Abstract
HIV-1 and its simian counterpart SIV have been exquisitely tailored by evolution to evade host immunity. By virtue of specific adaptations that thwart individual innate or adaptive immune mechanisms, and an overall replication strategy that provides for rapid establishment of a large, systemic viral population, capable of dynamic adaptation to almost all immune selection pressures, these viruses, once established, almost invariably stay one step ahead of the host, and in the vast majority of infected individuals, replicate indefinitely. Although many of vaccine approaches tested to date have been able to enhance the magnitude of the immune responses to HIV/SIV infection, most of these responses, whether cellular or humoral in nature, have largely failed to be both effectively anti-viral and targeted such that fully functional escape variants are not easily selected. Recent advances, however, have provided strong evidence that the initial stages of infection following mucosal transmission of these viruses are more vulnerable to immune intervention, and have led to the development of vaccine strategies that elicit responses able to effectively intervene in these early stages of infection, either preventing acquisition of infection or by establishing early, stringent, and durable control. Here, we place HIV/AIDS vaccine development in the context of the basic immunobiology of HIV/SIV, review the evidence for the vulnerability of early infection to immunity, and discuss how these newly recognized immune vulnerabilities might be exploited for the development of an effective HIV/AIDS vaccine.
Keywords: HIV/SIV, T cell vaccines, antibody vaccines, immune evasion, nonhuman primate models, persistent vectors, immune clearance
Introduction
HIV-1 infections of humans and pathogenic SIV infections of nonhuman primates (NHP) share a pattern of viral replication and a constellation of pathological features that in the absence of anti-retroviral therapy almost always result in unremitting infection and a progressive, ultimately fatal, immune deficiency (1-3). A striking feature of these pathologic lentiviral infections is their induction of robust cellular and humoral immune responses, which fail to clear, or in the vast majority of subjects, even effectively control viral replication (4, 5). Significantly, the AIDS-causing lentiviruses of humans and Asian NHP derive from SIVs endemic in African NHP populations that in their native hosts typically do not cause disease (6, 7). However, this lack of pathogenicity is not due to the ability of these natural hosts to immunologically control SIV replication, but rather to host adaptations that prevent the adverse effects of high viral replication on the immune system (7). The prevailing view is that extended co-evolution of these different viruses and their various natural hosts has resulted in variations on the common theme that pathogenesis is avoided through adaptive mechanisms other than the achievement of effective immune control of SIV replication. This is in striking contrast to the vast majority of viral pathogens, for which immune control of replication plays the major role in preventing pathogenic consequences (including even other persistent viruses, such as Herpes family viruses). These observations (along with the absence of precedent for natural immunity leading to clearance of HIV and durable immune protection from re-infection) indicate that development of an effective HIV/AIDS vaccine will not consist of identifying an immunization approach that mimics protective natural immunity, as has been the path to success for most vaccines, but rather, will require that vaccine developers achieve what nature and evolution could not -- a generally protective anti-HIV immune response (8). These considerations underscore the difficulty in developing an HIV/AIDS vaccine, and in large part, account for the disappointing progress in this development over the past 25 years. However, the substantial investment in basic and clinical research on the immunobiology of these AIDS-causing lentiviruses is clearly paying off. These investigations have painted an increasingly clear picture of the mechanisms used by pathogenic lentiviruses to evade immunity, and most importantly have revealed that these viruses appear to have an “Achilles heel”, immune vulnerabilities that may allow the host to prevent or control infection. Here, we will review the strategies used by HIV/SIV to avoid host immunity, as well as the weaknesses in these strategies that might be exploited for development of an effective HIV/AIDS vaccine. We will focus on the apparent window of viral vulnerability in the early stages of establishment of infection following mucosal transmission.
Immunobiology of HIV/SIV Infection
A crucial and slowly learned lesson of nearly 3 decades of HIV/SIV research has been the realization that the interaction of these viruses with the host immune system frequently does not follow the patterns established for other commonly studied viral pathogens. While all intra-cellular pathogens have to counter host immunity, especially innate immunity, to replicate and manifest disease, the breadth and depth of immune evasion achieved by HIV and SIV, given their comparatively meager ≤10 kb genomes, is astonishing. Specific viral adaptations targeting innate immunity include the viral vpu and vif genes countering the host anti-viral proteins tetherin and APOBECs, respectively (9). Cytotoxic T cell (CTL) responses are subverted by nef-mediated down-regulation of HLA-A and HLA-B, which prevents CTL recognition of infected cells. Notably, HLA-C expression is not down-regulated in such infected cells, preventing their efficient killing by NK cells (9). Humoral immune responses are countered by specific env adaptations that prevent the vast majority of Ab-specific antibodies from mediating neutralization, or even recognizing native env spikes on the HIV/SIV virion surface (10-12). However, while each of these specific mechanisms makes an important contribution to HIV/SIV immune evasion, they are but pieces of a larger overall strategy that starts with the transmission, target cell selection, and replication mechanisms of these viruses.
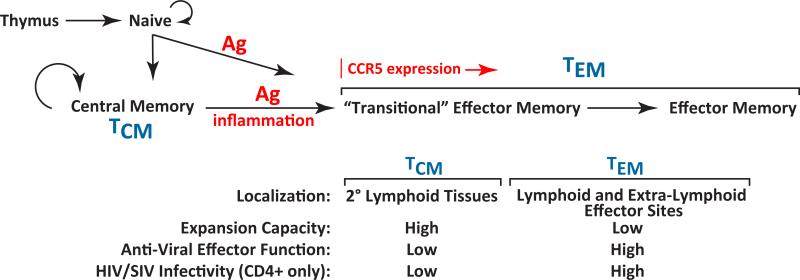
Early research identified the CD4 molecule as an entry receptor for HIV and SIV, explaining the loss of CD4+ T cells noted in infected subjects with AIDS (13). Depletion of this subset can subvert CD4+ T cell help, potentially leading to sub-optimal anti-viral immune responses that favor persistent viral replication rather than host immune control. However, maximal CD4+ T cell depletion typically occurs after failure of viral control and initial adaptive immune responses, particularly CD8+ T cell responses, to infection tend to be robust (4), suggesting loss of CD4+ T cell help does not solely account for the poor adaptive immune control of these viruses. The discoveries of the requirement for co-receptors for viral entry and that transmitted forms of these viruses utilize CCR5 [a chemokine receptor involved in T cell migration in effector/inflammatory sites (15)] as co-receptor (14), offered additional insight into the complex immunobiology of these infections. CCR5 co-receptor use, conferring “CCR5- tropism”, ingeniously focuses HIV/SIV infection on differentiated or differentiating “effector memory” CD4+ T cells (TEM) (Fig. 1), an enormous, widely distributed, and readily replenished reservoir of potential viral target cells (16, 17). This population constitutes the, major T cell population in gastrointestinal and genital mucosa, and thus the availability of these target cells in such mucosal tissues facilitates efficient transmission and the local spread of infection at these sites, with the latter augmented by the recruitment of additional target cells from the blood by the innate immune reaction to the initial local infection (18). Sufficient amounts of virus are produced in this early local infection to systemically seed lymphoid tissues and effector sites, leading to explosive systemic replication (18), and the establishment of latent infection in long-lived memory T cells that can serve as a quiescent life-long viral reservoir (19). Initial systemic HIV/SIV replication dramatically depletes primary viral target cells (CD4+, CCR5+ TEM) throughout the body (20, 21); however, this replication and depletion simultaneously elicits a potent inflammation- and homeostasis-driven regenerative response that produces new TEM target cells from less differentiated, CD4+ central memory T cell (TCM) precursors, which are relatively spared from destruction based on their limited expression of the CCR5 co-receptor required for infection (Fig. 1). This target cell regeneration process facilitates high levels of viral replication for long periods, although over time the persistent hyper-immune activation and inflammation inherent in pathogenic HIV/SIV infection eventually destroy both the CD4+ TCM precursors of the CD4+ TEM viral targets and the regenerative microenvironments necessary for their replenishment, leading to homeostatic failure of the CD4+ memory compartment and the onset of AIDS (16, 17, 22).
Figure 1.
Schema of peripheral T cell differentiation. The figure shows the coordinate changes in T cell homing behavior, expansion capacity, and effector function that occur with progressive differentiation in the peripheral immune system. Because of the CCR5 tropism of transmitted strains of HIV/SIV and the preferential expression of CCR5 in later stages of memory differentiation, this differentiation process is fundamental to HIV/SIV immunopathogenesis, providing for selective targeting of later stage (TEM) CD4+ memory cells while sparing a progenitor population (TCM) for continued production of new targets. Mechanisms of memory T cell differentiation are equally important to the activity of vaccine-elicited T cell responses as they determine whether such responses predominantly localize in lymphoid tissue and respond to infection with expansion, effector differentiation, and delayed migration to effector sites (TCM), or in extra-lymphoid effectors sites, allowing immediate interception of transmitted virus in such sites (TEM).
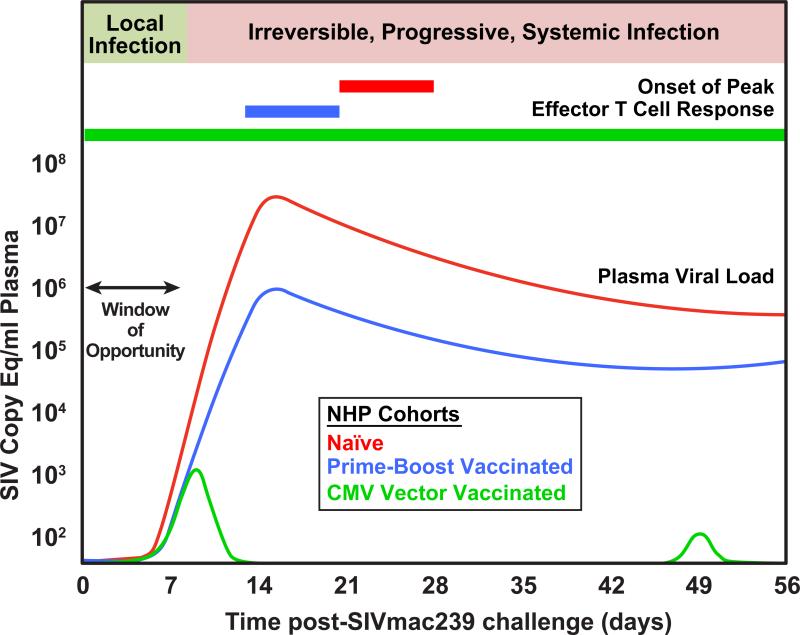
From an immunobiologic perspective, this infection strategy provides far more than an evolutionarily clever way to sustain target cells for HIV/SIV replication over long periods; it critically provides for the rapid establishment of massive systemic viral replication. The combination of this enormous, early onset of viral replication, employment of a replication strategy that includes an error prone reverse transcriptase and a consequently high mutation rate (leading to sequence diversity), and a high level of genetic malleability and functional plasticity results in an extraordinary ability to generate viral variants that can escape immune effector responses (4, 5, 23, 24). Once a rapidly replicating viral population reaches a certain size, capable of generating variants to overcome environmental selection pressures, the difficulty of controlling viral replication with anti-viral immunity- e.g., attacking the infection with an immune response that is both effectively anti-viral and targeted such that fully functional escape variants can't easily be selected—becomes especially daunting. Tellingly, in naïve hosts, adaptive immune anti-viral effector activity, both cellular and humoral, peak well after the peak in plasma viremia in acute HIV/SIV infection (4), and therefore, subsequent to establishment of this critical mass of viral adaptive flexibility (Fig. 2).
Figure 2.
The typical course of early SIVmac infection and kinetics of T cell response development in unvaccinated Indian-origin rhesus macaques is compared to animals vaccinated with TCM-generating prime-boost approaches using non-persistent vectors vs. TEM-generating CMV vectors. Note that only CMV vector-elicited responses can suppress infection during the window of opportunity, and thereby prevent the onset of irreversible, progressive systemic infection.
CD8+ T cell responses are the first adaptive immune effector responses to develop in acute HIV/SIV infection (4). These responses can exert demonstrable selective pressure on the virus population, leading to replacement of the founder virus sequence with escape variants. In some infected individuals, typically a subset of those with so-called “protective” MHC class I alleles that direct CD8+ T cell targeting to conserved regions of viral gene products (particularly gag), this can result in selection viral variants with significantly reduced replicative fitness, facilitating virologic control (25-27). While such individuals can manifest long-term “elite” virologic control (28), this outcome is uncommon in both humans and NHP. In most infected subjects, anti-viral CD8+ T cell responses are not effective, either selecting for viral variants with no apparent fitness defect, or are not associated with sequence changes at all (4, 29), suggesting that the responses have not exerted biologically meaningful selective pressure. Such “ignored” responses may represent dysfunctional CD8+ T cells (30), or inadequate TCR avidity (5), but more likely, may simply reflect an inability of these responses to achieve high enough effector to target ratios to impact viral replication (18).
The situation with anti-viral antibody responses in primary infection is even bleaker from the host's perspective. Due to intrinsic characteristics of the env protein (10-12), antibodies with the ability to neutralize autologous virus do not begin to appear until after approximately 12 weeks of infection (4). These antibody responses can also exert sufficient selective pressure to lead to escape mutations, and indeed the typical course of infection is characterized by the development of antibody responses capable of neutralizing virus, selection of escape variants, followed by development (after a delay) of new responses capable of neutralizing the new escape variants, followed by selection of new escape variants, with the host always playing catch up (31, 32). While these responses may select for escape variants, this immunologic pressure appears in most cases to have little effect on the course of infection (33). Thus, perhaps the most critical adaptive characteristic of HIV/SIV pathobiology is the speed and dynamism of these viruses (in ironic contrast to their group name of “lenti-viruses” or slow viruses, based on their typically protracted clinical course) in both the ability to rapidly establish a large, genetically flexible, adaptive viral population before the onset of effective anti-viral immunity, and the resulting ability to rapidly generate replication-competent viral variants in response to environmental selection pressures. This strategy almost invariably keeps the virus one step ahead of the host, and the infection permanent.
Lessons from Vaccines: Failures of Protection and Partial Successes
Although the de novo T cell response to primary HIV/SIV infection brings “too little” anti-viral activity “too late” to sites of viral replication to control infection in most naïve individuals (34), it remains possible that a prophylactic vaccine capable of eliciting memory responses that respond to HIV/SIV infection with earlier, stronger, and more broadly targeted CD8+ T cell effector responses than develop in naïve subjects might more effectively suppress viral replication in infected individuals, and thereby confer both individual and population benefits by limiting pathogenesis and reducing the likelihood of transmission (8, 35). Such anamnestic T cell responses would not be expected to prevent infection, but by bringing stronger immune pressure on more viral sequences (or by virtue of targeting more viral sequences, having a higher likelihood of targeting sequences critical to viral fitness) might reduce viral replication by direct suppression or by forcing genetic changes that compromise viral replication efficiency. Such suppression would be enhanced if these T cell responses were also able to intercept the infection prior to the massive systemic replication that facilitates immune escape.
This concept has guided development of T cell response-targeted HIV/AIDS vaccines over the past decade, and has been extensively evaluated in various NHP AIDS models using increasingly potent DNA and viral vector immunogens and combinations of these vectors in various prime-boost combinations. These vaccines have typically used replication-impaired or replication-incompetent viral vectors (particularly vectors based on poxviruses and adenoviruses) that safely provide high level, but relatively transient, Ag exposure, along with the necessary innate immune stimulation, to elicit potent conventional CD8+ T cell memory responses -- that is, TCM-biased populations capable of robust expansion and effector cell production after antigen re-stimulation (Fig. 1). Although the results of these studies vary somewhat with the vaccine approaches, NHP species and challenge viruses utilized, the experience is now extensive enough that a number of consistent themes have emerged. First, the best of these vaccines, particularly prime-boost combination vaccines, can in fact provide for a dramatic enhancement in the magnitude of anti-viral CD8+ T cell responses after infection (often > 10-fold) (36-45). Moreover, these anamnestic responses generally peak somewhat earlier than primary responses in naïve animals, although these peak responses still follow, rather than precede, peak viral replication (Fig. 2). Second, the most effective regimens can also reduce peak and early chronic phase viral loads and extend median survival after challenge with highly pathogenic, CCR5-tropic SIV (SIVmac and SIVsm derivatives), the challenge models thought to best recapitulate HIV infection of humans (35, 46). However, the protection observed with these vaccines has been found to be uneven within identically vaccinated RM cohorts (and often correlated with protective MHC alleles, and for SIVsm-derived viruses, TRIM5 alleles that influence target cell permissiveness for these viruses); usually limited to ~1.5-2 log median reduction in peak and plateau phase plasma viral loads with SIVmac challenge; and subject to loss of control over time (36-45, 47-50). This pattern of heterogeneous outcomes, with most vaccines manifesting limited and/or transient protection has been observed with both intravenous and mucosal challenges [the latter including both high (single) and low (repeated) doses]. This is the pattern expected of an antiviral response that depends on anamnestic expansion, effector differentiation, and homing of CD8+ memory T cells, and therefore can act to contain the infection only after systemic spread and massive viral replication have already occurred.
Arguably, infection with the highly pathogenic, CCR5-tropic SIV challenge used in these NHP vaccine efficacy studies (especially SIVmac-derived challenge viruses) is more aggressive than typical clinical HIV infections (attaining peak plasma viremia about a week earlier and manifesting 10-100-fold higher viral replication rates at set point), offering the possibility that such models may represent an unduly stringent challenge for vaccines, and that these vaccine approaches might be more effective against HIV infection in humans than in SIVmac infection in macaques. Unfortunately, this was not the case in the first clinical assessment of this CD8+ memory T cell vaccine concept, the Phase 2b Merck STEP trial (HVTN 502), using Adenovirus (Ad) 5 vectors expressing HIV gag, nef, and pol. Despite detection of HIV-specific CD8+ T cells in 73% of vaccinated subjects, this trial gave a clear negative result -- there was no evidence of vaccine-induced protection in terms of preventing acquisition of infection or facilitating enhanced control of post-infection HIV replication in the vaccinated group (51, 52). Post-hoc analysis revealed a statistically significant “sieving” effect of vaccine-elicited responses on the sequence of the viruses that established infection in vaccinees (53), suggesting that vaccine-elicited T cells did exert weak selection pressure, particularly in individuals with protective MHC I alleles, but this was much less than was hoped for with this vaccine. In a further complication, the results raised the question of whether the vaccine might have increased the incidence of infection in uncircumcised men (54). The vaccine used in the STEP trial (which consisted of Ad5 vectors only, not the more potent prime-boost approach) appears, in retrospect, to have been insufficiently potent in both the magnitude and breadth of responses induced to achieve significant protection (55). It is noteworthy that analogous “Ad5 vector only” vaccines did not provide significant protection in NHPs against intra-rectal SIVmac (44) or SIVsmE660 challenge (M. Reynolds and D. Watkins, personal communication). While it remains possible that a more potent prime-boost regimen would yield better results in a human trial, taken together, the NHP and human data illustrate the difficulty in attaining efficacy against HIV/SIV with a vaccine approach designed to elicit protection via an anamnestic CD8+ memory T cell response, as even the best of these responses still confront HIV/SIV infection only after its systemic establishment, and therefore must contend with immune evasion enabled by an established virus population capable of extensive adaptive variation.
The development of HIV/AIDS vaccines targeting humoral immunity has encountered equally formidable obstacles. The initial characterization of HIV-1 as a retrovirus with env protein-mediated entry brought high hopes that an env-based vaccination approach would yield neutralizing antibodies that would provide protection against either acquisition or progression of infection, a hope spurred on by the relative susceptibility of laboratory passage HIV strains to neutralization by anti-env antibodies. However, it was subsequently determined that clinical isolates were highly resistant to neutralization, and in fact that the vast majority of antibodies elicited by immunization with monomeric env proteins were directed at “decoy” epitopes with little or no neutralization activity (10-12). As might be expected from these findings, two large, well-conducted Phase 3 efficacy trials of alum-adjuvanted env protein (gp120; VAX003 and VAX004) showed no efficacy against HIV-1 acquisition or post-infection viremia (56, 57). Additional studies have demonstrated that neutralization sensitive epitopes (conserved functional parts of the env protein) are effectively concealed in native env trimers and are poorly immunogenic. Epitopes presented by denatured env dominate the anti-env response, but do not bind the critical regions of the trimeric env spikes of infectious virions required for neutralization (10-12, 58). As indicated above, HIV-infected individuals do start making neutralizing antibodies to their autologous virus after about 12 weeks of infection, and about 20% of infected individuals eventually develop HIV-specific antibodies capable of neutralizing heterologous HIV strains (4), with rare individuals manifesting antibodies that recognize conserved regions of env capable of broad neutralization (59). Although these antibodies, developing relatively late in infection, confer little or no benefit to these individuals due to viral escape (33), monoclonal antibodies with broadly neutralizing activity can, when present in appropriate concentration at the time of challenge, completely protect NHP from challenge with pathogenic SIV-HIV hybrid (SHIV) viruses expressing HIV env (60, 61). To date, however, despite extensive effort, no immunogen/vaccine approach has been capable of reliably eliciting such broadly neutralizing antibodies, and the prospects for such immunogens remain uncertain. Thus, HIV/AIDS vaccines targeting humoral immunity have been stymied by the inability to achieve responses with sufficient anti-viral activity, as defined by broad, high titer neutralization activity.
Immune Vulnerabilities Revealed
These considerations suggest that development of vaccines that prevent infection or consistently mediate effective immune control of HIV/SIV will be very difficult to achieve. Indeed, in the wake of the negative results from the STEP trial, there were calls from some quarters for abandoning vaccine development in favor of pursuing other HIV prevention strategies. However, more recent developments provide evidence of effective, vaccine-elicited anti-HIV/SIV immunity and point to brighter prospects for the development of an effective vaccine. Foremost among these developments is the outcome of the RV144 AIDS vaccine trial, which compared the ability of a canarypox virus prime (ALVAC-HIV)/env protein (gp120 subunit) boost vaccination vs. placebo to protect against HIV infection in 16,402 relatively low risk subjects in Thailand (62). Compared to the placebo group, the vaccinated group manifested a 31% reduction in infection acquisition, which was significant at a p value of 0.04 (modified intent-to-treat analysis). The apparent vaccination-associated efficacy seemed to be greatest in the first year after vaccination and then decreased thereafter. In study subjects that were infected with HIV, there was no difference between vaccinees and controls with respect to viral load. Immunologically, this vaccine approach elicited strong, but transient, env-specific antibody responses and env-specific CD4+ T cell lymphoproliferative responses, but no significant HIV-specific CD8+ T cell responses (55, 58, 62). The env-specific antibody responses included neutralization of tissue culture-adapted viruses, and the ability to mediate antibody-dependent cell-mediated cytotoxicity (ADCC), but not the broad, potent neutralization of transmitted HIV strains previously thought to be required for meaningful protection. Although the immunological correlate or correlates of the modest protection observed in the RV144 trial have not been precisely determined, the nature of protection (against acquisition of infection only, and not control of virus post-acquisition), and the nature of the elicited responses (humoral immune dominated) strongly suggest that antibody-mediated mechanisms may be responsible. This hypothesis is in keeping with an increasing body of data showing the ability of antibodies to mediate anti-HIV/SIV activity by mechanisms other than potent, broad virus neutralization, including ADCC, antibody-dependent cell-mediated viral inhibition (ADCVI), antibody-mediated trapping of virions in mucus, or inhibition of viral translocation across epithelia (63-67).
Importantly, RV144-like protection against acquisition of infection has been recapitulated in the NHP models of infection. In the largest NHP study, analogous to the RV144 trial, a prime-boost vaccination regimen protected ~50% of vaccinated monkeys from acquisition of SIVsmE660 after repeated low dose intra-rectal challenge, but had a minimal effect on peak viral loads of those animals that did become infected (50). Immunologic correlates of this protection included low titer neutralizing antibody titres and env-specific CD4+ T cell responses. In another recent study, mucosal vaccination of NHP with gp41 expressing virosomes showed protection against acquisition of simian-HIV (SHIV) virus infection after repeated, limiting dose intra-vaginal challenge. This protection correlated with anti-viral IgAs in the vagina, and occurred in the absence of plasma IgGs with neutralization activity (68).
The significance of these observations lies in the facts that: 1) for the first time, a vaccine has been shown to significantly, albeit modestly, impact acquisition of HIV infection in the field; 2) that such protection can be elicited by prime-boost vaccines and other approaches using relatively simple immunogens, almost certainly through an antibody-mediated mechanism; and 3) protection against infection acquisition appears to be achievable without the broad, potent, transmitted strain neutralization response that has been so difficult to achieve with vaccines. While these results are quite promising, caveats include the facts that the modest RV144 protection appeared to be time limited and achieved in a low-risk population (and may not be recapitulated in upcoming studies in higher risk populations such as those in sub-Saharan Africa), and that the acquisition protection achieved with SIVsmE660 was not recapitulated in a parallel study employing repeated low dose intra-rectal challenge of monkeys with a different SIV strain (SIVmac251) given the same vaccine (50). Nevertheless, taken together, these data indicate that significant protection against infection acquisition is attainable with vaccination, and strongly suggest that such protection can be mediated by anti-viral Abs that lack broadly neutralizing activity. A key implication of this latter conclusion is that the immunologic requirements for prevention or early control of infection may be less stringent than previously thought.
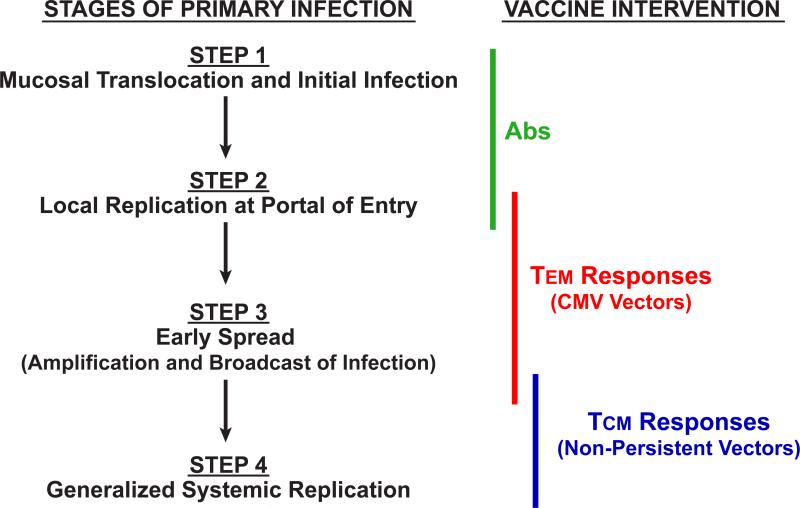
A second hopeful development for HIV/AIDS vaccine research is the demonstration that early SIV infection at mucosal portals of entry appears to be more vulnerable to T cell-mediated control than the massive systemic replication characteristic of later stages of infection. In this regard, it is now well documented that sexually/mucosally transmitted HIV infections in humans and experimental SIV infections in NHP after limiting dose mucosal challenge are typically initiated by one or very few transmitted/founder viral variants, and that in vaginal SIV transmission models in NHP, the establishment of systemic, progressive infection requires up to a week of local amplification and spread at the site of initial transmission before disseminated, systemic productive infection is established (18) (Fig. 2). Conceptually, if vaccine-elicited immune responses were able to intercept the developing infection in this early period (the “window of opportunity”), anti-viral effector mechanisms would act on a much smaller, localized, and less diverse viral population, likely one that is less able to evade T cell-mediated suppression.
As described above, almost all T cell-targeted HIV/SIV vaccines studied to date are based on non-persistent vectors and induce conventional (TCM-biased) CD8+ memory T cell responses that depend on an anamnestic expansion to provide anti-viral effectors in sufficient numbers to combat infection (Fig. 1,2). While for antibody-mediated vaccine approaches it is assumed that long-lived, effective antibody responses will be present at the time of exposure and able to deal directly with the incoming viral inoculum, the delay inherent in developing anti-viral effectors from the TCM populations induced by typical vaccines results in such anamnestic response-derived effectors only confronting primary HIV/SIV infection after systemic establishment of the infection. Thus, the responses elicited by most of the T cell-based vaccines studied to date have been unable to exploit the potential window of viral vulnerability in early infection. Advances in the understanding of the biology of memory T cells over the past decade have offered an alternative approach - development of vaccines that establish and maintain high frequency, tissue-based, functionally differentiated TEM, especially CD8+ TEM, at potential sites of infection (Fig. 1). As CD4+ TEM, the primary targets of CCR5-tropic HIV/SIV, are invariably co-localized with their CD8+ counterparts, this strategy would provide the potential to have in place anti-viral effectors in the immediate vicinity of the likeliest potential viral targets, allowing for immediate interception, and potentially, suppression of infection by either cytolysis of infected cells or suppression of viral spread by soluble factors. However, to the extent that virus-specific CD4+ TEM are also generated by a candidate TEM targeting vaccine, this approach does run the potential risk of providing a higher frequency of activated target cells at sites of early infection. Determination of the balance between these potentially infection-suppressing and infection-facilitating mechanisms and thus the potential efficacy of TEM responses in HIV/SIV infection requires a vaccine approach that can establish and maintain such responses.
TEM-biased responses are the hallmark of persistent pathogens and therefore the testing of this concept necessitates a change in vaccine strategy from vectors that provide high, but transient, antigen exposure to vectors capable of providing lower, but persistent, levels of antigen (Fig. 1). The first evidence that persistent vectors might have a superior ability to control pathogenic lentiviral infection came from analysis of live attenuated SIV vaccines. Although clinical development of attenuated HIV vaccines is precluded by the inherent potential of an attenuated primate lentivirus for pathogenicity (69), the ability of such vaccines to mediate stringent protection against pathogenic SIV challenge, especially homologous challenge, has been demonstrated by multiple groups (70, 71). Persistence of the vector and associated antigen exposure appear to be required for this type of protection, as too high of a level of attenuation results in vaccines that are considerably less effective (70, 72). Because these attenuated SIV vectors also infect CD4+ T cells and elicit both cellular and humoral adaptive immunity, as well as innate immunity, the mechanisms responsible for their high level protection are hard to define unambiguously and remain controversial, but persistent SIV-specific T cell responses have been implicated in several studies (73-77).
The ability of persistent vectors and their associated TEM responses to mediate a quantitatively and qualitatively unique pattern of protection consistent with control of early viral replication has been more definitively shown by the analysis of SIV protein-expressing vectors based on the persistent β-herpesvirus CMV [RhCMV in the RM model; (78)]. RhCMV/SIV vectors can repeatedly super-infect RhCMV+ monkeys in a clinically silent manner, and in the process of super-infection (unaffected by pre-existing CMV-specific immunity), reliably elicit and indefinitely maintain high frequency SIV-specific CD4+ and CD8+ TEM responses at potential sites of early viral replication after mucosal challenge (49, 79, 80). RhCMV/SIV vector designs analyzed to date elicit these TEM responses in the absence of a significant anti-SIV antibody response, and thus, appear to allow delineation of the efficacy of SIV-specific TEM without potentially confounding effects of humoral responses. Strikingly, about 50% of monkeys vaccinated with RhCMV/SIV vectors have manifested early, stringent control of intra-rectally administered highly pathogenic SIVmac. This protection was characterized by an initial peak of viremia of variable, but usually low, magnitude, followed by near immediate control to below quantifiable levels (Fig. 2). Although many of the protected monkeys showed periodic, low level viral “blips” of measurable viremia over the first 30 weeks of follow up (with blips approximately once every 6-7 weeks on average), these blips gradually waned (49). Overall viral control was sufficiently early and stringent to preclude any measurable CD4+ target cell depletion, as well as to prevent seroconversion to SIVenv. This early onset CMV vector-mediated protection occurred without an anamnestic response, and consistent with this, was all or none -- there was no peak or post-peak suppression of viral replication in non-protected monkeys. There was also no measureable enhancement of infection in these non-protected vaccinees, suggesting that vaccine-mediated induction of SIVspecific CD4+ T cells did not increase the pathogenicity of the infection.
The outcome of challenge in CMV vector-vaccinated monkeys (e.g., protection vs. non-protection) was predicted by peak SIV-specific CD8+ T cell response frequencies measurable in peripheral blood during the vaccine phase (pre-challenge), which likely reflects the degree of seeding of SIV-specific TEM in various effector sites, but was not predicted by CD8+ T cell responses associated with protective MHC I alleles. Most significantly, stringent SIV control has been stable for >1 year in all but 1 of the 17 protected monkeys with long-term follow-up (in association with stable RhCMV/SIV vector-maintained, SIV-specific TEM response frequencies). Comprehensive analysis of lymphoid tissues and effector sites of 4 long-term RhCMV/SIV vector-vaccinated controllers (necropsied after >1 yr. of control) with ultrasensitive nested PCR/RT-PCR assays has shown only rare detection of SIV nucleic acid at levels that are >3 logs lower than those measured in RM studied in parallel that had well-controlled SIVmac239 infection, as assessed by conventional criteria (49). Moreover, all lymphoid tissues from these RhCMV/SIV vector-vaccinated controllers were negative for “rescuable” SIV by co-culture (vs. easily co-culturable SIV in conventional controllers). Lastly, long-term CMV vector-mediated protection was unperturbed by CD8+ lymphocyte depletion - - in contrast to conventional protection, which is almost invariably diminished (e.g., viral replication increased) by this treatment. These data demonstrate an unprecedentedly low level of virus compared to any controlled or pharmacologically suppressed HIV or SIV infection reported to date, and raises the remarkable possibility that the SIV infection in RhCMV/SIV vector-vaccinated controllers may ultimately be cleared through ongoing immune surveillance.
The mechanisms by which CMV vector-elicited, TEM-dominated immune responses mediate this remarkable protection remain to be precisely determined, but the ability of these responses to arrest, control and perhaps even clear highly pathogenic SIV infection prior to irreversible systemic infection provides strong evidence that lentiviral immune evasion capability is limited when early stage infection is confronted by potent pre-existing anti-viral effector responses that do not require anamnestic expansion (49). A corollary of this premise is that the mechanisms involved in mediating elite HIV/SIV control and long-term nonprogression of established, chronic infection may not be required, or may be distinct from those necessary for early control and therefore should not serve as a strict guide for prophylactic vaccine development.
Prospects for an HIV/AIDS Vaccine -- Revisited
With the negative result of the STEP trial and the realization that even the most potent vaccines designed to elicit conventional CD8+ memory T cell responses were unlikely to confer elite controller status to the majority of infected vaccinees, the HIV/AIDS vaccine development field was left with no clear pathway to an effective vaccine -- only the hope that increasingly detailed and sophisticated structural analysis of the interaction between env and a growing panel of broadly neutralizing monoclonal antibodies isolated from HIV+ patients would lead to advances in env immunogen design, and eventually, a vaccine capable of eliciting high titer, broadly neutralizing antibodies (11, 12, 58). While much has been learned about the epitopes recognized by these different broadly neutralizing antibodies, applying this information on antigenicity to the development of useful immunogens capable of inducing similar responses has proven an especially daunting challenge. While there is little argument over the importance of fully exploring this strategy, at the time of this writing, the outcome of this effort remains uncertain. However, recent data suggesting that mucosal infection might be prevented by more prosaic antibody-targeted vaccines that induce antibody responses capable of virus or infected cell binding, but not necessarily broadly neutralizing activity, or alternatively, by stringent control of infection through TEM-generating vaccines, offers a new approach to HIV/AIDS vaccine development based on exploiting the immune vulnerabilities of the virus during the early stage of infection (Fig. 3). Although neither of these vaccine strategies is sufficiently optimized for clinical use in their current form, it is not unreasonable to suggest that both empirical optimization and rational design can improve the efficacy of each approach, and that the combination of these disparate and independent approaches might result in additive or potentially synergistic increases in overall efficacy. The RV144 vaccine approach might be empirically improved by optimization of priming vectors and their env inserts and the env protein immunogens used in the boost, as well as the use of more potent adjuvants with the protein boost (58, 81). Obviously, novel immunogens derived from structure-function analysis of broadly neutralizing monoclonal antibodies could be incorporated into these antibody-targeted prime-boost designs as they are shown to be effective. This optimization might be accomplished using both NHP models and adaptive clinical trials (82).
Figure 3.
Stages of early HIV/SIV infection and the point of effective intercept of the designated vaccine approaches.
The TEM vaccine concept, which, to date, has only been tested in an NHP-SIV model, has a more complex development path. CMV vectors, the prototype TEM vaccine approach, are species-specific, and therefore the translation of these vectors to the clinic requires development of human CMV/HIV vectors based on RhCMV designs validated in the NHP model. Although the rhesus and human forms of CMV are genetically distinct, they are closely related and there is sufficient functional homology among key genes to translate design concepts from the former to the latter (78). Although CMV infection is ubiquitous and nonpathogenic in the vast majority of infected individuals, otherwise wildtype CMV vectors would pose a possible risk to certain vulnerable populations such as pregnant women and CMV-naïve individuals with unsuspected immune deficiency, and would therefore not be ideal candidates for inclusion in a prophylactic vaccine. However, in both NHP and mouse models, CMV immunogenicity does not depend on full replication competence as genetically modified CMV constructs with an inability or a highly reduced ability to spread after the initial round of infection are capable of eliciting and maintaining high frequency TEM responses that are essentially indistinguishable from wildtype responses [(83) and L. Picker, S. Hansen and K. Frueh, unpublished data]. This capability likely reflects the ability of CMV-infected cells to avoid immune elimination and persist for prolonged periods despite ongoing antigen expression, a biology that strongly favors the development of safe, yet highly immunogenic, CMV vectors. Such vectors might be used subsequent to a heterologous prime, potentially increasing overall immunogenicity while retaining the TEM character of the elicited responses
Given variability in the magnitude, quality and duration of vaccine-elicited immune responses in humans and both the immune evasion capabilities and diversity of HIV, it is unlikely that any single vaccine approach (almost certainly including the HIV/AIDS vaccinologist's “Holy Grail” of an approach capable of eliciting potent, durable, broadly neutralizing antibodies) will be effective (e.g., prevent or stringently control infection) in all potential transmissions. And, of course, when infection occurs in the face of significant immunologic pressure that does not confer solid protection, it almost inevitably leads to immune escape, and potentially, generation of transmissible viruses that are no longer sensitive to the involved immunologic mechanism. While strategies such as mosaic vaccine insert/immunogen designs can broaden vaccine-elicited immune responses, and help overcome the sequence diversity of transmitted HIV strains (58), a more general solution to this issue may lie in the development of multimodal vaccines that target different immune vulnerabilities (much like the need for multiple, differentially-targeted anti-retroviral drugs in effective combination anti-retroviral chemotherapy regimens). Note that this strategy is subtly different than the oft-repeated conventional wisdom mantra that an optimally effective AIDS vaccine should induce both humoral and cellular immunity, in the hope that one arm of the adaptive immune system can confront the fraction of virus not effectively dealt with by the other. Here, we suggest combining independent vaccine elements (designed to be non-interfering) that will work together in a strategically complementary fashion for enhanced overall efficacy (Fig. 3). For example, an optimized prime-boost regimen focused on HIVenv and designed to generate acquisition-blocking antibodies might be combined with CMV vectors focused on the rest of the HIV proteome and designed to elicit long-lasting TEM based cellular responses for early control and long-term immune surveillance of any residual infection. If the former component is 50% efficacious in blocking acquisition, and the latter component is 50% effective in stringently controlling early infection, the overall efficacy, without synergy, would be a respectable 75%. However, it is plausible that synergy would occur - for example, among those vaccinees that acquire infection, the env-specific antibody response might decrease the number of infective foci and/or hamper early cell-to-cell transmission and thereby increase the likelihood that CMV vector-generated TEM would effectively control the infection. In addition, to the extent that initial protection is non-sterilizing, the ability of CMV vectors to maintain high frequency TEM over the long-term could potential clear residual infection or subject any rebound infection to stringent control, even if the antibody component eventually waned.
In conclusion, continued basic and clinical research on HIV/AIDS immunobiology and vaccinology has, in the wake of the disappointing outcome of the STEP trial, reinvigorated HIV/AIDS vaccine development. Coupled with the surprising positive results from the RV144 study, this work has unequivocally shown that the initial take of HIV/SIV infection after mucosal exposure and the early viral replication prior to irreversible disseminated infection are more vulnerable to immune invention than previously appreciated. Vaccines that can place appropriate immune effector responses at these early sites appear to provide meaningful protection. Although there remains much work to be done to optimize these approaches and translate this information to licensable vaccines, the HIV/AIDS vaccine field, for the first time, has a pathway to follow that is based on solid observations of efficacy and the foundation of an increasingly sophisticated understanding of lentiviral immunobiology.
ACKNOWLEDGMENTS
LJP and SGH acknowledge support from the National Institutes of Health (AI060392, AI095113, DE021291, RR00163); the International AIDS Vaccine Initiative (IAVI) and its donors, particularly the United States Agency for International Development (USAID), and the Bill & Melinda Gates Foundation-supported Collaboration for AIDS Vaccine Discovery (CAVD). JDL acknowledges support from the National Cancer Institute (#HHSN261200800001E).
Footnotes
DISCLOSURE STATEMENT
The authors declare the following competing financial interests: OHSU has licensed CMV vector technology, for which LJP and SGH are inventors, to the International AIDS Vaccine Initiative (IAVI).
LITERATURE CITED
- 1.Grovit-Ferbas K, Pappas T, O'Brien WA. Human Immunodeficiency Virus. In: Ahmed R, Chen AI, editors. Persistent Viral Infections. John Wiley & Sons; Chicester: 1999. pp. 3–45. [Google Scholar]
- 2.Cohen OJ, Fauci AS. Pathogenesis and Medical Aspests of HIV-1 Infection. In: Knipe DM, Howley PM, editors. Fields Virology. 4th ed. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 2043–2094. [Google Scholar]
- 3.Hirsch VM, Lifson JD. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and prevention. Adv. Pharmacol. 2000;49:437–477. doi: 10.1016/s1054-3589(00)49034-4. [DOI] [PubMed] [Google Scholar]
- 4.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat. Rev. Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Connor DH, Allen TM, Vogel TU, Jing P, DeSouza IP, Dodds E, Dunphy EJ, Melsaether C, Mothe B, Yamamoto H, Horton H, Wilson N, Hughes AL, Watkins DI. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 2002;8:493–499. doi: 10.1038/nm0502-493. [DOI] [PubMed] [Google Scholar]
- 6.Hahn BH, Shaw GM, De Cock KM, Sharp PM. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287:607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- 7.Brenchley JM, Silvestri G, Douek DC. Nonprogressive and progressive primate immunodeficiency lentivirus infections. Immunity. 2010;32:737–742. doi: 10.1016/j.immuni.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 9.Kirchhoff F. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe. 2010;8:55–67. doi: 10.1016/j.chom.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Burton DR, Stanfield RL, Wilson IA. Antibody vs. HIV in a clash of evolutionary titans. Proc. Natl. Acad. Sci. U S A. 2005;102:14943–14948. doi: 10.1073/pnas.0505126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu. Rev. Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 12.Hoxie JA. Toward an antibody-based HIV-1 vaccine. Annu Rev Med. 2010;61:135–152. doi: 10.1146/annurev.med.60.042507.164323. [DOI] [PubMed] [Google Scholar]
- 13.Levy JA. Pathogenesis of human immunodeficiency virus infection. Microbiol. Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simmons G, Reeves JD, Hibbitts S, Stine JT, Gray PW, Proudfoot AE, Clapham PR. Co-receptor use by HIV and inhibition of HIV infection by chemokine receptor ligands. Immunol. Rev. 2000;177:112–126. doi: 10.1034/j.1600-065x.2000.17719.x. [DOI] [PubMed] [Google Scholar]
- 15.Kunkel EJ, Boisvert J, Murphy K, Vierra MA, Genovese MC, Wardlaw AJ, Greenberg HB, Hodge MR, Wu L, Butcher EC, Campbell JJ. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am. J. Pathol. 2002;160:347–355. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 17.Grossman Z, Picker LJ. Pathogenic mechanisms in simian immunodeficiency virus infection. Curr. Opin. HIV AIDS. 2008;3:380–386. doi: 10.1097/COH.0b013e3282fbaae6. [DOI] [PubMed] [Google Scholar]
- 18.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu. Rev. Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 19.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc. Natl. Acad. Sci. U S A. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 21.Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Jr., Wang C, Allison DB, Maino VC, Lifson JD, Kodama T, Axthelm MK. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estes JD, Haase AT, Schacker TW. The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin. Immunol. 2008;20:181–186. doi: 10.1016/j.smim.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey J, Blankson JN, Wind-Rotolo M, Siliciano RF. Mechanisms of HIV-1 escape from immune responses and antiretroviral drugs. Curr. Opin. Immunol. 2004;16:470–476. doi: 10.1016/j.coi.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Boutwell CL, Rolland MM, Herbeck JT, Mullins JI, Allen TM. Viral evolution and escape during acute HIV-1 infection. J. Infect. Dis. 2010;202(Suppl 2):S309–314. doi: 10.1086/655653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goulder PJ, Watkins DI. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat. Rev. Immunol. 2008;8:619–630. doi: 10.1038/nri2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinges WL, Richardt J, Friedrich D, Jalbert E, Liu Y, Stevens CE, Maenza J, Collier AC, Geraghty DE, Smith J, Moodie Z, Mullins JI, McElrath MJ, Horton H. Virus-specific CD8+ T-cell responses better define HIV disease progression than HLA genotype. J. Virol. 2010;84:4461–4468. doi: 10.1128/JVI.02438-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins MA, Wilson NA, Reed JS, Ahn CD, Klimentidis YC, Allison DB, Watkins DI. T-cell correlates of vaccine efficacy after a heterologous simian immunodeficiency virus challenge. J. Virol. 2010;84:4352–4365. doi: 10.1128/JVI.02365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker BD. Elite control of HIV Infection: implications for vaccines and treatment. Top. HIV Med. 2007;15:134–136. [PubMed] [Google Scholar]
- 29.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, Ganusov VV, Keele BF, Learn GH, Turnbull EL, Salazar MG, Weinhold KJ, Moore S, Letvin N, Haynes BF, Cohen MS, Hraber P, Bhattacharya T, Borrow P, Perelson AS, Hahn BH, Shaw GM, Korber BT, McMichael AJ. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J. Exp. Med. 2009;206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu. Rev. Med. 2009;60:471–484. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 32.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. U S A. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Euler Z, van Gils MJ, Bunnik EM, Phung P, Schweighardt B, Wrin T, Schuitemaker H. Cross-reactive neutralizing humoral immunity does not protect from HIV type 1 disease progression. J. Infect. Dis. 2010;201:1045–1053. doi: 10.1086/651144. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds MR, Rakasz E, Skinner PJ, White C, Abel K, Ma ZM, Compton L, Napoe G, Wilson N, Miller CJ, Haase A, Watkins DI. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J. Virol. 2005;79:9228–9235. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat. Med. 2008;14:617–621. doi: 10.1038/nm.f.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, Furlott J, Gonzalez EJ, Yant LJ, Maness NJ, May GE, Soma T, Reynolds MR, Rakasz E, Rudersdorf R, McDermott AB, O'Connor DH, Friedrich TC, Allison DB, Patki A, Picker LJ, Burton DR, Lin J, Huang L, Patel D, Heindecker G, Fan J, Citron M, Horton M, Wang F, Liang X, Shiver JW, Casimiro DR, Watkins DI. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 2006;80:5875–5885. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosati M, von Gegerfelt A, Roth P, Alicea C, Valentin A, Robert-Guroff M, Venzon D, Montefiori DC, Markham P, Felber BK, Pavlakis GN. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J. Virol. 2005;79:8480–8492. doi: 10.1128/JVI.79.13.8480-8492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattapallil JJ, Douek DC, Buckler-White A, Montefiori D, Letvin NL, Nabel GJ, Roederer M. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J. Exp. Med. 2006;203:1533–1541. doi: 10.1084/jem.20060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, Landucci G, Forthal DN, Montefiori DC, Carville A, Mansfield KG, Havenga MJ, Pau MG, Goudsmit J, Barouch DH. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horton H, Vogel TU, Carter DK, Vielhuber K, Fuller DH, Shipley T, Fuller JT, Kunstman KJ, Sutter G, Montefiori DC, Erfle V, Desrosiers RC, Wilson N, Picker LJ, Wolinsky SM, Wang C, Allison DB, Watkins DI. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J. Virol. 2002;76:7187–7202. doi: 10.1128/JVI.76.14.7187-7202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hel Z, Nacsa J, Tryniszewska E, Tsai WP, Parks RW, Montefiori DC, Felber BK, Tartaglia J, Pavlakis GN, Franchini G. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J. Immunol. 2002;169:4778–4787. doi: 10.4049/jimmunol.169.9.4778. [DOI] [PubMed] [Google Scholar]
- 42.Hel Z, Tsai WP, Tryniszewska E, Nacsa J, Markham PD, Lewis MG, Pavlakis GN, Felber BK, Tartaglia J, Franchini G. Improved vaccine protection from simian AIDS by the addition of nonstructural simian immunodeficiency virus genes. J. Immunol. 2006;176:85–96. doi: 10.4049/jimmunol.176.1.85. [DOI] [PubMed] [Google Scholar]
- 43.Engram JC, Dunham RM, Makedonas G, Vanderford TH, Sumpter B, Klatt NR, Ratcliffe SJ, Garg S, Paiardini M, McQuoid M, Altman JD, Staprans SI, Betts MR, Garber DA, Feinberg MB, Silvestri G. Vaccine-induced, simian immunodeficiency virus-specific CD8+ T cells reduce virus replication but do not protect from simian immunodeficiency virus disease progression. J. Immunol. 2009;183:706–717. doi: 10.4049/jimmunol.0803746. [DOI] [PubMed] [Google Scholar]
- 44.Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, Tobery TW, Davies ME, McDermott AB, O'Connor DH, Fridman A, Bagchi A, Tussey LG, Bett AJ, Finnefrock AC, Fu TM, Tang A, Wilson KA, Chen M, Perry HC, Heidecker GJ, Freed DC, Carella A, Punt KS, Sykes KJ, Huang L, Ausensi VI, Bachinsky M, Sadasivan-Nair U, Watkins DI, Emini EA, Shiver JW. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 2005;79:15547–15555. doi: 10.1128/JVI.79.24.15547-15555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barouch DH, Liu J, Lynch DM, O'Brien KL, La Porte A, Simmons NL, Riggs AM, Clark S, Abbink P, Montefiori DC, Landucci G, Forthal DN, Self SG, Carville A, Mansfield K, Goudsmit J. Protective efficacy of a single immunization of a chimeric adenovirus vector-based vaccine against simian immunodeficiency virus challenge in rhesus monkeys. J. Virol. 2009;83:9584–9590. doi: 10.1128/JVI.00821-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valentine LE, Watkins DI. Relevance of studying T cell responses in SIV-infected rhesus macaques. Trends Microbiol. 2008;16:605–611. doi: 10.1016/j.tim.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDermott AB, O'Connor DH, Fuenger S, Piaskowski S, Martin S, Loffredo J, Reynolds M, Reed J, Furlott J, Jacoby T, Riek C, Dodds E, Krebs K, Davies ME, Schleif WA, Casimiro DR, Shiver JW, Watkins DI. Cytotoxic T-lymphocyte escape does not always explain the transient control of simian immunodeficiency virus SIVmac239 viremia in adenovirus-boosted and DNA-primed Mamu-A*01-positive rhesus macaques. J. Virol. 2005;79:15556–15566. doi: 10.1128/JVI.79.24.15556-15566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, Yang ZY, Chakrabarti B, Rao SS, Schmitz JE, Montefiori DC, Barker BR, Bookstein FL, Nabel GJ. Preserved CD4+ central memory T cells and survival in vaccinated SIVchallenged monkeys. Science. 2006;312:1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr., Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, Whitney JB, Seoighe C, Lacerda M, Keating S, Norris PJ, Hudgens MG, Gilbert PB, Buzby AP, Mach LV, Zhang J, Balachandran H, Shaw GM, Schmidt SD, Todd JP, Dodson A, Mascola JR, Nabel GJ. Immune and Genetic Correlates of Vaccine Protection Against Mucosal Infection by SIV in Monkeys. Sci. Transl. Med. 2011;3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rolland M, Tovanabutra S, deCamp AC, Frahm N, Gilbert PB, Sanders-Buell E, Heath L, Magaret CA, Bose M, Bradfield A, O'Sullivan A, Crossler J, Jones T, Nau M, Wong K, Zhao H, Raugi DN, Sorensen S, Stoddard JN, Maust BS, Deng W, Hural J, Dubey S, Michael NL, Shiver J, Corey L, Li F, Self SG, Kim J, Buchbinder S, Casimiro DR, Robertson MN, Duerr A, McElrath MJ, McCutchan FE, Mullins JI. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat. Med. 2011;17:366–371. doi: 10.1038/nm.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gray G, Buchbinder S, Duerr A. Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr. Opin. HIV AIDS. 2010;5:357–361. doi: 10.1097/COH.0b013e32833d2d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McElrath MJ. Immune responses to HIV vaccines and potential impact on control of acute HIV-1 infection. J. Infect. Dis. 2010;202(Suppl 2):S323–326. doi: 10.1086/655658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 57.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 58.McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity. 2010;33:542–554. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, Birungi J, Pozniak A, McPhee DA, Manigart O, Karita E, Inwoley A, Jaoko W, Dehovitz J, Bekker LG, Pitisuttithum P, Paris R, Walker LM, Poignard P, Wrin T, Fast PE, Burton DR, Koff WC. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 63.Florese RH, Demberg T, Xiao P, Kuller L, Larsen K, Summers LE, Venzon D, Cafaro A, Ensoli B, Robert-Guroff M. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J. Immunol. 2009;182:3718–3727. doi: 10.4049/jimmunol.0803115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forthal DN, Landucci G, Phan TB, Becerra J. Interactions between natural killer cells and antibody Fc result in enhanced antibody neutralization of human immunodeficiency virus type 1. J. Virol. 2005;79:2042–2049. doi: 10.1128/JVI.79.4.2042-2049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asmal M, Sun Y, Lane S, Yeh W, Schmidt SD, Mascola JR, Letvin NL. Antibody-Dependent Cell-Mediated Viral Inhibition Emerges after Simian Immunodeficiency Virus SIVmac251 Infection of Rhesus Monkeys Coincident with gp140-Binding Antibodies and Is Effective against Neutralization-Resistant Viruses. J. Virol. 2011;85:5465–5475. doi: 10.1128/JVI.00313-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hladik F, Hope TJ. HIV infection of the genital mucosa in women. Curr. HIV/AIDS Rep. 2009;6:20–28. doi: 10.1007/s11904-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 67.Tudor D, Derrien M, Diomede L, Drillet AS, Houimel M, Moog C, Reynes JM, Lopalco L, Bomsel M. HIV-1 gp41-specific monoclonal mucosal IgAs derived from highly exposed but IgG-seronegative individuals block HIV-1 epithelial transcytosis and neutralize CD4(+) cell infection: an IgA gene and functional analysis. Mucosal Immunol. 2009;2:412–426. doi: 10.1038/mi.2009.89. [DOI] [PubMed] [Google Scholar]
- 68.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, Devillier G, Cong Z, Wei Q, Gao H, Qin C, Yang GB, Zurbriggen R, Lopalco L, Fleury S. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 69.Ruprecht RM. Live attenuated AIDS viruses as vaccines: promise or peril? Immunol. Rev. 1999;170:135–149. doi: 10.1111/j.1600-065x.1999.tb01335.x. [DOI] [PubMed] [Google Scholar]
- 70.Johnson RP, Desrosiers RC. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr. Opin. Immunol. 1998;10:436–443. doi: 10.1016/s0952-7915(98)80118-0. [DOI] [PubMed] [Google Scholar]
- 71.Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, Hasenkrug KJ, McDermott AB, Schultz A, Zamb TJ, Boyle R, Desrosiers RC. HIV vaccine design: insights from live attenuated SIV vaccines. Nat. Immunol. 2006;7:19–23. doi: 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- 72.Jia B, Ng SK, DeGottardi MQ, Piatak M, Yuste E, Carville A, Mansfield KG, Li W, Richardson BA, Lifson JD, Evans DT. Immunization with single-cycle SIV significantly reduces viral loads after an intravenous challenge with SIV(mac)239. PLoS Pathog. 2009;5:e1000272. doi: 10.1371/journal.ppat.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Genesca M, McChesney MB, Miller CJ. Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6. J. Intern. Med. 2009;265:67–77. doi: 10.1111/j.1365-2796.2008.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmitz JE, Johnson RP, McClure HM, Manson KH, Wyand MS, Kuroda MJ, Lifton MA, Khunkhun RS, McEvers KJ, Gillis J, Piatak M, Lifson JD, Grosschupff G, Racz P, Tenner-Racz K, Rieber EP, Kuus-Reichel K, Gelman RS, Letvin NL, Montefiori DC, Ruprecht RM, Desrosiers RC, Reimann KA. Effect of CD8+ lymphocyte depletion on virus containment after simian immunodeficiency virus SIVmac251 challenge of live attenuated SIVmac239delta3-vaccinated rhesus macaques. J. Virol. 2005;79:8131–8141. doi: 10.1128/JVI.79.13.8131-8141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reynolds MR, Weiler AM, Weisgrau KL, Piaskowski SM, Furlott JR, Weinfurter JT, Kaizu M, Soma T, Leon EJ, MacNair C, Leaman DP, Zwick MB, Gostick E, Musani SK, Price DA, Friedrich TC, Rakasz EG, Wilson NA, McDermott AB, Boyle R, Allison DB, Burton DR, Koff WC, Watkins DI. Macaques vaccinated with live-attenuated SIV control replication of heterologous virus. J. Exp. Med. 2008;205:2537–2550. doi: 10.1084/jem.20081524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gauduin MC, Yu Y, Barabasz A, Carville A, Piatak M, Lifson JD, Desrosiers RC, Johnson RP. Induction of a virus-specific effector-memory CD4+ T cell response by attenuated SIV infection. J. Exp. Med. 2006;203:2661–2672. doi: 10.1084/jem.20060134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reynolds MR, Weiler AM, Piaskowski SM, Kolar HL, Hessell AJ, Weiker M, Weisgrau KL, Leon EJ, Rogers WE, Makowsky R, McDermott AB, Boyle R, Wilson NA, Allison DB, Burton DR, Koff WC, Watkins DI. Macaques vaccinated with simian immunodeficiency virus SIVmac239Delta nef delay acquisition and control replication after repeated low-dose heterologous SIV challenge. J. Virol. 2010;84:9190–9199. doi: 10.1128/JVI.00041-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Powers C, Fruh K. Rhesus CMV: an emerging animal model for human CMV. Med. Microbiol. Immunol. 2008;197:109–115. doi: 10.1007/s00430-007-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr., Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, Axthelm MK, Nelson JA, Jarvis MA, Picker LJ, Fruh K. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science. 2010;328:102–106. doi: 10.1126/science.1185350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim JH, Rerks-Ngarm S, Excler JL, Michael NL. HIV vaccines: lessons learned and the way forward. Curr. Opin. HIV AIDS. 2010;5:428–434. doi: 10.1097/COH.0b013e32833d17ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corey L, Nabel GJ, Dieffenbach C, Gilbert P, Haynes BF, Johnston M, Kublin J, Lane HC, Pantaleo G, Picker LJ, Fauci AS. HIV-1 Vaccines and Adaptive Trial Designs. Sci. Transl. Med. 2011;3:79ps13. doi: 10.1126/scitranslmed.3001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohr CA, Arapovic J, Muhlbach H, Panzer M, Weyn A, Dolken L, Krmpotic A, Voehringer D, Ruzsics Z, Koszinowski U, Sacher T. A spread-deficient cytomegalovirus for assessment of first-target cells in vaccination. J. Virol. 2010;84:7730–7742. doi: 10.1128/JVI.02696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]