Abstract
CFS (chronic fatigue syndrome) is commonly co-morbid with POTS (postural tachycardia syndrome). Individuals with CFS/POTS experience unrelenting fatigue, tachycardia during orthostatic stress and ill-defined neurocognitive impairment, often described as ‘mental fog’. We hypothesized that orthostatic stress causes neurocognitive impairment in CFS/POTS related to decreased CBFV (cerebral blood flow velocity). A total of 16 CFS/POTS and 20 control subjects underwent graded tilt table testing (at 0, 15, 30, 45, 60 and 75°) with continuous cardiovascular, cerebrovascular, and respiratory monitoring and neurocognitive testing using an n-back task at each angle. The n-back task tests working memory, concentration, attention and information processing. The n-back task imposes increasing cognitive challenge with escalating (0-, 1-, 2-, 3- and 4-back) difficulty levels. Subject dropout due to orthostatic presyncope at each angle was similar between groups. There were no n-back accuracy or RT (reaction time) differences between groups while supine. CFS/POTS subjects responded less correctly during the n-back task test and had greater nRT (normalized RT) at 45, 60 and 75°. Furthermore, at 75° CFS/POTS subjects responded less correctly and had greater nRT than controls during the 2-, 3- and 4-back tests. Changes in CBFV were not different between the groups and were not associated with n-back task test scores. Thus we conclude that increasing orthostatic stress combined with a cognitive challenge impairs the neurocognitive abilities of working memory, accuracy and information processing in CFS/POTS, but that this is not related to changes in CBFV. Individuals with CFS/POTS should be aware that orthostatic stress may impair their neurocognitive abilities.
Keywords: cerebral blood flow, chronic fatigue syndrome (CFS), graded tilt testing, neurocognition, orthostatic stress, postural tachycardia syndrome (POTS)
INTRODUCTION
CFS (chronic fatigue syndrome) is a clinical disorder of long-lasting self-reported fatigue that cannot be explained by any medical cause. Commonly, it has an insidious onset and the fatigue is not relieved by rest. As defined by the CDC (Center for Disease Control and Prevention) in 1994, the fatigue must last at least 6 months, cause substantial disability in a person’s life and be associated with at least four of the following symptoms: short-term memory or concentration impairment, pharyngitis, cervical or axillary lympadenopathy, muscle pain, joint pain without swelling or inflammation, headache, unrefreshing sleeping or post-exertional malaise [1]. CFS is severely disabling and limits an individual’s ability to attend school, work or social functions [2,3].
Additionally, CFS is frequently associated with autonomic nervous system dysfunction, in particular orthostatic intolerance in the form of POTS (postural tachycardia syndrome) [4,5]. POTS is defined as an increase in HR (heart rate) of more than 30 beats/min upon standing upright or a maximum HR of at least 120 beats/min, with associated signs and symptoms such as fatigue, nausea, headache, visual disturbances, tremulousness, diaphoresis, hypocapnia and/or peripheral venous pooling [6,7]. POTS is more common in the adolescent or young adult subgroup of CFS patients, but it may also affect older adults [8,9]. The symptoms of POTS are disabling and interfere with daily activities such as housekeeping, grocery shopping, showering and food preparation [7]. In both CFS and POTS, females are more predominately affected [2,7]. Furthermore, both CFS and POTS are associated with neurocognitive impairment [5,10]. Both syndromes share similar morbidities and frequently occur together with overlapping symptoms; thus for the purpose of this study, we have selected patients with both CFS and POTS.
Neurocognitive impairment in CFS/POTS is often subjectively described as ‘mental fog’ or ‘cloudiness’ by patients. Specific areas of impairment include concentration and memory [11]. Work by DeLuca et al. [12,13] showed that CFS patients have impaired working memory, concentration and difficulty processing complex information, as well as impaired speed and efficiency in information processing. Joyce et al. [14] showed that CFS patients have an inability to plan and order responses due to impairments in attention and working memory. Similarly, Dobbs et al. [15] reported working memory deficits in CFS patients during demanding tasks that required attention and switching between mental processing routines. Furthermore, cognitive deficits in memory recall, working memory and concentration are more severe in CFS patients who do not have psychiatric co-morbidity [13]. Patients with POTS also report cognitive impairment, especially during orthostatic stress [10]. Overall, neurocognitive impairments may be the most debilitating aspects of CFS/POTS [2,3,16,17].
A physiological cause for neurocognitive impairment in CFS/POTS has not been elucidated. One idea is that impaired cerebral perfusion, especially during orthostatic stress, may play a role in CFS/POTS dysfunction, but this has been controversial. Recently, our laboratory studied a subset of subjects with POTS who follow being placed upright during tilt-table testing were normocapnic, but had decreased cerebral blood flow, which may have been related to impaired cerebral autoregulation [18]. Similarly, Low et al. [19] found decreased cerebrovascular regulation in POTS. Yet, Schondorf et al. [20] found that cerebral autoregulation was preserved in POTS subjects during orthostatic challenge.
Early studies using SPECT (single-photon emission computed tomography) scanning found regional deficits of perfusion in the cerebral hemispheres and brain stem of CFS subjects [21–23]. More recently, the use of other techniques such as near-IR spectroscopy [24], xenon gas diffusion CT (computerized tomography) [25] and magnetic resonance arterial spin labelling [26] have found deficits in CFS cerebral blood flow and perfusion. However, work by Fischler et al. [27] and MacHale et al. [28] could not confirm any deficits in CFS cerebral perfusion. Additionally, a study by Rowe and co-workers [29] using transcranial Doppler sonography did not find any differences in CBFV (cerebral blood flow velocity) between CFS and control subjects during upright tilt. The results of these measurements of cerebral perfusion in CFS/POTS are controversial, and these differences may be methodological.
Since CFS patients commonly have POTS, and both syndromes have overlapping symptoms, neurocognitive impairment in both may be a consequence of orthostatic intolerance. We hypothesized that orthostatic stress is the cause of neurocognitive impairment in subjects with CFS/POTS. We tested neurocognition during a graded head-up tilt table test using an n-back task. The n-back task is a test of working memory, concentration, attention and information processing, and produces progressively increasing levels of cognitive challenge [30–32]. We also hypothesized that decreased cerebral blood flow induced by orthostatic stress may underlie the neurocognitive impairment.
MATERIALS AND METHODS
Subjects
A total of 16 subjects diagnosed previously with CFS using the 1994 CDC criteria [1] were enrolled in the present study. In addition, all CFS subjects had additionally been diagnosed as having co-morbid POTS during a preliminary 70° head-up tilt table test on a separate day. At total of 20 volunteer control subjects, who had no history of illness or orthostatic intolerance, were also enrolled. The age range for all subjects was 15–29 years. Subjects were recruited according to gender so that approximately two-thirds were female in accordance with the female predominance of CFS/POTS.
Prior to inclusion in the study, all CFS/POTS subjects were evaluated by their primary care physician or cardiologist and had a normal physical examination, ECG and echocardiograph. CFS/POTS subjects were free from all systemic illnesses and psychiatric disorders. If any CFS/POTS subjects were on medication, it was discontinued for at least 2 weeks prior to their study date.
Control subjects were recruited using internet and email advertisements, word of mouth and through a healthy volunteer database. Healthy controls had a normal physical examination, normal ECG, normal cardiovascular health and were free from all systemic or psychiatric illnesses, and from orthostatic intolerance. Use of medication was exclusionary for control subjects. If medication had been used, it was discontinued at least 2 weeks prior to their study date.
Prior to acceptance into the study, all subjects filled out a screening questionnaire concerning orthostatic intolerance, review of systems, and cognitive, physical and social functioning. Perfect vision or vision corrected by glasses or contacts and intact depth perception were necessary to complete the protocol, and uncorrected vision or depth perception disabilities were exclusionary.
The research was conducted in accordance with the Declaration of Helsinki of the World Medical Association. This study was approved by the Institutional Review Board of New York Medical College. All subjects 18 years and older signed informed consent prior to participation in the study. Subjects less than 18 years of age gave informed assent and their legal guardians signed informed consent.
Survey and written assessments
Prior to the start of the study all subjects filled out the Edinburgh Handedness Inventory [33] to determine their dominant hand, which was used for responding during the n-back task. In addition, all subjects completed the WTAR (Wechsler Test of Adult Reading) [http://www.pearsonassessments.com/HAIWEB/Cultures/en-us/Productdetail.htm?;Pid=015-8990-145]. This assessed baseline reading ability, intelligence and memory, and estimated any cognitive deficits prior to initiation of the study. Cognitive deficits were exclusionary for participation.
Instrumentation
Subjects were instrumented to monitor their cardiorespiratory parameters during tilt-table testing. Subjects rested supine on a tilt table (Colin Medical) and were instrumented with a Finometer and ECG (FMS) to measure MAP (mean arterial pressure) and HR, a respitrace to measure respiration (Respitrace; NIMS), a nasal cannula connected to a capnograph to measure ETCO2 (end-tidal CO2) (CapnoCheck 9004 Sleep IR capnography; BCI Tran-Anim Smiths Medical) and transcranial Doppler of the left MCA (middle cerebral artery) (Multigon) to measure CBFV. Additionally, subjects were instrumented with video screen eyewear (i-O Display Systems) and earplugs (Silent Ear; Earplug Superstore). The same operators made all measurements and instrumented all subjects for all experimental procedures.
n-back task general description and parameters
The parametric n-back task challenges working memory, attention, concentration and information processing [30,32,35,36]. The ‘n’ refers to a number that indicates increasing difficulty. We used 0-, 1-, 2-, 3- and 4-back levels as progressively increasing mental challenges. At each n-back level of testing, a sequence of alphabetical characters is displayed on a screen and the subject is asked to respond based on the following. During the 0-back level, the subject responds by activating a recording device when a particular character is displayed. We used the letter ‘O’ as the response character. During the 1-back level, the subject responds if the current character displayed on the screen is the same as that previously displayed ‘1’ character back. During the 2-back level, the subject responds if the current character displayed on the screen is the same as that previously displayed ‘2’ characters back. This scheme is repeated incrementally for the ‘3’ and ‘4’ back steps.
The n-back was viewed on the video screen eyewear. The stimulus duration (the amount of time the character was displayed on the screen) was 1 s, and the inter-stimulus duration (the amount of time in between the display of any character) was 1 s [30]. Between each n-back level, there was a 10 s pause [30]. To prevent confusion, the video screen displayed which n-back level was next during this pause. A computer generated a random sequence of 29 capital letters, excluding vowels, for each n-back level. Only the 0-back sequences contained the letter ‘O.’ Each level of the n-back had its own individual, non-repetitive sequence, and for continuity, all subjects saw identical sequences in identical order. n-back levels were presented in sequential order (0-back, then 1-back, then 2-back, then 3-back and then 4-back). Subjects responded during each n-back task by pressing a switch placed in their dominant hand.
Protocol
Throughout the entire protocol, a script was read to all subjects to assure standardization of testing. Following instrumentation, all subjects lay supine on a tilt table and awake with their eyes open for 5 min to accommodate. Subjects then practised responding with the hand-held switch to the beat of a metronome, and then rested for 5 min. Next, subjects underwent three n-back task practice sessions with a 2 min rest between sessions. Subjects were allowed to ask questions during these practice sessions and were monitored to assess if they correctly understood the n-back task. Following successful completion of the n-back practice sessions, no more questions were allowed.
After completion, subjects lay supine and awake with their eyes open for 15 min. Baseline measurements were taken at the last 3 min. The subjects then completed an n-back task while supine. Following the task, the subjects were tilted to 15°, 30°, 45°, 60° and 75° for 10 min at each angle. During each tilt angle, the first 1 min of data were excluded to allow for haemodynamic stabilization to occur. Minutes 2–4 of each tilt angle were taken as the baseline values for that angle. An n-back task was started at minute 4 of each tilt angle and completed. Subjects remained at each angle until the 10-min point was reached and were then tilted to the next angle. During head-up tilt, subjects were asked not to move, to breathe normally, to keep their eyes open and to remain awake. Subjects were instructed to speak if they felt ill, nauseated or presyncopal as a result of head-up tilt. Presyncope was defined as a fall in systolic blood pressure of 20 mmHg or a systolic blood pressure below 80 mmHg with signs and symptoms of orthostatic intolerance such as nausea, diaphoresis, pallor and/or headache. Each subject completed the full tilt unless they requested to be returned to the supine position, at which point the test was ended. If significant hypotension below 80 mmHg and/or bradycardia below 50 beats/min occurred, subjects were immediately lowered to the supine position and the test was ended. At the end of the tilt table testing, subjects returned to the supine position and remained still for 10 min.
Data analysis and statistics
For each n-back, the number of correct responses (C), missed responses and incorrect false responses (F) were recorded. Correct responses were defined as the subject appropriately responding to an n-back repeat. Missed responses were defined as a subject not responding to an n-back repeat when he/she should have. False responses were defined as a subject responding inappropriately when an n-back repeat was not presented. Absolute RT was calculated in milliseconds as the difference between the time the letter first appeared (t0) on the video screen and the time the subject responded (tr). Thus RT = tr − t0. For each level of the n-back, we report the mean RT. An nRT (normalized RT) was calculated as the mean absolute RT per total number of responses, or the mean time per response, and nRT = RT/(C + F). This took into account the number of times a subject responded.
Data were recorded continuously at 500 Hz. NCSS 2007 (LCC) statistical software was used. Demographic and WTAR were analysed using two-sided independent Student’s t tests. Additionally, group survival throughout tilt testing was analysed using the Kaplan–Meier (Logrank) test. Then 95% CIs (confidence intervals) were calculated using the log-transformation method because of the small sample size. Since each angle was maintained for the same length of time, the progressive change in angle was used as an index of time for the survival curve.
Data were analysed using a three-way ANOVA with Student–Newman–Keuls post-hoc tests to determine main effects and two-way interactions when appropriate. When the three-way interaction was significant, post-hoc Student’s t tests were used to determine differences only between groups [37]. In addition for the three-way interaction, standard errors used in the Student’s t tests were calculated as the square root of the mean square of the error term divided by the number of subjects. These standard errors were more conservative and more suitable for multiple comparison testing [38]. Results are reported as means ± S.E.M. Significance was set at P < 0.05.
RESULTS
Demographics
The mean age (21 ± 1 compared with 23 ± 1 years), height (171 ± 2 compared with 167 ± 2 cm), weight (63 ± 3 compared with 66 ± 4 kg) and body mass index (21 ± 1 compared with 23 ± 1 kg/m2) were not different between the CFS/POTS and control subjects respectively. Of the CFS/POTS patients, 14 were right-handed and two were left handed. A total of 18 control subjects were right-handed and two were left handed. The percentage of handedness between groups was not significantly different.
WTAR outcomes
As shown in Table 1, WTAR scores between the CFS/POTS and control subjects were not significantly different. In addition, in Table 1, the WTAR approximations of the WAIS III (Wechsler Adult Intelligence Scale III) and of the WMS III (Wechsler Memory Scale III) between the CFS/POTS and control subjects were not significantly different.
Table 1.
Baseline standardized intelligence and memory scores
| Measurement | Control | CFS/POTS |
|---|---|---|
| WTAR standard score | 118 ± 2 | 115 ± 2 |
| WAIS III subscales | ||
| Full scale IQ | 112 ± 1 | 111 ± 2 |
| Verbal IQ | 113 ± 2 | 111 ± 2 |
| Performance IQ | 110 ± 1 | 108 ± 1 |
| Verbal comprehension index | 113 ± 2 | 111 ± 1 |
| Perceptional organization index | 110 ± 1 | 108 ± 1 |
| Working memory index | 110 ± 1 | 109 ± 1 |
| Processing speed index | 108 ± 1 | 107 ± 1 |
| WMS III subscales | ||
| Immediate memory | 109 ± 1 | 107 ± 1 |
| General memory | 109 ± 1 | 108 ± 1 |
| Working memory | 109 ± 1 | 108 ± 1 |
Values are means ± S.E.M. There were no statistical differences between groups.
Subject dropout per angle
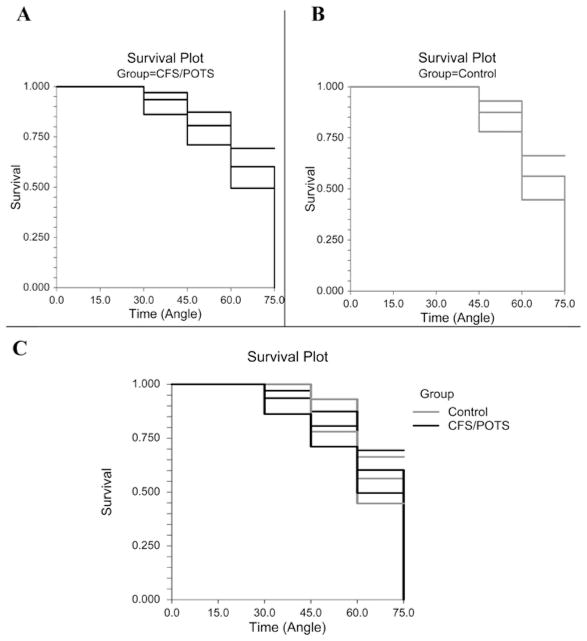
Figure 1 shows the survival curves or the ability of subjects to complete each successive tilt angle, for CFS/POTS and control subjects individually, as well as a combined curve of both groups. All 16 CFS/POTS and 20 control subjects completed the 0° and 15° tilts. A total of 15 CFS/POTS and 20 control subjects completed the 30° tilt. A total of 14 CFS/POTS and 18 control subjects completed the 45° tilt. A total of 13 CFS/POTS and 15 control subjects completed the 60° tilt. Seven CFS/POTS and 11 control subjects completed 75°. Kaplan–Meiers analysis using the logrank test found that survival throughout tilt was not significantly different between groups.
Figure 1. Survival analysis with 95 % CIs during graded tilt between CFS/POTS (black) and control (grey) subjects.
(A) The survival curve for the CFS/POTS subjects shows that the greatest amount of subject dropout occurred at 60° and 75°. (B) The survival curve for the control subjects shows that the greatest amount of subject dropout occurred at 60° and 75°. (C) The survival curve comparing CFS/POTS and control subjects shows that there was no difference between groups (P = 0.91).
Number of correct responses
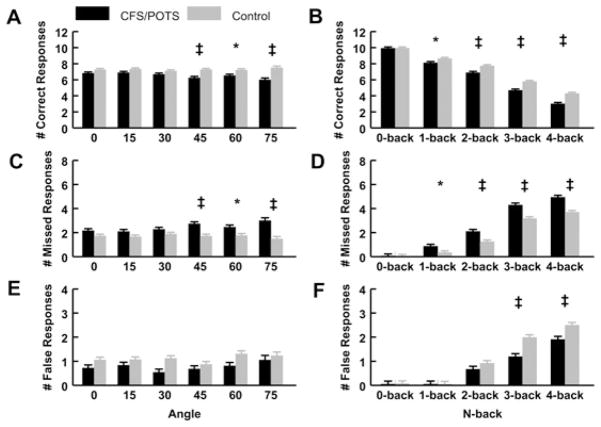
As shown in Figure 2(A), when evaluating the number of correct responses, the relationships between group and angle showed that the CFS/POTS subjects responded less correctly than controls at 45°, 60° and 75° (P < 0.05). Figure 2(B) shows that, in the two-way interaction between group and n-back, the CFS/POTS subjects responded less correctly than controls during the 1-, 2-, 3- and 4-back tests (P < 0.05).
Figure 2. Number of correct, missed and false responses during graded tilt and the n-back tasks between CFS/POTS (black) and control (grey) subjects.
(A) The analysis of group and angle illustrated that CFS/POTS had a lower number of correct responses than controls during 45°, 60° and 75°. (B) The analysis between group and n-back showed that CFS/POTS had a lower number of correct responses than controls during the 2-, 3- and 4-back. (C) The analysis of group and angle showed that CFS/POTS subjects missed more responses than controls at 45°, 60° and 75°. (D) The analysis between group and n-back showed that CFS/POTS subjects missed more responses than controls during the 1-, 2-, 3- and 4-back. (E) The analysis of group and angle illustrated that CFS/POTS and control subjects did not differ in the number of false responses as angle increased. (F) The analysis between group and n-back showed that CFS/POTS subjects had less false responses than controls during the 3- and 4-back. Values are means ± S.E.M. *P < 0.05, †P < 0.01 and ‡P < 0.001.
Analysis of the data as a function of group, angle and n-back found that CFS/POTS subjects responded less correctly than controls during the 15° 4-back, the 30° 4-back, the 45° 3-back and 4-back, the 60° 4-back, the 75° 2-back, 3-back and 4-back (CFS/POTS compared with control, P < 0.01 for all).
Number of missed responses
Figure 2(C) illustrates that CFS/POTS subjects missed significantly more responses than controls at 45°, 60° and 75° (P < 0.05). We found that CFS/POTS subjects missed significantly more responses than controls during the 1-, 2-, 3- and 4-back (P < 0.05), as shown in Figure 2(D).
Analysis of the data as a function of group, angle and n-back determined that CFS/POTS subjects also missed significantly more responses than controls during the 15° 4-back, the 45° 4-back, the 60° 4-back, the 75° 2-back, 3-back and 4-back (P < 0.05).
Number of false responses
There were no significant effects of angle between groups (see Figure 2E). Figure 2(F) shows that the number of false responses recorded for CFS/POTS subjects were significantly less than controls during the 3-back and 4-back (P < 0.05). Analysis of the data as a function of group, angle and n-back determined that CFS/POTS subjects responded falsely significantly less often than controls during the 0° 3-back, the 30° 3-back and 4-back and the 60° 4-back (CFS/POTS compared with control, P < 0.05).
nRT
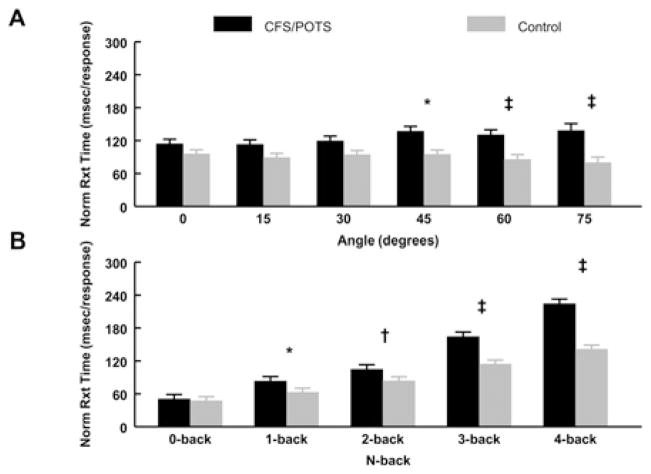
As shown in Figure 3(A), CFS/POTS subjects had a greater nRT compared with controls at 45°, 60° and 75°. Figure 3(B) shows that CFS/POTS subjects had a greater nRT than controls during the 1-, 2-, 3- and 4-back. When assessing group, angle and n-back, CFS/POTS subjects had significantly longer nRTs than controls during the 45° 3-back and 4-back, the 60° 3-back and 4-back, and the 75° 2-back, 3-back and 4-back (P < 0.05).
Figure 3. nRT during graded tilt and the n-back tasks between CFS/POTS (black) and control (grey) subjects.
(A) The analysis of group and angle showed that CFS/POTS had a greater nRT than controls at 45°, 60° and 75°. (B) The analysis between group and n-back showed that CFS/POTS had a greater nRT than controls during the 1-, 2-, 3- and 4-back. Values are means ± S.E.M. *P < 0.05, †P < 0.01 and ‡P < 0.001.
Baseline cardiorespiratory measurements
As shown in Table 2, CFS/POTS subjects had a significantly higher HR and respiratory rate than control subjects at baseline. MAP, ETCO2 and CBFV were not different between the groups at baseline.
Table 2.
Baseline cardiorespiratory and cerebrovascular values between the control and CFS/POTS groups
| Measurement | Control | CFS/POTS |
|---|---|---|
| MAP (mmHg) | 81 ± 2 | 81 ± 1 |
| HR (beats/min) | 66 ± 2 | 75 ± 4* |
| ETCO2 (mmHg) | 44 ± 1 | 44 ± 1 |
| Respiratory rate (breaths/min) | 16 ± 1 | 18 ± 1* |
| CBFV (cm/s) | 74 ± 3 | 73 ± 3 |
Values are means ± S.E.M. During supine baseline conditions, CFS/POTS subjects had a higher HR and a higher respiratory rate than control subjects.
P < 0.05 compared with control subjects.
MAP
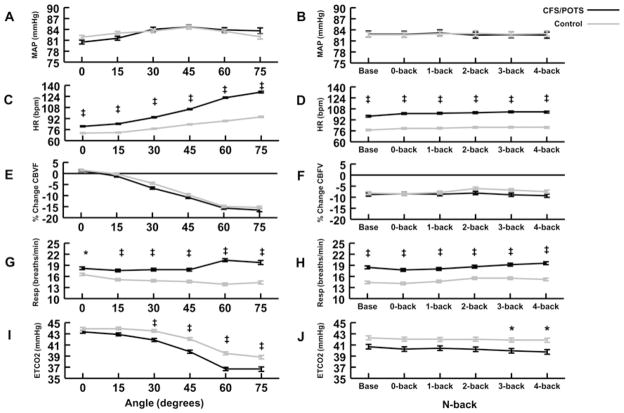
As shown in Figure 4(A), when MAP was analysed as a function of tilt angle, there were no significant differences comparing CFS/POTS subjects with controls. Figure 4(B) shows that, when MAP was analysed as a function of the n-back difficulty level, there were no differences between these two groups. In addition, there were no significant differences when the data were evaluated as a function of group, angle and n-back. Thus MAP was relatively maintained throughout tilt at all angles in both the CFS/POTS and control subjects and was not affected by the n-back testing.
Figure 4. Physiological changes during graded tilt and the n-back tasks between CFS/POTS (black) and control (grey) subjects.
(A) MAP was not different between groups as angle increased. (B) MAP was not different between groups as the n-back difficulty level increased. (C) HR was high in CFS/POTS subjects at all angles compared with control subjects. (D) HR was higher in CFS/POTS subjects at baseline and all n-back difficulty levels compared with control subjects. (E) The percentage change in CBFV was not different between groups as the angle increased. (F) The percent change in CBFV was not different between groups at baseline and as the n-back difficulty level increased. (G) Respiratory rate (Resp) was higher in CFS/POTS subjects at all angles compared with control subjects. (H) Respiratory rate was higher in CFS/POTS subjects at baseline and all levels of n-back difficulty. (I) ETCO2 was lower in CFS/POTS subjects at 30°, 45°, 60° and 75° compared with control subjects. ETCO2 was lower in CFS/POTS subjects at the 3- and 4-back n-back difficulty levels compared with controls. Values are means ± S.E.M. *P < 0.05 and ‡P < 0.001.
HR
As shown in Figure 4(C), as a function of tilt angle, CFS/POTS subjects had significantly higher HRs at 0, 15, 30, 45, 60 and 75° (P < 0.05). Figure 4(D) shows that CFS/POTS subjects had significantly higher HRs compared with controls during baseline, 0-, 1-, 2-, 3- and 4-back (P < 0.05).
Analysis of the data as a function of group, angle and n-back showed that CFS/POTS subjects had a higher HR than controls at all angles and all n-backs (P < 0.05). To determine whether the results of the three-way interaction was mainly due to the group differences at baseline, at each angle, or to the challenge of each n-back trial, a separate post-hoc ANOVA analysis between groups compared the percentage change in HR during each angle’s n-back trials to each angle’s baseline. This showed that HR progressively increased as the tilt angle increased in both groups, while there were no differences in the group–n-back interaction. Thus, for CFS/POTS subjects against controls, higher HR at baseline and the change in angle appear to be the main factors influencing HR between the groups rather than the challenge of the n-back trials.
CBFV
As shown in Figure 4(E), there were no significant differences between CBFV of the CFS/POTS and control subjects as a function of tilt angle. Figure 4(F) shows that there were no significant differences between groups comparing CBFV measured at different n-backs. No differences were found when the data were analysed as a function of group, angle and n-back.
Respiration rate
Figure 4(G) shows that CFS/POTS subjects had a significantly higher respiratory rate than controls at 0°, 15°, 30°, 45°, 60° and 75° (P < 0.05). Figure 4(H) demonstrates that CFS/POTS subjects had a higher respiratory rate than controls at baseline, 0-, 1-, 2-, 3-and 4-back (P < 0.05).
Analysis of the data as a function of group, angle and n-back showed that CFS/POTS subjects had a higher respiratory frequency than controls at 30° during baseline and all n-backs (P < 0.05), 45° during baseline and all n-backs (P < 0.05), 60° during baseline and all n-backs (P < 0.001), and 75° during baseline and all n-backs (P < 0.05). To determine whether the results of the three-way interactions was mainly due to the group differences at baseline, at each angle, or to the challenge of each n-back trial, a separate post-hoc ANOVA between groups compared the percentage change in respiratory frequency during each angle’s n-back trials with each angle’s baseline. We determined that the change in angle significantly affected respiratory rate. There was also a significant n-back effect showing that 2-, 3- and 4-back were greater than 0- and 1-back (P < 0.05 for both), but not different from each other.
CFS/POTS subjects had a higher percentage change in respiratory frequency during n-back compared with baseline than controls at 0°, 60° and 75° (all P < 0.05), and also had a higher percentage change in respiratory frequency during n-back compared with baseline than controls at 0-back (P < 0.05). Within-group analysis showed that both groups increased their respiratory rate at 4-back compared with 0- and 1-back (P < 0.05 for both). Thus the higher baseline respiratory rate and the change in angle appear to be the main factors influencing respiration between groups, but the increasing challenge of the n-back trials is influential at the 4-back for both groups.
ETCO2
As shown in Figure 4(I), CFS/POTS subjects had a significantly lower ETCO2 than controls at 30°, 45°, 60° and 75°. Figure 4(J), which shows the interaction between group and n-back, demonstrates that CFS/POTS subjects had significantly lower ETCO2 than controls during the 3- and 4-back.
Analysis of the data as a function of group, angle and n-back showed that CFS/POTS subjects had a significantly lower ETCO2 than controls during 45° baseline, 60° 1-, 2-, 3- and 4-back, and the 75° 4-back (P < 0.05).
DISCUSSION
Main findings
Our main finding was that the performance of CFS/POTS subjects worsened during the n-back task as orthostatic stress increased. The task performance of CFS/POTS subjects worsened further as the n-back difficulty increased, but only during imposition of an orthostatic challenge. In healthy controls, performance was not impaired. Therefore our study indicates, for the first time, that CFS/POTS subjects are neurocognitively impaired when exposed to orthostatic stress and difficult mental challenges. We also showed no CBFV differences between CFS/POTS subjects and controls and, therefore, could not associate altered CBFV with neurocognitive impairment.
CFS/POTS subjects have decreased accuracy and longer nRT during difficult tasks imposed on orthostatic stress
We measured the accuracy of each group’s n-back responses. During the more difficult neurocognitive tasks associated with a ‘higher’ n-back, CFS/POTS subjects were less accurate than controls. Also, the higher levels of orthostatic stress worsened the ability of CFS/POTS subjects to respond accurately during cognitive challenges. Therefore CFS/POTS subjects have impaired working memory, concentration and executive functioning during difficult tasks and high levels of orthostatic stress. These changes were not due to pre-existing deficits in reading, intelligence or memory (see Table 1).
Two-way analyses contrasted each group per angle and per n-back difficulty. These results show that CFS/POTS subjects are more susceptible to the adverse effects of combined mental and orthostatic stress than control subjects.
Complimentary to accuracy, RT assessed processing speed. Unexpectedly, absolute RT was not different between groups (results not shown), but nRT was. Adding the number of correct and false responses, CFS/POTS subjects did not respond as often as control subjects, and this created an analytical bias. When this was accounted for by nRT, group differences in response time became evident.
Comparing groups at each angle and n-back difficulty level, CFS/POTS subjects responded more slowly than controls. Moderate and severe orthostatic stress (45°, 60° and 75°) negatively affected the nRT of CFS/POTS subjects (see Figure 3A). In addition, as the difficulty of the n-back increased, the nRT of the CFS/POTS subjects progressively increased compared with controls (see Figure 3B). This is the first study to also show that increasing orthostatic stress has a negative impact on processing speed in response to a difficult cognitive challenge in CFS/POTS subjects.
The present work supports the findings in the literature. Considering the results we obtained at 0°, our CFS/POTS subjects responded similarly in accuracy and response time to the CFS subjects studied by Caseras et al. [31]. Furthermore, it has been reported previously that CFS subjects exhibit working memory deficits only during the most demanding tasks tested [15], which is in accord with our finding that CFS/POTS subjects had deficits during the 4-back. Similarly, Vollmer-Conna et al. [39] showed that CFS subjects had decreased accuracy and a slower RT on performance tests. In contrast, DeLuca et al. [13], using paced auditory serial addition task testing, did not find any accuracy deficits in CFS subjects compared with controls, yet found that CFS subjects had deficits in information processing speed [40,41]. The disparity between their study and ours concerning accuracy may be due to the difference in the tests applied, but both studies support decreased processing speed in CFS/POTS subjects. A recent meta-analysis of several studies concluded that information processing speed, but not RT, is impaired in CFS subjects in accord with our findings on CFS/POTS subjects [42].
CFS/POTS subjects respond falsely less often during orthostatic stress
Unexpectedly, CFS/POTS subjects responded falsely less often than controls (see Figures 2E and 2F). Increasing orthostatic stress did not have an effect on this type of response because CFS/POTS subjects responded falsely less often than control subjects at 0° (no orthostatic stress), at 30° (mild orthostatic stress) and 60° (moderate to high orthostatic stress); the main factor appeared to be n-back difficulty. At 0°, 30° and 60°, CFS/POTS subjects responded less falsely than controls during more difficult n-backs. The cause of CFS/POTS subjects responding falsely less often than controls may be that with increased difficulty, CFS/POTS subjects were cognitively impaired, attempted fewer responses, and thus had a smaller likelihood of responding falsely. Another possibility is that CFS/POTS subjects experienced neurocognitive impairment, ‘mental fog’, thus only responded when they believed they were correct.
CFS/POTS subjects do not have an increased incidence of presyncope
Graded tilt progressively increases the orthostatic challenge and provides prolonged stress that can lead to syncope. As the angle of tilt increases, the possibility of presyncope and subject dropout increases [43]. Survival analysis showed that CFS/POTS subjects and controls had similar subject dropout rates; thus, the incidence of presyncopal symptoms during graded tilt was similar between groups (see Figure 1). CFS/POTS subjects do not faint more often than controls, consistent with other studies describing syncope in adolescent/young adult CFS and/or POTS patients [10,44–47].
Physiological changes in CFS/POTS subjects during graded-tilt
At baseline, CFS/POTS subjects exhibited increased HR and respiratory rates, typical of this population due to decreased vagal tone at rest and/or cardiovascular deconditioning (see Table 2) [4,7]. In addition, the increased HR may be directly related to the increased respiratory drive via sinus arrhythmia due to vagal modulation. For both groups during graded tilt, MAP was relatively maintained, HR increased, and CBFV and ETCO2 decreased. For both groups, the cognitive challenge of the n-back did not produce significant physiological changes, except for increasing respiratory rate during the 3- and 4-backs. The higher HR in CFS/POTS subjects may be related to central hypovolaemia, decreased vagal tone, increased sympathetic activity or less effective reflex sympathetic influence on TPR (total peripheral resistance) [5,7]. Similar MAP between groups is not surprising because POTS patients tend not to experience syncope in daily life, and the rate of presyncope in this study was similar between groups [47]. Maintenance of MAP suggests proper functioning of the arterial baroceptors reflex in CFS/POTS subjects. Increased respiratory rate throughout graded tilt in CFS/POTS subjects probably produced the decreased ETCO2 compared with controls. The increased respiratory drive in CFS/POTS subjects is not fully understood. Respiratory changes in CFS/POTS subjects occur, as approximately 50% become hypocapnic and hyperventilate during head-up tilting; this may be related thoracic hypovolaemia, increased sympathetic activation, modulation of the peripheral chemoreceptors in response to hypoxia, and/or by higher CNS (central nervous system) influences [48,49]. In both groups, the decrease in ETCO2 can account for the decreased CBFV observed, since hypocapnia produces cerebrovascular vasoconstriction.
Neurocognitive impairment was not related to physiological changes or cerebral blood flow
Our hypothesis that neurocognitive impairment in CFS/POTS is related to CBFV was not supported by our results. We found no difference in CBFV between groups during any angle (see Figures 4E and 4F), and found no significant correlation between CBFV and n-back. We found no correlation between physiological variables and neurocognitive testing. This is at odds with our previous work, where we showed that POTS subjects had decreased CBFV compared with controls, but in that study subject selection criteria were different and a non-incremental tilt to 70° was used [18]. The findings of the present study that CBFV was not different between groups during tilt is consistent with the work of Rowe and co-workers [29]. During cognitive challenge, changes in CBFV are small and, therefore, these changes may well be obscured by the effects of orthostatic stress [50].
Thus the results of the present study give no clear indication of what causes the neurocognitive impairment frequently described by CFS/POTS subjects. We have, however, shown that high levels of orthostatic stress combined with cognitive challenges are directly associated with neurocognitive impairment, manifested by decreased accuracy and increased nRT. We also showed that changes in MAP and CBFV were not related to the induced neurocognitive impairment. In POTS, the characteristic tachycardia is thought to be due to central hypovolaemia and autonomic nervous system dysfunction [7]. Thus it is reasonable to speculate that these factors are also related to neurocognitive changes in CFS/POTS subjects, but future research is necessary to test whether any link is causal.
Limitations
Assessment of ‘neurocognition’ with one test is not possible. The n-back was chosen as an established way to assess working memory, attention, RT, information processing and increasing cognitive challenge rapidly and repeatedly over graded tilt because CFS/POTS subjects commonly described deficits in those areas [12,13,31].
Graded tilt table testing is not the same as standing. Therefore, how neurocognitive processes are affected by orthostatic stress in CFS/POTS during daily life was not directly measured.
Transcranial Doppler sonography only measures blood flow through a particular cerebral blood vessel, but has good temporal accuracy. We chose the MCA because it is the main vessel that perfuses the area of the brain that the n-back test activates [30]. The spatial accuracy of transcranial Doppler is not large and was limited to the perfusion region of the MCA. Also, our measurements of CBFV are not equivalent to cerebral blood flow, but changes in both cerebral blood flow and CBFV have been found to highly correlate with each other [51–53]. Additionally, the work by Serrador et al. [54] supports the linear relationship between CBFV and cerebral blood flow because they showed that the diameter of the MCA does not change during orthostatic stress. Future studies should include determination of cerebral autoregulation and may use SPECT and MRI (magnetic resonance imaging)-based scans in combination with an orthostatic and cognitive stressor. These may inform on local and regional changes in cerebral blood flow that transcranial Doppler cannot detect. Since we only measured CBFV though the MCA, future work should include measurements of the basilar and anterior cerebral arteries as well.
The n-back levels were presented in sequential order in attempt to eliminate subject confusion as to which n-back level came next. An alternative study design would have been to present the n-back levels in random order. Thus there could have been a learning effect, but all participants were subject to similar conditions. In addition, since subjects may have completed the n-back task up to six times (while supine and at each tilt angle), there was a chance for a learning effect to occur. If subjects learned how to successfully complete the n-back through repetition, it would have been expected that subjects would improve their accuracy and RT with each successive trial. This was not the case in either control or CFS/POTS subjects. Thus, although the present study design may have imparted a learning effect on the results, this was not measurable.
Summary and practical significance
The present study is the first to show that increasing orthostatic stress impairs cognitive performance in CFS/POTS subjects. The present study may have a strong practical application to those with CFS/POTS. Our results show that CFS/POTS subjects do not have differences in intelligence, but rather experience cognitive impairment mainly due to the effect of orthostatic stress, especially during difficult tasks.
In addition, we show that the information processing speed of CFS/POTS subjects may be affected by standing, especially during difficult tasks. In school, CFS/POTS subjects may require more time during testing, and tasks to be done while standing will be more difficult. During testing, an increased allotment of time may be beneficial to the performance of CFS/POTS subjects. Workplace arrangements that limit standing may improve performance for individuals with CFS/POTS.
Additional work is necessary to determine whether orthostatic stress and/or cognitive challenges have detrimental effects in CFS subjects without POTS or in POTS subjects without CFS. Although we would speculate that, in CFS subjects without POTS, cognitive challenges would correlate with decreased accuracy and RT, we are unsure about the effect of orthostatic stress on neurocognitive function in CFS subjects who are orthostatically tolerant. We would also assume that, in POTS subjects without CFS, increasing orthostatic stress would result in decreased accuracy and RT.
Overall, orthostatic stress impaired the cognitive abilities of CFS/POTS subjects compared with control subjects who were not affected. Changes in cerebral blood flow were not related to neurocognitive impairment. Future work is necessary to link physiological changes observed in CFS/POTS subjects with their cognitive deficits.
Acknowledgments
We thank the Departments of Physiology and Pediatric Cardiology for their scientific inspiration, support and encouragement.
FUNDING
This work was supported by the National Heart, Lung, and Blood Institute [grant numbers 1-F30-HL-097380 (to A.J.O.), 1-RO1-HL-074873 (to J.M.S.), 1-RO1-HL-087803 (to J.M.S.)], and the Chronic Fatigue and Immune Deficiency Syndrome Association of America (grant to M.S.M.).
Abbreviations
- CBFV
cerebral blood flow velocity
- CDC
Center for Disease Control and Prevention
- CFS
chronic fatigue syndrome
- CI
confidence interval
- ETCO2
end-tidal CO2
- HR
heart rate
- MAP
mean arterial pressure
- MCA
middle cerebral artery
- POTS
postural tachycardia syndrome
- RT
reaction time
- nRT
normalized RT
- SPECT
single-photon emission computed tomography
- WAIS III
Wechsler Adult Intelligence Scale II
- WMS III
Wechsler Memory Scale III
- WTAR
Wechsler Test of Adult Reading
Footnotes
AUTHOR CONTRIBUTION
Anthony Ocon, Zachary Messer, Marvin Medow and Julian Stewart all took part in the design and conduct of the study. Anthony Ocon and Zachary Messer were in charge of data collection, management and analysis. Anthony Ocon, Marvin Medow and Julian Stewart all took part in the interpretation of the data. Anthony Ocon, Zachary Messer, Marvin Medow and Julian Stewart all took part in the preparation, revision and approval of the paper.
References
- 1.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry. 2003;160:221–236. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 3.Jain SS, DeLisa JA. Chronic fatigue syndrome: a literature review from a physiatric perspective. Am J Phys Med Rehabil. 1998;77:160–167. doi: 10.1097/00002060-199803000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Freeman R, Komaroff AL. Does the chronic fatigue syndrome involve the autonomic nervous system? Am J Med. 1997;102:357–364. doi: 10.1016/s0002-9343(97)00087-9. [DOI] [PubMed] [Google Scholar]
- 5.Schondorf R, Freeman R. The importance of orthostatic intolerance in the chronic fatigue syndrome. Am J Med Sci. 1999;317:117–123. doi: 10.1097/00000441-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 7.Medow MS, Stewart JM. The postural tachycardia syndrome. Cardiol Rev. 2007;15:67–75. doi: 10.1097/01.crd.0000233768.68421.40. [DOI] [PubMed] [Google Scholar]
- 8.Hoad A, Spickett G, Elliott J, Newton J. Postural orthostatic tachycardia syndrome is an under-recognized condition in chronic fatigue syndrome. Q J Med. 2008;101:961–965. doi: 10.1093/qjmed/hcn123. [DOI] [PubMed] [Google Scholar]
- 9.Stewart JM, Gewitz MH, Weldon A, Arlievsky N, Li K, Munoz J. Orthostatic intolerance in adolescent chronic fatigue syndrome. Pediatrics. 1999;103:116–121. doi: 10.1542/peds.103.1.116. [DOI] [PubMed] [Google Scholar]
- 10.Karas B, Grubb BP, Boehm K, Kip K. The postural orthostatic tachycardia syndrome: a potentially treatable cause of chronic fatigue, exercise intolerance, and cognitive impairment in adolescents. Pacing Clin Electrophysiol. 2000;23:344–351. doi: 10.1111/j.1540-8159.2000.tb06760.x. [DOI] [PubMed] [Google Scholar]
- 11.Komaroff A. Clinical presentation of chronic fatigue syndrome. Ciba Found Symp. 1993;173:43–53. doi: 10.1002/9780470514382.ch4. [DOI] [PubMed] [Google Scholar]
- 12.DeLuca J, Johnson SK, Ellis SP, Natelson BH. Cognitive functioning is impaired in patients with chronic fatigue syndrome devoid of psychiatric disease. J Neurol Neurosurg Psychiatry. 1997;62:151–155. doi: 10.1136/jnnp.62.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLuca J, Christodoulou C, Diamond BJ, Rosenstein ED, Kramer N, Natelson BH. Working memory deficits in chronic fatigue syndrome: differentiating between speed and accuracy of information processing. J Int Neuropsychol Soc. 2004;10:101–109. doi: 10.1017/S1355617704101124. [DOI] [PubMed] [Google Scholar]
- 14.Joyce E, Blumenthal S, Wessely S. Memory, attention, and executive function in chronic fatigue syndrome. J Neurol Neurosurg Psychiatry. 1996;60:495–503. doi: 10.1136/jnnp.60.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobbs BM, Dobbs AR, Kiss I. Working memory deficits associated with chronic fatigue syndrome. J Int Neuropsychol Soc. 2001;7:285–293. doi: 10.1017/s1355617701733024. [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen R. Chronic fatigue: an evolutionary concept analysis. J Adv Nurs. 2008;63:199–207. doi: 10.1111/j.1365-2648.2008.04649.x. [DOI] [PubMed] [Google Scholar]
- 17.Natelson BH, Lange G. A status report on chronic fatigue syndrome. Environ Health Perspect. 2002;110 (Suppl 4):673–677. doi: 10.1289/ehp.02110s4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ocon AJ, Medow MS, Taneja I, Clarke D, Stewart JM. Decreased upright cerebral blood flow and cerebral autoregulation in normocapnic postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H664–H673. doi: 10.1152/ajpheart.00138.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Low PA, Novak V, Spies JM, Novak P, Petty GW. Cerebrovascular regulation in the postural orthostatic tachycardia syndrome (POTS) Am J Med Sci. 1999;317:124–133. doi: 10.1097/00000441-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Schondorf R, Benoit J, Stein R. Cerebral autoregulation is preserved in postural tachycardia syndrome. J Appl Physiol. 2005;99:828–835. doi: 10.1152/japplphysiol.00225.2005. [DOI] [PubMed] [Google Scholar]
- 21.Costa DC, Tannock C, Brostoff J. Brainstem perfusion is impaired in chronic fatigue syndrome. Q J Med. 1995;88:767–773. [PubMed] [Google Scholar]
- 22.Ichise M, Salit IE, Abbey SE, Chung DG, Gray B, Kirsh JC, Freedman M. Assessment of regional cerebral perfusion by 99Tcm-HMPAO SPECT in chronic fatigue syndrome. Nucl Med Commun. 1992;13:767–772. [PubMed] [Google Scholar]
- 23.Schwartz RB, Komaroff AL, Garada BM, Gleit M, Doolittle TH, Bates DW, Vasile RG, Holman BL. SPECT imaging of the brain: comparison of findings in patients with chronic fatigue syndrome, AIDS dementia complex, and major unipolar depression. Am J Roentgenol. 1994;162:943–951. doi: 10.2214/ajr.162.4.8141022. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka H, Matsushima R, Tamai H, Kajimoto Y. Impaired postural cerebral hemodynamics in young patients with chronic fatigue with and without orthostatic intolerance. J Pediatr. 2002;140:412–417. doi: 10.1067/mpd.2002.122725. [DOI] [PubMed] [Google Scholar]
- 25.Yoshiuchi K, Farkas J, Natelson BH. Patients with chronic fatigue syndrome have reduced absolute cortical blood flow. Clin Physiol Funct Imaging. 2006;26:83–86. doi: 10.1111/j.1475-097X.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- 26.Biswal B, Kunwar P, Natelson BH. Cerebral blood flow is reduced in chronic fatigue syndrome as assessed by arterial spin labeling. J Neurol Sci. 2011;301:9–11. doi: 10.1016/j.jns.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischler B, D’Haenen H, Cluydts R, Michiels V, Demets K, Bossuyt A, Kaufman L, De Meirleir K. Comparison of 99m Tc HMPAO SPECT scan between chronic fatigue syndrome, major depression and healthy controls: an exploratory study of clinical correlates of regional cerebral blood flow. Neuropsychobiology. 1996;34:175–183. doi: 10.1159/000119307. [DOI] [PubMed] [Google Scholar]
- 28.MacHale SM, Lawrie SM, Cavanagh JT, Glabus MF, Murray CL, Goodwin GM, Ebmeier KP. Cerebral perfusion in chronic fatigue syndrome and depression. Br J Psychiatry. 2000;176:550–556. doi: 10.1192/bjp.176.6.550. [DOI] [PubMed] [Google Scholar]
- 29.Razumovsky AY, DeBusk K, Calkins H, Snader S, Lucas KE, Vyas P, Hanley DF, Rowe PC. Cerebral and systemic hemodynamics changes during upright tilt in chronic fatigue syndrome. J Neuroimaging. 2003;13:57–67. [PubMed] [Google Scholar]
- 30.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 31.Caseras X, Mataix-Cols D, Giampietro V, Rimes KA, Brammer M, Zelaya F, Chalder T, Godfrey EL. Probing the working memory system in chronic fatigue syndrome: a functional magnetic resonance imaging study using the n-back task. Psychosom Med. 2006;68:947–955. doi: 10.1097/01.psy.0000242770.50979.5f. [DOI] [PubMed] [Google Scholar]
- 32.Gevins AS, Bressler SL, Cutillo BA, Illes J, Miller JC, Stern J, Jex HR. Effects of prolonged mental work on functional brain topography. Electroencephalogr Clin Neurophysiol. 1990;76:339–350. doi: 10.1016/0013-4694(90)90035-i. [DOI] [PubMed] [Google Scholar]
- 33.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 34.Reference deleted
- 35.Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 36.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen BH. Three-way ANOVA: Explaining Psychological Statistics. Wiley; Hoboken, NJ: 2004. pp. 688–746. [Google Scholar]
- 38.Hintze JL. NCSS statistical system for Windows. NCSS; Kaysville, UT: 2007. ( www.ncss.com) [Google Scholar]
- 39.Vollmer-Conna U, Wakefield D, Lloyd A, Hickie I, Lemon J, Bird KD, Westbrook RF. Cognitive deficits in patients suffering from chronic fatigue syndrome, acute infective illness or depression. Br J Psychiatry. 1997;171:377–381. doi: 10.1192/bjp.171.4.377. [DOI] [PubMed] [Google Scholar]
- 40.DeLuca J, Johnson SK, Natelson BH. Information processing efficiency in chronic fatigue syndrome and multiple sclerosis. Arch Neurol. 1993;50:301–304. doi: 10.1001/archneur.1993.00540030065016. [DOI] [PubMed] [Google Scholar]
- 41.DeLuca J, Johnson SK, Beldowicz D, Natelson BH. Neuropsychological impairments in chronic fatigue syndrome, multiple sclerosis, and depression. J Neurol Neurosurg Psychiatry. 1995;58:38–43. doi: 10.1136/jnnp.58.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cockshell SJ, Mathias JL. Cognitive functioning in chronic fatigue syndrome: a meta-analysis. Psychol Med. 2010;40:1253–1267. doi: 10.1017/S0033291709992054. [DOI] [PubMed] [Google Scholar]
- 43.Laszlo Z, Rossler A, Hinghofer-Szalkay HG. Cardiovascular and humoral readjustment after different levels of head-up tilt in humans. Aviat Space Environ Med. 2001;72:193–201. [PubMed] [Google Scholar]
- 44.Timmers HJ, Wieling W, Soetekouw PM, Bleijenberg G, van der Meer JW, Lenders JW. Hemodynamic and neurohumoral responses to head-up tilt in patients with chronic fatigue syndrome. Clin Auton Res. 2002;12:273–280. doi: 10.1007/s10286-002-0014-1. [DOI] [PubMed] [Google Scholar]
- 45.Stewart JM. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr Res. 2000;48:218–226. doi: 10.1203/00006450-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 46.Stewart JM, Gewitz MH, Weldon A, Munoz J. Patterns of orthostatic intolerance: the orthostatic tachycardia syndrome and adolescent chronic fatigue. J Pediatr. 1999;135:218–225. doi: 10.1016/s0022-3476(99)70025-9. [DOI] [PubMed] [Google Scholar]
- 47.Stewart JM. Postural tachycardia syndrome and reflex syncope: similarities and differences. J Pediatr. 2009;154:481–485. doi: 10.1016/j.jpeds.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taneja I, Medow MS, Clarke DA, Ocon AJ, Stewart JM. Baroreceptor unloading in postural tachycardia syndrome augments peripheral chemoreceptor sensitivity and decreases central chemoreceptor sensitivity. Am J Physiol Heart Circ Physiol. 2011;301:H173–H179. doi: 10.1152/ajpheart.01211.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart JM, Medow MS, Cherniack NS, Natelson BH. Postural hypocapnic hyperventilation is associated with enhanced peripheral vasoconstriction in postural tachycardia syndrome with normal supine blood flow. Am J Physiol Heart Circ Physiol. 2006;291:H904–H913. doi: 10.1152/ajpheart.01359.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moody M, Panerai RB, Eames PJ, Potter JF. Cerebral and systemic hemodynamic changes during cognitive and motor activation paradigms. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1581–R1588. doi: 10.1152/ajpregu.00837.2004. [DOI] [PubMed] [Google Scholar]
- 51.Aaslid R. Development and principles of transcranial Doppler. In: Newell DW, Aaslid R, editors. Transcranial Doppler. Raven Press Ltd; New York: 1992. pp. 1–8. [Google Scholar]
- 52.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 53.Bishop CC, Powell S, Rutt D, Browse NL. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke. 1986;17:913–915. doi: 10.1161/01.str.17.5.913. [DOI] [PubMed] [Google Scholar]
- 54.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]