunol. 2008;38:1138–1147. doi: 10.1002/eji.200737898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 92.Nayak D, et al. Sphingosine kinase 1 regulates the expression of proinflammatory cytokines and nitric oxide in activated microglia. Neuroscience. 2010;166:132–144. doi: 10.1016/j.neuroscience.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 93.Di A, et al. A novel function of sphingosine kinase 1 suppression of JNK activity in preventing inflammation and injury. J Biol Chem. 2010;285:15848–15857. doi: 10.1074/jbc.M109.075549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Samy ET, et al. Cutting edge: modulation of intestinal autoimmunity and IL-2 signaling by sphingosine kinase 2 independent of sphingosine 1-phosphate. J Immunol. 2007;179:5644–5648. doi: 10.4049/jimmunol.179.9.5644. [DOI] [PubMed] [Google Scholar]
- 95.Hait NC, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. This study demonstrated that S1P binds to and inhibits HDAC1 and HDAC2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nature Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Glauben R, Sonnenberg E, Zeitz M, Siegmund B. HDAC inhibitors in models of inflammation-related tumorigenesis. Cancer Lett. 2009;280:154–159. doi: 10.1016/j.canlet.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 98.Wang L, de Zoeten EF, Greene MI, Hancock WW. Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nature Rev Drug Discov. 2009;8:969–981. doi: 10.1038/nrd3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Igarashi N, et al. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J Biol Chem. 2003;278:46832–46839. doi: 10.1074/jbc.M306577200. [DOI] [PubMed] [Google Scholar]
- 100.Sankala HM, et al. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–10474. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 101.Hait NC, Bellamy A, Milstien S, Kordula T, Spiegel S. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J Biol Chem. 2007;282:12058–12065. doi: 10.1074/jbc.M609559200. [DOI] [PubMed] [Google Scholar]
- 102.Ding G, et al. Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. J Biol Chem. 2007;282:27493–27502. doi: 10.1074/jbc.M701641200. [DOI] [PubMed] [Google Scholar]
- 103.Brinkmann V, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nature Rev Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 104.Mullershausen F, et al. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nature Chem Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 105.Mehling M, et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008;71:1261–1267. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- 106.Choi JW, et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci USA. 2010;108:751–756. doi: 10.1073/pnas.1014154108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kulakowska A, et al. Intrathecal increase of sphingosine 1-phosphate at early stage multiple sclerosis. Neurosci Lett. 2010;477:149–152. doi: 10.1016/j.neulet.2010.04.052. [DOI] [PubMed] [Google Scholar]
- 108.Bagdanoff JT, et al. Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanoneoxime (LX2931) and (1R,2S,3R)-1-(2-(isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932) J Med Chem. 2010;53:8650–8662. doi: 10.1021/jm101183p. [DOI] [PubMed] [Google Scholar]