Abstract
The emergence of astroglia as an important participant of the synaptic machinery has led to the ‘tripartite synapse’ hypothesis. Recent findings suggest that synaptic signaling also involves the surrounding extracellular matrix (ECM). The ECM can incorporate and store molecular traces of both neuronal and glial activities. It can also modulate function of local receptors or ion channels and send diffuse molecular signals using products of its use-dependent proteolytic cleavage. Recent experimental findings implicate the ECM in mechanisms of synaptic plasticity and glial remodeling, thus lending support to the ‘tetrapartite synapse’ concept. This inclusive view might help to understand better the mechanisms underlying signal integration and novel forms of long-term homeostatic regulation in the brain.
Introduction
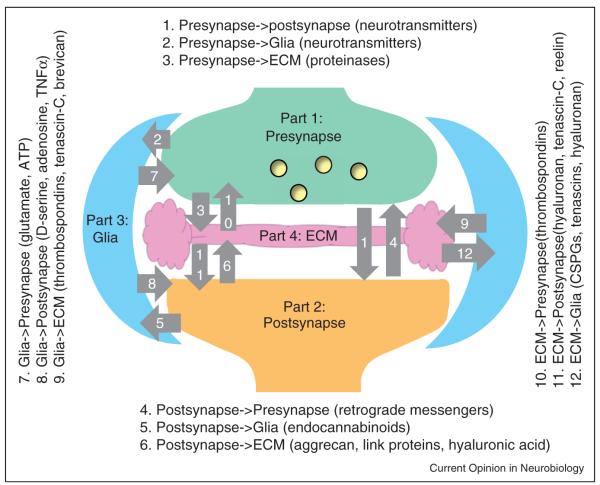
Chemical synapses are elemental units of information processing in the brain. By implication, signal transfer between presynaptic and postsynaptic cells has been considered as a bipartite mechanism. Over the last decade, however, the emergence of astrocytes as an important local player has led to the concept of the tripartite synapse [1]. Recent findings suggest that all parts of the tripartite synapse interact, either directly or through soluble signaling molecules, with the extracellular matrix (ECM) [2–5]. ECM structures are formed in an activity-dependent manner and incorporate molecular signatures of both glial and synaptic elements. In turn, ECM molecules modulate activities of pre- and postsynaptic receptors and ion channels. The ECM can respond to network activity either by incorporating secreted molecules and shed extracellular domains of transmembrane molecules, or by freeing products of its activity-dependent proteolytic cleavage as signaling messengers [6]. These observations have suggested that the ECM is a fourth essential element of what could be termed as the ‘synaptic quadriga’ [7] or the ‘tetrapartite synapse’ [8]. Theoretically, including the ECM as a fourth player increases the number of interaction pathways in a synapse from 6 to 12 (Figure 1). Here we briefly review the underlying mechanisms, focusing on interactions beyond the classical pre–postsynaptic exchange, namely on signals between neurons, astroglia and the ECM.
Figure 1.
The tetrapartite synapse and 12 possible signaling pathways among its four parts. Examples of signaling molecules are given in parentheses. Abbreviations: ATP, adenosine triphosphate; ECM, extracellular matrix; CSPGs, chondroitin sulfate proteoglycans; TNFα, tumor-necrosis factor α.
Presynaptic signals to glia and ECM
The bulk of information in the brain is processed via excitatory glutamatergic synapses. Once released, glutamate activates receptors inside the synaptic cleft but it can also reach some high-affinity receptors outside the cleft [9–11]. The excess of glutamate is rapidly buffered by high-affinity transporters [12–14] expressed in abundance by the surrounding astroglia [15,16]. Glial transporters EAAT1–EAAT2 account for >90% of brain glutamate uptake [17], and astrocytic protrusions can closely approach the synaptic cleft [18–20] altogether occupying 10–30% of the neuropil [21]. The uptake keeps the ambient glutamate concentration low, at ~25 nm in quiescent tissue [22]. Clearly, buffering and uptake of released glutamate is a major channel of signal exchange between synaptic terminals and glia.
Astrocytes in situ also express glutamate receptors, including Ca2+ permeable AMPA receptors (AMPARs), NMDA receptors (NMDARs), and group I metabotropic glutamate receptors [23–27], as reviewed in detail recently [28,29]. Because the same astrocytes also express high-affinity glutamate transporters, successful actions of glutamate seem to require spatial colocalization of receptors and release sites (or otherwise suppression of local glutamate uptake). Indeed, AMPAR-mediated currents in Bergmann glia have been related to neuronal ectopic release sites occurring in front of the target receptors [30]. Alternatively, glutamatergic signal transfer between neurons and glia may actually occur through the transporter-dependent activation of astrocytic Na+/Ca2+ exchanger leading to Ca2+ entry [31]. The inhibitory neurotransmitter GABA could activate metabotropic GABAB receptors in astrocytes resulting in Ca2+ rises which trigger release of glutamate [32] or ATP [33,34]. Unlike glutamatergic signals, this mode of diffuse communication is less restricted by high-affinity uptake: indeed, discharges of individual interneurons in situ could generate long-lasting, long-range extracellular GABA transients [35,36].
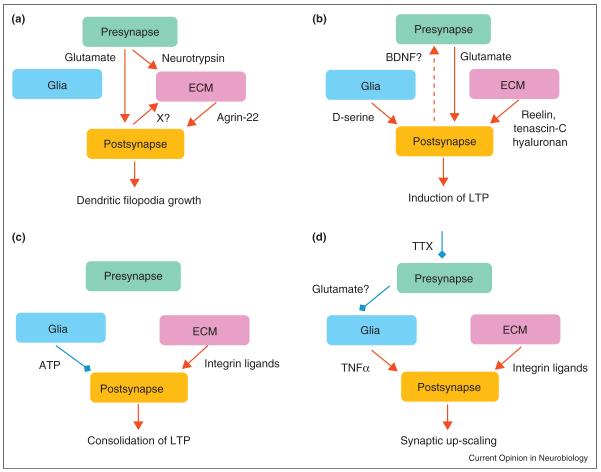
Presynaptic activity also controls the ECM. For instance, axonal terminals release the proteinase neurotrypsin [37], which cleaves the heparan sulfate proteoglycan agrin at central synapses [38••]. Earlier studies identified agrin as a major ECM player in the development and maintenance of the neuromuscular junction [39]. Neurotrypsin cleaves agrin at two homologous, highly conserved sites, releasing a 90-kDa (agrin-90) and a 22-kDa (agrin-22) fragment. The latter triggers the formation of dendritic filopodia after induction of NMDAR-dependent plasticity [38••]. Interestingly, agrin cleavage requires not only neurotrypsin exocytosis, but also activation of the postsynaptic neuron, suggesting a coincidence detection mechanism triggering structural plasticity in a Hebbian manner (Figure 2a).
Figure 2.
Mechanisms of plasticity at the tetrapartite synapse: where the ECM and glia get involved. (a) Co-activation of presynaptic and postsynaptic cells results in release and activation of neurotrypsin. The product of agrin cleavage by neurotrypsin, agrin-22, induces formation of dendritic filopodia. (b) Release of d-serine from astroglia and positive modulation of NMDA receptors and l-type Ca2+ channels by ECM molecules reelin, tenascin-C and hyaluronan supports induction of LTP. Activation of postsynaptic l-type Ca2+ channels may lead to retrograde signaling and an increase in efficacy of presynaptic release. (c) Astroglia-derived ATP is converted into adenosine which, by acting via A1 receptors, can destabilize new synaptic configurations during a consolidation phase of LTP (first 30 min after induction of LTP). Integrin signaling in contrast promotes stabilization of new synaptic configurations promoting polymerization of actin. (d) Presynaptic inactivity leads to release of TNFα from astrocytes that upregulates expression of β3 integrins, which signal to inhibit endocytosis of AMPA receptors, and thus increases their cell surface expression at synapses. Abbreviations: ATP, adenosine triphosphate; BDNF, brain-derived neurotrophic factor; CSPGs, chondroitin sulfate proteoglycans; ECM, extracellular matrix; LTP, long-term potentiation; TNFα, tumor-necrosis factorα; TTX, tetrodotoxin, a blocker of voltage-gated Na+ channels; X?, unknown factor(s); ↑, stimulation;  inhibition; – indirect evidence.
inhibition; – indirect evidence.
Postsynaptic signals to glia and ECM
Quantitative electron microscopy suggests that astroglia occur more frequently on the postsynaptic side of some excitatory synapses [21]. Several types of retrograde messengers are released from postsynaptic neurons, including neurotransmitters, endocannabinoids, gasses, and peptides, as detailed in a recent review [40]. Similar to axonal releases, ectopic release of glutamate and GABA from postsynaptic dendrites could exert receptor actions in astrocytes (see above), but little is known about other signaling channels. Recent evidence suggests that cannabinoid CB1 receptors are expressed by brain astrocytes and could be activated by endocannabinoids which are released from nearby neurons [41] through a Ca2+ and depolarization dependent mechanism [42,43]. This activation triggers Ca2+ waves in astroglia [41], which in turn initiates glial release of glutamate [44,45•]. The subcellular distribution and properties of CB1 receptors in astrocytes should provide important clues regarding the downstream mechanisms involved.
Cell bodies and proximal dendrites of many central neurons are surrounded by the so-called perineuronal nets (PNNs), which are a specialized form of the ECM containing hyaluronan, chondroitin sulfate proteoglycans (CSPGs), tenascin-R and link proteins Crtl1 and Bral2 [3,46]. Expression patterns of ECM proteins and enzymes involved in the synthesis of hyaluronan (hyaluronan synthases 1–3) indicate that CSPG aggrecan, link proteins and hyaluronan are secreted or exported from the somatodendritic domain of the PNN-covered neurons [47]. Enzymatic removal of hyaluronan impairs formation of PNNs [48], whereas ablation of Crtl1 results in abnormal structure of PNNs [49].
Astroglial signals
Ca2+ signals in astrocytes could trigger release of glutamate [32,44,50], ATP [33,34,51], d-serine [52,53,54•], the pro-inflammatory cytokine tumor-necrosis factor α (TNFα), and other signaling molecules. Whether and how the underlying mechanisms differ, in terms of their Ca2+-dependent molecular machinery and subcellular localization, remains intensely debated [29]. Presynaptic NMDARs and metabotropic glutamate receptors could be a target of glutamate released from astrocytes [44,45•] but further studies are required to understand how and where astrocytic glutamate can successfully compete with glutamate released by synapses. Indeed, a strategic juxtaposition of neuronal NMDARs and astroglial compartments featuring putative glutamate-containing vesicles has been identified [44].
ATP released from astrocytes [51] degrades in the extra-cellular space to adenosine which activates presynaptic A1 receptors inhibiting transmission in synaptic circuits [33,34]. This cascade may ultimately affect sleep [55] and prevent postsynaptic stabilization of LTP by interfering with actin polymerization [56](Figure 2c). In the retina, astrocytic ATP release activates A1 receptors coupled to K+ channels in postsynaptic neurons, and the ensuing hyperpolarization decreases neuronal excitability [57]. In the neuromuscular junction, differential Ca2+ signals in Schwann cells either depress or potentiate neurotransmission through activation of, respectively, adenosine A1 or A2A receptors [58•]. Release of ATP from brainstem astrocytes, in response to either physiological pH changes or the stimuli optogenetically targeted to astrocytes, regulates neural circuits controlling breathing [59••].
Substantial evidence points to the release of the NMDAR coagonist d-serine from astroglia. Because d-serine is not buffered by transporters, it enables astrocytes to regulate remotely NMDAR activation in neurons [52,60,61]. Release of d-serine from astroglia is critical for induction of LTP in cultures [53] and explains a correlation between glial coverage of synapses and LTP in the supra-optic nucleus [61]. In acute brain slices, induction of NMDAR-dependent LTP requires synthesis and supply of d-serine by astrocytes [54•](Figure 2b). Astrocytic release of TNFα helps to adjust synaptic strengths across the neural network, thus mediating a synaptic scaling phenomenon [62]. The underlying signaling cascade is initiated by a lack of transmitter release, which stimulates TNFα secretion from glia, which in turn upregulates postsynaptic expression of β3 containing integrins [63]. Since integrins are known to be major receptors to ECM molecules, the latter presumably enhances ECM signaling, with the net result being an increase in cell surface expression of postsynaptic AMPARs (Figure 2d).
In addition, astrocytes secrete ECM molecules, such as CSPG brevican and glycoprotein tenascin-C, and thrombospondins [4]. Brevican deficient mice show alterations in the expression of another ECM CSPG, neurocan, less prominent PNNs, and significant deficits in the maintenance of LTP [64]. Tenascin-C deficient mice also show impairment in LTP [65]. Examination of perisynaptic ECM composition in tenascin-C and brevican deficient mice could provide important clues regarding the interactions involved.
ECM signals
All members of the thrombospondin gene family trigger the formation of presynaptically active contacts in vitro, and double knockout mice deficient in thrombospondins 1 and 2 show reduced synaptic numbers [66]. This synaptogenic activity is mediated by the α2δ-1 Gabapentin receptor that is part of neuronal voltage-gated calcium channels (VGCCs) [67]. An extracellularly secreted protein, leucine-rich glioma-inactivated 1 (LGI1), has been found to interconnect presynaptic and postsynaptic complexes of proteins, including postsynaptic density proteins 95 and 93 and presynaptic K+ channels [68••]. Signaling via the major ECM receptors integrins control synaptic trafficking of NMDA and glycine receptors [69,70•], and the activity of matrix metalloproteinase-9 can stimulate this pathway [69]. Furthermore, ECM structures are thought to restrict mobility of neurotransmitters and their postsynaptic receptors [5,71•].
Several ECM molecules, such as tenascin-C, laminin, fibronectin, retinoschisin and hyaluronan, modulate activity of postsynaptic l-type VGCCs (L-VGCCs) in various types of cells (reviewed by [6]). Genetic ablation of tenascin-C or removal of hyaluronan by hyaluronidase impaired LTP at CA3-CA1 synapses; these effects are occluded by L-VGCC blockade, whereas synaptic plasticity can be restored by pharmacological potentiation of L-VGCC activity [65,72•]. Removal of hyaluronan reduces spike-induced postsynaptic Ca2+ transients in hippocampal pyramidal neurons during induction of LTP, also impairing hippocampus-dependent contextual fear conditioning [72•]. The ECM-related upregulation of postsynaptic L-VGCC could be important for retrograde signaling via BDNF (Figure 2b) [73,74]. Another ECM glycoprotein, reelin, boosts the activity of NMDARs [75], while HNK-1 carbohydrate carried by tenascin-R inhibits postsynaptic GABAB receptors [76]. Signaling via post-synaptic integrins promotes polymerization of actin and hence consolidation of LTP [77](Figure 2c).
In the neuromuscular junction, laminin 11 concentrates in the synaptic cleft and prevents Schwann cell processes from entering it [78]. It is plausible to assume that accumulations of perisynaptic ECM molecules and associated cytokines in central synapses also help to stabilize interactions between presynaptic and postsynaptic cells by restraining invasion of astrocytic and micro-glial processes. Conversely, remodeling of ECM and changes in the expression of secreted factors might prompt invasive astroglial and microglial enwrapping of postsynaptic cells following a loss of afferent inputs, as observed in the human facial nucleus [79,80]. In hypothalamic nuclei, astrocytic coverage of neurons strongly depends on physiological condition, such as lactation or dehydration [81,82], and this type of plasticity correlates with local expression of tenascin-C [83]. Furthermore, deficiency in tenascin-C or tenascin-R, or enzymatic removal of hyaluronan or chondroitin sulfates leads to astrogliosis [84], highlighting an inhibitory influence of the ECM on astrocytes.
Outlook
We are only beginning to appreciate the many forms of functional and structural synaptic changes that rely on interactions with the ECM and the surrounding astroglia. The relationship between perisynaptic ECM and local astrocytic processes remains particularly poorly understood even though it may be essential for the formation and use-dependent modification of synaptic environment. By restraining glial processes and by counteracting the effects of glia-derived adenosine [56], the ECM may contribute to the stabilization of new synaptic configurations. It would seem reasonable to consider remodeling of the ECM and glial processes as an important element of synaptic plasticity, yet the hierarchy of their relationships remains largely unexplored. We suggest therefore that the concept of the tetrapartite synapse might help to understand better the mechanisms underlying information processing in the brain. Indeed, astroglia have been thought to enable spatial integration of network activity, supporting conscious states [85,86]. Conversely, the mixed neuronal-glial origin of ECM suggests that it may contain memory traces of neural network activity and, in addition to the mechanisms discussed above, encompass novel forms of long-term homeostatic regulation.
Acknowledgements
A. Dityatev is supported by the Italian Institute of Technology and by a grant from the Government of the Russian Federation. D.A. Rusakov is supported by the Wellcome Trust and Medical Research Council (UK). This work was supported by the COST Action BM1001 ‘Brain Extracellular Matrix in Health and Disease’.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 2.Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci. 2003;4:456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- 3.Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Faissner A, Pyka M, Geissler M, Sobik T, Frischknecht R, Gundelfinger ED, Seidenbecher C. Contributions of astrocytes to synapse formation and maturation—potential functions of the perisynaptic extracellular matrix. Brain Res Rev. 2010;63:26–38. doi: 10.1016/j.brainresrev.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Gundelfinger ED, Frischknecht R, Choquet D, Heine M. Converting juvenile into adult plasticity: a role for the brain’s extracellular matrix. Eur J Neurosci. 2010;31:2156–2165. doi: 10.1111/j.1460-9568.2010.07253.x. [DOI] [PubMed] [Google Scholar]
- 6.Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010;11:735–746. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- 7.Dityatev A, Frischknecht R, Seidenbecher CI. Extracellular matrix and synaptic functions. Results Probl Cell Differ. 2006;43:69–97. doi: 10.1007/400_025. [DOI] [PubMed] [Google Scholar]
- 8.Dityatev A, Seidenbecher CI, Schachner M. Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci. 2010;33:503–512. doi: 10.1016/j.tins.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Lozovaya NA, Kopanitsa MV, Boychuk YA, Krishtal OA. Enhancement of glutamate release uncovers spillover-mediated transmission by N-methyl-d-aspartate receptors in the rat hippocampus. Neuroscience. 1999;91:1321–1330. doi: 10.1016/s0306-4522(98)00638-1. [DOI] [PubMed] [Google Scholar]
- 10.Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat Neurosci. 2002;5:325–331. doi: 10.1038/nn825. [DOI] [PubMed] [Google Scholar]
- 11.Scimemi A, Fine A, Kullmann DM, Rusakov DA. NR2B-containing receptors mediate cross talk among hippocampal synapses. J Neurosci. 2004;24:4767–4777. doi: 10.1523/JNEUROSCI.0364-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergles DE, Jahr CE. Glial contribution to glutamate uptake at Schaffer collateral—commissural synapses in the hippocampus. J Neurosci. 1998;18:7709–7716. doi: 10.1523/JNEUROSCI.18-19-07709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond JS. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. J Neurosci. 2001;21:8328–8338. doi: 10.1523/JNEUROSCI.21-21-08328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhry FA, Lehre KP, Campagne MV, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes—highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 16.Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danbolt NC. Glutamate uptake. Progr Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 18.Witcher MR, Kirov SA, Harris KM. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia. 2007;55:13–23. doi: 10.1002/glia.20415. [DOI] [PubMed] [Google Scholar]
- 19.Lushnikova I, Skibo G, Muller D, Nikonenko I. Synaptic potentiation induces increased glial coverage of excitatory synapses in CA1 hippocampus. Hippocampus. 2009;19:753–762. doi: 10.1002/hipo.20551. [DOI] [PubMed] [Google Scholar]
- 20.Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehre KP, Rusakov DA. Asymmetry of glia near central synapses favors presynaptically directed glutamate escape. Biophys J. 2002;83:125–134. doi: 10.1016/S0006-3495(02)75154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 24.Burnashev N, Khodorova A, Jonas P, Helm PJ, Wisden W, Monyer H, Seeburg PH, Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992;256:1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- 25.Evans PD, Reale V, Merzon RM, Villegas J. N-methyl-d-aspartate (NMDA) and non-NMDA (metabotropic) type glutamate receptors modulate the membrane potential of the Schwann cell of the squid giant nerve fibre. J Exp Biol. 1992;173:229–249. doi: 10.1242/jeb.173.1.229. [DOI] [PubMed] [Google Scholar]
- 26.Schipke CG, Ohlemeyer C, Matyash M, Nolte C, Kettenmann H, Kirchhoff F. Astrocytes of the mouse neocortex express functional N-methyl-d-aspartate receptors. FASEB J. 2001;15:1270–1272. doi: 10.1096/fj.00-0439fje. [DOI] [PubMed] [Google Scholar]
- 27.Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verkhratsky A, Kirchhoff F. Glutamate-mediated neuronal-glial transmission. J Anat. 2007;210:651–660. doi: 10.1111/j.1469-7580.2007.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD. What is the role of astrocyte calcium in neurophysiology? Neuron. 2008;59:932–946. doi: 10.1016/j.neuron.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsui K, Jahr CE, Rubio ME. High-concentration rapid transients of glutamate mediate neural-glial communication via ectopic release. J Neurosci. 2005;25:7538–7547. doi: 10.1523/JNEUROSCI.1927-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirischuk S, Kettenmann H, Verkhratsky A. Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signaling in Bergmann glial cells in situ. FASEB J. 1997;11:566–572. doi: 10.1096/fasebj.11.7.9212080. [DOI] [PubMed] [Google Scholar]
- 32.Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 33.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 34.Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci. 2006;26:5370–5382. doi: 10.1523/JNEUROSCI.5255-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P, Tamas G. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461:1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karayannis T, Elfant D, Huerta-Ocampo I, Teki S, Scott RS, Rusakov DA, Jones MV, Capogna M. Slow GABA transient and receptor desensitization shape synaptic responses evoked by hippocampal neurogliaform cells. J Neurosci. 2010;30:9898–9909. doi: 10.1523/JNEUROSCI.5883-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frischknecht R, Fejtova A, Viesti M, Stephan A, Sonderegger P. Activity-induced synaptic capture and exocytosis of the neuronal serine protease neurotrypsin. J Neurosci. 2008;28:1568–1579. doi: 10.1523/JNEUROSCI.3398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsumoto-Miyai K, Sokolowska E, Zurlinden A, Gee CE, Luscher D, Hettwer S, Wolfel J, Ladner AP, Ster J, Gerber U, et al. Coincident pre- and postsynaptic activation induces dendritic filopodia via neurotrypsin-dependent agrin cleavage. Cell. 2009;136:1161–1171. doi: 10.1016/j.cell.2009.02.034. [•• This work demonstrates an associative synaptic coincidence detection mechanism triggering structural plasticity of dendrites by involving the ECM.] [DOI] [PubMed] [Google Scholar]
- 39.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 40.Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navarrete M, Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57:883–893. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 42.Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- 43.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 44.Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- 45.Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 2010;68:113–126. doi: 10.1016/j.neuron.2010.08.043. [• This study shows that endocannabinoids released from neurons could either suppress transmission at local synapses through activation of presynaptic CB1 receptors or facilitate transmission at remote synapses through activation of astrocytic CB1 receptors.] [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carulli D, Rhodes KE, Brown DJ, Bonnert TP, Pollack SJ, Oliver K, Strata P, Fawcett JW. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol. 2006;494:559–577. doi: 10.1002/cne.20822. [DOI] [PubMed] [Google Scholar]
- 48.Dityatev A, Bruckner G, Dityateva G, Grosche J, Kleene R, Schachner M. Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol. 2007;67:570–588. doi: 10.1002/dneu.20361. [DOI] [PubMed] [Google Scholar]
- 49.Carulli D, Pizzorusso T, Kwok JC, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS, Glant TT, Fawcett JW. Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain. 2010;133:2331–2347. doi: 10.1093/brain/awq145. [DOI] [PubMed] [Google Scholar]
- 50.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 51.Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. d-Serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proc Natl Acad Sci U S A. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of d-serine. Proc Natl Acad Sci U S A. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of d-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [• This study shows that clamping Ca2+ or suppressing synthesis of d-serine inside individual astrocytes blocks induction of local LTP by reducing availability of NMDARs at nearby synapses.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newman EA. Glial modulation of synaptic transmission in the retina. Glia. 2004;47:268–274. doi: 10.1002/glia.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Todd KJ, Darabid H, Robitaille R. Perisynaptic glia discriminate patterns of motor nerve activity and influence plasticity at the neuromuscular junction. J Neurosci. 2010;30:11870–11882. doi: 10.1523/JNEUROSCI.3165-10.2010. [• The authors demonstrate that Schwann cells could translate patterns of presynaptic spikes in distinct internal Ca2+ signals which in turn differentially modulate transmission through activation of either ademosnie A1 or A2A receptors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [•• This study shows that physiological changes in pH induce Ca2+ waves in chemosensitive brainstem astrocytes leading to ATP-dependent modulation of neural network activity responsible for breathing. This effect can be reproduced using photoactivation of channel rhodopsins expressed specifically in the same target astrocytes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schell MJ, Molliver ME, Snyder SH. d-Serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived d-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 62.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 63.Cingolani LA, Thalhammer A, Yu LM, Catalano M, Ramos T, Colicos MA, Goda Y. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 2008;58:749–762. doi: 10.1016/j.neuron.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brakebusch C, Seidenbecher CI, Asztely F, Rauch U, Matthies H, Meyer H, Krug M, Bockers TM, Zhou X, Kreutz MR, et al. Brevicandeficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Biol. 2002;22:7417–7427. doi: 10.1128/MCB.22.21.7417-7427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evers MR, Salmen B, Bukalo O, Rollenhagen A, Bosl MR, Morellini F, Bartsch U, Dityatev A, Schachner M. Impairment of l-type Ca2+ channel-dependent forms of hippocampal synaptic plasticity in mice deficient in the extracellular matrix glycoprotein tenascin-C. J Neurosci. 2002;22:7177–7194. doi: 10.1523/JNEUROSCI.22-16-07177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 67.Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fukata Y, Lovero KL, Iwanaga T, Watanabe A, Yokoi N, Tabuchi K, Shigemoto R, Nicoll RA, Fukata M. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc Natl Acad Sci U S A. 2010;107:3799–3804. doi: 10.1073/pnas.0914537107. [•• This work shows that LGI1 may serve to cross-link pre- and postsynaptic machineries and that ablation of LGI1 leads to lethal epilepsy in mice.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Michaluk P, Mikasova L, Groc L, Frischknecht R, Choquet D, Kaczmarek L. Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin beta1 signaling. J Neurosci. 2009;29:6007–6012. doi: 10.1523/JNEUROSCI.5346-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Charrier C, Machado P, Tweedie-Cullen RY, Rutishauser D, Mansuy IM, Triller A. A crosstalk between beta1 and beta3 integrins controls glycine receptor and gephyrin trafficking at synapses. Nat Neurosci. 2010;13:1388–1395. doi: 10.1038/nn.2645. [• This study highlights a possibility that ECM-induced signaling via integrins may control efficacy of inhibitory transmission via glycine receptors.] [DOI] [PubMed] [Google Scholar]
- 71.Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12:897–904. doi: 10.1038/nn.2338. [• This study shows that removal of hyaluronic acid changes diffusion of synaptic molecules and paired-pulse facilitation in hippocampal cultures.] [DOI] [PubMed] [Google Scholar]
- 72.Kochlamazashvili G, Henneberger C, Bukalo O, Dvoretskova E, Senkov O, Lievens PM-J, Westenbroek R, Engel AK, Catterall WA, Rusakov D, et al. The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic l-type Ca2+ channels. Neuron. 2010;67:116–128. doi: 10.1016/j.neuron.2010.05.030. [• This study demonstrates the importance of hyaluronic acid in signaling via the l-type calcium channels and long-term potentiation in hippocampal slices.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bayazitov IT, Richardson RJ, Fricke RG, Zakharenko SS. Slow presynaptic and fast postsynaptic components of compound long-term potentiation. J Neurosci. 2007;27:11510–11521. doi: 10.1523/JNEUROSCI.3077-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1–CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- 75.Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- 76.Saghatelyan AK, Snapyan M, Gorissen S, Meigel I, Mosbacher J, Kaupmann K, Bettler B, Kornilov AV, Nifantiev NE, Sakanyan V, et al. Recognition molecule associated carbohydrate inhibits postsynaptic GABA(B) receptors: a mechanism for homeostatic regulation of GABA release in perisomatic synapses. Mol Cell Neurosci. 2003;24:271–282. doi: 10.1016/s1044-7431(03)00163-5. [DOI] [PubMed] [Google Scholar]
- 77.Kramar EA, Lin B, Rex CS, Gall CM, Lynch G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci U S A. 2006;103:5579–5584. doi: 10.1073/pnas.0601354103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patton BL, Chiu AY, Sanes JR. Synaptic laminin prevents glial entry into the synaptic cleft. Nature. 1998;393:698–701. doi: 10.1038/31502. [DOI] [PubMed] [Google Scholar]
- 79.Graeber MB, Bise K, Mehraein P. Synaptic stripping in the human facial nucleus. Acta Neuropathol. 1993;86:179–181. doi: 10.1007/BF00334886. [DOI] [PubMed] [Google Scholar]
- 80.Cullheim S, Thams S. The microglial networks of the brain and their role in neuronal network plasticity after lesion. Brain Res Rev. 2007;55:89–96. doi: 10.1016/j.brainresrev.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 81.Langle SL, Poulain DA, Theodosis DT. Neuronal-glial remodeling: a structural basis for neuronal–glial interactions in the adult hypothalamus. J Physiol Paris. 2002;96:169–175. doi: 10.1016/s0928-4257(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 82.Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- 83.Theodosis DT, Pierre K, Cadoret MA, Allard M, Faissner A, Poulain DA. Expression of high levels of the extracellular matrix glycoprotein, tenascin-C, in the normal adult hypothalamoneurohypophysial system. J Comp Neurol. 1997;379:386–398. doi: 10.1002/(sici)1096-9861(19970317)379:3<386::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 84.Dityatev A, Fellin T. Extracellular matrix in plasticity and epileptogenesis. Neuron Glia Biol. 2008;4:235–247. doi: 10.1017/S1740925X09000118. [DOI] [PubMed] [Google Scholar]
- 85.Robertson JM. The astrocentric hypothesis: proposed role of astrocytes in consciousness and memory formation. J Physiol Paris. 2002;96:251–255. doi: 10.1016/s0928-4257(02)00013-x. [DOI] [PubMed] [Google Scholar]
- 86.Pereira A, Jr, Furlan FA. Astrocytes and human cognition: modeling information integration and modulation of neuronal activity. Prog Neurobiol. 2010;92:405–420. doi: 10.1016/j.pneurobio.2010.07.001. [DOI] [PubMed] [Google Scholar]