Abstract
Intercalated discs (ICDs) are cardiac-specific structures responsible for mechanical and electrical communication among adjacent cardiomyocytes and are implicated in signal transduction. The striated muscle-specific Xin repeat-containing proteins localize to ICDs and play critical roles in ICD formation and cardiac function. Knocking down the Xin gene in chicken embryos collapses the wall of developing heart chambers and leads to abnormal cardiac morphogenesis. In mammals, a pair of paralogous genes, Xinalpha and Xinbeta exist. Ablation of the mouse Xinalpha (mXinalpha) does not affect heart development. Instead, mXinalpha-deficient mice show adult late-onset cardiac hypertrophy and cardiomyopathy with conduction defects. The mXinalpha-deficient hearts up-regulate mouse Xinbeta (mXinbeta), suggesting a partial compensatory role of mXinbeta. Complete loss of mXinbeta, however, leads to failure of forming ICD, mis-localization of mXinalpha, and early postnatal lethality. In this review, we will briefly discuss recent advances in the anatomy and function of ICDs. We will then review what we know about Xin repeat-containing proteins and how this protein family promotes ICD maturation and stability for normal cardiac function.
Keywords: Xin Repeats, Intercalated Disc Formation, Severe Growth Retardation, Postnatal Lethality, Cardiomyopathy With Conduction Defects, Review
2. INTRODUCTION
Intercalated discs (ICDs) are essential structures unique to cardiac muscle (1–4); they enable mechanical coupling and chemical communications among adjacent cardiomyocytes to achieve regulated contraction essential for cardiac function. Recent evidence also points to the involvement of ICD components in transducing signals important for cardiac remodeling in either the healthy or diseased state (5–10). The structure of the ICD and its function, deduced from electron microscopic studies, have been comprehensively summarized in a seminal review paper by Forbes and Sperelakis (1). In this classical description, the function of ICDs was assigned to three types of intercellular junctions: (i) gap junctions are responsible for electrical and chemical communications between cardiomyocytes; (ii) adherens junctions (fasciae adhaerentes) connect the myofibrils from neighboring cardiomyocytes, thus transmitting the contractile force; and (iii) desmosomes (maculae adhaerentes) anchor the desmin intermediate filament to provide mechanical strength to the ICDs. This classic view of the structure and function of ICDs has been supported by recent studies that employ genetic, biochemical, physiological and cell biological approaches in several animal models and cardiac diseases. In general, mutations or deficiencies in ICD components give rise to many types of cardiomyopathy, arrhythmias and other fatal heart diseases (for references see recent reviews (3, 7, 9–11)). Conversely, progression of cardiac disease to heart failure is generally associated with various degrees of ICD structural disruption.
Recent surveys from the human protein atlas web site (http://www.proteinatlas.org), ExPASY protein binding data (http://ca.expasy.org/sprot/) and published papers reveal nearly 200 proteins associated with ICDs (12), about 40% of them are altered in their expression and/or location in various cardiac diseases (12). The discovery of a subcellular domain (termed transitional junction (13), review in Bennett, this issue 2011) between the ICDs and the myofibrils, further increase the numbers of ICD-associated proteins. Thus, the inventory of ICD molecular components is far from complete and the molecular mechanisms by which these components support normal cardiac function remain to be elucidated. Adding to this list, in 1996, we identified a family of Xin repeat-containing proteins in the heart, which co-localize with adherens junction proteins to the ICDs and play an important role in cardiac morphogenesis and function (14–18). In this review, we will briefly discuss recent advances in the anatomy of ICDs and in the functions (signaling) of adhering junctions (adherens junctions and desmosomes), and then focus on the recent advances in our understanding of how the Xin repeat-containing protein family promotes ICD maturation and stability for normal cardiac function.
2.1. Advances in the anatomy of ICD
Using immunogold electron microscopy and immunofluorescence microscopy, two new structures/domains, area composita and transitional junction (Figure 1), at the ICDs were recently identified (13, 19). Furthermore, characterizations of ICD components with molecular, cellular and genetic approaches revealed intricate connections among the intercellular junctions in the ICD.
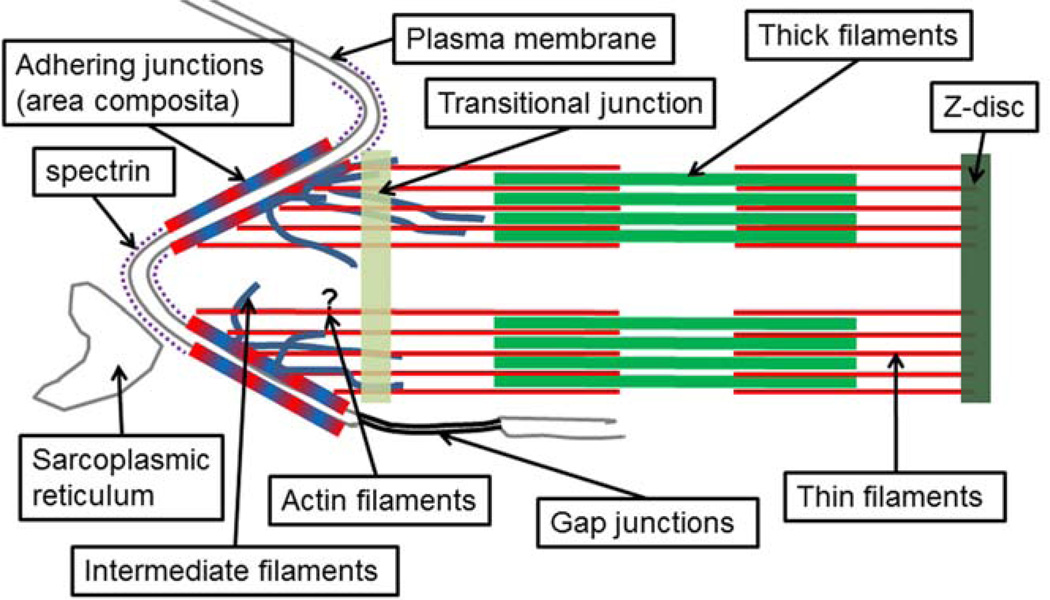
Figure 1.
Diagram of an adult mammalian ICD. The adhering junctions (adheres junctions and desmosomes) intermix to form the area composita. Both actin filaments and intermediate filaments insert into the area composita. The transitional junctions are located at the level of the apexes of the membrane folds and mediate the transition between the thin filaments and the actin filaments in the ICD. The question mark indicates that the organization of the actin filaments in the ICDs is not well understood. SR, sarcoplasmic reticulum.
2.1.1. Area composita (mixed type of junctions) exist in mammalian ICDs
The adhering junctions (adherens junctions and desmosomes) of the ICD are traditionally defined based on their morphological resemblance under transmission electron microscopy to corresponding junctions in epithelial cells. The adherens junction is characterized by a fuzzy electron dense plaque underlining the plasma membrane. Actin filaments extending from myofibril thin filaments apparently insert into the adherens junction, suggesting that these junctions are the anchorage sites of the termini of myofibrils. Adherens junctions of the ICD consist of N-cadherin as the transmembrane component, whose highly conserved cytoplasmic domain interacts with β-catenin, plakoglobin (γ-catenin), α-catenin (αE-catenin and αT-catenin), p120-catenin, vinculin and other actin-binding proteins to link to the actin filaments (Figure 2). Conversely, the desmosomes are characterized by straighter membranes, intermembrane bridges that form a line in the middle of the gap between two membranes, and the two-layered intracellular (cytoplasmic) plaques. Intermediate filaments insert into the cytoplasmic plaques. Desmosomes in the ICD consist of the desmosomal cadherins (desmoglein 2 and desmocollin 2) and intracellular linker proteins such as desmoplakin and plakophillin 2 (PKP2) (Figure 2). Such strict distinction between adherens junctions and desmosomes in the ICD has been challenged by recent work from Werner W. Franke and his colleagues with immunoelectron microscopy and immunofluorescence microscopy on mammalian hearts from different species (19, 20). These studies demonstrated in the adult mammalian ICDs, but not in the non-mammalian ICDs, that the molecular composition of adherens junctions and desmosomes are less exclusive than those in the epithelial junctions (21, 22). Adherens junctions of the ICD contain not only the typical adherens junctional components, but also desmosomal cadherins and cytoplasmic plaque proteins. Conversely, desmosomes in the ICDs contains not only typical desmosomal proteins, but also N-cadherin, β-catenin and α-catenin. Based on these observations, a new type of intercellular junction, area composita, was proposed. The formation of area composita by fusing adherens junctions and desmosomes appears to be a late process both in ontogenesis and in evolution (21); the area composita is only found in cardiomyocytes of maturing and adult mammalian hearts (23). The significance of area composita in mammalian hearts remains to be determined; however, it may strengthen mechanical coupling among neighboring cardiomyocytes and may enhance crosstalk among different types of junctions. On the other hand, the absence of the area composita found in the ICDs of non-mammalian hearts may advantageously assist in the regeneration of damaged hearts. This possibility is supported by the recent finding that the mouse heart retains impressive regenerative capacity at birth but not at one week of age (24). The timing of the loss of regenerative capacity coincides with the maturation of area composita.
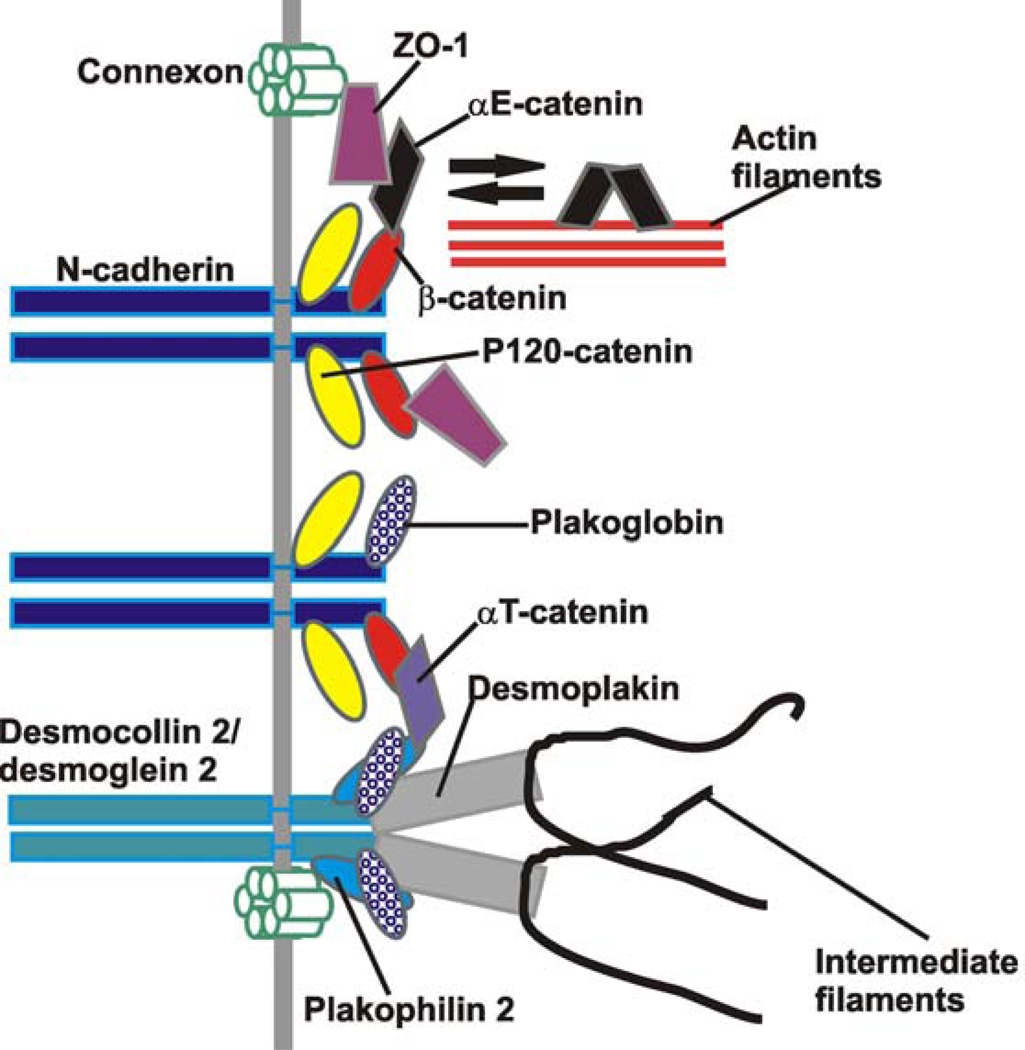
Figure 2.
Major molecular components of the ICDs. Proteins that may shuttle between different intercellular junctions are shown. ZO-1 interacts with Cx43, αE-catenin and β-catenin and thus it may provide a link between the gap junctions and the adherens junctions. Plakoglobin is known to interact with both N-cadherin and desmosomal cadherins (desmocollin 2 & desmoglein 2) and thus it may play an important role in the association between adherens junctions and the desmosomes. αT-catenin may be a direct link between the adherens junctions and desmosomes through its interaction with the β-catenin and plakophilin 2. p120-catenin is a critical component of the adherens junctions and it has been shown to associate with the desmosomes. However, the mechanism of p120-catenin’s association with the desmosomes is not clear; thus such association is not shown. Plakophilin 2 links the desmosomes and the gap junctions together. P0071 is not shown because its interaction partner in the ICDs has not been defined.
2.1.2. Defective adhering junctions generally lead to gap junction remodeling
Despite the apparent mixing of the molecular components in different types of junctions of mammalian ICDs, the morphology of adherens junctions and desmosomes are nevertheless discernable and the associated filament systems are clearly defined. The targeted deletion/disruption of mouse genes encoding ICD-associated and actin-interacting proteins such as Ena/VASP, mXinα, non-muscle myosin IIB, αE-catenin or vinculin, seems to affect the morphologically defined adherens junctions specifically and spare the desmosomes (17, 25–28). In addition to alterations in the expression levels of adherens junctional components, most of these hearts from the above mutant animals exhibit reduced levels of connexin 43 (Cx43) expression and altered localization of Cx43 (gap junction remodeling).
Gap junction remodeling is also commonly observed in human patient and animal model hearts with mutations in desmosomal protein components. Desmoplakin is one of the major components of desmosomes and is capable of interacting with many other desmosomal components, including PKP2 and plakoglobin, as well as with desmin intermediate filaments (29). The global or cardiac restricted deletion of desmoplakin in mice results in embryonic lethality, and mutant embryos display a severe deficiency of desmosomes (5, 30); unfortunately, effects on the structures of adherens junctions and gap junctions of ICD cannot be examined in these mutant lines. However, cardiac-restricted desmoplakin heterozygous mice recapitulate the phenotype of human arrhythmogenic right ventricular cardiomyopathy (ARVC), a major cause of sudden cardiac death, ventricular tachycardia and heart failure (5). Gap junction remodeling has also been observed in human patients with ARVC (31, 32) due to desmoplakin mutations (33, 34), plakoglobin deletion (Naxos disease) (35) or PKP2 mutations (36). Similarly, cardiac-restricted overexpression of a desmoplakin missense mutation (disrupting the binding of desmin) results in changing the expression and localization of Cx43 as well as widening the gaps of ICDs (34). It is also known that gap junction remodeling can occur in human patients with ischemic cardiomyopathy, dilated cardiomyopathy and heart failure (10, 37–44).
In summary, the defective linkage between adhering junctions (adherens junctions and desmosomes) and the cytoskeleton (actin and intermediate filaments) affects formation and maintenance of gap junctions (for a more extensive discussion see (7)). As will be described below, gap junction remodeling was detected in mouse hearts completely lacking one of the Xin repeat-containing and adherens junction-associated proteins, mXinα. Whether mXinα can directly or indirectly interact or associate with gap junctional components remains to be determined. Following is a brief review of what is known about junctional proteins that can co-exist in more than one junction of the ICD.
2.1.3. Linkers involved in molecular crosstalk among different junctions of ICDs
Junctional proteins shuttled and/or linked among different junctions of the ICD could potentially play important roles in the formation of area composita and/or in molecular crosstalk among the ICD junctions. Recent studies have revealed that many junctional proteins can coexist in different junctions, potentially providing linkers to strengthen the mechanical coupling and to enhance molecular crosstalk (Figure 2). In addition, many protein components of one type of junction can associate with protein components of another junction (Figure 2). Understanding these associations and interactions may unveil underlying mechanisms for pathogenesis of many cardiac diseases, such as cardiomyopathy, arrhythmias and heart failure.
2.1.3.1 Plakoglobin (γ-catenin)
Plakoglobin is the first known junctional component present in both adherens junctions and desmosomes of the ICD (45). Plakoglobin-null mice die between E12 and E16 due to severe heart defects (46, 47). In these mutant mice, typical desmosomes are no longer detectable in the heart but are still present in epithelial organs, and the desmosomal cadherin, desmoglein 2, becomes diffusely distributed. The extended adherens junctions of mutant ICDs contain desmoplakin, most of which co-localizes with β-catenin, thus prematurely forming a “mixed type” of adhering junction (47). Furthermore, similar phenotypes have been observed in mice with targeted deletion of PKP2, another armadillo protein plaque constituent of desmosomes (48). These results suggest that both plakoglobin and PKP2 are not only essential for the formation of cardiac desmosomes, but also critically involved in the segregation of the two sets of molecules into desmsomes and adherens junctions. Studies with desmoplakin heterozygous knockout mice and cardiomyocytes to understand the pathogenesis of ARVC have suggested that desmoplakin deficiency leads to mis-localization of plakoglobin from the ICD to the nucleus. Plakoglobin has structural and functional similarity to β-catenin, and is able to compete with β-catenin to suppress the Wnt/β-catenin signaling pathway through T cell factor/lymphoid-enhancer factor (Tcf/Lef) (49–51). Suppression of Wnt/β-catenin signaling could promote adipogenic and fibrogenic gene expression in cardiomyocytes, leading to adipocytic replacement of cardiomyocytes, the hallmark of ARVC (5). These results suggest that plakoglobin can function as a signaling protein in addition to a linking protein between cadherins and the cytoskeleton. Recent studies with cardiac-restricted overexpression and deletion of plakoglobin further support this signaling (crosstalk) function of plakoglobin (52, 53).
2.1.3.2. Plakophilin 2 (PKP2)
Mutations in both plakoglobin and PKP2 have been identified in ARVC patients (35, 54–58); about 70% of familial ARVC is caused by a PKP2 mutation. Using small interference RNA (siRNA) techniques, it has been shown that inhibition of PKP2 expression in primary cultures of neonatal rat ventricular myocytes leads to progressive loss of area composita-like structures and undetectable desmoplakin in the residual ICD-like structures (58). In addition, knocking down PKP2 by siRNA causes gap junction remodeling (a reduction in Cx43 expression, a decrease in dye coupling between cells, and a significant redistribution of Cx43), further suggesting intra-ICD crosstalk (59). Similar to that observed in PKP2-null cardiomyocytes (48), knocking down PKP2 also results in the loss of desmoplakin in residual junctions; instead, there is an accumulation of many cytoplasmic vesicles/aggregates containing desmoplakin, PKP2 and desmoglein 2 (58). Thus, PKP2 acts not only as an “organizer” protein in the formation, stabilization and function of the area composita, but also functions in the molecular crosstalk between desmosomes and gap junctions (8, 11). The exact mechanism mediating junctional organization and molecular crosstalk likely involves the multiple functional domains of PKP2, the only plakophilin isoform expressed in the heart (60). It is known that PKP2 can bind to a large number of desmosomal proteins, including desmoplakin, plakoglobin, desmoglein and desmocollin (61). Through these interactions, PKP2 may zip up desmosomal cadherins and tighten the desmosomal plaque. In addition, PKP2 is capable of interacting with αT-catenin but not αE-catenin; αT-catenin is a component of the adherens junctions which co-localizes with αE-catenin at the ICD (62). Through its interaction with αT-catenin, PKP2 could link components of adherens junctions and desmosomes to form and/or stabilize mixed type adhering junctions (area composita). Moreover, it has been shown by pull down and co-immunoprecipitation assays from rat heart lysates that PKP2 and Cx43 coexist in the same macromolecular complex; the head domain of PKP2 appears to be sufficient for this association (59). Through this head domain, PKP2 is also able to associate with β-catenin and up-regulate the signaling activity of Wnt/β-catenin/Tcf in an overexpression system (61). Furthermore, the Cx43 gene is known to be a functional target of Wnt/β-catenin signaling and its C-terminus can associate with β-catenin, which can account, in part, for the crosstalk (63, 64).
2.1.3.3. p0071 (sometimes referred to as PKP4)
Another junctional protein having dual localization in desmosomes and adherens junctions on epithelial and endothelial cells is p0071 (65, 66). Although dual localization of p0071 has not been demonstrated in cardiomyocytes, the p0071 message is detected in mouse hearts (67), and its protein product is localized to ICDs (20). p0071 (PKP4) belongs to a member of the p120-catenin subfamily of armadillo related proteins; p120-catenin is the prototype of this subfamily that comprises p0071, ARVC protein, NPRAP/δ-catenin and the more distantly related plakophilins 1–3 (68). Structurally different from p120-catenin, p0071 contains a PDZ domain-binding motif at its C-terminus. The head domain of p0071 interacts with desmocollin and desmoplakin, whereas the armadillo repeat domain binds to classical cadherins (65, 66). In addition, both head and armadillo repeat domains interact with plakoglobin (65). Moreover, p0071 and p120-catenin can bind to the same region of the cytoplasmic tail of VE-cadherin, and thus, p0071 can compete p120-catenin off from intercellular junctions (66). Functionally similar to p120-catenin, p0071 can organize small Rho-GTPase signaling, in particular, spatially increasing RhoA activity via its interaction with Ect2, a guanine nucleotide exchange factor (GEF) for Rho, and subsequently regulating cell adhesion, cytokinesis and motility (69). However, the exact roles of p0071 in ICD maturation, stability and function remain to be determined.
2.1.3.4. p120-catenin
p120-catenin is an armadillo-repeat protein that directly binds to the juxtamembrane region of classical cadherins and regulates cadherin-based adhesion, cell shape determination and migration. Recent evidence suggests that like plakoglobin, p120-catenin is another component common to adherens junctions and desmosomes, at least in epithelial cells. In addition to binding E-cadherin, p120-catenin can associate with desmoglein 1 and desmoglein 3 when desmosomes are assembled in high Ca2+ medium but not in low Ca2+ medium (70). These observations of conditional dual localization suggest that p120-catenin may play an important role both in the regulation of desmosome assembly and disassembly, as well as in junctional crosstalk. The region required for the association of p120-catenin with desmosomes has been identified to aa#758-773 of desmoglein 3, which is different from the plakoglobin-binding site (71). However, results from in vitro pull-down assays and yeast two hybrid assays suggest that p120-catenin cannot directly interact with desmoglein 3 (71, 72). Similar to p0071, p120-catenin can induce Rac1 and Cdc42 activation via its interaction with Vav2 (a GEF for Rac1 and Cdc42) and subsequently regulate cell adhesion, shape and motility (73, 74). However, this regulatory role of p120-catenin has not been demonstrated in cardiomyocytes. We have previously reported that the loss of the Xin repeat-containing and ICD-associated protein, mXinβ, in the developing heart impairs N-cadherin clustering during the formation of mature ICDs, alters the expression and localization of p120-catenin, and reduces Rac1 activity (18). How mXin proteins influence p120-catenin and Rac1 remains to be determined. Finally, p120-catenin has been shown to link the adherens junctions to the minus end of microtubules through PLEKHA7 and Nezha, which is required for the establishment and maintenance of the zonula adherens/adherens junctions (75).
2.1.3.5. ZO-1 (zonula occludens-1)
ZO-1 is a member of the membrane-associated guanylate kinase family of proteins and originally discovered in association with the tight junction (76). In the heart, ZO-1 is expressed in endothelial cells, interstitial cells and cardiomyocytes and localized to ICDs of cardiomyocytes (37, 77, 78). The N-terminal half of ZO-1 contains 3 PDZ domains, a SH3 domain and a catalytically inactive guanylate kinase domain (78, 79). Through its second PDZ (PDZ-2) domain, ZO-1 binds to the extreme C-terminus of Cx43 (78, 80, 81). The N-terminal fragment of ZO-1 can also bind directly to α-catenin, whereas the C-terminal specifically co-sediments with actin filaments in vitro and localizes to microfilament bundles in non-muscle cells (79). Therefore, ZO-1 appears to be able to link between gap junctions and adherens junctions. These associations between adherens junctional proteins and Cx43 are shown to be required for the development/formation of gap junctions in non-muscle cells (82, 83) and in cardiomyocytes (84). In addition, as described above, depletion of the Cx43-associated desmosomal protein, PKP2, by siRNA treatment of cultured cardiomyocytes leads to gap junction remodeling and a decrease in dye coupling between cells (58, 59). Therefore, molecular crosstalk/associations between adhering junction (adherens junction and desmosome) components and gap junction proteins (α-catenin, ZO-1, Cx43 and PKP2) at the ICD likely account for the underlying mechanisms for gap junction remodeling observed in many human cardiac diseases and heart failure.
In the heart, Cx43-associated ZO-1 may also play a key role in regulating size, number and distribution of gap junctions (85–87). ZO-1 was found to preferentially localize to the periphery of gap junction plaques, presumably either to inhibit further recruitment of connexons or to favor their removal from gap junctions on reaching a certain size (37, 77, 86). Within the ICDs in vivo, only low level co-localization between ZO-1 and Cx43 is found, as compared with the relatively high level co-localization between ZO-1 and N-cadherin (77). However, during remodeling of cardiac gap junctions, such as in the enzymatically isolated cardiomyocytes (77) or in the human failing heart (37), co-localization and interaction between ZO-1 and Cx43 strikingly increase. This increased interaction of Cx43 with ZO-1 could constrain the growth of gap junctions and contribute to reduction in the Cx43 levels observed in the human failing heart (10, 37, 40, 41). Further support of this hypothesis comes from studies of both in vitro cell systems (86) and in vivo hearts of mice expressing C-terminally truncated Cx43 (K258stop/KO) (88). Specific disruption of the interaction between ZO-1 and Cx43 leads to increased size, decreased number, and altered localization of gap junction plaques. Thus, both ZO-1 and the C-terminal domain of Cx43 are involved in regulating the organization of Cx43 plaques. As will be described below, mXinα-null mouse hearts display gap junction remodeling (17). Like ZO-1, mXinα has been shown to be a β-catenin-binding and actin-binding protein located at ICDs (89). It would be of interest to investigate whether mXinα and ZO-1 may cooperatively be involved in the assembly and maintenance of gap junction plaques in the heart.
2.1.3. Transitional junction near ICD
A recent study has also advanced our understanding of how sarcomeres are connected to the ICD. It has long been noticed that in addition to the intercellular junction-covered membrane, the ICD membrane also contains regions free of gap junctions, adherens junctions and desmosomes (1). Bennett and coworkers observed that these regions are mainly located at the apex of the membrane interdigitations and are associated with spectrins (13). It has been shown that this junction-free region of the ICD membrane is at the level where the Z-disc of the last sarcomere would have been located, if the last sarcomere formed a Z-disc close to the ICD. Consistently, some of typical Z-disc proteins such as α-actinin, titin, ZASP are identified in this region. In addition, although the thin filaments extend from the sarcomeres into the ICD seamlessly, the ICD actin seems to be β-actin instead of the sarcomeric α-actin (90). This isoform switch appears at the Z-disc-like region, where non-muscle myosin IIB (26) and NRAP (91, 92) are also found; this specialized Z-disc-like structure was thus defined as the transitional junction (13). The finding not only explains how the last sarcomere retains regular organization even though its thin filaments insert into the highly convoluted ICD, but also suggests that new sarcomeres can be added onto the end of the myofibril in the convoluted region of ICDs without disturbing the overall organization of the myofibrils. Indeed, addition of new sarcomeres in the ICD was recently observed in cardiomyocytes whose myofibrils are elongating under volume overload (93). The ICD has been shown to change the organization of its interdigitation to accommodate the addition of forming sarcomeres without disrupting the overall organization of the myofibrils, supporting an important role for the ICD in myofibril formation.
In this special issue, the article “From myofibril to membrane: the transitional junction at the intercalated disc” by Pauline Bennett reviews new and known information on the structure and function of the transitional junction. Xufei Ma and Robert Adelstein review the role of non-muscle myosin II in cardiac development and function. Particularly relevant to this issue is that similar to mXinα-null mice (17), myosin IIB conditional knockout mouse hearts develop cardiomyopathy and fibrosis (26). In addition, the loss of myosin IIB in the heart results in a significant reduction in mXinα protein. How the actin-binding protein, mXinα, collaborates with non-muscle myosin IIB in such cardiac defects remains to be determined.
2.2. The involvement of ICD in signaling
Recent evidence from studies with transgenic overexpressing and knockout animals clearly points to the involvement of ICD components in transducing signals important for cardiac remodeling in both physiological and pathological states (see references in (5–8, 10, 52, 53, 94)). Here, we only briefly discuss signaling relevant to β-catenin in the heart, because the β-catenin-binding domain is present in Xin repeat-containing proteins (89). β-catenin is a multifunctional protein and plays a central role in regulating both canonical Wnt (Wnt/β-catenin) signaling and cadherin-mediated (cadherin/β-catenin) signaling in many cell types and tissues (95–97). The interplay between these two signaling pathways has been shown to be crucial in the process of epithelial-mesenchymal transition (EMT), which occurs not only in normal embryonic development such as cardiac morphogenesis, but also in tumor formation and metastasis (98). Both Wnt/β-catenin and N-cadherin-mediated signaling pathways likely operate in postnatal and adult hearts, and a faulty component of these pathways could result in cardiac hypertrophy and cardiomyopathy (5, 6, 52, 53, 99, 100). In the presence of canonical Wnt signaling, cytoplasmic β-catenin is stabilized and enters the nucleus, where it interacts with TCFs, such as Lef1, to regulate gene expression. In the absence of Wnt signaling, cytoplasmic β-catenin is targeted for destruction by the APC, axin, and GSK3β complex that phosphorylates β-catenin and directs it to a destruction pathway (95). β-catenin is known to bind N-cadherin at ICDs to regulate N-cadherin-mediated adhesion. Therefore, canonical Wnt and N-cadherin-mediated signaling pathways potentially compete for the same pool of β-catenin.
2.2.1. N-cadherin/β-catenin signaling in the heart
It has been shown that either too much or too little N-cadherin in the heart leads to dilated cardiomyopathy, suggesting that delicate signaling through N-cadherin is required for normal adult heart function. Transgenic mice over-expressing N-cadherin in the heart develop cardiomyopathy, whereas ectopic expression of E-cadherin in the heart leads to a much more severe cardiomyopathy (101). Ectopic expression of E-cadherin in the heart would interfere with N-cadherin-mediated signaling and result in a more severe cardiomyopathy. Conditional deletion of N-cadherin in the adult heart leads to a complete dissolution of ICD structure and a significant decrease in the gap junction protein, Cx43 (102). Consequently, N-cadherin-deficient mice exhibit dilated cardiomyopathy, impaired cardiac function, ventricular arrhythmias and sudden death (103, 104). These results suggest that N-cadherin-mediated adhesion and signaling pathway are essential for the structural integrity and function of the heart.
The most characterized cellular signals involving cadherin/catenin complexes are those generated locally upon cadherin-cadherin engagement during cell-cell contact formation. In non-cardiomyocytes, small GTPases (Rho, Rac and Cdc42) have been shown to transduce such local signals to control cell adhesion, survival, shape change and motility (105–107). Following the engagement, the juxtamembrane domain of cadherin interacts with p120-catenin, which can activate Rac1 and Cdc42, by binding to Vav2, a GEF for these GTPases (73). In addition to initiating cellular signals during contact formation, cadherin/catenin complexes in established junctions are also involved in mediating signal transduction. The adherens junctions are recognized as a sensor for mechanical forces and transduce signals that influence the actin cytoskeleton, which relies on the actin cytoskeleton and the proteins linking adherens junctions to the actin cytoskeleton (108, 109). Such mechanical signal transduction likely involves small GTPases (110). Interestingly, we have shown that mXinα is capable of interacting not only with β-catenin but also with p120-catenin (89). Furthermore, mXinβ-null hearts show a significant decrease in active Rac1, a failure to form mature ICD and a misaligned myocardium (18). These results together suggest an involvement of Xin repeat-containing proteins in the N-cadherin-mediated signaling pathway.
2.2.2. Wnt/β-catenin signaling in the heart
The role of Wnt/β-catenin signaling in cardiac development has been intensively studied in a variety of organisms, although controversy remains. During early cardiac development, Wnt/β-catenin signaling appears to have developmental stage-specific biphasic effects on cardiogenesis (111, 112). Activation of Wnt/β-catenin signaling before gastrulation promotes mesoderm formation and cardiogenesis, whereas signaling during and after gastrulation inhibits cardiomyocyte differentiation by opposing bone morphogenetic protein (BMP) signaling (113, 114). However, the hypothesis that Wnt actively inhibits cardiogenesis is still too simple. Recent studies using both gain and loss of Wnt/β-catenin function have shown that the Wnt/β-catenin pathway acts cooperatively with fibroblastic growth factor (FGF) and BMP signaling to promote expansion of the second heart field progenitors (115–117), which contribute to outflow tract and right ventricle (118, 119). In postnatal and adult hearts, the importance of Wnt/β-catenin signaling for cardiac remodeling under physiological and pathological conditions has also been intensively addressed. Activation of β-catenin in cultured rat neonatal cardiomyocytes was found to be not only sufficient but also necessary to induce cardiomyocyte hypertrophy (120, 121). In vivo studies using inducible cardiac-specific knockout or transgenic mice to modulate the expression levels of Wnt/β-catenin signaling components or their mutants have further confirmed that stabilization of β-catenin or activation of Wnt signaling is required for both physiological and pathological cardiac hypertrophy (99, 122–124). However, conflicting results have also been reported (125). The precise reason for such discrepancy is unknown but may reflect the pleiotropic effects of Wnt signaling depending on the experimental conditions. Recently, studies with conditional transgenic mice expressing either no β-catenin or stabilized β-catenin generated by using a ventricle-specific driver (MLC2v-Cre) have revealed that mice lacking β-catenin in the adult ventricles do not have an overt phenotype (100), due to an up-regulation of plagkoglobin, as suggested previously (126). In contrast, mice expressing stabilized β-catenin develop cardiac hypertrophy and dilated cardiomyopathy at 2 months of age, and do not survive beyond 5 months (100). Furthermore, stabilized β-catenin was only found at the ICDs but never detected in the nucleus (100). These results suggest that β-catenin’s role in the nucleus may be of little significance in the healthy adult heart, and that similar to N-cadherin, too much β-catenin at the ICD may critically affect the N-cadherin/β-catenin signaling and subsequently lead to dilated cardiomyopathy. It should be noted that increased β-catenin levels were also detected in the hypertrophic hearts from human cardiomyopathy patients and from spontaneously δ-sarcoglycan-deficient hamsters (127). The accumulation of β-catenin at the ICD, but not in the nucleus, is accompanied by an increase in Wnt5a (a noncanonical Wnt) expression, a decrease in GSK3β expression and differential expression of APC isoforms. The existence of multiple Wnt signaling pathways in the heart has added another level of complexity to Wnt signaling related to cardiac remodeling.
2.2.3. Interplay between Wnt/β-catenin signaling and adhering junction-mediated signaling
Down-regulation of Wnt/β-catenin signaling by nuclear plakoglobin as detected in ARVC hearts might be part of the molecular mechanism for the pathogenesis of ARVC (5, 53). Adult mice heterozygous for the deletion of desmoplakin in the heart recapitulate the phenotype of ARVC (5). Apparently, desmoplakin deficiency leads to an impaired desmosome assembly, which could free plakoglobin from the desmosomes and increase its nuclear localization in cardiomyocytes. Plakoglobin is known to be able to compete with β-catenin at multiple cellular levels with a net negative effect on the Wnt/β-catenin signaling pathway (50, 51). Thus, increasing plakoglobin nuclear localization in desmoplakin heterozygous mice should suppress Wnt/β-catenin signaling, which in turn would promote adipogenesis, fibrogenesis and apoptosis (128–130), the characteristic hallmarks of human ARVC. This mechanism has been further supported by two recent studies with transgenic mice over-expressing plakoglobin in cardiomyocytes as well as mice with a conditional knockout of plakoglobin in cardiomyocytes. Over-expressed plakoglobin translocated to the nucleus and suppressed Wnt/β-catenin signaling. The association of plakoglobin, instead of β-catenin, with Tcf712 increases the expression of Wnt5b and BMP7, which promotes adipogenesis, and decreases the expression of connective tissue growth factor, which is an inhibitor of adipogenesis (53). The adipocytes in mouse and human ARVC hearts were identified to originate from the second heart field progenitors, accounting for a predominant involvement of the right ventricle in human ARVC (53). On the other hand, Wnt/β-catenin signaling was activated in the hearts of mice with inducible cardiac-restricted plakoglobin deletion (52). Upon deletion of plakoglobin, expression levels of Wnt/β-catenin target genes, such as c-Myc and c-Fos, were increased significantly. Stabilization of β-catenin following the loss of plakoglobin may be due to activation of Akt and inhibition of GSK3 (52), which could affect the β-catenin phosphorylation state/stability and promote cardiac hypertrophy. Interestingly, stabilized β-catenin in plakoglobin-null hearts became associated with Tcf4, a transcription factor primarily binding to plakoglobin. These results are consistent with the idea that β-catenin directly competes with plakoglobin for Tcf4 binding. The mutant mice exhibit progressive loss of cardiomyocytes, extensive inflammation, fibrosis, altered desmosome structure and cardiac dysfunction similar to ARVC patients. However, in contrast to the desmoplakin heterozygous hearts, neither adipocyte replacement nor lipid droplet accumulation was observed in the conditional plakoglobin knockout hearts, suggesting that plakoglobin itself might be required for the full spectrum of ARVC phenotype.
Thus, based on mouse models with various manipulations of the components of adhering junctions (adherens junctions and desmosomes), it seems that the molecular mechanism of ARVC consists of both nuclear and desmosomal signaling pathways. In one mechanism, elevated plakoglobin in the nuclei alters Wnt/β-catenin signaling, which appears to be critical for the manifestation of ARVC phenotypes such as apoptosis, fibrosis and adipogenesis. On the other hand, signals generated by desmosome likely play a role in the pathology of ARVC, because down-regulation of Wnt/β-catenin signaling by simple conditional deletion of β-catenin in the postnatal heart does not lead to ARVC. The specific involvement of signals from the desmosomes in ARVC is further supported by the lack of ARVC phenotype in mice with conditional deletion of adherens junction components, such as N-cadherin (102), αE-catenin (27), mXinα (17) and β-catenin (99, 100, 126). These mice exhibit dilated cardiomyopathy with neither myocyte loss nor inflammation, which is different from the conditional plakoglobin knockout mice (52) and other animal models of ARVC (34, 53, 131). These differences suggest that different signaling may transduce through adherens junctions versus desmosomes.
2.2.4. ICD influences ion channel surface expression
As a functional unit, the ICD also plays important roles in organizing and/or regulating surface ion channels and receptors. Previous studies have shown that the pore-forming α-subunit, Nav1.5, of the voltage-gated sodium channel is preferentially localized to the ICD (132–136). This population of sodium channel complexes is composed of Nav1.5, tyrosine-phosphorylated β1 subunit, and ankyrin G in close association with both N-cadherin and Cx43 (137, 138). A recent study has shown that Nav1.5 can be pulled down by the head domain of PKP2 from heart lysates, suggesting that PKP2 participates in the same molecular complex at ICDs. Knockdown of PKP2 expression in cultured cardiomyocytes by siRNA leads to a decrease in peak current density, changes in the current kinetics (inactivation and recovery from inactivation), and a slower velocity of action potential propagation (139). In this special issue, Dr. Cheng-I Lin’s group presents evidence that ICD-associated mXinα protein influences surface expression of transient outward potassium current (ITO) through its ability to interact with Kv channel interacting protein 2 (KChIP2) (140), an auxiliary subunit of ITO, and filamin, an actin-crosslinking protein. Taken together, these results further suggest a link among all 4 components of the ICD: desmosomes, adherens junctions, gap junctions and the voltage-gated channel complex. It seems relevant to consider the ICD as an overall functional unit when seeking to understand the pathogenesis of cardiac disease.
2.3. Discovery of the Xin repeat-containing and ICD-associated protein family
Prior to the availability of genome-wide microarray and functional genomics, we used differential mRNA display screening in conjunction with whole-mount in situ hybridization to clone novel genes that are temporally and spatially expressed during cardiac morphogenesis (14). Cardiac cushion formation and valvuloseptal morphogenesis are essential for a four-chambered heart. These processes involve inductive interaction between myocardium and endocardium as well as EMT, which occur temporally in chicken embryos between Hamburger and Hamilton (HH) stage 15 and 21 and spatially at the future atrioventricular canal (AVC) and future outflow tract of the linear heart tube (141–143). Therefore, we performed differential display cloning on the total RNAs prepared from the AVC region of stage 15 and 21 chicken hearts. Whole-mount in situ hybridization was used as a secondary screening method to confirm the temporal and spatial expression patterns of isolated genes. From this screen a novel gene, 21C, among others was identified. Subsequently, we used antisense oligonucleotide treatment of culture chicken embryos to show that this 21C, later called chicken Xin (cXin), plays a very important function in cardiac morphogenesis and looping (15). Furthermore, we cloned mouse homologs of cXin and identified the gene as a downstream target of Mef2C and Nkx2.5 (15). Because of its critical role for normal heart development, but not because of its strong cardiac expression as wrongly cited in Otten et al.(144), we then called this gene Xin (a Chinese word meaning heart). Xin encodes a modular protein that contains a N-terminal 16 amino acid repeating unit (called Xin repeat) with a consensus sequence of GDV(K/Q/R/S)XX(R/K/T)WLFET(Q/R/K/T)PLD (15, 145, 146). Since the initial discovery of cXin, two homologous genes, each containing a Xin repeat region, have been identified in mammals: mXinα and mXinβ (also called Myomaxin) in mouse (15, 17, 18, 147, 148) as well as hXinα (also called cardiomyopathy associated 1, CMYA1, or Xin actin-binding repeat containing 1, XIRP1) and hXinβ (also called CMYA3 or XIRP2) in humans (145, 146, 149).
3. EXPRESSION, DOMAIN STRUCTURES AND FUNCTION OF XIN
3.1. Xin is a striated muscle-restricted gene and a downstream target of Nkx2.5 and Mef2
Multiple tissue Northern blot analyses revealed that cXin (9.0kb), mXinα (5.8kb) and mXinβ (12kb) messages were detected only in heart and skeletal muscle (15, 145, 147). Occasionally, low level Xin expression could be detected in the pulmonary vein of lung tissue, which may represent associated cardiomyocytes in this tissue. Whole-mount in situ hybridization revealed that expression patterns of cXin and mXinα in the developing heart and somites (15) are very similar to that of Nkx2.5 and Mef2C (150, 151). In an anterior medial mesoendoderm explant system, the induction of cXin expression by BMP-2 followed activation of Nkx2.5 and Mef2C, but preceded expression of myosin heavy chain (15). Similar effects on cardiac looping morphogenesis observed in embryos after Nkx2.5 (152) or Mef2C (153) deletion or cXin antisense treatment (15) further suggest that cXin participates in a BMP-Nkx2.5-Mef2C pathway to regulate chicken cardiac morphogenesis. Either Nkx2.5 or Mef2C alone is able to trans-activate the expression of a luciferase reporter gene driven by the mXinα promoter in non-muscle cells. These results together with drastically down-regulated mXinα messages in Nkx2.5-null and Mef2C-null mouse embryos (145) suggest that mXinα also participates in a Nkx2.5-Mef2C pathway to control cardiac differentiation and morphogenesis in the mouse. Moreover, mXinβ has been shown to be a direct target of Mef2A (147) and to function downstream of angiotensin II (AngII) signaling to modulate pathological cardiac remodeling (154). Therefore, the Xin repeat-containing protein family plays an important role in cardiac development and function through the Nkx2.5-Mef2 pathway.
3.2. Two phases of Xin up-regulation during development correlate with chamber/valve formation and postnatal heart growth
During embryogenesis, the cXin transcript is first detected at HH stage 8 in the paired lateral plate mesoderm that forms the primordial of the heart (15). At stage 9, cXin expression increases substantially in the heart forming fields, which migrate anteriorly and ventrally toward the midline of the embryo. At stage 10, cXin is expressed exclusively in the linear heart tube. Cardiac specific expression continues until stage 15, when somite expression can be detected. Skeletal and cardiac muscle-restricted expression of cXin continues throughout development and into adulthood. The relative expression level of cXin/glyceraldehyde 3 phosphate dehydrogenase (GAPDH) determined from Northern blot analysis of developing hearts reveals that two major phases of cXin up-regulation exist at stage (st.) 16–25 and post-hatch day (D)12–14 (Figure 3). These two peaks of cXin up-regulation appears to coincide with the timing for chamber/valve formation and postnatal heart growth, respectively (141), suggesting a role for cXin in normal cardiac morphogenesis. Supporting this role, knocking down cXin by antisense oligonucleotide collapses the heart chamber wall, leading to abnormal cardiac morphology (15).
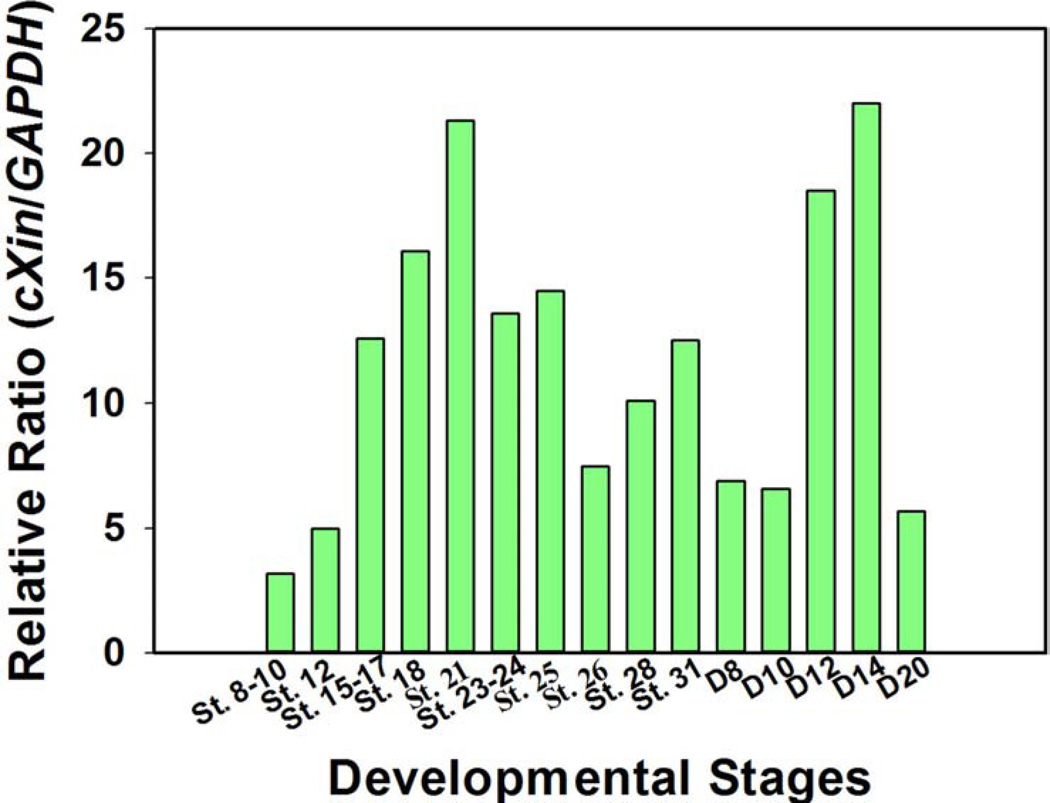
Figure 3.
cXin expression profile during cardiac development. Developing hearts were dissected from chicken embryos at various Hamburger-Hamilton Stages (St.) and from chicks at various post-hatch day (D) and used for total RNA isolation and Northern blot analysis. After scanning the blots, the expression levels of cXin message were normalized with the expression levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) at respective time point, and the ratios were plotted against developmental stage. Two Xin up-regulation peaks correlate with valve/chamber formation (St. 26~25) and postnatal heart growth (D10~20).
The mXin protein was first detected in the developing linear heart tube of E8.0 mice by whole-mount immunofluorescence microscopy with the antibody recognizing both mXinα and mXinβ (148). At this stage, one to seven somites are present but contain no mXin protein. At E10 (equivalent to HH st. 17 in chick), mXin is expressed throughout the myocardium but not the endocardium of the truncus arteriosus, common atrial chamber and ventricle; mXin expression is also detected within the myotome of the first two rostral most somites (148). As development progresses to E13.5 (equivalent to HH st. 30 in chick), staining detects strong mXin expression throughout the myocardium of the ventricle and atria. Co-localization of mXin with both β-catenin and N-cadherin is observed in the cell periphery but not the nucleus of early mouse embryonic hearts (148). Later in embryogenesis co-localization becomes punctate structures around the cardiomyocytes. During the first 3 weeks of postnatal life, these co-localized structures gradually move into the termini of elongated cardiomyocytes to form mature ICDs. Similar to the second phase of cXin up-regulation, peaking at D14, expression of mXinα and mXinβ in postnatal mouse hearts increase significantly from newborn to postnatal day 13.5 (P13.5) (18). During this developmental period, N-cadherin expression in the mouse heart also exponentially increases, reaching a plateau at two weeks of age and maintaining this level throughout adulthood. The timing of the Xin up-regulation coincides with the maturation of ICD (3, 148), T-tubule and sarcoplasmic reticulum (155), as well as diastolic function (156), suggesting a vital role for Xin in cardiac development and function. Supporting this role, the mXinβ-null mouse hearts fail to form mature ICDs, exhibit diastolic dysfunction and premature death (18).
3.3. Xin expression is significantly up-regulated in animal models of cardiac hypertrophy and hypertension
In response to abnormal stresses, such as hypertension, pressure overload, endocrine disorders and myocardial infarction, adult cardiomyocytes undergo pathological hypertrophy. Hypertrophy can be a compensatory mechanism that helps to preserve pump function in pathological conditions. Frequently, this hypertrophy progresses to dilated cardiomyopathy. Using pressure overload-induced hypertrophy by thoracic aortic banding (157–159), we detected up-regulation of both mXinα and mXinβ messages in the banded hearts, as compared with sham-operated controls (Figure 4A). In addition, immunofluorescence microscopy showed that banded hypertrophic hearts had thickening ICDs containing much more mXin and N-cadherin proteins (Figure 4B), suggesting that mXin may play an important role in modulating hypertrophy/stress responses in the adult heart. The up-regulation of mXinβ has also been observed in hypertensive hearts induced only by Ang II infusion (within 6 hours) but not by salt, suggesting that its up-regulation is due to Ang II-induced myocardial damages and not to blood pressure elevation per se (160). Thus, up-regulation of mXinβ appears to be one of the earliest molecular events triggered by Ang II. It is likely that mXinα up-regulation would also be observed in this Ang II-induced hypertension/myocardial damage model, because both mXinα and mXinβ are transcriptional targets of MEF2 (15, 145, 147). It has recently been shown that mXinβ hypomorphic mice with 80% reduction in mXinβ message results in cardiac hypertrophy (154). Hearts from these hypomorphic mice display less myocardial damage when exposed to Ang II (154). These results suggest that mXinβ functioning downstream of Ang II signaling can modulate cardiac function in health and disease.
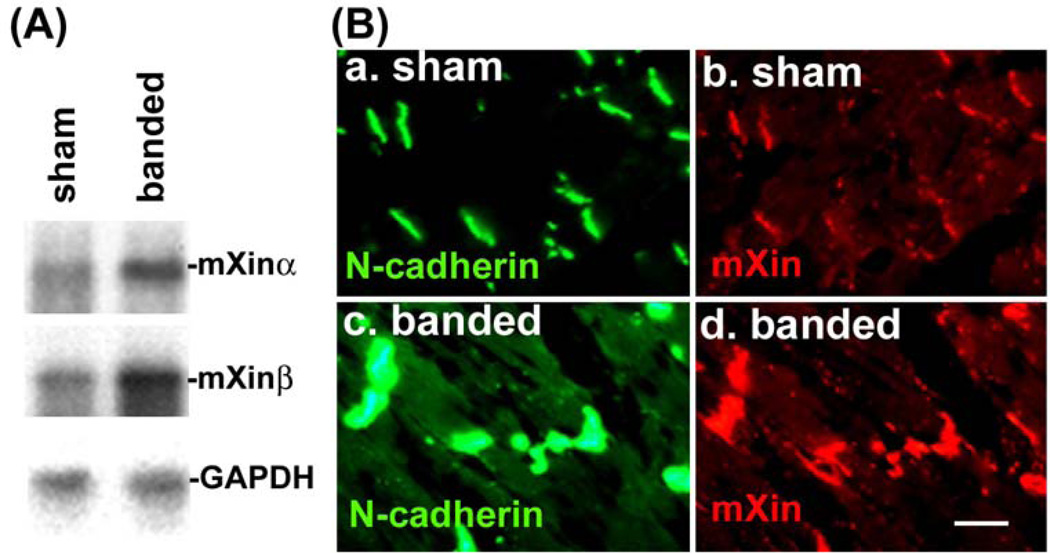
Figure 4.
Up-regulation of both messages and proteins of mXinα and mXinβ in stressed hearts. (A) Northern blot analysis of total RNAs prepared from sham-operated and thoracic aortic banded hearts. GAPDH blot was used as a control for RNA loading. (B) Double-label immunofluorescence microscopy of heart sections from sham-operated and thoracic aortic banded mice with anti-N-cadherin antibody (in green) and anti-Xin antibody (in red). N-cadherin and Xin co-localize to ICD of both sham-operated and banded hearts. The thickening ICD in banded hearts supports the up-regulation of both N-cadherin and mXin proteins.
3.4. The origin of Xin coincides with the first appearance of the true heart chamber, and mXinβ is phylogenetically closer to the ancestral Xin protein than mXinα
A phylogenetic analysis has been performed with 40 vertebrate Xins to elucidate the evolutionary relationship between Xin proteins and to identify the origin of Xin (16). Multiple sequence alignment (161, 162) of the Xin repeats from vertebrates was analyzed in maximum likelihood and Bayesian analyses (163–165). The constructed evolutionary tree replicates the phylogeny of taxa with mammals, other land vertebrates and teleost phylum-level groups. Clearly, whole genome duplication that occurred early in evolution to produce both Xinα and Xinβ (16). The additional gene duplication of Xinβ was detected in teleosts. BLAST searches only detect Xin in chordates but not in other organisms such as Saccharomyces cerevisiae, Candida albicans, Arabidopsis thaliana, Dictyostelium discoideum, Caenorhabditis elegans, Anopheles gambiae (mosquito) and Drosophila melanogaster. Further analyses failed to identify Xin repeat-containing proteins in the Urochordate tunicate (Ciona savignyi) or the Cephalochordate amphioxus (Branchiostoma floridae). A Xin protein, defined as a protein containing Xin repeating units, was first identified in the Craniate lamprey (evolved about 550 million years ago). Importantly, both the Urochodate tunicate and the Cephalochordate amphioxus have hearts with only a single layer of contracting mesoderm or contracting vessel coupled with an incomplete endothelial cell layer, whereas the Craniate lamprey has a true chambered heart with complete endothelial and myocardial layers. Thus, the origin of Xin proteins coincides with a critical evolutionary modification of the heart, namely, the origin of true chambers. This finding is consistent with the chamber genesis role for Xin repeat-containing proteins in vertebrates.
In the avian lineage, there is a loss of Xinβ, however, its sole Xin protein still retains 27 Xin repeats (compared to 28 repeats in the ancestral lamprey Xin) in order to carry out the essential functions of Xin (16). Therefore, chicken embryos treated by antisense oligonucleotide targeting cXin showed defects in cardiac looping and morphogenesis (15). In the mammalian lineage, Xinα contains a reduced number of Xin repeating units with 17 repeats in opossum and 15 in placental mammals. This strongly suggests that there was selective pressure within the mammalian lineage that resulted in the reduction of Xin repeats. We previously found that mXinα-null mice are viable but develop cardiac defects in adults (17), and mXinβ-null mice die postnatally with chamber defects (18). Thus, it is likely that mammalian Xinαs evolved quickly to form many unique traits for neofunction in the adult heart, whereas all mammalian Xinβs are highly conserved with the ancestral lamprey Xin and retain its function in embryonic development (16). Evolutionary studies have also identified a putative DNA-binding domain conserved in the N-terminus of all Xins, in addition to a highly conserved β-catenin binding domain within the Xin repeat region (16). In the C-terminus, Xinαs and Xinβs are divergent relative to each other but each isoform from mammals shows a high degree of within isoform sequence identity (16). These results suggest different but conserved functions for mammalian Xinα and Xinβ.
3.5. mXinβ messages are preferentially localized to ICDs and enriched in cells of the left ventricle, interventricular septum and apex, particularly near the base of the aorta and pulmonary artery of the heart
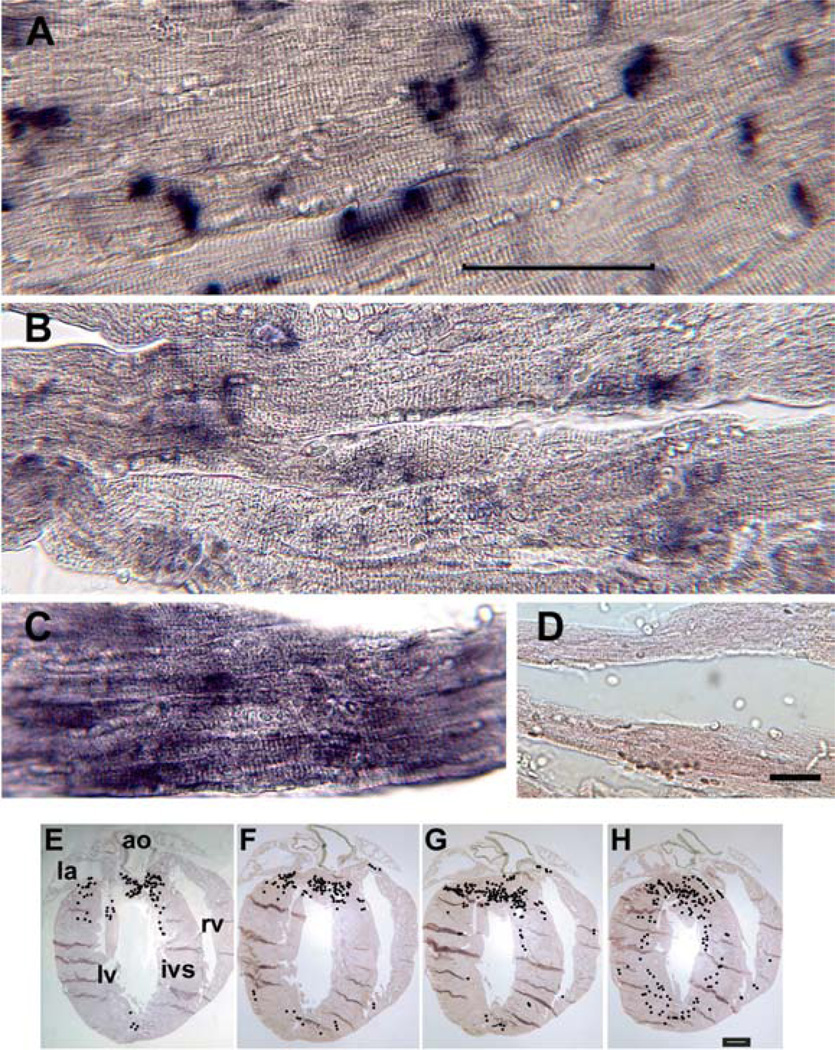
We have previously shown by in situ hybridization that mXinβ mRNAs are specifically expressed at discrete regions in cardiomyocytes that highly resemble ICDs (17). On the other hand, only a less extent of mXinα mRNAs are localized to the ICD-like structures, whereas cardiac troponin T messages are never found at ICDs (Figure 5). The localization of mXinβ mRNAs to the ICD, where the protein is utilized, may provide an efficient way for mXin protein localization, given the fact that mXin is also capable of binding to actin filaments. Although the molecular mechanism for this ICD localization of mXinβ RNAs remains to be determined, it may suggest an initiating role for mXinβ protein in the formation of ICDs (more discussion below).
Figure 5.
mXinβ RNA was specifically expressed at the ICD-like region. In situ hybridization of heart sections was performed with digoxigenin-labeled mXinβ antisense riboprobe (A), mXinα antisense riboprobe (B), cardiac troponin T antisense riboprobe (C), or mXinβ control sense riboprobe (D). bar in A = 50μm for A~C, bar in D = 25μm. (E~H) In situ hybridization was performed with mXinβ antisense riboprobe on serial 15μ-sections of an entire heart (total 264 sections/heart). The locations of mXinβ RNAs were carefully and correspondently mapped by black dots on the low-magnification images of serial sections after examining each section at high magnification. (E~H) represents sections from 22 to 25. Bar in H = 0.5mm for E~H. la, left atrium; ao, aorta; lv, left ventricle; rv, right ventricle; ivs, interventricular septum.
To further identify which region of the heart mXinβ is preferentially expressed, serial sections of the entire hearts were prepared for in situ hybridization. At high magnification, positive hybridization signals were again found at discrete ICD-like regions; however, mXinβ expression is not uniformly distributed throughout the heart. The location of mXinβ mRNAs were carefully and correspondently mapped by “black” dots on the low-magnification images of serial sections while examining each section at high-magnification, examples are shown in Figure 5E-H. Overall, mXinβ messages were enriched in the left ventricle (lv), interventricular septum (ivs) and apex, particularly concentrated at the base of aorta (ao) and pulmonary artery, where cardiomyocytes may be subjected to relatively high stress. Relatively low levels of mXinβ expression were found in the right ventricle (rv), right atrium (ra) and left atrium (la). The significance of this expression pattern remains to be determined.
3.6. Domain structures of Xin proteins
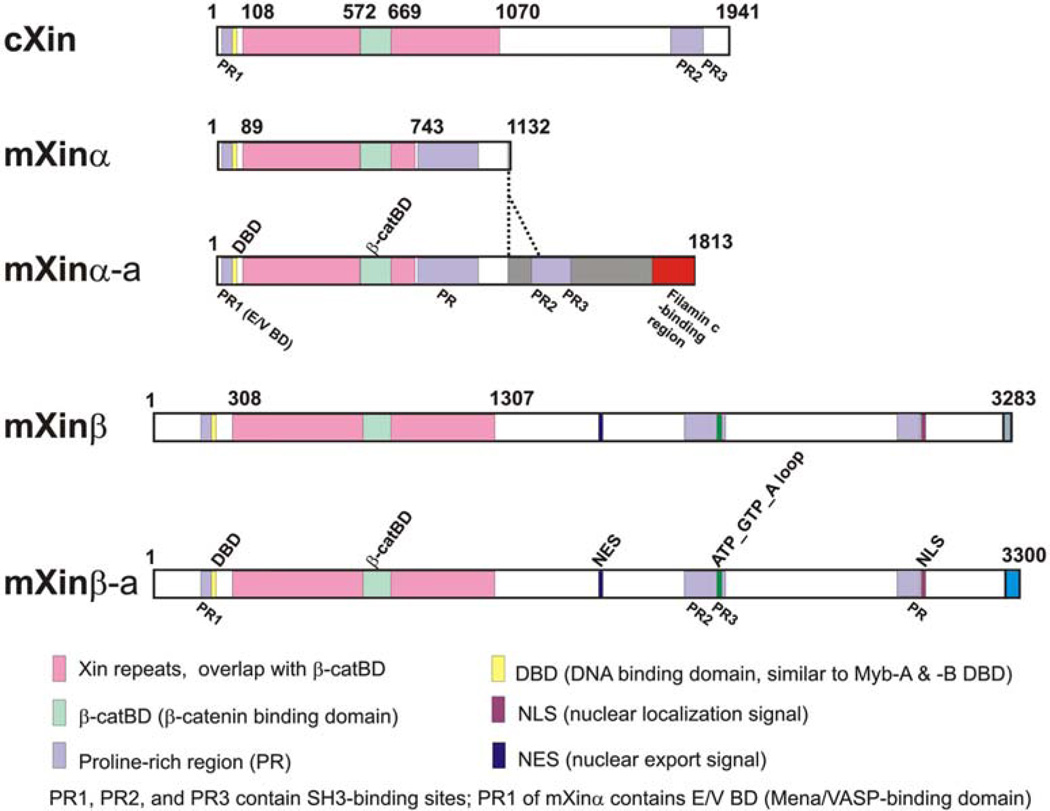
The Xin gene encodes a striated muscle-specific protein containing a region with 15~28 Xin repeats. The Xin repeat defines a new class of actin binding domains with a minimum of 3 repeats required to bind actin filaments (146). The mXinα is further shown to not only bind but also bundle actin filaments (89). The pink box shown in Figure 6 represents the Xin repeat region found in all Xin proteins from chick and mouse. Within the Xin repeat region, there is a highly conserved β-catenin-binding domain (β-catBD, indicated by a light green box), which has been mapped previously on mXinα (17). Similar to hXinα (149), mXinα undergoes an unusual intraexonic splicing of exon 2 to generate two variants differing in its C-terminus (17). The larger variant is termed mXinα-a, which contains a region homologous to the filamin c-binding motif (red box in Figure 6) identified in hXinα (149); however, this filamin c-binding motif is not found in cXin and mXinβ (16). Another feature of all Xin repeat-containing proteins is the existence of multiple proline-rich (PR) regions. The highly conserved PR1 or Mena/VASP-binding domain (E/V BD in Figure 6) at the N-terminus has been shown to bind to Mena/VASP proteins (16, 149). Interestingly, the sequences downstream of the E/V BD are highly conserved among all Xin proteins (indicated by yellow box in Figure 6) and homologous to the Myb DNA-binding domain, despite that the function of this putative DNA binding domain (DBD) is unknown. The roles of the other PR regions including PR2, PR3 and PR distributed at the C-terminus of the protein remain unclear.
Figure 6.
Domain structures of Xin from chick and mouse. Amino acid residue numbers are labeled above on each Xin protein diagrams. Through alternative splicing, both mXinα and mXinβ genes encode two protein variants, which differ only in the very C-terminal sequences. The actin-binding motifs are contained in the Xin repeat region (indicated by pink box), within which a conserved β-catenin binding domain (β-catBD, indicated by light green box) is located. Other binding domains defined on large variant of human Xinα (hXinα-a) include filamin c-binding region (indicated by red box in mXinα-a) and Mena/VASP-binding domain (PR1, E/V BD, indicated by purple box in all Xin proteins). The functions of other proline-rich regions (PR, PR2, PR3) and other consensus sequences such as DNA-binding domain (DBD), nuclear export
In mXinβ, alternative splicing of the primary transcript leads to an inclusion or exclusion of exon 8 and results in two protein variants differing in its very Cterminus (18). The large variant is called mXinβ-a. The significance of this difference remains to be determined. Both mXinβ and mXinβ-a also possess consensus sequences for nuclear export signal (NES), nuclear localization signal (NLS) and ATP/GTP-binding domain (ATP_GTP_A loop) (18); however, the functions of these domains are still unknown.
3.7. ICDs are formed postnatally
Adult ventricular cardiomyocytes are rod-shaped, highly bi-polarized cells. A structural and functional segregation exists between parts of the plasma membrane that are either parallel or perpendicular to the long axis of the cell. The membrane that is parallel to the long axis (lateral membrane) associates with the extracellular matrix through costameres. On the other hand, the membrane that is perpendicular to the long axis (terminal membrane) forms the highly specialized ICDs that mediate cell-cell adhesion and communication (3). Early observations with transmission electron microscopy had hinted that formation of ICDs is a late event of cardiac development. With the electron microscopy, Legato found that in neonatal dog hearts, cardiomyocytes were tightly packed together and extensively contacted each other, contrasting with the limited cell-cell contacts at the ICDs in adult hearts (166). Forbes and Sperelakis noticed that the ICDs of the P2 mouse hearts were more primitive than adult ICDs, in that the former had less inter-digitation and less dense cytoplasmic plaques (1). However, electron microscopy could only reveal ICD components that are already incorporated into specialized intercellular junctions; thus the extent of developmental changes during ICD formation had largely been overlooked until specific antibodies against ICD components were utilized to study the development of ICDs.
Although all three types of intercellular junctions localized to the adult ICDs could be identified by electron microscopy in mouse cardiomyocytes at embryonic day 10 (E10) (1), recent studies with specific antibodies demonstrated that ICDs are formed through a series of events that happen mainly postnatally. In mice and rats, typical adult ICDs are not completely formed until P90, while in humans, maturation of ICDs continues until age 7 (21, 167–169). The formation and maturation of ICDs, in essence, is a process of specialization of the subdomains of the cardiomyocytes’ membrane. During the formation of ICDs, cardiomyocytes redistribute the adhering cell-cell junctions (adherens junctions and desmosomes) and gap junctions to the terminal ends, and through poorly understood processes, further recruit an extensive panoply of junctional, channel, signaling and auxiliary proteins to the ICDs.
3.7.1. Redistribution of Junctional components during ICD formation
3.7.1.1. Adherens Junctions
The leading role of adherens junctions in the hierarchy of establishment and maintenance of different intercellular junctions has been demonstrated in various in vitro and in vivo systems, including cardiomyocytes (102, 170–172). Thus, the developmental changes of adherens junctions are particularly important when studying ICD formation. Indeed, the components of adherens junctions are extensively redistributed during embryonic and postnatal development in the heart. Consistent with the tight packing and extensive contacting of cardiomyocytes in embryonic hearts (166), immunofluorescence staining localizes N-cadherin to the cardiomyocyte surface almost homogeneously at E10.5 (148). Later during embryonic development, N-cadherin staining becomes heterogeneous and the cardiomyocyte surface is characterized by bright spots interspaced by weakly and diffusely stained areas (148). These bright spots likely represent N-cadherin clusters (and their intracellular partners) involved in strong homophilic interaction with opposing cells. On the other hand, the adherens junctional components not incorporated in such bright spots may represent N-cadherin molecules that are yet to be clustered and engaged in strong cell-cell adhesion. Consistent with this idea, adherens junctions identifiable in transmission EM micrographs only occupy a small fraction of the cell-cell contacting interface (166), despite the extensive staining of N-cadherin on the surface of cardiomyocytes during these embryonic stages.
The extensive coexistence of brightly stained spots and more diffusely distributed N-cadherin signals can be found on virtually the entire surface of cardiomyocytes until P3.5 (21, 148, 167, 168). The trend for N-cadherin to become heterogeneously distributed continues after P3.5, leading to the loss of N-cadherin staining at some lateral regions in the P5.5 mouse heart. However, at P5.5, very few ICD-like features can be found and the N-cadherin spots are still extensively scattered on the entire surface of cardiomyocytes, suggesting that ICD formation requires further steps.
At P7.5, a few contacts between the longitudinal termini of cardiomyocytes are strongly stained for N-cadherin, which also adopt a transverse orientation relative to the long axis, resembling the ICDs in adult hearts. Compared to P3.5, the number of N-cadherin spots and the intensity of the N-cadherin signal in them decrease at the lateral surface of the cardiomyocytes. The diffusely stained N-cadherin at the lateral membrane of the cardiomyocytes also occupies smaller areas. By P15.5, a majority of the ventricular cardiomyocytes form ICD-like structures, suggesting ICDs are established rapidly between P7.5 and P15.5. Coinciding with the intense staining of N-cadherin at the cell termini, the diffuse staining at the lateral surface disappears and the number and intensity of the lateral spots greatly decreases.
The switch of N-cadherin distribution from the lateral membrane to maturing ICDs is also supported by quantification of the N-cadherin signal at these loci by confocal imaging. The proportion of the integrated density of the N-cadherin signal (pixel number×intensity) at the maturing ICDs increases slowly during the first week of postnatal development and then rapidly in the second week and reaches a plateau after one month of ages (our unpublished observation).
3.7.1.2. Desmosomes
The dependence of desmosomes on the adherens junctions for their formation and maintenance has been well documented. Consistent with this, the time course of incorporation of desmosomal components into the ICDs seems to follow that of adherens junctions (167). However, the establishment of area composita and thus the almost perfect co-localization between adherens junctions and desmosomes by immunostaining seems to be a late event during postnatal development. Indeed, the amalgamation of the transmembrane proteins N-cadherin and desmoglein-2 is an ongoing process even at 3 weeks postnatal in mice, even though both proteins are highly localized to the cell termini at earlier stages (21).
3.7.1.3. Gap junctions
The developmental redistribution of gap junctions is one of the first phenomena noticed by researchers demonstrating the late maturation of ICDs (169). The incorporation of gap junctions into the cell termini seems to happen much later than the adherens junctions and desmosomes, and this phenomenon is shared by different mammalian species examined so far. In the mouse, at 3 weeks postnatal development, prominent Cx43 staining is seen as large puncta located at both the ICD and the lateral surface (167). Quantitative analysis of the distribution of adherens junctions and gap junctions confirmed delayed incorporation of gap junctions to the cardiomyocyte termini (167, 169). Interestingly, through double immunostaining, we have noticed that at three weeks of age the lateral gap junction puncta are associated with weakly labeled N-cadherin spots (unpublished observation). This observation suggests that the delayed incorporation of gap junctions may in fact be a manifestation of the N-cadherin mediated cell-cell contacts that persist on the lateral surface of cardiomyocytes at this time.
3.7.2. Molecular mechanisms of ICD formation
As reviewed above, the components of adherens junctions seem to undergo two phases of redistribution in order for ICDs to form. During embryonic and early postnatal development, adherens junction components form discrete spots from a diffusely localized pool. Next, the contacts at the cell termini expand to form mature ICDs while the lateral spots reduce both in number and signal intensity. Little is known about the molecular mechanisms controlling these processes. However, since cadherin mediated cell-cell interaction is fundamental in multicellular organisms, many principles learned from other models of adherens junction establishment, maintenance and regulation likely hold true for cardiomyocytes.
The process of forming brightly stained spots and the reduction of diffusely-stained N-cadherin on the lateral surface of cardiomyocytes might be mediated by cadherin clustering. Cadherin clustering is a critical aspect in adherens junction establishment and maintenance; however, its mechanism is still under debate. One model suggests that the cadherin extracellular domain interacts weakly with neighboring cadherin molecules and the intracellular domain is required for lateral clustering (173, 174). Yet studies with tailless E-cadherin mutants indicate that the extracellular domain of cadherin can form strong dimers; clustering and incorporation into cell-cell junctions might be an intrinsic property of cadherin’s extracellular domain (175, 176) and the clustering of cadherin at the initial stage of junction formation is mediated by a diffusion-mediated trapping mechanism (177). Since extensive evidence supports a critical role of the cytoplasmic tail of cadherin in clustering, it is likely that for intact cadherin molecules, their intrinsic clustering tendency is tightly regulated by intracellular mechanisms. Supporting this idea, it was shown that in 4-cell stage embryos, E-cadherin and other adherens junctional components are diffusely distributed on the entire surface of each blastomere. Junctional assembly is initiated at the 8-cell stage when compaction starts and protein kinase C plays a critical role through regulating β-catenin and other factors (178). Interestingly, the formation of N-cadherin clusters in the embryonic heart is akin to E-cadherin clusters in the compacting embryo. Therefore, it might be worthwhile investigating if similar mechanisms exist in the heart for the formation of N-cadherin clusters.
In epithelial cells, it has been shown that E-cadherin clusters are highly dynamic structure. New E-cadherin molecules are continuously added to the clusters and ATP-dependent mechanisms maintain the size of clusters by actively removing E-cadherin molecules from the cluster (175). Depletion of ATP in cells leads to very large cadherin clusters, suggesting cadherin cluster size is a result of dynamic equilibrium (175). The active removal of cadherins from clusters requires both endocytosis and other unidentified mechanisms (175, 179). If similar mechanisms exist for N-cadherin clusters in the heart, they could have important implications for the formation of ICDs. In principle, tipping the balance between N-cadherin clustering and dissociation could account for both the reduction of N-cadherin spots at the lateral surface of cardiomyocytes and the expansion of adherens junctions at the cell termini. Indeed, the role of cadherin endocytosis and recycling has been shown to be critical for epithelial junction formation and remodeling (180, 181). It has been well recognized that p120-catenin is the central player regulating internalization of cadherins (182). Thus, molecular partners of p120-catenin are also likely to be important regulators of cadherin stability.
The actin cytoskeleton plays an important role in the establishment and maintenance of adherens junctions. Therefore, regulating the interaction between cadherin-catenin complexes with the actin cytoskeleton may also be an important aspect in adherens junction remodeling during ICD formation. Although it has been shown that a direct and stable link between the cadherin-β-catenin complex and actin filaments mediated solely by α-catenin is unlikely (183, 184), emerging evidence indicates that molecules other than or in addition to α-catenin can stably couple the cadherin complex to the underlining actin filaments. Cavey and colleagues demonstrated in Drosophila embryonic epithelial cells that spot adherens junctions (SAJs) mediated by DE-cadherin is tightly coupled to very stable, actin depolymerizing drug-resistant actin patches (need to add ref 183 here). Since SAJ’s stability is α-catenin independent, Cavey et al. suggested that a factor “X” is responsible for connecting SAJs to the actin patches (185, 186). One candidate for the factor X in mammalian epithelial cells might be the protein EPLIN, which binds to both α-catenin and actin (187). Abe and Takeichi showed that EPLIN directly couples the E-cadherin-β-catenin-α-catenin complex to actin filaments (need to add ref 184 here). Interestingly, after treating the cells with actin depolymerizing drugs, the adhesion belt became fragmented and formed spots resembling the SAJs in Drosophila. It was shown that EPLIN stabilized the actin patches underling the cadherin clusters in drug treated cells (187). One difference between Abe and Cavey’s studies is that in order to stabilize adherens junctions, EPLIN requires α-catenin but the factor X does not. Potentially, the factor X in Drosophila has a partner in the cadherin complex other than α-catenin. Thus, these studies suggest that direct coupling to the actin cytoskeleton is critical for the stability of the cadherin complex and the specific molecular link might be cell type-dependent. In the heart, we have shown that mXinα can stabilize adherens junctions. Because mXinα directly interacts with β-catenin and actin filaments (89), mXinα may realize this function by acting as the factor X in the cardiomyocytes. Considering that mXinβ also contains the highly conserved β-catenin interaction domain (16), it is possible that mXinβ may also act as a stable link between the adherens junctions and actin filaments.
In addition to regulating adherens junction stability at the lateral surface and in maturing ICDs, cardiomyocytes likely promote adherens junction expansion at the maturing ICDs through active mechanisms. In epithelial cells, Rho family small GTPases have been shown not only to mediate signals initiated by cadherin engagement (177) but also to drive adherens junction initiation and expansion (188). Rac activity promotes lamellipodia formation, which promotes contact formation, whereas RhoA regulates contact expansion through regulating actomyosin contractility underlining the forming adherens junctions (188). It has been shown in non-muscle cells, that the small GTPase, Rap1, acts upstream of Rac and Rho to regulate adherens junctions (189, 190). Interestingly, Rap1 mediated signals have been shown to enhance adherens junction formation followed by gap junction establishment in cardiomyocytes (191), suggesting that the roles of these small GTPases are likely conserved in cardiomyocytes.
An important question about ICD formation is how do cardiomyocytes establish polarity? Cardiomyocytes are highly polarized cells with distinct lateral and terminal membrane domains. The mechanism that defines these membrane domains is poorly understood. One possibility is that epithelial apical-basal polarity regulators also function in cardiomyocytes. Indeed, the polarity protein Scribble was localized to the entire lateral surface but was excluded from ICDs in adult cardiomyocytes (192). It was shown that mutation of Scribble led to a temporary mislocalization of N-cadherin in embryonic hearts of mice. Unfortunately, the role of Scribble in ICD formation was not studied due to the early lethality of Scribble mutants (192).
On the other hand, it was firmly established that mechanical stretch plays critical roles in establishing and maintaining polarized positioning of junctional components in cultured cardiomyocytes and the small GTPase, Rac1, is an important mediator in this process (193–195). Nevertheless, it is still unclear how cardiomyocytes transduce mechanical stretch to localized signaling that dictates the positioning of cell-cell junctions.
3.7.3. Roles of mXinβ in ICD formation
3.7.3.1. mXinβ is required for ICD formation
Previously, our studies indicate that mXinβ is an important factor for ICD maturation (18). In the mXinβ-null heart at P16.5, ICDs fail to form. In contrast to the terminal localization of N-cadherin in wild-type cardiomyocytes, mutant cardiomyocytes exhibit numerous N-cadherin puncta on the lateral surface. Accompanying the mis-localization of N-cadherin, gap junctions and desmosomes are also scattered on the lateral surface and fail to concentrate at the termini. Our recent time course observation of N-cadherin distribution between P3.5 and P24.5 in the mutant heart showed that N-cadherin localization is indistinguishable from the wild-type heart up to P7.5, at which time cardiomyocytes are characterized by N-cadherin spots all over the surface and patches of diffusely localized N-cadherin. Therefore, mutant cardiomyocytes likely preserve the ability to carry out such process up to P7.5. However, at P13.5 when wild-type cardiomyocytes form morphologically distinguishable ICDs at their termini, very few ICDs can be found in the mutant heart; instead, the N-cadherin spots are still scattered on the surface of the mutant cardiomyocytes. Quantification showed that the proportion of N-cadherin localized to the termini of cardiomyocytes remain largely unchanged in mutant cardiomyocytes from P13.5 to P24.5, while wild-type cardiomyocytes exhibit a continuous increase of N-cadherin at the termini. These observations suggest that loss of mXinβ results in a failure to localize N-cadherin at the termini of cardiomyocytes as well as defects in the reduction of laterally localized N-cadherin spots. However, N-cadherin clustering into spots seems to be independent of mXinβ.
Distribution of desmoplakin and connexin43 in developing hearts indicated that both desmosomes and gap junctions are scattered on the surface of cardiomyocytes by P24.5. Interestingly, double labeling showed that the associations between desmosomes and adherens junctions as well as between gap junctions and adherens junctions are not different between wild-type and mutant hearts, suggesting that the laterally localized N-cadherin clusters were capable of organizing desmosomes and gap junctions.
Supporting the role of mXinβ in the redistribution of N-cadherin to the cell termini and the expansion of ICDs, a process that is most rapid during the second postnatal week, we showed that mXinβ expression is significantly upregulated at P7.5 and P13.5 (18). We have further extended this observation with quantitative Western blot analysis and showed that two expression peaks exist for mXinβ postnatally. The level of mXinβ protein reached the first peak at P13.5, followed by a significant reduction at P15.5 only to increase again to reach adult level. Most importantly, we found that at P7.5, mXinβ was highly localized to the cardiomyocytes’ termini where it is colocalized with N-cadherin. On the other hand, the lateral surface of cardiomyocytes contains little mXinβ. From P7.5 to adulthood, mXinβ is always localized to the termini of cardiomyocytes. Taken together, restricted localization of mXinβ to the termini of cardiomyocytes proceeds that of N-cadherin, suggesting that mXinβ is required for forming ICDs at the cell termini.
3.7.3.2. Potential mechanism by which mXinβ mediates ICD formation
How can mXinβ mediate the redistribution of N-cadherin to form the ICDs? As described in section 3.5 (Figure 5) and Supplemental Figure 2B in (17), mXinβ but not mXinα messages are preferentially localized to the ICDs, suggesting an efficient way for mXinβ protein to initiate ICD localization. This process is further enhanced by the postnatal surge of mXinβ expression at the onset of redistribution of N-cadherin clusters/puncta to cell termini (18). Through its Xin repeats and conserved β-catenin binding domain (16), mXinβ could directly interact with actin filaments and β-catenin, linking N-cadherin clusters to the underlying actin cytoskeleton. Further stabilization of N-cadherin clusters at the cell termini to form ICDs may require a collaborative role for mXinα, because mXinα, similar to N-cadherin, is mis-localized in mXinβ-null hearts (18). Previously, we have also shown that mXinα can directly interact with both β-catenin and p120-catenin (89) and both catenins are known to be necessary for assembling and maintaining cadherin on the cell surface (182, 196). Thus, by collaborating with mXinα at the cell termini, mXinβ may further enlist the catenins for the localized stabilization of adherens junctions and thus facilitate ICDs to be formed. Moreover, our observations that local activity of Rac1 at the forming ICD was significantly down-regulated in the mXinβ-null hearts may suggest an additional role of mXinβ in regulating Rac1 activity for ICD expansion (18). In summary, mXinβ may mediate ICD formation by initiating the redistribution of N-cadherin to cell termini, collaborating with mXinα to further stabilize the forming adherens junctions, and regulating Rac1 activity to expand the ICD membrane.
3.8. mXin plays important roles in the surface expression of ion channels
mXin proteins are co-localized with N-cadherin and Cx43 in the myocardium of the mouse (148). Loss of mXinα causes a decrease in the expression level of β-catenin, N-cadherin, and desmoplakin in the adult heart, weakening cardiomyocyte adhesion and compromising ICD integrity. Consequently, Cx43 in mXinα-null hearts is mis-localized, which may lead to cardiac gap junction remodeling and conduction defects (17).
Studies have revealed a more detailed mechanism by which mXinα affects the electrophysiology of the heart. Whole-cell patch-clamp recordings on ventricular myocytes obtained from 10~20-week-old mice reveal that mXinα-null cells have an increased inward Na+ current (INa), a reduced transient outward K+ current (ITO), a weaker L-type Ca2+ current (ICa,L) and a smaller inward rectifier K+ current (IK1) densities (140, 197, 198). In addition, the amplitude of the intracellular Ca2+ transient decreases significantly in mXinα-null myocytes prepared from ventricles and left atrial-pulmonary veins (LA-PV) (140). Optical mapping analyses reveal that the conduction velocity is significantly slower in mXinα-null ventricles and LA-PV as compared to wild-type (198, 199). These data suggest that mXinα plays an important role in regulation of channel activity in both ventricular and atrial myocytes.
mXinα interacts with different proteins that regulate the surface expression of ion channels, which may account for the altered electrophysiological properties of mXinα-null cardiomyocytes. Yeast two-hybrid interaction assays reveal that mXinα interacts with KChIP2, an auxiliary subunit of the ITO channel (140). It is also known that KChIP2 interacts with Kv4.2, the pore-forming α- subunit of the ITO channel, and maintains the surface expression of ITO (200, 201). Consistently, loss of mXinα decreases the expression of KChIP2 protein and KChIP2 message, leading to significant reduction in the surface expression of the ITO channel (140). Furthermore, mXinα has been shown to bind to filamins and actin filaments (89, 149); thus, mXinα affects the ITO current activity by interacting with and stabilizing the KChIP2 and filamin proteins.
The role of mXinα in regulating ion channel surface expression and function may be analogous to that of the actin-binding protein ankyrins. The ankyrins interact with actin filaments and spectrin, and together with their associated proteins form a membrane cytoskeleton, which plays an important role in the targeting of ion channels, transporters and cell adhesion molecules to specialized compartments within the plasma membrane. Mutations affecting the association between ankyrins and ion channels alter the functions of ion channels and result in cardiac arrhythmia. For example, the human SCN5A missense mutation which is defective in binding to ankyrin-G leads to reductions of INa at T-tubes and ICDs and results in Brugada syndrome (202). mXinα interacts with actin filaments and maintains the structure of ICDs; thus, the increase of INa current density in mXinα-null ventricular myocytes may result from an altered cytoskeletal structure of the ICDs.
Alterations of channel activity may induce cardiac electrical remodeling. Our previous studies using optical mapping (198) found that the mXinα-deficient mice had a hypertrophied ventricular myocardium with reduced conduction velocity. Similarly, the conduction velocity was also reduced in LA-PV tissues from mXinα-null hearts as compared to wild-type hearts (199). The latter study also found that mXinα-null LA-PV have a larger area of conduction block than the control (defined as the area with a conduction velocity ≤ 10 cm/s; (203)). Finally, when we used isoproterenol to activate the β–adrenergic receptors, mXinα-null LA-PV tissues were less responsive to the adrenergic induction of atrial fibrillation as compared to wild-type LA-PVs.
It is known that loss of Cx43 or N-cadherin decreased the degree of coupling and conduction characteristics. Thus in mXinα-null mice, decreased cardiac conduction velocity may partly result from decreased expression of N-cadherin and Cx43 proteins.
4. XIN AND CARDIAC DISEASES
ICDs are essential structures unique to cardiac muscle, which enable mechanical coupling and chemical communication between cardiomyocytes (3). It is known that mutations or deficiencies in its components give rise to many types of cardiomyopathy and other fatal heart diseases (40, 102, 204–207). However, the molecular mechanisms for most of the proteins responsible for normal cardiac function remain to be elucidated. The human ortholog, hXinα or Cardiomyopathy-associated 1 (CMYA1), of mXinα has been mapped to chromosome 3p21.2-22.2 by radiation hybrid analysis (145) or to 3p22.2 by DNA sequence, which is near the loci for dilated cardiomyopathy with conduction defect-2 (3p22-25) (208) and ARVD-5 (3p23) (209). Genome-wide association studies suggest a locus in or adjacent to ULK4 (3p22.1) associated with diastolic blood pressure (210). mXinα-null mice are viable and fertile, but develop adult late-onset cardiomyopathy with conduction defects as well as up-regulation of mXinβ (17). Despite being viable, mXinα-deficient mouse hearts exhibit progressive ICD structure defects, which may account for the late-onset cardiac hypertrophy and cardiomyopathy. However, juvenile mXinα-null mouse hearts show neither hypertrophy nor cardiomyopathy, but electrophysiological studies on these mutant cardiomyocytes detect alterations in their channel properties, particularly for the depression of the ITo current density (140). The underlying mechanism for this depression likely involves the ability of mXinα to interact with KChIP2, an auxiliary subunit of the transient outward potassium channel, and filamin, an actin-crosslinking protein (140). Therefore, the mXinα knockout mouse may represent a model for human dilated cardiomyopathy with conduction defect-2.
Ablation of mXinβ can lead to ventricular septal defects, cardiac diastolic dysfunction, severe growth retardation and postnatal lethality (18). The human ortholog, CMYA3 (XIRP2), of mXinβ is mapped to 2q24.3, whose nearby disease-associated loci (Online Mendelian Inheritance in Man) include dilated cardiomyopathy 1H (2q14-q22), 1G (2q24.3) (211) and ARVD-4 (2q32.1-32.3) (212). Human patients with monosomy 2q24 (chromosome band 2q24 deletion) also exhibit severe growth retardation and heart anomalies, including ventricular septal defects (http://www.orpha.net/data/patho/GB/uk-2q24.pdf). The genome-wide linkage analysis of a large Kyrgyz family with premature hypertension reveals candidate localization on 2q24.3-q31.1 for a gene conferring susceptibility to this disorder (213). Further studies are warranted to characterize the involvement of mXinβ in cardiac development, function and disease.
5. CONCLUSIONS AND PERSPECTIVES
The Xin proteins are modular in nature, contain many copies of a 16-amino acid “Xin” repeating unit, which defines a novel actin binding domain, and localize to the ICDs. Together with their ability to interact with Mena/VASP, filamin b & c, gelsolin, tropomyosin, and vinculin (89), the Xin proteins likely provide an effective way to regulate actin dynamics underneath the ICD membrane. The highly conserved β-catenin-binding domain maps within the Xin repeat region (16, 89) and the ability to bind and bundle actin filaments can be enhanced by the presence of β-catenin (89). These findings further suggest that the Xin proteins play a novel role in linking the actin cytoskeleton to N-cadherin-mediated adhesions as well as in regulating ICD stability in the heart. The existence of a p120-catenin interacting domain distinct from the β-catenin binding domain on mXinα provides another level of regulation for the Xin proteins in N-cadherin turnover and signaling pathways through ICDs (unpublished observations). In addition, mXinα is capable of interacting with KChIP2, filamin and actin filaments (89, 140) and then influencing surface expression of the ITO current (140). ITO depression detected in mXinα-deficient ventricular myocytes appears to be independent of cardiac hypertrophy and/or ICD structural defects (140). Therefore, the effects of mXinα on the channel activity of ITO and IK likely provide a reasonable account for the conduction defects observed in the mXinα-deficient mice. Interestingly, a complete loss of mXinα prevents the induction of atrial fibrillation in the LA-PV tissues (199). A slower conduction velocity and many conduction blocks observed in the mXinα-null LA-PV tissues (199) may account for the lack of re-entrance rhythms and thus atrial fibrillation. However, the molecular mechanism of how mXinα is involved in the genesis of atrial fibrillation remains to be elucidated. Accumulated lines of evidence suggest the existence of a functional hierarchy between mXinα and mXinβ. Hearts without mXnβ fail to form mature ICDs and result in a mis-localization of mXinα. On the other hand, hearts without mXinα develop late-onset ultrastructural ICD defects, but mXinβ is up-regulated and localized normally to ICDs. The molecular basis of this functional hierarchy remains to be determined.
Based on our studies of mXin knockout mouse lines and other molecular characterizations of mXin proteins, we hypothesize that mXinβ initiates the formation of ICDs, whereas mXinα further stabilizes the ICD. Future detailed analysis of the binding/interacting domains on Xin proteins should advance our understanding toward the mechanisms by which mXin proteins function. For example, in addition to binding to β-catenin and p120-catenin (89), mXinα may interact with and recruit p0071, another member of the p120-catenin subfamily of armadillo related proteins found in the heart, to facilitate the ICD maturation and the stability of the N-cadherin-based adhesion. The potential interactions of Xin proteins with p120-catenin and/or p0071 may modulate effectors such as Vav2 (73, 74) and Ect2 (69) to regulate Rac1 and Rho activity, respectively. Further studies of whether mXinα directly interacts with Cx43 and/or ZO-1 may explain the gap junction remodeling (decrease in Cx43 amounts and altered localization) observed in the mXinα-null hearts.
Generation and characterization of conditional mXinβ knockout mice will allow us to further examine the role of mXinβ in maintaining ICD integrity in the adult heart. Considering that conditional deletion of N-cadherin in the adult heart leads to total dissolution of ICDs (102), and that postnatal hearts without mXinβ fail to form ICDs (18), we anticipate compromised ICD integrity in cardiac-specific mXinβ conditional knockout mice. The information obtained may also reveal the molecular mechanisms underlying the adult ICD (and/or gap junction) remodeling often associated with cardiomyopathy and heart failure.
mXinα-deficient mice exhibit similar cardiac phenotypes to human dilated cardiomyopathy with conduction defect-2, whereas mXinβ-null mice die around weaning and exhibit congenital heart defects and severe growth retardation. The future study of single nucleotide polymorphisms on CMYA1 and CMYA3 from human populations with cardiomyopathy and conduction defects or with congenital heart defects may potentially define these genes as disease-causing genes. Therefore, it is therefore conceivable that the knowledge gained on the role of Xin proteins in cardiac development and function will provide new insights for improved therapeutic strategies for human cardiomyopathy, arrhythmias and heart failure.
ACKNOWLEDGEMENTS
This work was supported, in part, by NIH grants HL075015 and HL107383 (to JJCL) and by National Science council (Taiwan) grants NSC96-2320-B016-013 and NSC98-2320-B016-010-MY3 (to CIL).
Abbreviations
- ICD
intercalated disc
- mXinα and mXinβ
mouse Xinα and Xinβ
- PKP2/4
plakophilin 2/4
- Cx43
connexin 43
- ARVC(/D)
arrhthymogenic right ventricular cardiomyopathy (/dysplasia)
- Tcf/Lef
T cell factor/lymphoid enhancer factor
- siRNA
small interference RNA
- GEF
guanine nucleotide exchange factor
- ZO-1
zonula occludens-1
- EMT
epithelial-mesenchymal transition
- BMP
bone morphogenetic protein
- FGF
fibroblast growth factor
- ITO
transient outward potassium current
- KChIP2
Kv channel interacting protein 2
- HH st.
Hamberger and Hamilton stage
- AVC
atrioventricular canal
- hXinα/CMYA1/XIRP1
human Xinα/cardiomyopathy associated 1/Xin actin-binding repeat containing 1
- hXinβ/CMYA3/XIRP2
human Xinβ/cardiomyopathy associated 3/Xin actin-binding repeat containing 2
- cXin
chicken Xin
- Ang II
angiotensin II
- GAPDH
glyceraldehyde 3 phosphate dehydrogenase
- D
posthatch day
- P
postnatal day
- E
embryonic day
- lv and rv
left and right ventricles
- la and ra
left and right atria
- ivs
interventricular septum
- ao
aorta
- β-catBD
β-catenin-binding domain
- PR
proline-rich region
- E/V BD (PR1)
Mena/VASP-binding domain
- DBD
DNA-binding domain
- NLS
nuclear localization signal
- NES
nuclear export signal
- ATP_GTP_A loop
ATP/GTP-binding domain
- SAJ
spot adherens junction
- INa
inward sodium current
- ICa,L
L-type Ca2+ current
- IK1
inward rectifier potassium current
- LA-PV
left atrial-pulmonary vein
REFERENCES
- 1.Forbes MS, Sperelakis N. Intercalated discs of mammalian heart: a review of structure and function. Tissue Cell. 1985;17:605–648. doi: 10.1016/0040-8166(85)90001-1. [DOI] [PubMed] [Google Scholar]
- 2.Severs NJ. The cardiac gap junction and intercalated disc. Int J Cardiol. 1990;26:137–173. doi: 10.1016/0167-5273(90)90030-9. [DOI] [PubMed] [Google Scholar]
- 3.Perriard JC, Hirschy A, Ehler E. Dilated cardiomyopathy: a disease of the intercalated disc? Trends Cardiovasc Med. 2003;13:30–38. doi: 10.1016/s1050-1738(02)00209-8. [DOI] [PubMed] [Google Scholar]
- 4.Palatinus JA, Rhett JM, Gourdie RG. The connexin43 carboxyl terminus and cardiac gap junction organization. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbamem.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ. Suppression of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–2021. doi: 10.1172/JCI27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Patel VV, Radice GL. Dysregulation of cell adhesion proteins and cardiac arrhythmogenesis. Clin Med Res. 2006;4:42–52. doi: 10.3121/cmr.4.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noorman M, van der Heyden MA, van Veen TA, Cox MG, Hauer RN, de Bakker JM, van Rijen HV. Cardiac cell-cell junctions in health and disease: Electrical versus mechanical coupling. J Mol Cell Cardiol. 2009;47:23–31. doi: 10.1016/j.yjmcc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Rohr S. Molecular crosstalk between mechanical and electrical junctions at the intercalated disc. Circ Res. 2007;101:637–639. doi: 10.1161/CIRCRESAHA.107.161901. [DOI] [PubMed] [Google Scholar]
- 9.Sheikh F, Ross RS, Chen J. Cell-cell connection to cardiac disease. Trends Cardiovasc Med. 2009;19:182–190. doi: 10.1016/j.tcm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Radice GL. A new perspective on intercalated disc organization: implications for heart disease. Dermatol Res Pract. 2010;2010:207835. doi: 10.1155/2010/207835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estigoy CB, Ponten F, Odeberg J, Herbert B, Guilhuas M, Charleston M, Ho JWK, Cameron D, dos Remedies CG. Intercalated discs: multiple proteins perform multiple functions in non-failing and failing human hearts. Biophys Rev. 2009;1:43–49. doi: 10.1007/s12551-008-0007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett PM, Maggs AM, Baines AJ, Pinder JC. The transitional junction: a new functional subcellular domain at the intercalated disc. Mol Biol Cell. 2006;17:2091–2100. doi: 10.1091/mbc.E05-12-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang DZ, Hu X, Lin JL, Kitten GT, Solursh M, Lin JJ. Differential displaying of mRNAs from the atrioventricular region of developing chicken hearts at stages 15 and 21. Front Biosci. 1996;1:a1–a15. doi: 10.2741/a100. [DOI] [PubMed] [Google Scholar]
- 15.Wang DZ, Reiter RS, Lin JL, Wang Q, Williams HS, Krob SL, Schultheiss TM, Evans S, Lin JJ. Requirement of a novel gene, Xin, in cardiac morphogenesis. Development. 1999;126:1281–1294. doi: 10.1242/dev.126.6.1281. [DOI] [PubMed] [Google Scholar]
- 16.Grosskurth SE, Bhattacharya D, Wang Q, Lin JJ. Emergence of Xin demarcates a key innovation in heart evolution. PLoS ONE. 2008;3:e2857. doi: 10.1371/journal.pone.0002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustafson-Wagner EA, Sinn HW, Chen YL, Wang DZ, Reiter RS, Lin JL, Yang B, Williamson RA, Chen J, Lin CI, Lin JJ. Loss of mXinα, an intercalated disk protein, results in cardiac hypertrophy and cardiomyopathy with conduction defects. Am J Physiol Heart Circ Physiol. 2007;293:H2680–H2692. doi: 10.1152/ajpheart.00806.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Lin JL, Reinking BE, Feng HZ, Chan FC, Lin CI, Jin JP, Gustafson-Wagner EA, Scholz TD, Yang B, Lin JJ. Essential roles of an intercalated disc protein, mXinβ, in postnatal heart growth and survival. Circ Res. 2010;16:1468–1478. doi: 10.1161/CIRCRESAHA.109.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franke WW, Borrmann CM, Grund C, Pieperhoff S. The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur J Cell Biol. 2006;85:69–82. doi: 10.1016/j.ejcb.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Borrmann CM, Grund C, Kuhn C, Hofmann I, Pieperhoff S, Franke WW. The area composita of adhering junctions connecting heart muscle cells of vertebrates. II. Colocalizations of desmosomal and fascia adhaerens molecules in the intercalated disk. Eur J Cell Biol. 2006;85:469–485. doi: 10.1016/j.ejcb.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Pieperhoff S, Franke WW. The area composita of adhering junctions connecting heart muscle cells of vertebrates - IV: coalescence and amalgamation of desmosomal and adhaerens junction components - late processes in mammalian heart development. Eur J Cell Biol. 2007;86:377–391. doi: 10.1016/j.ejcb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Pieperhoff S, Franke WW. The area composita of adhering junctions connecting heart muscle cells of vertebrates. VI. Different precursor structures in nonmammalian species. Eur J Cell Biol. 2008;87:413–430. doi: 10.1016/j.ejcb.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Franke WW, Rickelt S, Barth M, Pieperhoff S. The junctions that don't fit the scheme: special symmetrical cell-cell junctions of their own kind. Cell Tissue Res. 2009;338:1–17. doi: 10.1007/s00441-009-0849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eigenthaler M, Engelhardt S, Schinke B, Kobsar A, Schmitteckert E, Gambaryan S, Engelhardt CM, Krenn V, Eliava M, Jarchau T, Lohse MJ, Walter U, Hein L. Disruption of cardiac Ena-VASP protein localization in intercalated disks causes dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2003;285:H2471–H2481. doi: 10.1152/ajpheart.00362.2003. [DOI] [PubMed] [Google Scholar]
- 26.Ma X, Takeda K, Singh A, Yu ZX, Zerfas P, Blount A, Liu C, Towbin JA, Schneider MD, Adelstein RS, Wei Q. Conditional ablation of nonmuscle myosin II-B delineates heart defects in adult mice. Circ Res. 2009;105:1102–1109. doi: 10.1161/CIRCRESAHA.109.200303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheikh F, Chen Y, Liang X, Hirschy A, Stenbit AE, Gu Y, Dalton ND, Yajima T, Lu Y, Knowlton KU, Peterson KL, Perriard JC, Chen J. alpha-E-catenin inactivation disrupts the cardiomyocyte adherens junction, resulting in cardiomyopathy and susceptibility to wall rupture. Circulation. 2006;114:1046–1055. doi: 10.1161/CIRCULATIONAHA.106.634469. [DOI] [PubMed] [Google Scholar]
- 28.Zemljic-Harpf AE, Miller JC, Henderson SA, Wright AT, Manso AM, Elsherif L, Dalton ND, Thor AK, Perkins GA, McCulloch AD, Ross RS. Cardiacmyocyte-specific excision of the vinculin gene disrupts cellular junctions, causing sudden death or dilated cardiomyopathy. Mol Cell Biol. 2007;27:7522–7537. doi: 10.1128/MCB.00728-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnenberg A, Liem RK. Plakins in development and disease. Exp Cell Res. 2007;313:2189–2203. doi: 10.1016/j.yexcr.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 30.Gallicano GI, Kouklis P, Bauer C, Yin M, Vasioukhin V, Degenstein L, Fuchs E. Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J Cell Biol. 1998;143:2009–2022. doi: 10.1083/jcb.143.7.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basso C, Czarnowska E, Della Barbera M, Bauce B, Beffagna G, Wlodarska EK, Pilichou K, Ramondo A, Lorenzon A, Wozniek O, Corrado D, Daliento L, Danieli GA, Valente M, Nava A, Thiene G, Rampazzo A. Ultrastructural evidence of intercalated disc remodelling in arrhythmogenic right ventricular cardiomyopathy: an electron microscopy investigation on endomyocardial biopsies. Eur Heart J. 2006;27:1847–1854. doi: 10.1093/eurheartj/ehl095. [DOI] [PubMed] [Google Scholar]
- 32.Delmar M, McKenna WJ. The cardiac desmosome and arrhythmogenic cardiomyopathies: from gene to disease. Circ Res. 2010;107:700–714. doi: 10.1161/CIRCRESAHA.110.223412. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan SR, Gard JJ, Carvajal-Huerta L, Ruiz-Cabezas JC, Thiene G, Saffitz JE. Structural and molecular pathology of the heart in Carvajal syndrome. Cardiovasc Pathol. 2004;13:26–32. doi: 10.1016/S1054-8807(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 34.Yang Z, Bowles NE, Scherer SE, Taylor MD, Kearney DL, Ge S, Nadvoretskiy VV, DeFreitas G, Carabello B, Brandon LI, Godsel LM, Green KJ, Saffitz JE, Li H, Danieli GA, Calkins H, Marcus F, Towbin JA. Desmosomal dysfunction due to mutations in desmoplakin causes arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Res. 2006;99:646–655. doi: 10.1161/01.RES.0000241482.19382.c6. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan SR, Gard JJ, Protonotarios N, Tsatsopoulou A, Spiliopoulou C, Anastasakis A, Squarcioni CP, McKenna WJ, Thiene G, Basso C, Brousse N, Fontaine G, Saffitz JE. Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (Naxos disease) Heart Rhythm. 2004;1:3–11. doi: 10.1016/j.hrthm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Fidler LM, Wilson GJ, Liu F, Cui X, Scherer SW, Taylor GP, Hamilton RM. Abnormal connexin43 in arrhythmogenic right ventricular cardiomyopathy caused by plakophilin-2 mutations. J Cell Mol Med. 2009;13:4219–4228. doi: 10.1111/j.1582-4934.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruce AF, Rothery S, Dupont E, Severs NJ. Gap junction remodelling in human heart failure is associated with increased interaction of connexin43 with ZO-1. Cardiovasc Res. 2008;77:757–765. doi: 10.1093/cvr/cvm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaprielian RR, Gunning M, Dupont E, Sheppard MN, Rothery SM, Underwood R, Pennell DJ, Fox K, Pepper J, Poole-Wilson PA, Severs NJ. Downregulation of immunodetectable connexin43 and decreased gap junction size in the pathogenesis of chronic hibernation in the human left ventricle. Circulation. 1998;97:651–660. doi: 10.1161/01.cir.97.7.651. [DOI] [PubMed] [Google Scholar]
- 39.Smith JH, Green CR, Peters NS, Rothery S, Severs NJ. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am J Pathol. 1991;139:801–821. [PMC free article] [PubMed] [Google Scholar]
- 40.Dupont E, Matsushita T, Kaba R, Vozzi C, Coppen SR, Khan N, Kaprielian R, Yacoub MH, Severs NJ. Altered connexin expression in human congestive heart failure. J Mol Cell Cardiol. 2001;33:359–371. doi: 10.1006/jmcc.2000.1308. [DOI] [PubMed] [Google Scholar]
- 41.Kostin S, Rieger M, Dammer S, Hein S, Richter M, Klovekorn WP, Bauer EP, Schaper J. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem. 2003;242:135–144. [PubMed] [Google Scholar]
- 42.Kostin S, Dammer S, Hein S, Klovekorn WP, Bauer EP, Schaper J. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc Res. 2004;62:426–436. doi: 10.1016/j.cardiores.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 43.Kitamura H, Ohnishi Y, Yoshida A, Okajima K, Azumi H, Ishida A, Galeano EJ, Kubo S, Hayashi Y, Itoh H, Yokoyama M. Heterogeneous loss of connexin43 protein in nonischemic dilated cardiomyopathy with ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13:865–870. doi: 10.1046/j.1540-8167.2002.00865.x. [DOI] [PubMed] [Google Scholar]
- 44.Yamada KA, Rogers JG, Sundset R, Steinberg TH, Saffitz JE. Up-regulation of connexin45 in heart failure. J Cardiovasc Electrophysiol. 2003;14:1205–1212. doi: 10.1046/j.1540-8167.2003.03276.x. [DOI] [PubMed] [Google Scholar]
- 45.Cowin P, Kapprell HP, Franke WW, Tamkun J, Hynes RO. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell. 1986;46:1063–1073. doi: 10.1016/0092-8674(86)90706-3. [DOI] [PubMed] [Google Scholar]
- 46.Bierkamp C, McLaughlin KJ, Schwarz H, Huber O, Kemler R. Embryonic heart and skin defects in mice lacking plakoglobin. Dev Biol. 1996;180:780–785. doi: 10.1006/dbio.1996.0346. [DOI] [PubMed] [Google Scholar]
- 47.Ruiz P, Brinkmann V, Ledermann B, Behrend M, Grund C, Thalhammer C, Vogel F, Birchmeier C, Gunthert U, Franke W, Birchmeier WW. Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J Cell Biol. 1996;135:215–225. doi: 10.1083/jcb.135.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grossmann KS, Grund C, Huelsken J, Behrend M, Erdmann B, Franke WW, Birchmeier W. Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J Cell Biol. 2004;167:149–160. doi: 10.1083/jcb.200402096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhurinsky J, Shtutman M, Ben-Ze'ev A. Plakoglobin and beta-catenin: protein interactions, regulation and biological roles. J Cell Sci. 2000;113(Pt 18):3127–3139. doi: 10.1242/jcs.113.18.3127. [DOI] [PubMed] [Google Scholar]
- 50.Zhurinsky J, Shtutman M, Ben-Ze'ev A. Differential mechanisms of LEF/TCF family-dependent transcriptional activation by beta-catenin and plakoglobin. Mol Cell Biol. 2000;20:4238–4252. doi: 10.1128/mcb.20.12.4238-4252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klymkowsky MW, Williams BO, Barish GD, Varmus HE, Vourgourakis YE. Membrane-anchored plakoglobins have multiple mechanisms of action in Wnt signaling. Mol Biol Cell. 1999;10:3151–3169. doi: 10.1091/mbc.10.10.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Swope D, Raess N, Cheng L, Muller EJ, Radice GL. Cardiac-restricted deletion of plakoglobin results in progressive cardiomyopathy and activation of {beta}-catenin signaling. Mol Cell Biol. 2011;31:1134–1144. doi: 10.1128/MCB.01025-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lombardi R, Dong J, Rodriguez G, Bell A, Leung TK, Schwartz RJ, Willerson JT, Brugada R, Marian AJ. Genetic fate mapping identifies second heart field progenitor cells as a source of adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res. 2009;104:1076–1084. doi: 10.1161/CIRCRESAHA.109.196899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffery S, McKenna WJ. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease) Lancet. 2000;355:2119–2124. doi: 10.1016/S0140-6736(00)02379-5. [DOI] [PubMed] [Google Scholar]
- 55.Asimaki A, Syrris P, Wichter T, Matthias P, Saffitz JE, McKenna WJ. A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet. 2007;81:964–973. doi: 10.1086/521633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, Lerman BB, Markowitz SM, Ellinor PT, MacRae CA, Peters S, Grossmann KS, Drenckhahn J, Michely B, Sasse-Klaassen S, Birchmeier W, Dietz R, Breithardt G, Schulze-Bahr E, Thierfelder L. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–1164. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 57.van Tintelen JP, Entius MM, Bhuiyan ZA, Jongbloed R, Wiesfeld AC, Wilde AA, van der Smagt J, Boven LG, Mannens MM, van Langen IM, Hofstra RM, Otterspoor LC, Doevendans PA, Rodriguez LM, van Gelder IC, Hauer RN. Plakophilin-2 mutations are the major determinant of familial arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2006;113:1650–1658. doi: 10.1161/CIRCULATIONAHA.105.609719. [DOI] [PubMed] [Google Scholar]
- 58.Pieperhoff S, Schumacher H, Franke WW. The area composita of adhering junctions connecting heart muscle cells of vertebrates. V. The importance of plakophilin-2 demonstrated by small interference RNA-mediated knockdown in cultured rat cardiomyocytes. Eur J Cell Biol. 2008;87:399–411. doi: 10.1016/j.ejcb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res. 2007;101:703–711. doi: 10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- 60.Mertens C, Kuhn C, Franke WW. Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol. 1996;135:1009–1025. doi: 10.1083/jcb.135.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X, Bonne S, Hatzfeld M, van Roy F, Green KJ. Protein binding and functional characterization of plakophilin 2. Evidence for its diverse roles in desmosomes and beta -catenin signaling. J Biol Chem. 2002;277:10512–10522. doi: 10.1074/jbc.M108765200. [DOI] [PubMed] [Google Scholar]
- 62.Goossens S, Janssens B, Bonne S, De Rycke R, Braet F, van Hengel J, van Roy F. A unique and specific interaction between alphaT-catenin and plakophilin-2 in the area composita, the mixed-type junctional structure of cardiac intercalated discs. J Cell Sci. 2007;120:2126–2136. doi: 10.1242/jcs.004713. [DOI] [PubMed] [Google Scholar]
- 63.van der Heyden MA, Rook MB, Hermans MM, Rijksen G, Boonstra J, Defize LH, Destree OH. Identification of connexin43 as a functional target for Wnt signalling. J Cell Sci. 1998;111(Pt 12):1741–1749. doi: 10.1242/jcs.111.12.1741. [DOI] [PubMed] [Google Scholar]
- 64.Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. 2000;105:161–171. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hatzfeld M, Green KJ, Sauter H. Targeting of p0071 to desmosomes and adherens junctions is mediated by different protein domains. J Cell Sci. 2003;116:1219–1233. doi: 10.1242/jcs.00275. [DOI] [PubMed] [Google Scholar]
- 66.Calkins CC, Hoepner BL, Law CM, Novak MR, Setzer SV, Hatzfeld M, Kowalczyk AP. The Armadillo family protein p0071 is a VE-cadherin- and desmoplakin-binding protein. J Biol Chem. 2003;278:1774–1783. doi: 10.1074/jbc.M205693200. [DOI] [PubMed] [Google Scholar]
- 67.Hatzfeld M, Nachtsheim C. Cloning and characterization of a new armadillo family member, p0071, associated with the junctional plaque: evidence for a subfamily of closely related proteins. J Cell Sci. 1996;109(Pt 11):2767–2778. doi: 10.1242/jcs.109.11.2767. [DOI] [PubMed] [Google Scholar]
- 68.Hatzfeld M. The p120 family of cell adhesion molecules. Eur J Cell Biol. 2005;84:205–214. doi: 10.1016/j.ejcb.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 69.Keil R, Wolf A, Huttelmaier S, Hatzfeld M. Beyond regulation of cell adhesion: local control of RhoA at the cleavage furrow by the p0071 catenin. Cell Cycle. 2007;6:122–127. doi: 10.4161/cc.6.2.3741. [DOI] [PubMed] [Google Scholar]
- 70.Kanno M, Aoyama Y, Isa Y, Yamamoto Y, Kitajima Y. P120 catenin is associated with desmogleins when desmosomes are assembled in high-Ca2+ medium but not when disassembled in low-Ca2+ medium in DJM-1 cells. J Dermatol. 2008;35:317–324. doi: 10.1111/j.1346-8138.2008.00480.x. [DOI] [PubMed] [Google Scholar]
- 71.Kanno M, Isa Y, Aoyama Y, Yamamoto Y, Nagai M, Ozawa M, Kitajima Y. P120-catenin is a novel desmoglein 3 interacting partner: identification of the p120-catenin association site of desmoglein 3. Exp Cell Res. 2008;314:1683–1692. doi: 10.1016/j.yexcr.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 72.Bonne S, Gilbert B, Hatzfeld M, Chen X, Green KJ, van Roy F. Defining desmosomal plakophilin-3 interactions. J Cell Biol. 2003;161:403–416. doi: 10.1083/jcb.200303036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–580. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276:33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- 75.Meng W, Mushika Y, Ichii T, Takeichi M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell. 2008;135:948–959. doi: 10.1016/j.cell.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 76.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barker RJ, Price RL, Gourdie RG. Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res. 2002;90:317–324. doi: 10.1161/hh0302.104471. [DOI] [PubMed] [Google Scholar]
- 78.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- 79.Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc Res. 2004;62:233–245. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 81.Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 82.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei CJ, Francis R, Xu X, Lo CW. Connexin43 associated with an N-cadherin-containing multiprotein complex is required for gap junction formation in NIH3T3 cells. J Biol Chem. 2005;280:19925–19936. doi: 10.1074/jbc.M412921200. [DOI] [PubMed] [Google Scholar]
- 84.Wu JC, Tsai RY, Chung TH. Role of catenins in the development of gap junctions in rat cardiomyocytes. J Cell Biochem. 2003;88:823–835. doi: 10.1002/jcb.10390. [DOI] [PubMed] [Google Scholar]
- 85.Palatinus JA, Gourdie RG. Xin and the art of intercalated disk maintenance. Am J Physiol Heart Circ Physiol. 2007;293:H2626–H2628. doi: 10.1152/ajpheart.00954.2007. [DOI] [PubMed] [Google Scholar]
- 86.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011;22:1516–1528. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maass K, Shibayama J, Chase SE, Willecke K, Delmar M. C-terminal truncation of connexin43 changes number, size, and localization of cardiac gap junction plaques. Circ Res. 2007;101:1283–1291. doi: 10.1161/CIRCRESAHA.107.162818. [DOI] [PubMed] [Google Scholar]
- 89.Choi S, Gustafson-Wagner EA, Wang Q, Harlan SM, Sinn HW, Lin JL, Lin JJ. The intercalated disc protein, mXinα, is capable of interacting with β-catenin and bundling actin filaments. J Biol Chem. 2007;282:36024–36036. doi: 10.1074/jbc.M707639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Balasubramanian S, Mani SK, Kasiganesan H, Baicu CC, Kuppuswamy D. Hypertrophic stimulation increases beta-actin dynamics in adult feline cardiomyocytes. PLoS ONE. 2010;5:e11470. doi: 10.1371/journal.pone.0011470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Manisastry SM, Zaal KJ, Horowits R. Myofibril assembly visualized by imaging N-RAP, alpha-actinin, and actin in living cardiomyocytes. Exp Cell Res. 2009;315:2126–2139. doi: 10.1016/j.yexcr.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang JQ, Elzey B, Williams G, Lu S, Law DJ, Horowits R. Ultrastructural and biochemical localization of N-RAP at the interface between myofibrils and intercalated disks in the mouse heart. Biochemistry. 2001;40:14898–14906. doi: 10.1021/bi0107445. [DOI] [PubMed] [Google Scholar]
- 93.Yoshida M, Sho E, Nanjo H, Takahashi M, Kobayashi M, Kawamura K, Honma M, Komatsu M, Sugita A, Yamauchi M, Hosoi T, Ito Y, Masuda H. Weaving hypothesis of cardiomyocyte sarcomeres: discovery of periodic broadening and narrowing of intercalated disk during volume-load change. Am J Pathol. 2010;176:660–678. doi: 10.2353/ajpath.2010.090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bass-Zubek AE, Godsel LM, Delmar M, Green KJ. Plakophilins: multifunctional scaffolds for adhesion and signaling. Curr Opin Cell Biol. 2009;21:708–716. doi: 10.1016/j.ceb.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nelson WJ, Nusse R. Convergence of Wnt, β-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kwiatkowski AV, Weis WI, Nelson WJ. Catenins: playing both sides of the synapse. Curr Opin Cell Biol. 2007;19:551–556. doi: 10.1016/j.ceb.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2:a002915. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen X, Shevtsov SP, Hsich E, Cui L, Haq S, Aronovitz M, Kerkela R, Molkentin JD, Liao R, Salomon RN, Patten R, Force T. The beta-catenin/T-cell factor/lymphocyte enhancer factor signaling pathway is required for normal and stress-induced cardiac hypertrophy. Mol Cell Biol. 2006;26:4462–4473. doi: 10.1128/MCB.02157-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hirschy A, Croquelois A, Perriard E, Schoenauer R, Agarkova I, Hoerstrup SP, Taketo MM, Pedrazzini T, Perriard JC, Ehler E. Stabilised beta-catenin in postnatal ventricular myocardium leads to dilated cardiomyopathy and premature death. Basic Res Cardiol. 2010;105:597–608. doi: 10.1007/s00395-010-0101-8. [DOI] [PubMed] [Google Scholar]
- 101.Ferreira-Cornwell MC, Luo Y, Narula N, Lenox JM, Lieberman M, Radice GL. Remodeling the intercalated disc leads to cardiomyopathy in mice misexpressing cadherins in the heart. Journal of Cell Science. 2002;115:1623–1634. doi: 10.1242/jcs.115.8.1623. [DOI] [PubMed] [Google Scholar]
- 102.Kostetskii I, Li J, Xiong Y, Zhou R, Ferrari VA, Patel VV, Molkentin JD, Radice GL. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ Res. 2005;96:346–354. doi: 10.1161/01.RES.0000156274.72390.2c. [DOI] [PubMed] [Google Scholar]
- 103.Li J, Patel VV, Kostetskii I, Xiong Y, Chu AF, Jacobson JT, Yu C, Morley GE, Molkentin JD, Radice GL. Cardiac-specific loss of N-cadherin leads to alteration in connexins with conduction slowing and arrhythmogenesis. Circ Res. 2005;97:474–481. doi: 10.1161/01.RES.0000181132.11393.18. [DOI] [PubMed] [Google Scholar]
- 104.Li J, Levin MD, Xiong Y, Petrenko N, Patel VV, Radice GL. N-cadherin haploinsufficiency affects cardiac gap junctions and arrhythmic susceptibility. J Mol Cell Cardiol. 2008;44:597–606. doi: 10.1016/j.yjmcc.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arulanandam R, Vultur A, Cao J, Carefoot E, Elliott BE, Truesdell PF, Larue L, Feracci H, Raptis L. Cadherin-cadherin engagement promotes cell survival via Rac1/Cdc42 and signal transducer and activator of transcription-3. Mol Cancer Res. 2009;7:1310–1327. doi: 10.1158/1541-7786.MCR-08-0469. [DOI] [PubMed] [Google Scholar]
- 106.Raptis L, Arulanandam R, Vultur A, Geletu M, Chevalier S, Feracci H. Beyond structure, to survival: activation of Stat3 by cadherin engagement. Biochem Cell Biol. 2009;87:835–843. doi: 10.1139/o09-061. [DOI] [PubMed] [Google Scholar]
- 107.Watanabe T, Sato K, Kaibuchi K. Cadherin-mediated intercellular adhesion and signaling cascades involving small GTPases. Cold Spring Harb Perspect Biol. 2009;1:a003020. doi: 10.1101/cshperspect.a003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 109.le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smutny M, Yap AS. Neighborly relations: cadherins and mechanotransduction. J Cell Biol. 2010;189:1075–1077. doi: 10.1083/jcb.201005151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marvin MJ, di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes & Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tzahor E, Lassar AB. Wnt signals from the neural tube block ectopic cardiogenesis. Genes & Dev. 2001;15:255–260. doi: 10.1101/gad.871501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci U S A. 2007;104:9319–9324. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci U S A. 2007;104:18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 119.Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 120.Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, Woodgett J, Kilter H, Force T. Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci U S A. 2003;100:4610–4615. doi: 10.1073/pnas.0835895100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Force T, Woulfe K, Koch WJ, Kerkela R. Molecular scaffolds regulate bidirectional crosstalk between Wnt and classical seven-transmembrane-domain receptor signaling pathways. Sci STKE. 2007;2007:pe41. doi: 10.1126/stke.3972007pe41. [DOI] [PubMed] [Google Scholar]
- 122.Qu J, Zhou J, Yi XP, Dong B, Zheng H, Miller LM, Wang X, Schneider MD, Li F. Cardiac-specific haploinsufficiency of beta-catenin attenuates cardiac hypertrophy but enhances fetal gene expression in response to aortic constriction. J Mol Cell Cardiol. 2007;43:319–326. doi: 10.1016/j.yjmcc.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van de Schans VA, van den Borne SW, Strzelecka AE, Janssen BJ, van der Velden JL, Langen RC, Wynshaw-Boris A, Smits JF, Blankesteijn WM. Interruption of Wnt signaling attenuates the onset of pressure overload-induced cardiac hypertrophy. Hypertension. 2007;49:473–480. doi: 10.1161/01.HYP.0000255946.55091.24. [DOI] [PubMed] [Google Scholar]
- 124.Malekar P, Hagenmueller M, Anyanwu A, Buss S, Streit MR, Weiss CS, Wolf D, Riffel J, Bauer A, Katus HA, Hardt SE. Wnt signaling is critical for maladaptive cardiac hypertrophy and accelerates myocardial remodeling. Hypertension. 2010;55:939–945. doi: 10.1161/HYPERTENSIONAHA.109.141127. [DOI] [PubMed] [Google Scholar]
- 125.Baurand A, Zelarayan L, Betney R, Gehrke C, Dunger S, Noack C, Busjahn A, Huelsken J, Taketo MM, Birchmeier W, Dietz R, Bergmann MW. Beta-catenin downregulation is required for adaptive cardiac remodeling. Circ Res. 2007;100:1353–1362. doi: 10.1161/01.RES.0000266605.63681.5a. [DOI] [PubMed] [Google Scholar]
- 126.Zhou J, Qu J, Yi XP, Graber K, Huber L, Wang X, Gerdes AM, Li F. Upregulation of gamma-catenin compensates for the loss of beta-catenin in adult cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;292:H270–H276. doi: 10.1152/ajpheart.00576.2006. [DOI] [PubMed] [Google Scholar]
- 127.Masuelli L, Bei R, Sacchetti P, Scappaticci I, Francalanci P, Albonici L, Coletti A, Palumbo C, Minieri M, Fiaccavento R, Carotenuto F, Fantini C, Carosella L, Modesti A, Di Nardo P. β-catenin accumulates in intercalated disks of hypertrophic cardiomyopathic hearts. Cardiovasc Res. 2003;60:376–387. doi: 10.1016/j.cardiores.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 128.Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152:87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Longo KA, Kennell JA, Ochocinska MJ, Ross SE, Wright WS, MacDougald OA. Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J Biol Chem. 2002;277:38239–38244. doi: 10.1074/jbc.M206402200. [DOI] [PubMed] [Google Scholar]
- 130.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 131.Pilichou K, Remme CA, Basso C, Campian ME, Rizzo S, Barnett P, Scicluna BP, Bauce B, van den Hoff MJ, de Bakker JM, Tan HL, Valente M, Nava A, Wilde AA, Moorman AF, Thiene G, Bezzina CR. Myocyte necrosis underlies progressive myocardial dystrophy in mouse dsg2-related arrhythmogenic right ventricular cardiomyopathy. J Exp Med. 2009;206:1787–1802. doi: 10.1084/jem.20090641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kucera JP, Rohr S, Rudy Y. Localization of sodium channels in intercalated disks modulates cardiac conduction. Circ Res. 2002;91:1176–1182. doi: 10.1161/01.res.0000046237.54156.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maier SK, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation. 2004;109:1421–1427. doi: 10.1161/01.CIR.0000121421.61896.24. [DOI] [PubMed] [Google Scholar]
- 134.Maier SK, Westenbroek RE, Schenkman KA, Feigl EO, Scheuer T, Catterall WA. An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc Natl Acad Sci U S A. 2002;99:4073–4078. doi: 10.1073/pnas.261705699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, Priori SG, Keating MT, Bennett V. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci U S A. 2004;101:9137–9142. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cohen SA. Immunocytochemical localization of rH1 sodium channel in adult rat heart atria and ventricle. Presence in terminal intercalated disks. Circulation. 1996;94:3083–3086. doi: 10.1161/01.cir.94.12.3083. [DOI] [PubMed] [Google Scholar]
- 137.Malhotra JD, Thyagarajan V, Chen C, Isom LL. Tyrosine-phosphorylated and nonphosphorylated sodium channel beta1 subunits are differentially localized in cardiac myocytes. J Biol Chem. 2004;279:40748–40754. doi: 10.1074/jbc.M407243200. [DOI] [PubMed] [Google Scholar]
- 138.Meadows LS, Isom LL. Sodium channels as macromolecular complexes: implications for inherited arrhythmia syndromes. Cardiovasc Res. 2005;67:448–458. doi: 10.1016/j.cardiores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 139.Sato PY, Musa H, Coombs W, Guerrero-Serna G, Patino GA, Taffet SM, Isom LL, Delmar M. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ Res. 2009;105:523–526. doi: 10.1161/CIRCRESAHA.109.201418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chan FC, Cheng CP, Wu KH, Chen YC, Hsu CH, Gustafson-Wagner EA, Lin JL, Wang Q, Lin JJ, Lin CI. Intercalated disc-associated protein, mXin-alpha, influences surface expression of ITO currents in ventricular myocytes. Front Biosci (Elite Ed) 2011;3:1425–1442. doi: 10.2741/e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Butcher JT, Markwald RR. Valvulogenesis: the moving target. Philos Trans R Soc Lond B Biol Sci. 2007;362:1489–1503. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77:1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- 143.Markwald RR, Norris RA, Moreno-Rodriguez R, Levine RA. Developmental basis of adult cardiovascular diseases: valvular heart diseases. Ann N Y Acad Sci. 2010;1188:177–183. doi: 10.1111/j.1749-6632.2009.05098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Otten J, van der Ven PF, Vakeel P, Eulitz S, Kirfel G, Brandau O, Boesl M, Schrickel JW, Linhart M, Hayess K, Naya FJ, Milting H, Meyer R, Furst DO. Complete loss of murine Xin results in a mild cardiac phenotype with altered distribution of intercalated discs. Cardiovasc Res. 2010;85:739–750. doi: 10.1093/cvr/cvp345. [DOI] [PubMed] [Google Scholar]
- 145.Lin JJ-C, Gustafson-Wagner EA, Sinn HW, Choi S, Jaacks SM, Wang DZ, Evans S, Lin JL-C. Structure, expression, and function of a novel intercalated disc protein, Xin. J Med Sci. 2005;25:215–222. doi: 10.1901/jaba.2005.25-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pacholsky D, Vakeel P, Himmel M, Lowe T, Stradal T, Rottner K, Furst DO, van der Ven PFM. Xin repeats define a novel actin-binding motif. J Cell Sci. 2004;117:5257–5268. doi: 10.1242/jcs.01406. [DOI] [PubMed] [Google Scholar]
- 147.Huang HT, Brand OM, Mathew M, Ignatiou C, Ewen EP, McCalmon SA, Naya FJ. Myomaxin is a novel transcriptional target of MEF2A that encodes a Xin-related alpha-actinin-interacting protein. J Biol Chem. 2006;281:39370–39379. doi: 10.1074/jbc.M603244200. [DOI] [PubMed] [Google Scholar]
- 148.Sinn HW, Balsamo J, Lilien J, Lin JJ. Localization of the novel Xin protein to the adherens junction complex in cardiac and skeletal muscle during development. Dev Dyn. 2002;225:1–13. doi: 10.1002/dvdy.10131. [DOI] [PubMed] [Google Scholar]
- 149.van der Ven PFM, Ehler E, Vakeel P, Eulitz S, Schenk JA, Milting H, Micheel B, Furst DO. Unusual splicing events result in distinct Xin isoforms that associate differentially with filamin c and Mens/VASP. Exp Cell Res. 2006;312:2154–2167. doi: 10.1016/j.yexcr.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 150.Edmondson DG, Lyons GE, Martin JF, Olson EN. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development. 1994;120:1251–1263. doi: 10.1242/dev.120.5.1251. [DOI] [PubMed] [Google Scholar]
- 151.Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:969. doi: 10.1242/dev.119.3.969. [DOI] [PubMed] [Google Scholar]
- 152.Lyons I, Parsons LM, Hartley L, Li R, Andrews JE, Robb L, Harvey RP. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995;9:1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- 153.Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McCalmon SA, Desjardins DM, Ahmad S, Davidoff KS, Snyder CM, Sato K, Ohashi K, Kielbasa OM, Mathew M, Ewen EP, Walsh K, Gavras H, Naya FJ. Modulation of angiotensin II-mediated cardiac remodeling by the MEF2A target gene Xirp2. Circ Res. 2010;106:952–960. doi: 10.1161/CIRCRESAHA.109.209007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hirakow R, Gotoh T. Ontogenetic implication of the myocardial ultrastructure in the development of mammalian heart. In: van Praagh R, Takao A, editors. Etiology and morphogenesis of congenital heart disease. Mount Kisco, NY: Futura Publishing Co.; 1980. pp. 99–108. [Google Scholar]
- 156.Zhou YQ, Foster FS, Parkes R, Adamson SL. Developmental changes in left and right ventricular diastolic filling patterns in mice. Am J Physiol Heart Circ Physiol. 2003;285:H1563–H1575. doi: 10.1152/ajpheart.00384.2003. [DOI] [PubMed] [Google Scholar]
- 157.Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 158.Rockman HA, Knowlton KU, Ross JJ, Chien KR. In vivo murine cardiac hypertrophy. Circulation. 1993;87(suppl VII):VII14–VII21. [Google Scholar]
- 159.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr, Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Duka A, Schwartz F, Duka I, Johns C, Melista E, Gavras I, Gavras H. A novel gene (Cmya3) induced in the heart by angiotensin II-dependent but not salt-dependent hypertension in mice. Am J Hypertens. 2006;19:275–281. doi: 10.1016/j.amjhyper.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 161.Rambaut A. Version 1.D1. Oxford, England: Department of Zoology, University of Oxford; 1995. SE-AL sequence alignment program. [Google Scholar]
- 162.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignments through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Abascal F, Zardoya R, Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics. 2005;21:2104–2105. doi: 10.1093/bioinformatics/bti263. [DOI] [PubMed] [Google Scholar]
- 164.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 165.Stamatakis A, Ludwig T, Meier H. RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics. 2005;21:456–463. doi: 10.1093/bioinformatics/bti191. [DOI] [PubMed] [Google Scholar]
- 166.Legato MJ. Cellular mechanisms of normal growth in the mammalian heart. I. Qualitative and quantitative features of ventricular architecture in the dog from birth to five months of age. Circ Res. 1979;44:250–262. doi: 10.1161/01.res.44.2.250. [DOI] [PubMed] [Google Scholar]
- 167.Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res. 1997;80:88–94. doi: 10.1161/01.res.80.1.88. [DOI] [PubMed] [Google Scholar]
- 168.Hirschy A, Schatzmann F, Ehler E, Perriard JC. Establishment of cardiac cytoarchitecture in the developing mouse heart. Dev Biol. 2006;289:430–441. doi: 10.1016/j.ydbio.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 169.Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation. 1994;90:713–725. doi: 10.1161/01.cir.90.2.713. [DOI] [PubMed] [Google Scholar]
- 170.Eppenberger HM, Zuppinger C. In vitro reestablishment of cell-cell contacts in adult rat cardiomyocytes. Functional role of transmembrane components in the formation of new intercalated disk-like cell contacts. Faseb J. 1999;(13 Suppl):S83–S89. doi: 10.1096/fasebj.13.9001.s83. [DOI] [PubMed] [Google Scholar]
- 171.Hertig CM, Butz S, Koch S, Eppenberger-Eberhardt M, Kemler R, Eppenberger HM. N-cadherin in adult rat cardiomyocytes in culture. II. Spatio-temporal appearance of proteins involved in cell-cell contact and communication. Formation of two distinct N-cadherin/catenin complexes. J Cell Sci. 1996;109(Pt 1):11–20. doi: 10.1242/jcs.109.1.11. [DOI] [PubMed] [Google Scholar]
- 172.Hertig CM, Eppenberger-Eberhardt M, Koch S, Eppenberger HM. N-cadherin in adult rat cardiomyocytes in culture. I. Functional role of N-cadherin and impairment of cell-cell contact by a truncated N-cadherin mutant. J Cell Sci. 1996;109(Pt 1):1–10. doi: 10.1242/jcs.109.1.1. [DOI] [PubMed] [Google Scholar]
- 173.Kusumi A, Suzuki K, Koyasako K. Mobility and cytoskeletal interactions of cell adhesion receptors. Curr Opin Cell Biol. 1999;11:582–590. doi: 10.1016/s0955-0674(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 174.Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hong S, Troyanovsky RB, Troyanovsky SM. Spontaneous assembly and active disassembly balance adherens junction homeostasis. Proc Natl Acad Sci U S A. 2010;107:3528–3533. doi: 10.1073/pnas.0911027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Troyanovsky RB, Laur O, Troyanovsky SM. Stable and unstable cadherin dimers: mechanisms of formation and roles in cell adhesion. Mol Biol Cell. 2007;18:4343–4352. doi: 10.1091/mbc.E07-01-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Perez TD, Tamada M, Sheetz MP, Nelson WJ. Immediate-early signaling induced by E-cadherin engagement and adhesion. J Biol Chem. 2008;283:5014–5022. doi: 10.1074/jbc.M705209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol. 2010;2:a000125. doi: 10.1101/cshperspect.a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.de Beco S, Gueudry C, Amblard F, Coscoy S. Endocytosis is required for E-cadherin redistribution at mature adherens junctions. Proc Natl Acad Sci U S A. 2009;106:7010–7015. doi: 10.1073/pnas.0811253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 181.Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xiao K, Oas RG, Chiasson CM, Kowalczyk AP. Role of p120-catenin in cadherin trafficking. Biochim Biophys Acta. 2007;1773:8–16. doi: 10.1016/j.bbamcr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 183.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cavey M, Lecuit T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb Perspect Biol. 2009;1:a002998. doi: 10.1101/cshperspect.a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cavey M, Rauzi M, Lenne PF, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 2008;453:751–756. doi: 10.1038/nature06953. [DOI] [PubMed] [Google Scholar]
- 187.Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A. 2008;105:13–19. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007;120:17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- 190.Pannekoek WJ, Kooistra MR, Zwartkruis FJ, Bos JL. Cell-cell junction formation: the role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim Biophys Acta. 2009;1788:790–796. doi: 10.1016/j.bbamem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 191.Somekawa S, Fukuhara S, Nakaoka Y, Fujita H, Saito Y, Mochizuki N. Enhanced functional gap junction neoformation by protein kinase A-dependent and Epac-dependent signals downstream of cAMP in cardiac myocytes. Circ Res. 2005;97:655–662. doi: 10.1161/01.RES.0000183880.49270.f9. [DOI] [PubMed] [Google Scholar]
- 192.Phillips HM, Rhee HJ, Murdoch JN, Hildreth V, Peat JD, Anderson RH, Copp AJ, Chaudhry B, Henderson DJ. Disruption of planar cell polarity signaling results in congenital heart defects and cardiomyopathy attributable to early cardiomyocyte disorganization. Circ Res. 2007;101:137–145. doi: 10.1161/CIRCRESAHA.106.142406. [DOI] [PubMed] [Google Scholar]
- 193.Matsuda T, Takahashi K, Nariai T, Ito T, Takatani T, Fujio Y, Azuma J. N-cadherin-mediated cell adhesion determines the plasticity for cell alignment in response to mechanical stretch in cultured cardiomyocytes. Biochem Biophys Res Commun. 2005;326:228–232. doi: 10.1016/j.bbrc.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 194.Salameh A, Wustmann A, Karl S, Blanke K, Apel D, Rojas-Gomez D, Franke H, Mohr FW, Janousek J, Dhein S. Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein connexin43. Circ Res. 2010;106:1592–1602. doi: 10.1161/CIRCRESAHA.109.214429. [DOI] [PubMed] [Google Scholar]
- 195.Yamane M, Matsuda T, Ito T, Fujio Y, Takahashi K, Azuma J. Rac1 activity is required for cardiac myocyte alignment in response to mechanical stress. Biochem Biophys Res Commun. 2007;353:1023–1027. doi: 10.1016/j.bbrc.2006.12.144. [DOI] [PubMed] [Google Scholar]
- 196.Wahl JK, 3rd, Kim YJ, Cullen JM, Johnson KR, Wheelock MJ. N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J Biol Chem. 2003;278:17269–17276. doi: 10.1074/jbc.M211452200. [DOI] [PubMed] [Google Scholar]
- 197.Cheng CP, Loh YX, Lin CI, Lai YJ, Chen YC, Sytwn HK, Gustafson-Wagner EA, Lin JJ-C. Electrophysiological characteristics of ventricular myocytes of Xinα-deficient mice. In: Kimchi A, editor. International Proceedings: Advances in heart disease. Bologna, Italy: MEDIMOND; 2005. pp. 25–29. [Google Scholar]
- 198.Lai YJ, Chen YY, Cheng CP, Lin JJ, Chudorodova SL, Roshchevskaya IM, Roshchevsky MP, Chen YC, Lin CI. Changes in ionic currents and reduced conduction velocity in hypertrophied ventricular myocardium of Xinα-deficient mice. Anatol J Cardiol. 2007;7(Suppl 1):90–92. [PMC free article] [PubMed] [Google Scholar]
- 199.Lai YJ, Huang EY, Yeh HI, Chen YL, Lin JJ, Lin CI. On the mechanisms of arrhythmias in the myocardium of mXinalpha-deficient murine left atrial-pulmonary veins. Life Sci. 2008;83:272–283. doi: 10.1016/j.lfs.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Guo W, Li H, Aimond F, Johns DC, Rhodes KJ, Trimmer JS, Nerbonne JM. Role of heteromultimers in the generation of myocardial transient outward K+ currents. Circ Res. 2002;90:586–593. doi: 10.1161/01.res.0000012664.05949.e0. [DOI] [PubMed] [Google Scholar]
- 201.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 202.Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, Bennett V. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci U S A. 2004;101:17533–17538. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Eijsbouts SC, Houben RP, Blaauw Y, Schotten U, Allessie MA. Synergistic action of atrial dilation and sodium channel blockade on conduction in rabbit atria. J Cardiovasc Electrophysiol. 2004;15:1453–1461. doi: 10.1046/j.1540-8167.2004.04326.x. [DOI] [PubMed] [Google Scholar]
- 204.Ehler E, Horowits R, Zuppinger C, Price RL, Perriard E, Leu M, Caroni P, Sussman M, Eppenberger HM, Perriard JC. Alterations at the intercalated disk associated with the absence of muscle LIM protein. J Cell Biol. 2001;153:763–772. doi: 10.1083/jcb.153.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fujio Y, Yamada-Honda F, Sato N, Funai H, Wada A, Awata N, Shibata N. Disruption of cell-cell adhesion in an inbred strain of hereditary cardiomyopathic hamster (Bio 14.6) Cardiovasc Res. 1995;30:899–904. [PubMed] [Google Scholar]
- 206.Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–379. doi: 10.1016/s0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 207.Wang X, Gerdes AM. Chronic pressure overload cardiac hypertrophy and failure in guinea pigs: III. intercalated disc remodeling. J Mol Cell Cardiol. 1999;31:333–343. doi: 10.1006/jmcc.1998.0886. [DOI] [PubMed] [Google Scholar]
- 208.Olson TM, Keating MT. Mapping a cardiomyopathy locus to chromosome 3p22–p25. J Clin Invest. 1996;97:528–532. doi: 10.1172/JCI118445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ahmad F, Li D, Karibe A, Gonzalez O, Tapscott T, Hill R, Weilbaecher D, Blackie P, Furey M, Gardner M, Bachinski L, Roberts R. Localization of a gene responsible for arrhythmogenic right ventricular dysplasia to chromosome 3p23. Circulation. 1998;98:2791–2795. doi: 10.1161/01.cir.98.25.2791. [DOI] [PubMed] [Google Scholar]
- 210.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, Glazer NL, Morrison AC, Johnson AD, Aspelund T, Aulchenko Y, Lumley T, Kottgen A, Vasan RS, Rivadeneira F, Eiriksdottir G, Guo X, Arking DE, Mitchell GF, Mattace-Raso FU, Smith AV, Taylor K, Scharpf RB, Hwang SJ, Sijbrands EJ, Bis J, Harris TB, Ganesh SK, O'Donnell CJ, Hofman A, Rotter JI, Coresh J, Benjamin EJ, Uitterlinden AG, Heiss G, Fox CS, Witteman JC, Boerwinkle E, Wang TJ, Gudnason V, Larson MG, Chakravarti A, Psaty BM, van Duijn CM. Genome-wide association study of blood pressure and hypertension. Nat Genet. 2009;41:677–687. doi: 10.1038/ng.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jung M, Poepping I, Perrot A, Ellmer AE, Wienker TF, Dietz R, Reis A, Osterziel KJ. Investigation of a family with autosomal dominant dilated cardiomyopathy defines a novel locus on chromosome 2q14–q22. Am J Hum Genet. 1999;65:1068–1077. doi: 10.1086/302580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rampazzo A, Nava A, Miorin M, Fonderico P, Pope B, Tiso N, Livolsi B, Zimbello R, Thiene G, Danieli GA. ARVD4, a new locus for arrhythmogrnic right ventricular cardiomyopathy, maps to chromosome 2 long arm. Genomics. 1997;45:259–263. doi: 10.1006/geno.1997.4927. [DOI] [PubMed] [Google Scholar]
- 213.Kalmyrzaev B, Aldashev A, Khalmatov M, Polupanov A, Jumagulova A, Mamanova L, Wilkins MR, Town M. Genome-wide scan for premature hypertension supports linkage to chromosome 2 in a large Kyrgyz family. Hypertension. 2006;48:908–913. doi: 10.1161/01.HYP.0000244107.13957.2b. [DOI] [PubMed] [Google Scholar]