Abstract
Background
Children with sickle cell disease and abnormal transcranial Doppler (TCD) ultrasonography have a high risk of stroke, but this risk is greatly reduced when chronic transfusion therapy is administered. The change in TCD velocities during chronic transfusion therapy and rate and frequency of normalization of TCD findings have not been studied extensively.
Procedures
Using data from children with sickle cell disease enrolled as potential subjects in the Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) trial, we characterized the change in TCD velocities on transfusion therapy and identified predictors of developing a normal TCD.
Results
Among 88 children with serial TCD data after starting transfusions for abnormal TCD 46 (52%) converted to normal TCD after a mean of 4.3 months (median 3.0; range 0.85-14.3 months) of transfusions. TCD studies remained abnormal in 19/88 (21.6%) after a mean of 2.4 years of transfusion. The median TCD velocity was lowered by 38 cm/s within three months of initiating transfusions, followed by a more gradual decline then stabilization of velocities, although with significant individual variation. Factors associated with conversion to normal TCD included lower initial TCD velocity, younger age, and higher pre-transfusion hemoglobin level during transfusion therapy.
Conclusion
Younger children with higher pre-transfusion hemoglobin levels and lower abnormal TCD velocities are most likely to have rapid normalization of TCD on transfusions. Long-term follow-up of children with persistently abnormal exams or worsening velocities on transfusion is needed to determine if these children are at higher risk of stroke.
Keywords: sickle cell disease, transcranial Doppler ultrasonography, red blood cell transfusions
Background
Overt stroke is a potentially devastating complication of sickle cell disease (SCD). Stroke risk can be predicted using transcranial Doppler (TCD) ultrasonography. The risk of stroke is highest, with a rate of approximately 10%/year, in children with velocities of 200 cm/s or greater (abnormal) in the internal carotid or middle cerebral arteries (ICA/MCA) compared with a rate of less than 0.5% per year in those with velocities less than 170 cm/sec (normal) [1-3]. The Stroke Prevention Trial in Sickle Cell Anemia (STOP) showed that regular red cell transfusions administered to maintain the hemoglobin S level below 30% of total hemoglobin reduce the risk of stroke in those with abnormal TCD by more than 90% [3]. In clinical practice, TCD monitoring is often discontinued after transfusion therapy is initiated; thus, the change in TCD velocities with chronic transfusion therapy and the rate of normalization of TCD have not been well documented. Based on the rise in hemoglobin level with transfusion and perhaps other factors such as reduced hemolysis and vascular changes, the ICA/MCA velocities should improve in children who are receiving red cell transfusions for abnormal TCD unless, perhaps, vascular stenosis is severe. In one published report, just over half of 24 children with abnormal TCD without prior history of stroke developed normal TCD while receiving transfusions for a median of nine months [5]. In a second report of six children receiving transfusions for primary stroke prevention showed a mean reduction in TCD velocity of 26 cm/s after an unspecified period of time [4]. Two of the six children reduced blood flow velocity to normal (<170 cm/sec) on transfusions, while three reduced the velocity to conditional (170-199 cm/sec) and one had persistently abnormal TCD.
Persistently abnormal TCD velocities despite transfusion may indicate more severe vasculopathy and/or higher stroke risk. In post-trial follow-up of STOP subjects previously randomized to transfusion or standard care [6], the last interpretable TCD was abnormal in all six subjects who developed stroke. Two of these six were actively receiving transfusions, generally quite protective, at the time of the stroke, suggesting that persistently elevated TCD velocities on transfusion therapy may indicate a worse prognosis.
Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) was a follow-up study to STOP, designed to assess whether transfusion therapy could be safely halted after at least 30 months of adequate transfusion therapy in those who reverted from abnormal to normal TCD while receiving transfusions [7]. Over 200 children not yet eligible for randomization in STOP 2 were enrolled in a prospective observational substudy and identified as STOP 2 “potentials.” Consent was obtained to monitor their clinical treatment based on STOP recommendations and to approach them for STOP 2 randomization if/when they met TCD normalization and other criteria. Data from this substudy are utilized in this paper to characterize the effect of transfusion therapy on TCD velocities and to identify predictors of normalization of TCD velocity with transfusion therapy.
Methods
Subjects and Monitoring
Children with SCD-SS or SCD-S-beta0-thalassemia ages 2 to 20 years receiving transfusion therapy for confirmed abnormal TCD defined as 2 studies with velocity in the MCA and/or ICA of 200 cm/s or greater or a single study greater than 220 cm/s, were enrolled in the STOP 2 study as potential subjects. These subjects were identified in one of three ways: 1) prior participation in the STOP study, 2) no prior participation in STOP, but transfused for abnormal TCD as part of clinical care and 3) screened and found to have abnormal TCD during the early phase of STOP 2 and placed on transfusion therapy. All groups were followed as potential subjects while receiving transfusion therapy prior to meeting eligibility criteria for randomization. Laboratory studies (complete blood count, hemoglobin S quantitation) were obtained prior to each transfusion and lactate dehydrogenase (LDH) levels were measured annually. TCD examinations were performed every three months. It was recommended, but not mandated, that TCD studies be performed at least two weeks after transfusion. Interim TCD results as of June, 2000, have been previously reported for STOP study participants (group 1)[6]; the current study includes additional follow-up from July 2000 through late 2004. In the current study, the third group of children (identified with abnormal TCD during STOP 2) was utilized to characterize changes in TCD velocity over time from initiation of therapy because TCD studies were obtained at specified time points from the start of transfusions in these children, while the entire cohort of potential subjects was assessed to determine factors associated with normalization of TCD. To be eligible for randomization in STOP 2, potential subjects had to have 1) received 30 months or more of a regular red cell transfusion protocol (simple, partial exchange, or automated exchange), 2) maintained pre-transfusion HbS levels < 30% in at least 20 of the last 30 months, 3) reverted to normal TCD (two normal TCD exams obtained at least two weeks apart), and 4) had no evidence of moderate to severe vessel stenosis by magnetic resonance angiography [7]. During the last 18 months of the STOP 2 study, study participation was terminated for potential subjects whose TCD remained in the abnormal range after 30 months of transfusion because it was felt that they would be unlikely to achieve eligibility for randomization.
TCD ultrasound
A standard TCD protocol was used at all study sites and all ultrasound examiners were trained for this protocol [7]. Similar equipment and software were utilized (2-MHz pulsed-wave Doppler, Nicolet/EME Companion or Nicolet/EME Pioneer). Using right and left temporal approaches, the highest time-averaged mean of the maximum velocity (TAMMvel) was recorded in 2 mm increments in the MCA, ICA, anterior cerebral artery, and posterior cerebral artery and the basilar artery velocity was recorded using the suboccipital approach. TCD studies were transmitted to central readers unaware of the subject’s transfusion history. Using the highest TAMMvel in the MCA/ICA, studies were classified as normal (< 170 cm/s), conditional (170 – 199 cm/s), abnormal (200 cm/s or higher), or inadequate (information not available from either an ICA or MCA, unless one side was abnormal).
Statistical Analysis
Subjects were divided into two groups by their TAMMvel values: 1) converted to normal TCD group defined as having two consecutive TAMMvel below 170 cm/sec and otherwise 2) did not convert to normal TCD group. Baseline characteristics of subjects, including demographics and laboratory values before starting transfusion in the two groups were compared using Student’s t-tests for continuous variables and chi-square test or Fisher’s exact test for categorical variables. Mean pre-transfusion laboratory values during transfusion period were compared by Student’s t-tests. All reported p-values are two-sided and were not adjusted for multiple testing.
Kaplan-Meier analyses were performed to determine the proportion of participants converting to normal TCD over time (months) for the overall sample. We explored the individual effects of converting to normal TCD with Cox proportional hazards analyses. All factors with a p-value of 0.25 or less in the univariate analysis were included in multivariate analyses using Cox proportional Hazard model. A combination of backward elimination and stepwise regression method was used to find the significance of factors including their interactions and confounding effects. SAS version 9.1.3 was used for all statistical analyses.
Results
Subjects
A total of 205 potential subjects were enrolled in STOP 2. Of these, 10 were excluded from this analysis because they did not have any adequate follow-up TCDs. Of the 195 evaluable subjects, 48 had participated in STOP, including 29 in the transfusion arm who were receiving transfusions for a mean of 62.8 months (range, 52.3 – 71.2 months) and 19 in the standard care arm who started transfusions after STOP trial closure and were receiving transfusions for a mean of 40.1 months (range 34.6 – 44.1 months) at the time of enrollment in STOP 2. Fifty-nine subjects identified through routine clinical care had been receiving transfusions for a mean of 23 months (range, 0.7 – 55.4 months) at STOP 2 enrollment. An additional 88 subjects were identified to have abnormal TCD through screening for STOP 2 and began transfusions during STOP 2. The baseline characteristics of this subgroup of 88 compared with the remainder of the “potential” subjects is shown in the Table. Ultimately, 79 of the potential subjects subsequently were randomized to participate in STOP 2, including 17 of the 88 identified during STOP 2 screening. No children developed stroke while receiving transfusion therapy as potential subjects during STOP 2.
TABLE I.
Characteristics of Subjects Identified With Abnormal TCD in STOP 2 Compared With Subjects Identified in STOP or Through Routine Clinical Screen*
| Factor | STOP 2 (N = 88) | STOP and clinical screen (N=106a) | P-value |
|---|---|---|---|
| Age (years) | 7.6 (3.1) | 10.9 (3.8) | <0.0001 |
| Sex (n, % male) | 41 (46.6) | 50 (47.0) | 0.93 |
| Transfusion duration at the beginning of STOP2 (months) | 0.58 (1.5) | 36.5 (19.8) | <0.000l |
| Initial TCD velocity (cm/sec) | 227 (28) | 218 (17) | 0.012 |
| Baseline laboratory (before starting transfusion) | |||
| Hemoglobin (g/dl) | 7.4 (0.74) | 7.7 (1.15) | 0.317 |
| White blood cell count (109/L) | 14.1 (4.1) | 14.8 (5.4) | 0.557 |
| Platelet count (109/L) | 464 (104) | 404 (71) | 0.232 |
| Reticulocyte count (%) | 9.7 (2.6) | 8.2 (7.7) | 0.652 |
| Fetal hemoglobin (%) | 2.9 (1.9) | 3.8 (3.4) | 0.574 |
| LDH (U/L) | 442 (104) | 431 (850) | 0.953 |
| Laboratory on transfusions—pre-transfusion value | |||
| Hemoglobin (g/dl) | 9.1 (1.0) | 9.3 (1.1) | 0.222 |
| HbS (%) | 37 (20) | 33 (18.1) | 0.153 |
| Reticulocyte count (%) | 9.1 (3.0) | 9.1 (4.1) | 0.959 |
Change in TCD velocity on transfusion therapy
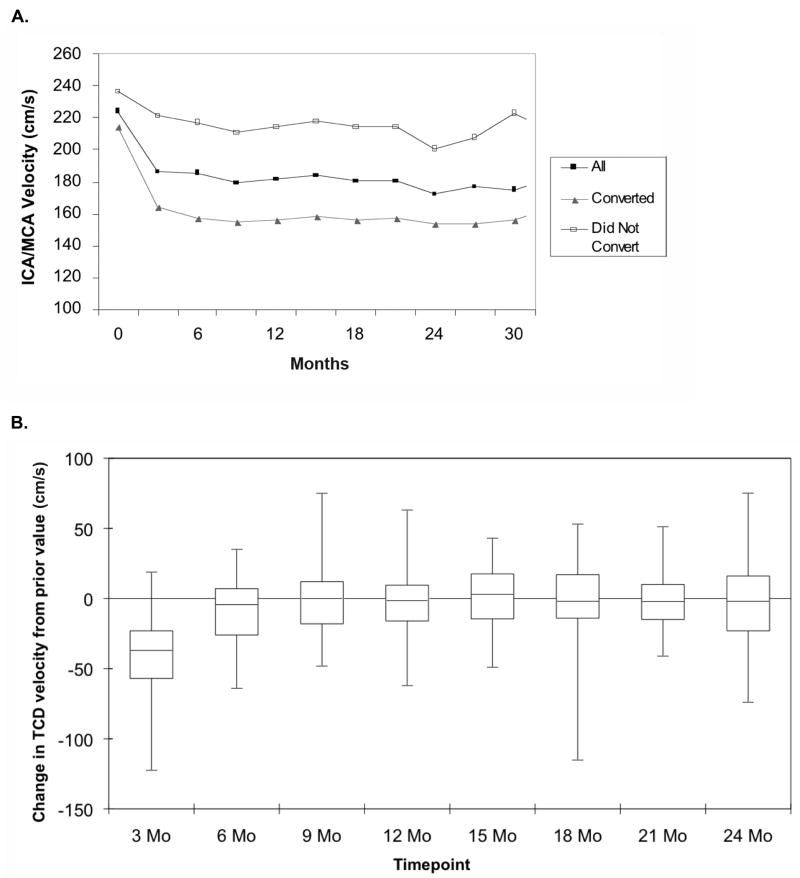
The change in TCD velocity over time following initiation of transfusion therapy for the group of 88 subjects who began transfusions during STOP 2 is shown in Figure 1. There is a rapid initial decline in TCD velocity with the initiation of transfusion therapy, followed by an additional, much lower decline over the next three months. At 3 months of transfusion therapy, the median reduction in TCD velocity from the pre-transfusion value was 38 cm/s, while a further reduction of 5.1 cm/s was seen at six months. Children with higher initial TCD velocities experienced a greater absolute magnitude of reduction in TCD velocity on transfusion therapy with a positive correlation of initial TCD velocity with mean reduction in final TCD velocity (r=0.24, p=0.0014); however, these children were less likely to convert to normal TCD. There was little change in median TCD velocity for the overall group beyond six months of treatment; however, there was significant variability among patients (Figure 1B).
Fig. 1.
(A) The change in TCD velocity following initiation of transfusion therapy for abnormal TCD is shown for all children (■), children who converted to normal TCD (▲) and children who never converted to normal TCD (□). Follow-up was censored at the time of randomization into the STOP 2 trial (17 subjects). (B) The change in TCD velocity over time from the prior value (three months before) is shown in the box and whisker plot (N = 88).
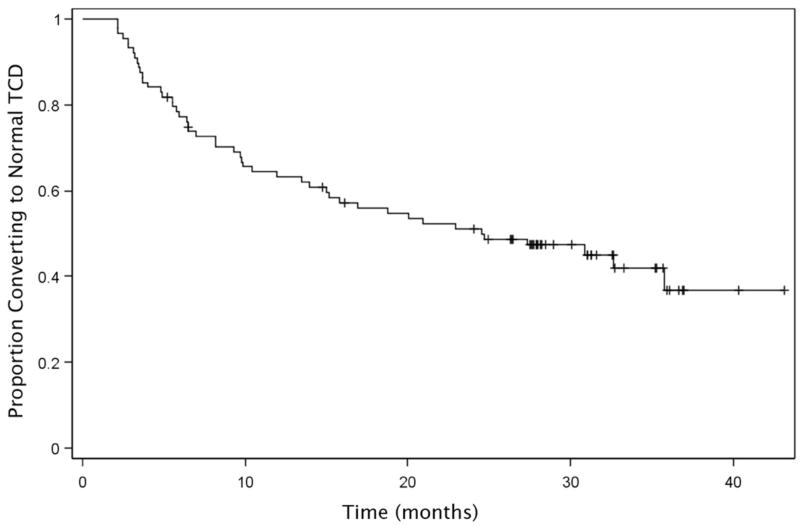
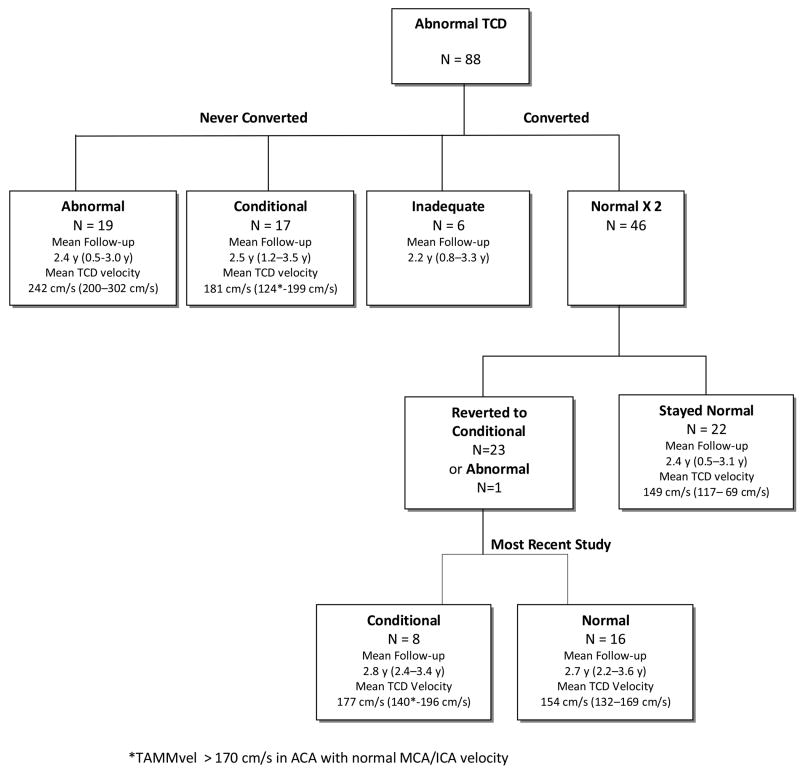
In this subgroup of 88 children, 46 (52%) converted to normal TCD after a mean of 4.3 months (median, 3.0 months and range 0.9.-14.3 months) of transfusion therapy (Figure 2). The majority (67%) of conversions occurred within the first 12 months of starting transfusions. Twenty-four children who had two sequential normal TCD studies subsequently had fluctuations in their TCD velocities to the abnormal (N=1, TAMMvel = 202cm/s) or conditional (N=23, mean TAMMvel = 181 cm/s) range despite continued transfusions, but the TCD returned to normal in the majority of these children (Figure 3). More than half (14 /23, 60%) of the transient TCD elevations into the conditional range, remained in the low conditional (TAMMvel < 185 cm/s) range. Nineteen children (21.6%) with a mean follow-up of 2.4 y of transfusion therapy had TCD velocities that remained in the abnormal range (≥ 200 cm/s). None of these children had developed stroke while receiving continued transfusion therapy at the time of STOP 2 study termination. These children had significantly higher baseline (pretreatment) TCD velocities (244 cm/s) compared with children whose TCD fell to the normal (220 cm/s, p= 0.0005) range.
Fig. 2.
Kaplan Meier estimate of the proportion of children with abnormal TCD who converted to normal TCD while receiving transfusions is shown (N = 88).
Fig. 3.
Follow-up TCD results for the 88 children who began transfusion therapy for abnormal TCD during STOP 2 are shown.
Factors associated with conversion to normal TCD
Of the 195 evaluable potential subjects, 128 (66%) developed normal TCD on transfusion. In univariate analysis, lower initial TCD velocity (218 ± 16 vs. 230 ± 30 cm/s, p=0.0004) and younger age (8.6 ± 4 vs. 9.9 ± 3.7 y, p=0.029) were associated with an increased probability of normalizing TCD, while there was a trend towards higher pre-transfusion hemoglobin levels during chronic transfusion therapy (9.2 ± 0.8 vs. 9 ± 0.7 g/dL, p = 0.083) in those who converted to normal. Other CBC parameters, both at baseline and during transfusion, LDH, and HbS levels, as well as sex, were not significantly different between the groups. In multivariate analysis, only the pre-transfusion hemoglobin level (p=0.05) and age (p=0.0023) remained significant. The probability of converting to normal TCD on transfusion therapy increased by 26.6% for each 1 g/dL rise in pre-transfusion hemoglobin level and decreased by 8.5% for each one-year increase in age after controlling for initial TCD velocity, reticulocyte count, and average pre-transfusion Hb S level. In the subset of 88 children who began transfusions during STOP 2, only lower initial TCD velocity was significantly associated with a higher probability of conversion to normal TCD (p=0.015) in multivariate analysis.
Discussion
In this large cohort of children with SCD and abnormal TCD, followed with close, standard monitoring, we describe the changes in TCD velocity with transfusion therapy. TCD velocities generally improved rapidly after initiation of transfusion therapy and, overall, approximately two-thirds of children receiving transfusions for abnormal TCD ultimately converted to normal TCD velocities, higher than reported previously [4,5]. Children with TCD velocities in the lower abnormal range were more likely to develop normal TCD on transfusion therapy. In addition, in the entire cohort, younger children and those whose pre-transfusion hemoglobin levels were higher were most likely to develop normal TCD. Age effect was not significant in the subgroup of 88 children who started transfusions during STOP 2, likely because this was a younger group of children (Table) and the hemoglobin effect also was not apparent, possibly due to the smaller sample size.
Intermittent fluctuations in TCD velocities, usually into the low conditional range, occurred even following two normal TCD studies; however, in children whose TCD studies normalized, reversion to abnormal TCD with continued transfusion therapy was extremely uncommon, occurring in only one child in this study. Although the STOP 2 screening protocol recommended obtaining TCD at least two weeks after transfusion, the timing of TCD screening in relation to the transfusion was variable. Thus, it is possible that some of these fluctuations in TCD velocities could have been related to varying time intervals between transfusion and TCD. Future studies that mandate that TCD be obtained immediately prior to transfusion might address this issue.
The improvement in TCD velocities in response to transfusion is rapid and the magnitude of reduction in TCD velocity is proportional to the pre-transfusion TCD velocity. Children with the highest baseline TCD velocities show the greatest change, though this reduction is often not substantial enough to reduce velocity to the normal range. A reduction in TCD velocity and especially conversion to normal TCD may be surrogate markers for reduction in stroke risk, although the number of children who develop stroke while receiving transfusions for abnormal TCD is too small to establish this definitively. Although overall TCD velocities stabilize after about six months of transfusion, there is significant variability and fluctuations in TCD velocity are common with continued transfusion. The average standard deviation of TCD velocity within individuals with repeat TCD testing has been reported to be approximately 15 cm/sec [8], and thus, some fluctuation in TCD velocity over time is expected. Nonetheless, given the relative stability of TCD velocities after six months of transfusions, in the occasional patient with persistent larger changes in TCD velocity beyond this time point, these changes might have clinical significance, such as indicating worsening vasculopathy and/or higher stroke risk; further evaluation such as magnetic resonance angiography (MRA) might be warranted. An understanding of the natural history of changes in TCD velocities also may aid clinicians or researchers in monitoring patients who are treated with alternative therapies, as fluctuations beyond that expected with transfusion therapy may indicate poor treatment response.
The underlying mechanisms by which transfusion therapy lowers TCD velocity and protects against stroke risk are not fully understood but likely involve a number of factors. Transfusions raise the hemoglobin level, which is associated with a reduction in cerebral blood flow velocity. Of note, 19 of 88 children (21.6%) followed closely with TCD screening in STOP 2 had persistently abnormal TCD velocities despite optimal transfusion therapy and none of these children had stroke during approximately two and a half years of therapy, suggesting that reduction in TCD velocity, alone, is not the only protective mechanism of transfusion. The reduction in abnormal HbS containing red blood cells with transfusion leads to a reduction in hemolysis and plasma free hemoglobin, which may protect against stroke; although, interestingly, in the current study, we did not find an association with HbS level or LDH levels and probability of converting to normal TCD. Levels of vascular adhesion molecule-1 (VCAM-1), which mediates erythrocyte/leukocyte/endothelial interactions and is thought to contribute to vasculopathy, also are lower in transfused compared with non-transfused patients with SCD at steady state [9]. Finally, transfusions may have a protective effect on the vessel wall and prevent progression of vasculopathy and brain parenchymal injury, particularly in children with normal MRA findings prior to initiation of transfusions. This theory is supported by a recent publication that showed that only three of nine children receiving transfusions for abnormal TCD showed progression of magnetic resonance imaging (MRI) and/or MRA findings and two of these three had MRI/A abnormalities prior to starting transfusions [10].
A subset of children with abnormal TCD has rapid normalization of TCD velocities following initiation of transfusion therapy. These children are younger and perhaps have not yet developed more extensive vascular changes. While STOP 2 clearly showed that transfusion therapy cannot be discontinued safely in children with a history of abnormal TCD whose TCD studies normalize on transfusion therapy [7], the group of children whose TCD velocities normalize rapidly may be especially good candidates for studies of alternative therapies for primary stroke prevention, such as hydroxyurea therapy, which has been shown to lower TCD velocities [11]. In one report, four of seven children who developed normal TCD velocities on transfusions and switched to hydroxyurea did not revert to abnormal TCD [5]. It is unclear, however, if the children who successfully switched to hydroxyurea had more rapid improvement in TCD velocities on transfusions compared with those who reverted to abnormal TCD on hydroxyurea. Larger studies are needed to determine if rapid normalization of TCD and other factors can predict which children may be managed with hydroxyurea therapy.
Conversely, a subset of children, usually with very high pre-transfusion TCD velocities, maintains persistently abnormal TCD on transfusion therapy. Brain MRI/A studies were not obtained routinely on potential subjects in STOP 2, and thus we cannot address whether MRA or MRI abnormalities were associated with a failure to normalize the TCD on transfusions. Nonetheless, children with higher pre-transfusion TCD velocities are more likely to have severe vessel stenosis than those with velocities in the lower abnormal range and, in the absence of treatment, these children have an increased risk of stroke than children with TCD velocities in the low abnormal range and normal MRA [12]. Furthermore, in the Bernaudin, et al, report of 24 children receiving transfusions for primary stroke prevention, children whose TCD velocities remained elevated despite transfusions were more likely to have stenosis detected by brain MRA than those whose TCD studies normalized [5].
Presumably, children with persistently abnormal TCD velocities remain at higher risk of stroke than children whose TCD studies normalize, although this needs to be tested in a long-term follow-up study. Unfortunately, such long-term follow-up was not included in the STOP 2 study, but an ongoing follow-up study of STOP and STOP 2 study participants is currently underway which may address this issue. Nonetheless, more rigorous follow-up of children with persistently abnormal TCD using TCD and brain magnetic resonance angiography (MRA) as well as careful neurological examination should be considered to help to monitor vasculopathy and cerebral injury in these children. If progression of vasculopathy is detected, these children may benefit from further intensification of treatment. Thus, follow-up TCD studies on children who are receiving transfusions for abnormal TCD may be beneficial in guiding future treatments.
In summary, transfusion therapy reduces TCD velocities in children receiving transfusions for abnormal TCD. The majority of children with prior abnormal TCD develop normal TCD while receiving transfusions, while a small number of children have persistently abnormal TCD. Longer follow-up of stroke risk in these children with persistently abnormal TCD studies is needed to determine if their long-term risk of stroke is higher than children whose TCD normalizes. Monitoring with TCD during transfusion therapy may alert clinicians to potential worsening of cerebral vessel disease and may be helpful in tailoring treatment for these children, a concept that deserves further study. The pattern of change in TCD velocities also may be useful in the design of future studies of alternative treatment strategies.
Acknowledgments
This study was supported by grants (U01 052193 and U01 HL 052016) from the National Heart Lung and Blood Institute.
Appendix
The STOP 2 team of principal investigators is listed according to site (in descending order of the number of patients who underwent randomization): M. Abboud, J. Barredo, C. Brown, Medical University of South Carolina, Charleston; O. Alvarez, C. Pegelow, University of Miami School of Medicine, Miami; V. McKie, K. McKie, Medical College of Georgia, Augusta; E. Vichinsky, K. Quirolo, Children’s Hospital of Oakland, Oakland, Calif.; C. Driscoll, Children’s National Medical Center, Washington, D.C.; C. Daeschner, East Carolina University, Greenville, N.C.; S. Piomelli, M. Lee, Columbia University, New York; R. Iyer, University of Mississippi Medical Center, Jackson; P. Lane, B. Gee, B. Files, T. Adamkiewicz, C. Davis, Emory University School of Medicine, Grady Health System, Morehouse School of Medicine, and Children’s Healthcare of Atlanta, Atlanta; M. Kirby, Hospital for Sick Children, Toronto; N. Olivieri, University Health Network, Toronto; B. Berman, A. Villella, Rainbow Babies and Children’s Hospital, Cleveland; G. Woods, Children’s Mercy Hospital, Kansas City, Mo.; W. Wang, St. Jude Children’s Research Hospital, Memphis, Tenn.; J. Kwiatkowski, The Children’s Hospital of Philadelphia, Philadelphia; Baltimore-Washington Sickle Cell Research Consortium (J.F. Casella, Johns Hopkins University School of Medicine, Baltimore; J. Wiley, Sinai Hospital of Baltimore, Baltimore; N. Grossman, University of Maryland, Baltimore; A. Shad, Georgetown University, Washington, D.C.); L. Hilliard, University of Alabama at Birmingham, Birmingham; A. Provisor, Columbus Regional-The Medical Center, Columbus, Ga.; S.T. Miller, SUNY Downstate Medical Center, Kings County Hospital Center, Brooklyn, N.Y.; T. Coates, University of Southern California, Los Angeles; R. Warrier, D. Ode, Louisiana State University, New Orleans; C. Scher, Tulane University Medical School, New Orleans; K. Kalinyak, Children’s Hospital Medical Center, Cincinnati; National Heart, Lung, and Blood Institute (NHLBI): D.R. Bonds (program officer), R.B. Moore, M. Mathis, L. Barbosa, M. Waclawiw.
NHLBI-appointed data safety and monitoring board: V. Mankad (chair), A. Dyer, T. Kinney, L. McMahon, S. Pavlakis, P. Roberson, J. Seibert, F.W. Schmidt, Jr.; Data coordinating center at New England Research Institutes: D. Brambilla (principal investigator), S. McKinlay, D. Gallagher, T. Mansolf, S. Granger, S. Harkness, M. Therencial, M. Pouliot, C. Pollari, M. Berkman, S. Della Grotta, L. Enos, R. Glauber, S. Harter, R. Lagos, K. Morales, M. Pare, T. Wiegand; Central administrative center, Medical College of Georgia, Augusta: R.J. Adams (principal investigator), M. Good, N. Odo, D. Ramsingh, E. Rohde, J.S. Schweitzer, R.K. Wright; Transcranial Doppler (TCD) training center, Medical College of Georgia, Augusta: F.T. Nichols (director), A. Jones, M. Sahm; Core laboratory, Medical College of Georgia, Augusta: A. Kutlar (director), J. Harbin, L. Holley; Clinical and data coordinators: K. Allen, L. Ash, S. Bankston, D. Barnes, S. Bergeron, M. Blumenstein, C. Brown, S. Carson, J. Chow, M. D’Angelo, L. Dabbar, E. Dackiw, M. DeBarr, S.M. Dixon, D. Dodge, K. Doig, M. Doyle, E. Eckroth, H. Enninful-Eghan, T. Faircloth, G. Fortner, D. Gordon, B. Gould, H. Gutin, E. Hackney-Stephens, J. Handy, D. Harris, D. Haughey, E. Hirsch, D. Jack, S. Johnson, C. Kendig, J. Luden, H. Machen, J. Marasciulo, A. Marra, B. Martin, M. Merelles-Pulcini, K. Murch, T. Murdock, A. Mynatt-Norman, C. O’Haver, H. Poplick, E. Randall, B. Record, K. Rey, C. Rhoad, G. Roath, J. Routhieux, K. Ruff, S. Somjee, A. Stevens, K. Stewart, G. Taplin, I. Tillman, T. Walker, D. Wright, A. Zaki; TCD examiners: J. Adams, K. Allen, N. Anderson, G. Bell, L. Bowman, I. Campo-Bustillo, M. DeBarr, R. DeJong, K. Doig, G. Fortner, B. Gould, D. Griffith, T. Hogan, A. Jones, A. Lester, J. Luden, A. Mann, L. Mollo, B. Perret, K. Rey, A. Spinks, K. Stewart, S. Trocio, L. Utley, A. Wong; Neurologists: G. Chari, R. Curless, R. Khan, K. Krohn, R. Lopez Alberola, D. MacGregor, J. Murphy, Y. Park, M. Patterson, B. Philbrook, A. Reddy, A. Rose, F. Silver, V. Vedanaraynan, M. Wiznitzer, K. Yohay; Radiologists, neuroradiologists, and ultrasound radiologists: S. Blaser, B. Bowen, R. Figueroa-Ortiz, D. Greer, K. Helton, G. Hotson, A. Khandji, L. Lowe, J. Nath, M. Nelson, B. McCarville, S. Palas, G. Vezina; Stroke adjudication panel: S. Roach (chair), L. Caplan, D. DeWitt; Magnetic resonance reading panel: R. Zimmerman (chair), J. Bello, F. Moser; Manuscript preparation committee: R.J. Adams (chair), D. Brambilla, S. Miller, D. Bonds.
Footnotes
Presented in abstract form at the American Society of Pediatric Hematology/Oncology Annual Meeting, San Diego, CA. May 23, 2009
The authors state that there are no conflicts of interest relevant to this work.
References
- 1.Adams R, McKie V, Nichols F et al. The use of transcranial ultrasonography to predict stroke in sickle cell disease. N Engl J Med. 1992;326:605–610. doi: 10.1056/NEJM199202273260905. [DOI] [PubMed] [Google Scholar]
- 2.Adams RJ, McKie VC, Carl EM et al. Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Ann Neurol. 1997;42:699–704. doi: 10.1002/ana.410420505. [DOI] [PubMed] [Google Scholar]
- 3.Adams RJ, McKie VC, Hsu L et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 4.Minniti CP, Gidvani VK, Bulas D et al. Transcranial Doppler changes in children with sickle cell disease on transfusion therapy. J Pediatr Hematol Oncol. 2004;26:626–630. doi: 10.1097/01.mph.0000141350.39662.5f. [DOI] [PubMed] [Google Scholar]
- 5.Bernaudin F, Verlhac S, Coic L et al. Long-term follow-up of pediatric sickle cell disease patients with abnormal high velocities on transcranial Doppler. Pediatr Radiol. 2005;35:242–248. doi: 10.1007/s00247-005-1419-5. [DOI] [PubMed] [Google Scholar]
- 6.Lee MT, Piomelli S, Granger S et al. Stroke Prevention Trial in Sickle Cell Anemia (STOP): extended follow-up and final results. Blood. 2006;108:847–852. doi: 10.1182/blood-2005-10-009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams RJ, Brambilla D. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353:2769–2778. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]
- 8.Brambilla DJ, Miller ST, Adams RJ. Intra-individual variation in blood flow velocities in cerebral arteries of children with sickle cell disease. Pediatr Blood Cancer. 2007;49:318–322. doi: 10.1002/pbc.21142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakhalkar VS, Rao SP, Weedon J et al. Elevated plasma sVCAM-1 levels in children with sickle cell disease: impact of chronic transfusion therapy. Am J Hematol. 2004;76:57–60. doi: 10.1002/ajh.20016. [DOI] [PubMed] [Google Scholar]
- 10.Brousse V, Hertz-Pannier L, Consigny Y et al. Does regular blood transfusion prevent progression of cerebrovascular lesions in children with sickle cell disease? Ann Hematol. 2009;88:785–788. doi: 10.1007/s00277-008-0670-x. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman SA, Schultz WH, Burgett S et al. Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood. 2007;110:1043–1047. doi: 10.1182/blood-2006-11-057893. [DOI] [PubMed] [Google Scholar]
- 12.Abboud MR, Cure J, Granger S et al. Magnetic resonance angiography in children with sickle cell disease and abnormal transcranial Doppler ultrasonography findings enrolled in the STOP study. Blood. 2004;103:2822–2826. doi: 10.1182/blood-2003-06-1972. [DOI] [PubMed] [Google Scholar]