Abstract
Implant surface topography influences osteoblastic proliferation, differentiation and extracellular matrix protein expressions. Previous researches proved that chemical surface modification of titanium implants could be used to improve Bone-to-implant contact. In this study, the surface topography, chemistry and biocompatibility of polished titanium surfaces treated with mixed solution of three acids containing HCl, HF and H3PO4 with different etched conditions for example concentration, time and addition of calcium chloride were studied. Osteoblast cells (MG-63) were cultured on different groups of titanium surfaces. In order to investigate titanium surfaces, SEM, AFM and EDS analyses were carried out. The results showed that surfaces treated with HCl–HF–H3PO4 had higher roughness, lower cytotoxicity level and better biocompatibility than controls. Moreover, addition of calcium chloride into mixed solution of three acids containing HCl, HF and H3PO4 is an important, predominant and new technique for obtaining biofunction in metals for biomedical use including dentistry.
1 Introduction
Titanium has been generally used as biomaterial for the manufacture of artificial joints and dental implants because of its excellent biocompatibility and mechanical properties [1–7]. The early osseointegration of titanium oral implants plays an important role in their clinical applications. However, as a biomaterial, unlike bioactive ceramics, bioglass and bioglass-ceramic, titanium cannot bond directly to the living bone [8–11]. Bioinert titanium substrate materials are commonly encapsulated after implantation into the living body by fibrous tissue that isolates them from the surrounding bone. The fixation of an implant to bone is influenced by various factors such as surface chemistry and surface topography [12]. These surface properties are important in the bone healing phase in the peri-implant. After implantation, implant surfaces are in contact with body fluids and interact with a number of proteins and different cell types. Osteoblasts that produce a bone extracellular matrix, which will ensure a high bone-implant contact. Cell adhesion is one of the initial stages for consequent proliferation of osteoblastic cells producing bone tissue. It has been confirmed that osteoblastic cell adhesion, growth and proliferation are correlated to surface roughness [10]. So various surface treatment techniques such as chemical treatment, mechanical treatment and deposition methods was employed to modify titanium surface for improving its bioactivity [13–15]. Chemical surface modification of titanium implant is particular useful. Recently, acid-etching processes have been emphasized. Because they increase cell adhesion and bone formation on the surface of titanium [16–22]. It has been known that acid etched surfaces improve the osteoconductive process due to the attachment of osteogenic cells. As a result, bone cell is formed on the surface of the implant directly [23]. It is obvious that bone growths into porous implant surfaces improved osseointegration and mechanical stability by the interlock between surrounding bone tissue and implant [24]. Etching with strong acids such as HCl, H2SO4, HNO3 and HF is another method for roughening titanium dental implants [23].
The aim of this study was to see if the structural and chemical changes of the titanium surfaces after different etched conditions in HCl-HF-H3PO4 could be related with the biological performance. Surface topography and chemistry were linked to treatment condition. The HCl–HF–H3PO4 concentration and the immersion time were selected based on previous experiments [8].
In vitro experiments were performed to evaluate the biological response of osteoblast cell (MG-63) to the modified titanium surfaces.
2 Materials and methods
2.1 Titanium plates preparation and surface modification
Commercially available pure (n = 80) titanium plates (KS-50, JIS type 2, Kobelco, Kobe, Japan) with size of 4 mm × 4 mm × 1 mm were used as substrate. They were polished with sandpaper of 600 grits [26]. After polishing, the specimens were washed with NaOH at 40 vol% and HNO3 at 50 vol% in an ultrasonic bath to remove contaminants, then washed with deionised water to reach a neutral pH, and stored at room temperature in 70 vol% ethanol [25].
70 polished and cleaned titanium plates were sent to chemical surface modification by HCl–HF–H3PO4 treatment [26], whereas 10 plates were kept non-modified as control group. By changing the surface treatment conditions, seven groups of sample were obtained, as shown in Table 1.
Table 1.
Samples treatment conditions
| Group | Acid solution | Time (sec) |
|---|---|---|
| A (Control) | – | – |
| B | HF | 120 |
| C | %85HCl–%6HF–%9H3PO4 | 120 |
| D | %80HCl–%6HF–%14H3PO4 | 120 |
| E | %80HCl–%10HF–%10H3PO4 | 120 |
| F | %80HCl–%10HF–%10 H3PO4 | 30 |
| G | %80HCl–%10HF–%10 H3PO4 | 210 |
| H | %80HCl–%10HF–%10 H3PO4–1.7gr CaCl2. 2H2O | 120 |
The aim of treatment H was to create surface roughness and presence of phosphorous and calcium in the titanium surface did positively affect their biocompatibility. This is a known bio-mimetice phenomena. For this purpose, the acidic solution %80HCl–%10HF–%10 H3PO4–1.7gr CaCl2. 2H2O was used. Calcium ions are incorporated in the hydrated Ti–OH layer. The positively charged Ca2+ may act as nucleation sites for HCA by attaching to negatively charged (PO4)3− and (CO3)2− to form Ca–P enriched surface layer which crystallizes to bone-like apatite (HCA).
2.2 Surface analysis
2.2.1 Surface topography
The micro and nano-level surface topography of the HCl–HF–H3PO4 modified titanium plates were characterized by using two non-destructive techniques [25].
Scanning Electron Microscopy (Philips XL 30 and VEGA\\TESCAN) was used to detect the surface changes induced by the aforesaid acidic solution in micro level (1000× of magnification).
An Atomic Force Microscope (AUTO PROBE, PARK SCIENTIFIC INSTRUMENTS, USA) was used to evaluate the surface changes in micro and nano-level (scan size: 2 × 2 µm2 and 500 × 500 nm2).
2.2.2 Surface chemistry
The presence of fluorine, phosphor and calcium, on the surface of titanium, as a result of the different chemical surface treatments, was analyzed by energy dispersion spectroscopy (VEGA\\TESCAN).
2.3 Biological analyses
2.3.1 Cell culture
Human osteosarcoma cells, MG-63, were obtained from the National Cell Bank of Iran (NCBI), Pasteur Institute of Iran (NCBI, C555). Cells were routinely cultured at 37 in a humidified atmosphere of 5% CO2, and maintained in Dulbecco’s Modified Eagle Medium (DMEM, GIBCO, Scotland) containing 10% of fetal calf serum (FCS, Seromed, Germany) and 100 IU penicillin/ml and 100 mg streptomycin/ ml. Cells were sub-cultured 1:4 before reaching confluence using PBS and trypsin/EDTA. To test the effect of surface modification of titanium implants with HCl–HF–H3PO4 at different concentrations and exposure times, plates from each group (n = 9) were placed in a 96-well plate and 104 cells were seeded on all the plates. The same number of cells were cultured in parallel in other 96 plate’s wells in all experiments [13].
2.3.2 Preparation of samples for SEM
SEM analyses were performed to study the morphology of MG-63 cells grown on the surface of both control and treated Ti plates. For this purpose, after cell culture for 16 h, the samples were washed with phosphate-buffered saline (PBS) and then fixing with 0.25% glutaraldehyde in 0.1 M cacodylic acid buffer (pH 7.4) and then kept for 15 minutes to ensure complete dissolution of crystal. After fixation, the samples were sequentially dehydrated in a graded series of ethanol (70, 80, 90, 95 and 100%) for 10 min each, immersed in isoamyl acetate for 1.5 min, treated in a critical point dryer and sputter-coated with a thin layer of Au. Morphologies of the cells were observed by SEM [25].
2.3.3 Proliferation assay
The cells were cultured for 3, 7 and 14 days on titanium specimens. Cell viability and proliferation were evaluated by the MTT (Sigma, St. Louis, MD, USA) assay. This method is based on the reduction of 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide by viable cells. The cells were incubated with 50 µl of MTT for 4 h at 37°C. The dark blue formazan crystals were dissolved with DMSO (Merck, Darmstadt, Germany) and then kept in the acid solution overnight to ensure complete dissolution of crystals. The absorbance was evaluated at 570 nm in an ELISA reader (organon- Teknika, Netherland) [8].
2.3.4 Cell attachment
Cell attachment was observed by fluorescence microscopy 16 h after incubation. Titanium substrates (n = 3 for each treatment) were stained with 4′-6-diamidine-2′ phenylindol dihydrochloride (DAPI, Sigma, St. Louis, MO, USA), and the number of adherent cells was determined for each of the eight specimens [8].
2.4 Statistical analysis
The results were expressed as means with standard errors. The data were then analyzed by one-way ANOVA, followed by the post hoc paired Student’s two-tailed t-test. The significance levels were notified significant (*, if P < 0.05) and highly significant (**, if P < 0.01) in the figures and tables [25].
3 Results
3.1 Surface topography
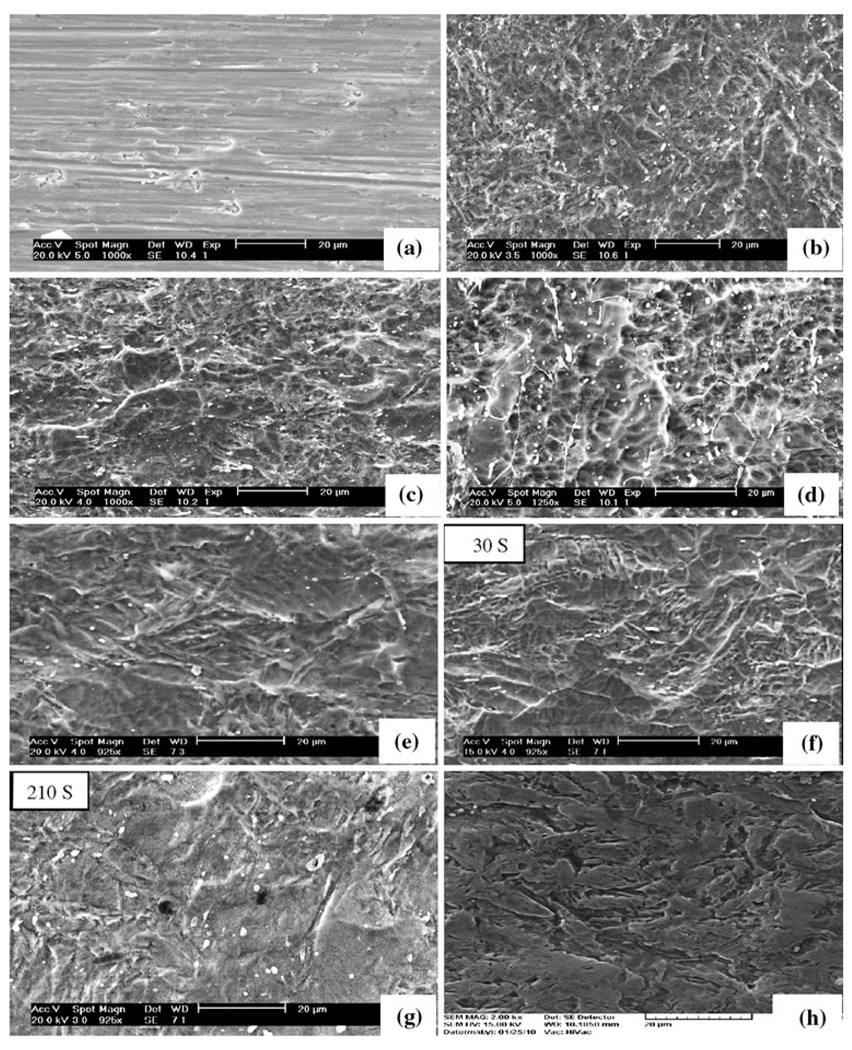
Figure 1 shows SEM photomicrographs of polished titanium surfaces treated with different acid solutions. Smooth-Ti surfaces of control samples present parallel grooves, the typical character resulted from grinding process using silicon carbide papers (Fig. 1a). Plate surfaces etched with acid (HF) present poor microtexture without micropits an irregular rough morphology (Fig. 1b). Plate etched with %80HCl–%6HF–%14H3PO4 presents a rather rough surface with few micro pits and smooth waviness (Fig. 1c). Surface etched with %85HCl–%6HF–%9H3PO4 shows significant roughness with micro pits of 0.5–3 µm. The surface spreads with waviness and roughness and without intact areas (Fig. 1d). The surface etched with %80HCl–%10HF–%10H3PO4 has a microtexture without micropits (Fig. 1e). The coarse surface was characterized by its peaks and valleys like structure, but several flat facets could be observed. The facets also had small irregularities appearance such as pits and stripes. The effect of etching time was also studied for the acidic solution of 80%HCl–10%HF–10%H3PO4 That Fig. 1f–g shows SEM photomicrographs of time-related changes. SEM of group F specimen etched for 30 s (Fig. 1f) showed an regular texture surface with micropits approximately the same size and similar. No significant difference in the surface height deviation was detected between group F (Fig. 1f) and group D (Fig. 1d). However, there were visible differences in surface configuration between group F and group E/G because the latter endured longer exposure time in 80%HCl–10% HF–10% H3PO4 (120 and 210 s, respectively). Figure 1h shows heterogeneous surface topographic feature with variations of peaks and valleys. This surface appeared course, but several flat facets could be observed. The facets also had small irregularities appearing as pits and stripes. The surface roughness of both the untreated and surface treated samples was measured using AFM by contact mode and the AFM images are shown in Fig. 2.
Fig. 1.
SEM micrographs showing the surface roughness of the tested titanium plates that a Treatment A, b Treatment B, c Treatment C, d Treatment D, e Treatment E, f Treatment F, g Treatment G and h Treatment H. Original magnification ×1000; bar = 20 µm
Fig. 2.
AFM micrographs show the surface topography of the tested titanium plates that a Treatment A, b Treatment B, c Treatment C, d Treatment D and e Treatment E, f Treatment F, g Treatment G. bar = 2 × 2 µm2, h Treatment F, i Treatment E, j Treatment G, k Treatment H. bar = 500 × 500 µm2
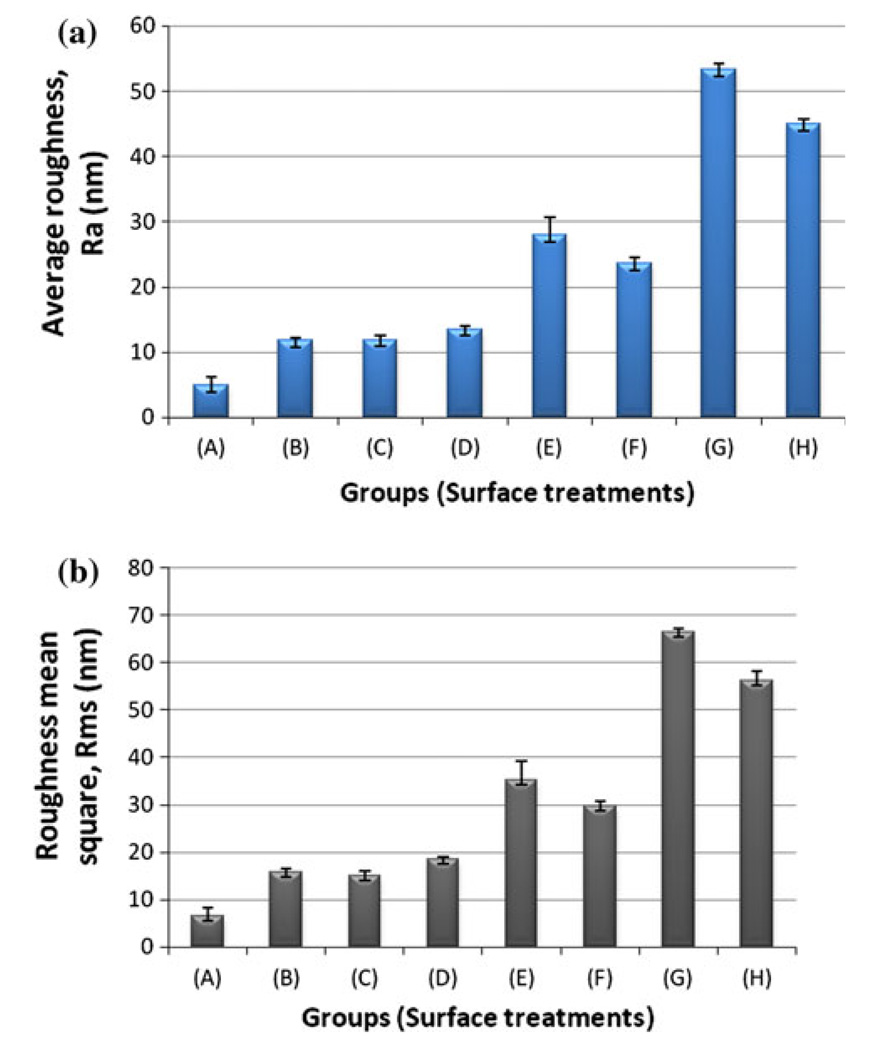
AFM images exhibited differences in surface topography due to various treatments (Fig. 2). The control surface had parallel grooves along the polishing direction (Fig. 2a). All the treated surfaces demonstrated roughened oxide topography with sharply crests and pits (Fig. 2b–e). Figure 2f–j shows AFM images of time-related changes. Plate etched with acid of %80HCl–%10HF–%10H3PO4 for 30 s exhibited an irregular rough morphology at the micro and nano-level (Fig. 2h). Plates etched with %80HCl–%10HF–%10H3PO4 had a nanotexture without nanopits (Fig. 2i–j). The facets also had small irregularities appearing as stripes. The surface analysis showed that these implants had surface parameters with the high roughness values. Figure 2k showed significant surface roughness with micro pits of 0.05–0.1 µm. The waviness and roughness of the surface was regular without intact areas. Figure 3 shows the surface roughness values (Ra and Rms) of commercially pure titanium (cpTi) plates with different surface modification treatment. Polished titanium surface (A) showed the lowest roughness values for Ra (4.87 ± 1.39) and Rms (6.43 ± 1.91), while Group G showed the highest Ra (53.25 ± 1.03) and Rms (66.48 ± 0.64) values. The Ti surface of group E showed moderate values. Moreover, Groups B, C and D had similar average surface roughness. Between the specimens of group G and group H with other groups (A, B, C and D) there were significant differences in surface roughness. The results revealed that acid concentrations variation in aforementioned mixed solution of three acids had a significant effect on the morphology and surface roughness. Furthermore, the Ra value was found to be higher for acid treated samples as expected when compared to the untreated sample.
Fig. 3.
Surface roughness values a Average surface roughness (Ra) and b roughness mean square (Rms) of polished titanium surfaces treated with different acid solutions. All data are reported as mean ± standard deviation (n = 3)
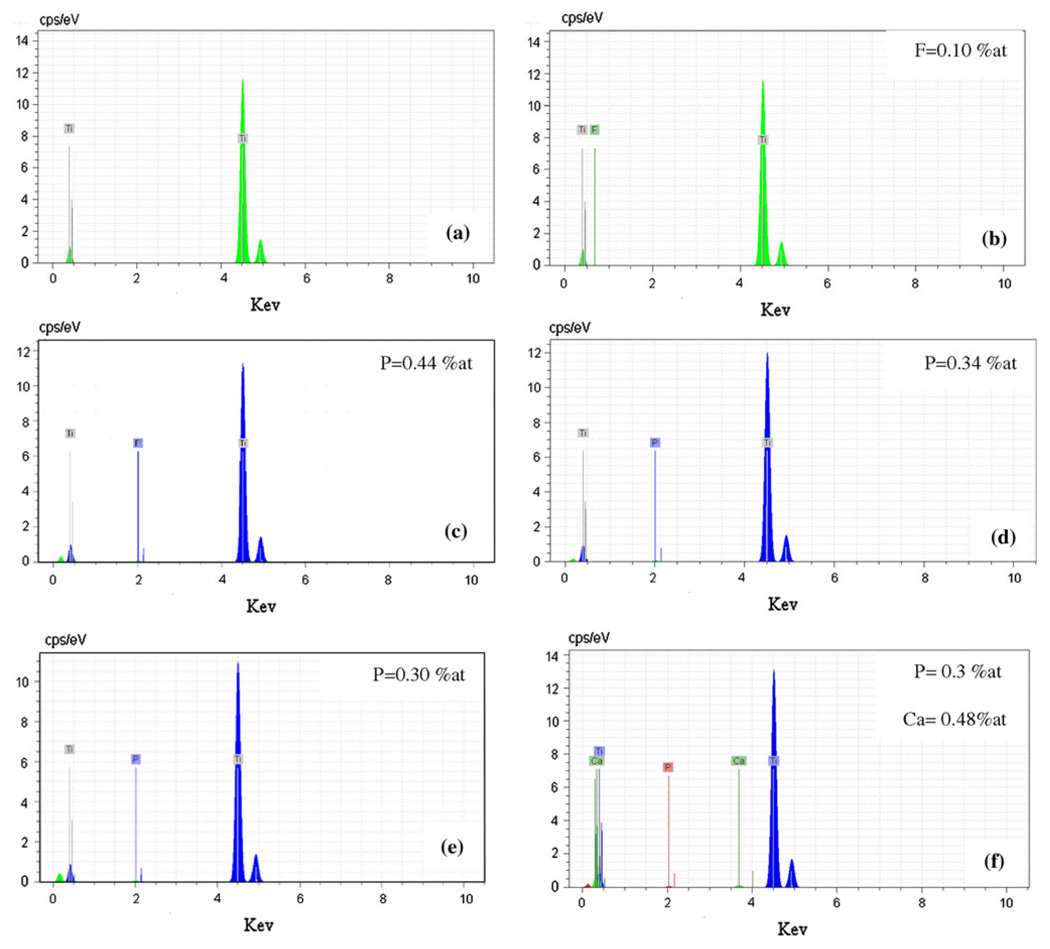
Figure 4 shows EDS of polished titanium surfaces treated with different etched conditions. Phosphorous peaks were observed in addition to the peaks of titanium for surfaces treated with mixed solution of three acids, in accordance with the literature [5]. The surface of titanium without chemical treatment exhibited only titanium and no traces of oxygen peaks were observed (Fig. 4a). The surface exposed to HF did present traces of fluorine while no fluorine could be detected for surfaces treated with mixed solution of three acids. In addition, EDS analysis exhibited the presence of phosphorous and calcium element on the surfaces of titanium implant after chemical treatment with %80HCl–%10HF–%10H3PO4–1.7gr CaCl2. 2H2 O (Fig. 4f). The evaluation of Ca/P ratio of samples was made by EDS analysis to get information of the Ca/P ratio. The values obtained for the apatite were in the range of 1.6, which is near to the stoichiometric value of apatite.
Fig. 4.
Energy dispersive spectroscopy of a Untreated titanium, b Treatment B, c Treatment C, d Treatment D, e Treatment E and f Treatment H
3.2 Cell morphology, viability and attachment
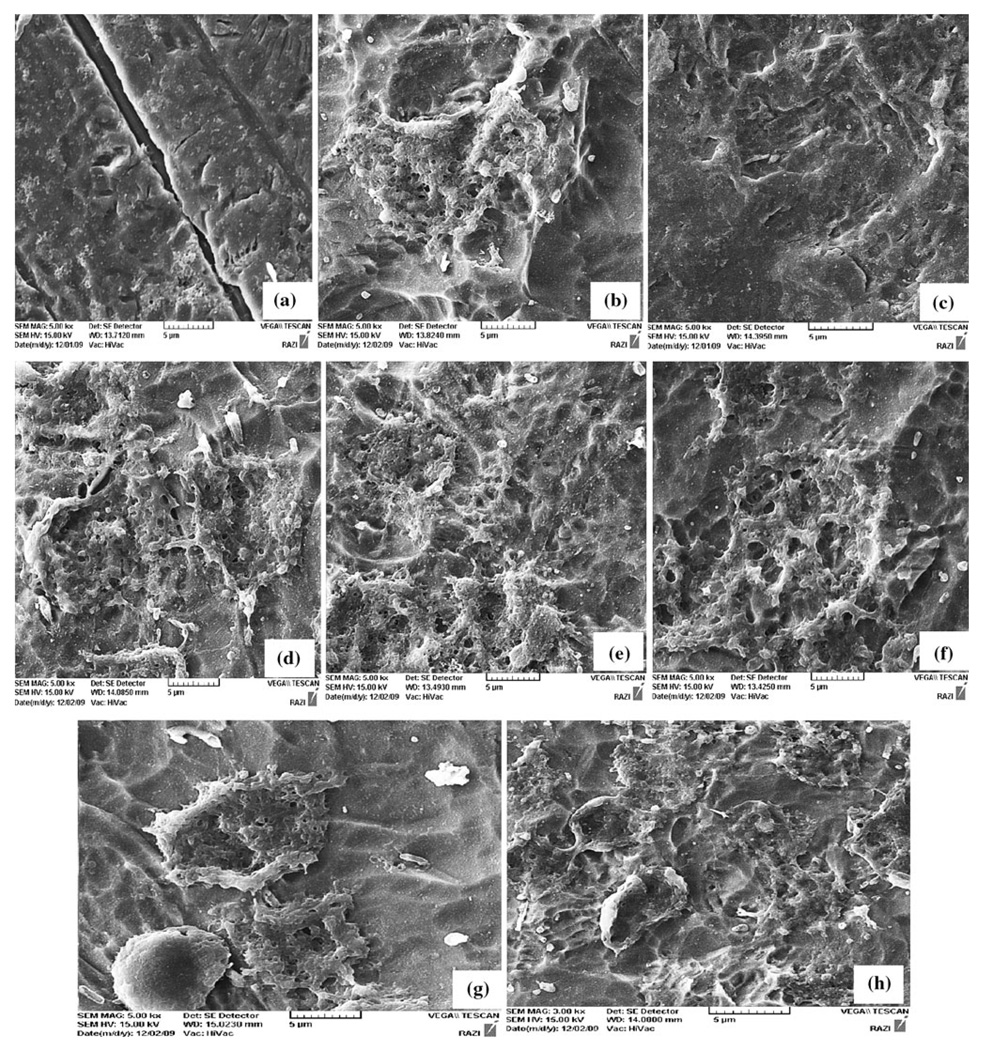
The morphology of osteoblastic cells MG-63 after culturing of 16 h on the different surfaces are shown in Fig. 5. The osteoblastic cells attached on the rough titanium surfaces. The different substrates were uniformly covered with a layer of osteoblastic cells after culturing of 16 h using an initial cell density of 10,000 cells. It was observed that the cells on the HCl–HF–H3PO4 modified surfaces had more visible nuclei than on the cells attaching on the control surface. However, on the mirror polished surface (Fig. 5a) no cells could be detected. On the surfaces (5d), (5e), (5g) and (5h) presented cell attachment could be seen, this images indicates that compared to the surface (5a) the chemical surface modification was optimal for the cell attachment.
Fig. 5.
Morphological assessment of MG-63 cells cultured on test implant surfaces. Scanning electron micrographs of a control (A), b Surfaces treated of B, c Treatment C, d Treatment D, e Treatment E, f Treatment F, g Treatment G, h Treatment H; are shown after 16 h in culture
Figure 6 shows the cell viability measured by MTT assay after 3, 7 and 14 days of culture on the chemical modified titanium surfaces in comparison with polished titanium (control). For chemical modified titanium surfaces with mixed solution of three acids containing HCl–HF–H3PO4, the results show an increase in cell viability indicating cell proliferation during the culture period. Results concerning cell viability/proliferation after 3 days of culture evaluated by MTT assay showed that cell growth was not affected by surface treatment, and osteoblastic cells proliferated very well on all substrates. The statistical analysis of MTT data did show highly significant differences between groups H and G when compared to control (P < 0.001, n = 3 at day 7 and P < 0.01, n = 3 at day 14). In addition, samples D, E and F exhibited a greater cell proliferation at day 7 when compared to control (P < 0.01 n = 3). Treatment B and C resulted in a increase in the cell growth rate after 14 days of culture (P < 0.05; n = 3). Samples G and H exhibited a greater cell proliferation after 3, 7 and 14 days of culture on the surfaces in comparison with polished titanium (control) and chemical modified titanium surfaces.
Fig. 6.
Viability/proliferation (MTT assay) of osteoblastic cells on control and treated surfaces after 3, 7 and 14 days. All data are reported as mean ± standard deviation (n = 3)
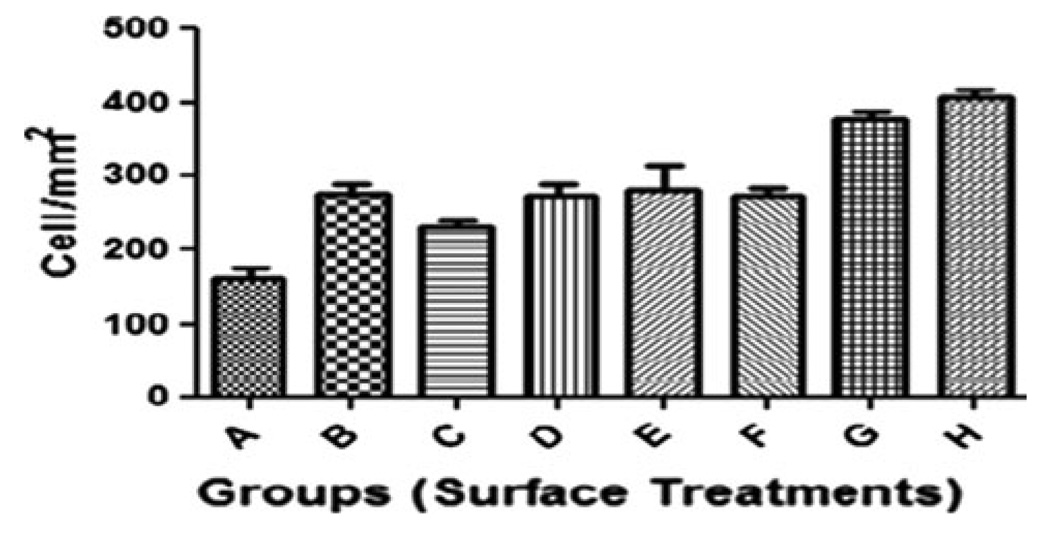
Data on cell attachment are presented in Fig. 7. The statistical analysis of cell attachment data did show highly significant differences between groups H and G when compared to control (P < 0.01, n = 3 at 16 h).
Fig. 7.
Number of adherent osteoblast cells after 16 h in culture on treated Ti and control surfaces. All data are reported as mean ± standard deviation (n = 3)
4 Discussion
The surface properties of an implant have determinant effects on the biological behavior. Chemical modification made the surface have rough surface topography (Figs. 1, 2), and the associated advantage on these early stages of healing was that the rough surface promoted contact osteogenesis [21]. Development of bone–implant interfaces depends directly on the interactions between osteoblasts and biomaterial. Osteoblastic adhesion then becomes an essential phenomenon for the first bone–biomaterial interaction. Adhesion by itself is essential for embryogenesis, maintenance of tissue integrity, wound healing, immune response, and biomaterial tissue integration. Numerous proteins are involved in adhesion to the ECM proteins (fibronectin, collagen, laminin, vitronectin), cytoskeletal proteins (actin, talin, vinculin), and membrane receptors (integrins). Interactions between these proteins and their specific receptors induce signal transduction which consequently influences cell growth and differentiation [4]. A number of studies using MG63 cells suggest that cell growth and attachment are influenced by surface roughness [22].
Various methods have been developed to create rough implant surfaces in order to improve the clinical performance of implants and to form a stable mechanical bone-implant interface [23, 24]. Etching with strong acids such as HCl, H2SO4, HNO3 and HF is another method for roughening titanium surface of dental implants. Acid-etching produces micro pits on titanium surfaces with sizes ranging from 0.5 to 2 µm in diameter (Figs. 1, 2). Several experimental studies have reported higher bone-to-implant contact and less bone resorption on acid-etched surfaces compared to manufactured or TPS surfaces [24]. Recently, acid-etching methods have been improved in order to increase cell adhesion and bone formation.
The study presented here has shown a new approach to investigate the improvement of a surface texture using acid etching technology. Thus, the surface topography and biocompatibility of titanium surfaces treated by mixed solution of HCl–HF–H3PO4 with different concentration, treatment time and addition of calcium chloride. The results revealed that acid concentration variations in the aforementioned mixed solution has a significant effect on the morphology and surface roughness, and the greatest R.M.S and Ra was attributed to 80%HCl–10% HF–10% H3PO4, (Fig. 3). Definitely, the surface roughness showed a significant increase when the exposure time increased (Figs. 1, 2).
In addition, it was found that after a culture period of 7 days the cell viability/proliferation on the etched surfaces with HCl–HF–H3PO4 was higher than on the surfaces polished and etched with HF, resulting in a higher surface coverage by the cells (Fig. 6). Rough surfaces encourage the entrapment of fibrin protein, adhesion of osteogenic cells and mechanical stability of implants in host bone [23–29]. Results from in vitro studies suggested a positive correlation between surface roughness and cellular attachment and cell proliferation. Moreover, that the modified sample with HF presented a feature of weaker cell growth and proliferation than that treated with mixed solution of three acids can be thought as the evidence that the two other acids (HCl and H3PO4) play an important role in improving this feature. The same relationship also can be obtained from that the results of EDS analysis exhibited the presence of phosphorous element on the surfaces etched with 80%HCl–10%HF–10%H3PO4, due to phosphoric acid (Fig. 4). Polished titanium surfaces (control) is poor in cell growth and proliferation in comparison with samples modified by chemical treatment. From the results of cell culture it was determined that sample G with the highest surface roughness (RMS) had a high ability in cell proliferation and cell attachment (Figs. 6, 7).
It has been found that acid etched surfaces enhance the osteoconductive process through the attachment of fibrin and osteogenic cells, resulting in bone formation directly on the surface of the implant. Adhesive ability of cells to the surface of titanium treated by the mixture solution with higher percent of HF, showed a higher value because HF in the three acid solution can further increased surface roughness [25]. The present study showed that precise selection of the composition ratio of mixture solution played the main role in preparation of the rough titanium surface. In general, the surfaces of our experimental samples were rougher compared to commercially available implants.
Another approach involves treating titanium dental implants in fluoride solutions. Titanium acutely reacts to fluoride ions, forming soluble TiF4 species. The surface produced has microrough topography as shown in (Fig. 1b). This chemical treatment of the titanium created both a surface roughness and fluoride incorporation favorable to the osseointegration of dental implants [25]. This chemical treatment may have the potential to improve implant anchorage in bone by rendering the implant surface bioactive. Therefore, in our study we used one method acid etching to create a new titanium surface combining all the aforementioned surface texture features.
In this study, a positive correlation between material surface properties (surface roughness and surface chemistry) and biological responses was observed, and the results of EDS exhibited the presence of phosphorous element, due to phosphoric acid (Fig. 4), on the surfaces of samples. The penetration of phosphorous and calcium to the Ti structure could induce apatite formation and improving the bone-to-implant contact [19]. Calcium ions are incorporated (Fig. 4) in the hydrated Ti–OH layer. The positively charged Ca2+ may act as nucleation sites for HCA by attaching to negatively charged (PO4)3− and (CO3)2− to form Ca–P enriched surface layer which crystallizes to bone-like apatite (HCA). The apatite formation on chemically treated titanium seems to be similar to that on bioactive glasses [9, 26, 30–33, ].
These results suggest that presence of phosphorous and calcium in the titanium surface did positively affect their biocompatibility. This is a known bio-mimetice phenomena. In addition, nanoscale topography is a powerful way of altering protein interactions with a surface. Webster and colleagues observed an increased vitronectin adsorption on nanostructured surfaces when compared to conventional surfaces. They also found an increased osteoblast adhesion when compared to other cell types, such as fibroblasts, on the nanosurfaces [20]. Another study suggested higher adsorption of fibronectin on hydrophilic SAMs surfaces with greater focal adhesion formation (integrin binding) evident in the osteoblast cells adhered to the hydrophilic SAM treated surfaces [20].
5 Conclusion
In this study, pure titanium was subjected to chemical surface modifications and examined in terms of morphology, chemical characteristics. The results have shown that the modification of titanium surface when exposed to a mixed solution of three acids influenced by both etched conditions (concentration, time and addition of calcium chloride) and the initial surface topography. A good control of these parameters gives the best reproducibility of this surface modification method. Different rough titanium surfaces were correlated to osteoblastic cell adhesion, viability and morphology in comparison with smooth titanium.
Cell proliferation/viability/was higher for Ti samples with treatments H and G. Therefore, these treated surfaces seem to provide a better environment for osteoblastic cell integration. These results suggest that the treatments used in the present study may support favorable biological responses in vivo. This method is easy to obtain porous surface of titanium such as in biomaterial applications.
Acknowledgments
Authors, gratefully thank Semnan University, Bio nano Materials Research Center at Semnan University and Pasteur Institute of Iran for the research support and Prof. J. Chen in Sichuan University.
Contributor Information
Amir Zareidoost, Email: a.zareidoost@gmail.com, Department of Materials Science and Engineering, University of Semnan, Semnan, Iran; Bio-Nano-Materials Research Center, University of Semnan, Semnan, Iran.
Mardali Yousefpour, Email: myousefpor@semnan.ac.ir, mardaliyousefpour@yahoo.com, Department of Materials Science and Engineering, University of Semnan, Semnan, Iran; Bio-Nano-Materials Research Center, University of Semnan, Semnan, Iran; Nano Nafez Company, Science and Technology Park of University of Semnan, Semnan University, Semnan, Iran.
Behrooz Ghaseme, Department of Materials Science and Engineering, University of Semnan, Semnan, Iran.
Amir Amanzadeh, Email: amira@pasteur.ac.ir, National Cell Bank of Iran, Pasteur Institute of Iran, Tehran, Iran.
References
- 1.Ohtsu N, Abe C, Ashino T, Semboshi S, Wagatsuma K. Calcium-hydroxide slurry processing for bioactive calcium-titanate coating on titanium. Sur Coat Tech. 2008;202:5110–5115. [Google Scholar]
- 2.Ban S, Iwaya Y, Kono H, Sato H. Surface modification of titanium by etching in concentrated sulfuric acid. Den Mat. 2006;22:1115–1120. doi: 10.1016/j.dental.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Giordano C, Sandrini E, Curto BD, Signorellie E, Rondelli G, Sillvio LD. Titanium for osteointegration: comparison between a novel biomimetic treatment and commercially exploited surfaces. J App Biomat Biomech. 2004;2:35–44. [PubMed] [Google Scholar]
- 4.Jayaraman M, Meyer U, Bühner M, Joos U, Wiesmann HP. Influence of titanium surfaces on attachment of osteoblast-like cells in vitro. Biomaterials. 2004;25:625–631. doi: 10.1016/s0142-9612(03)00571-4. [DOI] [PubMed] [Google Scholar]
- 5.Variola F, Yi JH, Richert L, Wuest JD, Rosei F, Nanci A. Tailoring the surface properties of Ti6Al4V by controlled chemical oxidation. Biomaterials. 2008;29:1285–1298. doi: 10.1016/j.biomaterials.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 6.Ueda M, Ikeda M, Ogawa M. Chemical–hydrothermal combined surface modification of titanium for improvement of osteointegration. Mat Sci Eng C. 2009;29:994–1000. [Google Scholar]
- 7.Yukari I, Machigashira M, Kanbara K, Miyamoto M, Noguchi K, Izumi Y, Ban S. Surface properties and biocompatibility of acid-etched titanium. Den Mat J. 2008;27(3):415–421. doi: 10.4012/dmj.27.415. [DOI] [PubMed] [Google Scholar]
- 8.Santiago AS, Santos EA, Sader MS, Santiago MF, Soares Gde A. Response of osteoblastic cells to titanium submitted to three different surface treatments. Braz Oral Res. 2005;19(3):203–208. doi: 10.1590/s1806-83242005000300009. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Chu P, Ding C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mat Sci Eng R. 2004;47:49–121. [Google Scholar]
- 10.Guehennec Laurent L, Lopez-Heredia MA, Enkel B, Weiss P, Amouriq Y, Layrolle P. Osteoblastic cell behaviour on different titanium implant surfaces. Acta Biomat. 2008;4:535–543. doi: 10.1016/j.actbio.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Weichang X, Xuanyong L, XueBin Z, Chuanxian D. In vivo evaluation of plasma-sprayed titanium coating after alkali modification. Biomaterials. 2005;26:3029–3037. doi: 10.1016/j.biomaterials.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Gabbi C, Cacchioli A, Ravanetti F, Spaggiari B, Borghetti P, Martini FM, Leonardi F. Osteogenesis and Bone Integration: The Effect of new Titanium Surface Treatments. Ann Fac Medic Vet. di Parma. 2005;XXV:307–318. [Google Scholar]
- 13.Montanaro L, Arciola CR, Campoccia D, Cervellati M. In vitro effects on MG63 osteoblast-like cells following contact with two roughness-differing fluorohydroxyapatite-coated titanium alloys. Biomaterials. 2002;23:3651–3659. doi: 10.1016/s0142-9612(02)00098-4. [DOI] [PubMed] [Google Scholar]
- 14.Jonasova L, Muller AF, Helebrant A, Strnad J, Greil P. Biomimetic apatite formation on chemically treated titanium. Biomaterials. 2004;25:1187–1194. doi: 10.1016/j.biomaterials.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Lee BH, Lee C, Kim DG, Choi K, Lee KH, Kim YD. Effect of surface structure on biomechanical properties and osseointegration. Mat Sci Eng C. 2008;28:1448–1461. [Google Scholar]
- 16.Hamada K, Kon M, Hanawa T, Yokoyama K, Miyamoto Y, Asaoka K. Hydrothermal modification of titanium surface in calcium solutions. Biomaterials. 2002;23:2265–2272. doi: 10.1016/s0142-9612(01)00361-1. [DOI] [PubMed] [Google Scholar]
- 17.Juodzbalys G, Sapragoniene M, Wennerberg A. New acid etched titanium dental implant surface. Stomatologija Bal Den Maxillofac J. 2003;5:101–105. [Google Scholar]
- 18.Yousefpour M, Afshar A, Chen J, Zhang X. Electrophoretic deposition of porous hydroxyapatite coatings using polyterafluoroethylene particles as templates. Mat Sci Eng C. 2007;27(5–8):1482–1487. [Google Scholar]
- 19.Vanzillotta SP, Sader SM, Bastos NI, de Almeida SG. Improvement of in vitro titanium bioactivity by three different surface treatments. Den Mat. 2006;22:275–282. doi: 10.1016/j.dental.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Mendonca G, Mendonca DB, Aragao FJ, Cooper LF. Advancing dental implant surface technology: from micronto nanotopography. Biomaterials. 2008;29:3822–3835. doi: 10.1016/j.biomaterials.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Cooper LF, Zhou Y, Takebe J, Guo J, Abron A, Holmen A, Ellingsen JE. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials. 2006;27:926–936. doi: 10.1016/j.biomaterials.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Rausch-fan X, Zhe Q, Marco W, Michael M, Andreas S. Differentiation and cytokine synthesis of human alveolar osteoblasts compared to osteoblast-like cells (MG63) in response to titanium surfaces. Den Mat. 2008;24:102–110. doi: 10.1016/j.dental.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Poon Ray WY, Kwok Sunny CH, Chu Paul K, Chuanxian D. Plasma surface modification of titanium for hard tissue replacements. Sur Coat Tech. 2004;186:227–233. [Google Scholar]
- 24.Wei M, Kim HM, Kokubo T, Evans JH. Optimising the bioactivity of alkaline-treated titanium alloy. Mat Sci Eng C. 2002;20:125–134. [Google Scholar]
- 25.Lamolle SF, Monjo M, Rubert M, Haugen HJ, Lyngstadaas SP, Ellingsen JE. The effect of hydrofluoric acid treatment of titanium surface on nanostructural and chemical changes and the growth of MC3T3-E1 cells. Biomaterials. 2009;30:736–742. doi: 10.1016/j.biomaterials.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 26.Yousefpour M, Afshar A, Chen J, Xingdong Z. Bioactive layer formation on alkaline-acid treated titanium in simulated body fluid. Mat Des. 2007;28:2154–2159. [Google Scholar]
- 27.Geetha M, Singh AK, Asokamani R, Gogia AK. Ti based biomaterials, the ultimate choice for orthopaedic implants: a review. Prog Mat Sci. 2009;54:397–425. [Google Scholar]
- 28.Elias Carlos N, Oshida Y, Lima Jose Henrique C, Muller Carlos A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J Mecha Behavior Biomedi Mat. 2008;1:234–242. doi: 10.1016/j.jmbbm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Ellingsen JE, Johansson CB, Wennerberg A, Holman A. Improved retention and bone-to-implant contact with fluoride-modified titanium implants. Int J Oral Maxillo Imp. 2004;19(5):659–666. [PubMed] [Google Scholar]
- 30.Thirugnanam A, Sampath Kumar TS, Chakkingal U. Bioactivity enhancement of commercial pure titanium by chemical treatments. Trends Biomat Artif Organs. 2009;32(2):76–85. [Google Scholar]
- 31.Yousefpour M, Afshar A, Yang X, Li X, Yang B, Wu Y, Chen J, Zhang X. Nano-crystalline growth of electrochemically deposited apatite coating on pure titanium. J Electroanal Chem. 2006;6589:96–105. [Google Scholar]
- 32.Yousefpour M, Afshar A, Yang X, Li X, Yang B, Wu Y, Chen J, Zhang X. Investigation the morphology and bioactive properties of composite coating of HA/vinyl acetate on pure titanium. Mat Sci Eng B. 2006;128:243–249. [Google Scholar]
- 33.Muller FA, Bottino MC, Muller L, Henriques VAR, Lohbauer U, Bressiani AHA, Bressiani JC. In vitro apatite formation on chemically treated (P/M) Ti–13Nb–13Zr. Den mat. 2008;24:50–56. doi: 10.1016/j.dental.2007.02.005. [DOI] [PubMed] [Google Scholar]