Abstract
It is estimated that one billion people around the world are vitamin D deficient. Vitamin D deficiency has been linked to various inflammatory diseases. However, the mechanism by which vitamin D reduces inflammation remains poorly understood. In this study, we investigated the inhibitory effects of physiologic levels of vitamin D on lipopolysaccharide (LPS)-stimulated inflammatory response in human blood monocytes, and explored potential mechanisms of vitamin D action. We observed that two forms of the vitamin D, 1,25(OH)2D3, and 25(OH)D3, dose dependently inhibited LPS-induced p38 phosphorylation at physiologic concentrations, IL-6 and TNF-α production by human monocytes. Upon vitamin D treatment, the expression of mitogen-activated protein kinase phosphatase-1 (MKP-1) was significantly upregulated in human monocytes and murine bone marrow-derived macrophages (BMM). Increased binding of the vitamin D receptor and increased histone H4 acetylation at the identified vitamin D response element of the murine and human MKP-1 promoters were demonstrated. Moreover, in BMM from MKP1−/− mice, the inhibition of LPS-induced p38 phosphorylation by vitamin D was completely abolished. Vitamin D inhibition of LPS-induced IL-6 and TNF-α production by BMM from MKP-1−/− mice was significantly reduced as compared to wild type mice. In conclusion, this study identified the upregulation of MKP-1 by vitamin D as a novel pathway by which vitamin D inhibits LPS-induced p38 activation and cytokine production in monocytes/macrophages.
INTRODUCTION
Vitamin D is well known for its role in calcium homeostasis and maintenance of bone metabolism (1). However, recent evidence suggests that vitamin D plays important roles in both innate and adaptive immunity (2). Vitamin D levels are routinely tested by assessing the concentration of the major circulating form of the vitamin D, 25(OH)D3, in serum; this form of vitamin D has a half-life of 15 days, while the active form of vitamin D, 1,25(OH)2D3, has a short half-life of approximately 15 h (3-5).
1,25(OH)2D3 acts as a ligand for the vitamin D receptor (VDR), a member of the nuclear receptors superfamily (6). VDR forms a heterodimer with retinoid X receptor (RXR) and regulates gene expression by binding to the Vitamin D Response Element (VDRE). VDRE had been shown to be predominantly located in introns and intergenic intervals (7). VDRE is characterized by direct repeats of two hexameric core-binding motifs (preferentially being AGTTCA) spaced by three nucleotides (8, 9). The binding of VDR to VDRE recruits co-activators and enzymes with histone acetylation activity, causing the structural changes in chromatin, therefore facilitating gene transcription (10).
Lipopolysaccharide (LPS), a component of the Gram-negative bacterial cell wall, induces cytokine production by monocytes/macrophages. LPS had been implicated in sepsis caused by Gram-negative bacteria, and induces intense inflammatory and procoagulant responses, which can be lethal (11). LPS is recognized by cell surface Toll-like receptor 4 (TLR4) which initiates intracellular signal transduction cascades(12). The MAP kinases activated by LPS (ERK, JNK and p38(12)) are critical regulators of pro-inflammatory cytokine production, including TNF-α and IL-6 (13, 14). Although these pro-inflammatory cytokines enhance host defense, excessive production leads to unresolved inflammation(15). Therefore, feed-back control of MAP kinase activation is necessary. Mitogen-activated protein kinase phosphatases (MKP) inactivate MAP kinases by dephosphorylating conserved threonine and tyrosine residues of the activated MAP kinases(16). MKP-1 is known to preferentially inactivate p38 and JNK, leading to subsequent inhibition of proinflammatory cytokines production (17, 18). In the current study we examined mechanisms of the vitamin D-mediated suppression of LPS-activated monocytes/macrophages. We found that vitamin D inhibits LPS-induced cytokine production by up-regulating MKP-1 thereby attenuating p38 activation.
MATERIAL AND METHODS
Materials
1,25(OH)2D3, 25(OH)D3, and monoclonal anti-β-actin antibody were purchased from Sigma (St. Louis, MO). HyQTase was purchased from HyClone Laboratories, Inc. (Logan, UT). TrypLE Express was purchased from Invitrogen Corporation (Carlsbad, CA). Phospho-p38 and p38 antibodies were purchased from Cell Signaling (Danvers, MA). Anti-mouse and anti-rabbit horseradish peroxidase (HRP)-labeled IgG were purchased from Amersham Biosciences (Piscataway, NJ). Rabbit polyclonal antibody to VDR, Rabbit polyclonal antibody to MKP-1, RIPA Lysis Buffer and Protein A/G PLUS-Agarose beads were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Rabbit polyclonal antibody to histone H4 and acetylated histone H4, and Magna ChIP A/G Chromatin Immunoprecipitation Kit were purchased from Millipore (Temecula, CA). Chemiluminescent reagents were purchased from Perkin Elmer Life Sciences (Waltham, MA). All the reagents and conjugated antibodies against phospho-p38, phospho-ERK1/2, phospho-JNK and IL-6 in flow cytometry analysis were purchased from BD Biosciences (San Diego, CA), while the TLR4 antibody was purchased from eBioscience (San Diego, CA).
Study subjects
Blood samples were collected from normal healthy adults. Approval was received from the National Jewish Health Institutional Review Board (Denver, CO) for the study.
Mice
C57BL6×129 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). MKP-1−/− mice were provided by Bristol-Myers Squibb (19). Six-eight week old males were used in the experiments. All experiments using these animals were approved by the Institutional Animal Care and Use Committee at National Jewish Health. This institution has an animal welfare assurance number (A3026-1) on file with the Office of Protection and Research Risks.
Cell Culture and Treatment
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized, venous blood of healthy donors by Ficoll-Hypaque density gradient centrifugation as described elsewhere. PBMC were cultured in hormone-free medium (phenol-red free RPMI containing 5% charcoal stripped FCS, 50 μg/ml streptomycin, and 50 U/ml penicillin) with a range of doses of 1,25(OH)2D3 or 25(OH)D3 for 24 h at 37°C with 5% CO2. Equal volume of ethanol was used as vehicle control. Following pretreatment with vitamin D the cells were stimulated with 10 ng/ml of LPS and the effects of vitamin D on LPS responses in CD14+ monocytes were examined.
Bone marrow cells from wild type (C57BL6×129) and MKP−/− mice were isolated as previously described(20, 21), cultured in DMEM containing 10% FBS, 10% L929 cell conditioned medium (as a source of CSF-1), 50 μg/ml streptomycin, and 50 U/ml penicillin at 37°C with 10% CO2 for 5 days to produce bone marrow macrophages (BMM).
Flow Cytometry Analysis
To analyze the effects of vitamin D on p38 activation by LPS, PBMC were pre-incubated in hormone free medium containing 1,25(OH)2D3 or 25(OH)D3 for 24 h, followed by stimulation with 10 ng/ml LPS for 10 min. Cells were then fixed with 2% formaldehyde at 37°C for 10 min. Adherent cells were collected by using HyQTase and TrypLE consecutively and combined with suspension cells. Cells were permeabilized in 500 μl 1X Perm/Wash Buffer I (BD Pharmingen) at 4°C for 30 min, incubated in 100 μl 1X Perm/Wash Buffer I containing FITC-conjugated anti-CD14 and APC-conjugated anti-phospho-p38 antibodies at 4°C for 1 h, and washed with 1X Perm/Wash Buffer I. The samples were then analyzed by flow cytometry (FacsCalibur Becton-Dickinson Cytometer, Franklin Lakes, NJ) and CellQuest Pro software. The flow data was displayed as % of CD14+ cells that express p-p38 (the gate for p-p38+ cells was set based on isotype control binding).
To examine the effects of vitamin D on IL-6 production by LPS-activated monocytes, PBMC were cultured in hormone-free medium containing 1,25(OH)2D3 or 25(OH)D3 for 24 h, followed by 10 ng/ml LPS stimulation for 6 h with GolgiPlug™ added for the last 4 h of the incubation. Cells were collected as above and stained with APC-conjugated anti-CD14 antibody in staining buffer at 4°C for 30 min. The cells were permeabilized in 500 μl 1X Perm/Wash Buffer at 4°C for 30 min, incubated in 100 μl 1X Perm/Wash Buffer containing FITC-conjugated anti-IL-6 antibody at 4°C for 30 min, and washed with 1X Perm/Wash Buffer before testing on FacsCalibur as above.
Real-Time PCR
Total RNA from human PBMC and murine BMM at the specified time point was prepared using RNeasy Mini kit (Qiagen, Valencia, CA). After reverse transcription, 500 ng cDNA from each sample were analyzed by real-time PCR using the dual-labeled fluorogenic probe method on an ABI Prism 7300 real time PCR system (Applied Biosystems). The expression of human and murine MKP-1, beta-actin, TNF-α, IL-6, and human MKP-5 mRNA was determined. All primers were purchased from Applied Biosystems (Foster City, CA).
Western blot
Whole cell extracts were prepared by incubating cells with RIPA Lysis Buffer with protease inhibitors on ice for 30 min, followed by centrifugation at 4°C for 10 min and collection of supernatants. Protein samples from vitamin D/LPS-treated PBMC and murine bone marrow cultures were resolved on Invitrogen 4-12% Bis-tris gel and transferred to PVDF membranes. The membranes were incubated in PBS containing specific antibodies, 5% dry milk, and 0.1% Tween 20 at 4°C overnight. Subsequently, membranes were washed in PBS/0.1% Tween 20, incubated for 1 h at room temperature with horseradish peroxidase (HRP)-labeled secondary antibodies, washed, incubated with chemiluminescent reagent and processed for immunodetection. Densitometry was used to evaluate the intensity of the bands.
ELISA analysis
PBMC and murine BMM were treated with 1,25(OH)2D3 for 24 h, followed by 10 ng/ml LPS stimulation for 24 h. Supernatants were collected, human IL-6 levels from PBMC were tested using Human IL-6 ELISA ready-set-go! Kit, and murine IL6 and TNF-α levels from BMM were tested using Mouse IL-6 ELISA ready-set-go! Kit and Mouse TNF alpha ELISA ready-set-go! Kit respectively. All the ELISA kits were purchased from eBioscience, Inc. (San Diego, CA).
Chromatin Immunoprecipitation (ChIP) Assay
VDR binding to VDRE and histone H4 acetylation at the human MKP-1 promoter were assessed using Magna ChIP A/G Chromatin Immunoprecipitation Kit following manufacturer’s instructions. VDR binding to VDRE and histone H4 acetylation at the murine MKP-1 promoter were assessed by ChIP assay as previously described (22) with modifications. Briefly, 2×106 cells were used in each precipitation. After sonication, chromatin solution was pre-cleared with 50 μl Protein A/G PLUS-Agarose beads and 10 μg herring DNA at 4°C for 2 h, incubated with specific antibody or isotype control at 4°C overnight, followed by precipitation with 50 μl Protein A/G PLUS-Agarose beads at room temperature for 2 h. Precipitated chromatin complexes were removed from the beads through incubation at 65°C for 30 min with Elution Buffer (50 mM Tris pH 8.0, 1 mM EDTA, 1% SDS). Two-hundred μl of eluate was mixed with 10 μl 5 M NaCl, 0.5 μl RNase A (10 mg/ml, DNase-free) and incubated at 65°C overnight. Precipitated DNA was quantified by real time PCR using SYBR green (Applied Biosystems). Primers used to detect the potential VDRE in the human MKP-1 promoter were: 5′-AGCTGGGATTCTAATCCAGGCAGT-3′ (E4.7 forward), 5′-CTTTGGAAGGTGGAGTTCCTCTCA-3′ (E4.7 reverse); Primers used to detect the potential VDRE in the murine MKP-1 promoter were: 5′-ACCCTTGCTCTCTCCCAAGTTCAT-3′ (E33 forward), 5′-AGAGTGTACCCAACACGACCCAAT-3′ (E33 reverse); 5′-ACGAGGGCAGATGCCTTTAATCCT-3′ (E0.9 forward), 5′-TGCTGTGTAGCTCTGGCTAGTCTT-3′ (E0.9 reverse).
Statistical analyses
Results were expressed as the mean ± SEM. Statistical analysis was conducted using GraphPad Prism, version 5 (GraphPad Software, La Jolla, CA). The data were analyzed by the paired Student’s t test, pairing by experiment. Before testing, paired difference distributions were examined for outliers, which can indicate violation to the normality assumption of the t test. No outliers were apparent. Tests were performed between specific treatments and LPS treatments or between vitamin D treatment and control. Unpaired t-test was used for comparison of responses between cells from wild type and MKP-1−/− mice. Differences were considered significant at p<0.05. A minimum of three independent experiments was conducted to allow for statistical comparisons.
RESULTS
Pretreatment of human PBMC with vitamin D inhibits LPS-induced p38 phosphorylation in human monocytes
In this study, we examined the role of vitamin D in the regulation of LPS responses. Human PBMC were pretreated with vitamin D for 24 h, followed by stimulation with 10 ng/ml of LPS. Both forms of the vitamin D, an active form, 1,25(OH)2D3, and 25(OH)D3 were tested. Monocytes/macrophages have previously been shown to be able to locally convert 25(OH)D3 into an active form (23). Vitamin D levels are normally measured by serum 25(OH)D3 levels; as this form of vitamin D is more stable, while the active form of vitamin D has a short half-life (5). In these experiments, we assessed the anti-inflammatory effects of 25(OH)D3 doses that are related to vitamin D deficiency (15 ng/ml) and vitamin D sufficiency (30 ng/ml – lower normal range, 50 ng/ml and 70 ng/ml – upper normal range for the serum vitamin D levels) (1, 23, 24). The binding of LPS to TLR4 on monocytes triggers immediate activation of MAP kinases, which together with activation of the canonical IKK pathway regulate NFκB activation, to induce proinflammatory cytokine production (25). We investigated whether the pretreatment of cells with vitamin D affects the activation of MAP kinases. The phosphorylation of three subfamilies of MAP kinases, ERK, JNK, and p38, was examined by flow cytometry. Human PBMC were pretreated with vitamin D for 24 h followed by 10 min of treatment with 10 ng/ml of LPS. After short-term stimulation with LPS, phosphorylation of p38 MAP kinase in CD14+ cells was induced. Monocytes cultured in media for 24h had high level of p-JNK, which was not changed after LPS stimulation for 10 min. No ERK phosphorylation was observed 10 min after LPS treatment in these cells. As expected LPS treatment did not activate CD3+ T cells.
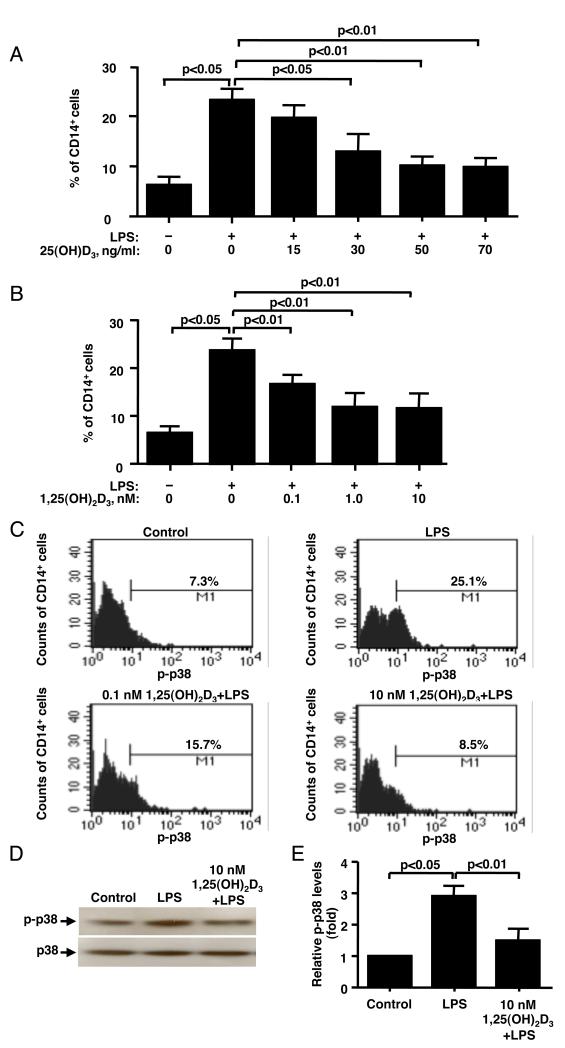
As shown by flow cytometry (Fig. 1A) 6.5±1.2% CD14+ cells expressed phosphorylated p38 MAP kinase (p-p38) prior to LPS treatment, and 23.6±2.5% CD14+ cells expressed p-p38 after 10 min of LPS treatment (n=4, p<0.05). While after LPS stimulation a significant increase in the % of CD14+ cells that express p-p38 was observed, there was no change in p-p38 mean fluorescence intensity (MFI) (Fig. 1C). It was found that 15 ng/ml of 25(OH)D3, a concentration corresponding to the insufficient serum vitamin D levels in humans(24), did not suppress LPS-induced p38 phosphorylation (Fig. 1A); while significant inhibition of LPS-induced p38 phosphorylation was achieved with 30 ng/ml and higher of 25(OH)D3 (Fig. 1A). Maximum inhibition was achieved with 50 ng/ml of 25(OH)D3 [a mean inhibition of 78% (n=4, p<0.01)]. Similarly, a dose-dependent inhibition of LPS-induced p38 activation was observed in human monocytes when the cells were pretreated with active vitamin D (Fig. 1B, 1C). The maximum inhibitory effect was achieved when the cells were preincubated with 0.4 ng/ml (1 nM) 1,25(OH)2D3 (mean inhibition of 70% (n=4, p<0.01). Inhibition of LPS-induced p38 phosphorylation by vitamin D was confirmed by Western blot, as 10 nM 1,25(OH)2D3 significantly suppressed LPS-induced p38 phosphorylation by 75% in adherent fraction of the PBMC (n=4, p<0.01) (Fig. 1D, 1E). As confirmed by flow cytometry this adherent fraction of PBMC consisted of 85-90% CD14+ monocytes. Both flow cytometry and Western blot evaluations demonstrated that in vitamin D pretreated cells LPS-induced p-38 phosphorylation was inhibited to the level of p-p38 phosphorylation observed in cells cultured with media alone.
Figure 1. Vitamin D inhibits LPS-induced p38 phosphorylation in human monocytes.
PBMC were cultured in hormone-free medium containing 25(OH)D3 (A) or 1,25(OH)2D3, (B) for 24 h, followed by stimulation with 10 ng/ml LPS for 10 min. (A, B) As shown by flow cytometry, vitamin D pretreatment inhibits LPS-induced p38 phosphorylation in CD14+ cells (n=4). (C) Representative flow cytometry data on the effects of LPS and vitamin D/LPS on p38 activation in human monocytes is shown. (D) Pretreatment with vitamin D significantly inhibits p-p38 expression by the cells as shown by Western blot. Whole cell extracts from LPS or vitamin D/LPS treated adherent PBMC fraction were prepared, and blotted against phosphorylated p38 and total p38. (E) Fold changes in the densitometry of phosphorylated p38 normalized to total p38 MAPK expression are provided. Values represent mean ± SEM (n=4 experiments).
Pretreatment with vitamin D inhibits LPS-induced IL-6 and TNF-α production by human monocytes
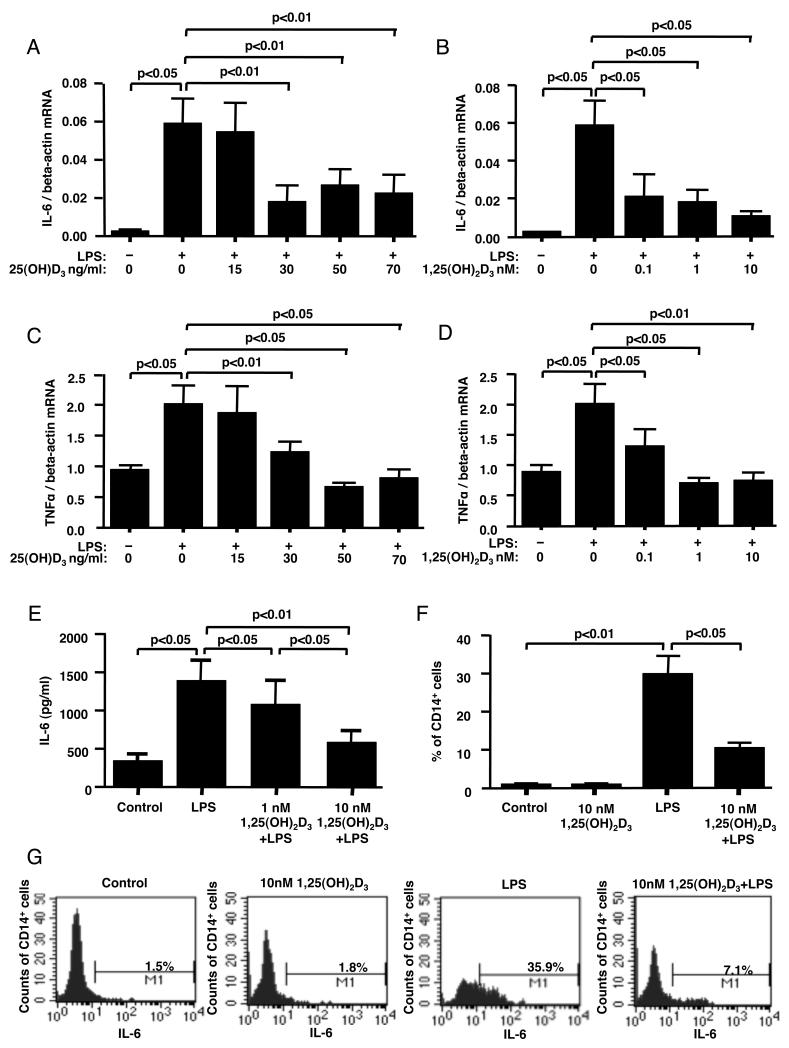
Upon LPS stimulation, monocytes produce pro-inflammatory cytokines, such as TNF-α and IL-6 (26). Persistent inflammatory responses can damage host tissues(27, 28). To examine whether changes in LPS-induced p38 activation in the presence of vitamin D influenced cytokine production, human PBMC were preincubated as above with either 1,25(OH)2D3 or 25(OH)D3 for 24 h followed by stimulation with 10 ng/ml of LPS for 24 h. LPS treatment significantly induced IL-6 mRNA production by the cells (p<0.05). When the cells were preincubated with 30 ng/ml and greater of 25(OH)D3, a significant inhibition of LPS-induced IL-6 mRNA expression was observed (p<0.01) (Fig. 2A). No inhibition of LPS-induced IL-6 expression was observed when the cells were cultured with 15 ng/ml of 25(OH)D3 (Fig. 2A). All doses of the active form of vitamin D significantly inhibited LPS-induced IL-6 mRNA expression (Fig. 2B). The degree of suppression of IL-6 mRNA by 30 ng/ml (70 nM) of 25(OH)D3 was comparable to the inhibition achieved with 0.04 ng/ml (0.1 nM) of the active form of vitamin D (Fig. 2A, 2B). Similar vitamin D effects were observed in LPS-induced TNF-α mRNA expression (Fig. 2C, 2D).
Figure 2. Vitamin D inhibits LPS-induced cytokine production in human monocytes.
PBMC were cultured in hormone-free medium containing 25(OH)D3, (A, C) or 1,25(OH)2D3, (B, D, E) for 24 h, followed by 24 h of treatment with 10 ng/ml of LPS. IL-6 (A, B) and TNF-a (C, D) mRNA levels in the total PBMC were detected by real-time PCR after 24 h of stimulation with LPS (n=4). IL-6 protein levels (E) in the culture supernatants following LPS stimulation were detected by ELISA (n=4). (F) IL-6 expression in CD14+ cells was detected in human monocytes by flow cytometry after 24 h of pretreatment with 10 nM 1,25(OH)2D3 followed by 6 h of stimulation with LPS. The percentage of CD14+ cells expressing IL-6 was calculated. Values represent mean±SEM (n=4 experiments). (G) Representative flow cytometry data on the effects of LPS and vitamin D/LPS on IL-6 production by human monocytes is shown.
Upon stimulation with LPS the amounts of IL-6 protein in culture supernatants increased from a basal level of 330 ± 96 pg/ml to 1385 ± 277 pg/ml (p<0.05). When the cells were pretreated with 10 nM 1,25(OH)2D3 for 24 h, LPS-induced IL-6 production by the cells was significantly inhibited by a mean of 77% (n=4, p<0.01) (Fig. 2E). The inhibition of IL-6 production was more efficient with 10 nM 1,25(OH)2D3 as compared to 1 nM 1,25(OH)2D3 (p<0.05) (Fig. 2E). These data were confirmed by flow cytometry as the amount of IL-6-producing monocytes was significantly increased after the stimulation with LPS from basal 0.8 ± 0.4% to 29.7 ± 4.9% (n=4, p<0.01) (Fig. 2F, 2G). However, when the cells were pretreated with 10 nM 1,25(OH)2D3 for 24 h, the amount of IL-6-producing CD14+ cells induced by LPS was significantly inhibited by a mean of 67% (n=4, p<0.05) (Fig. 2F, 2G).
Vitamin D pretreatment induces MKP-1 expression
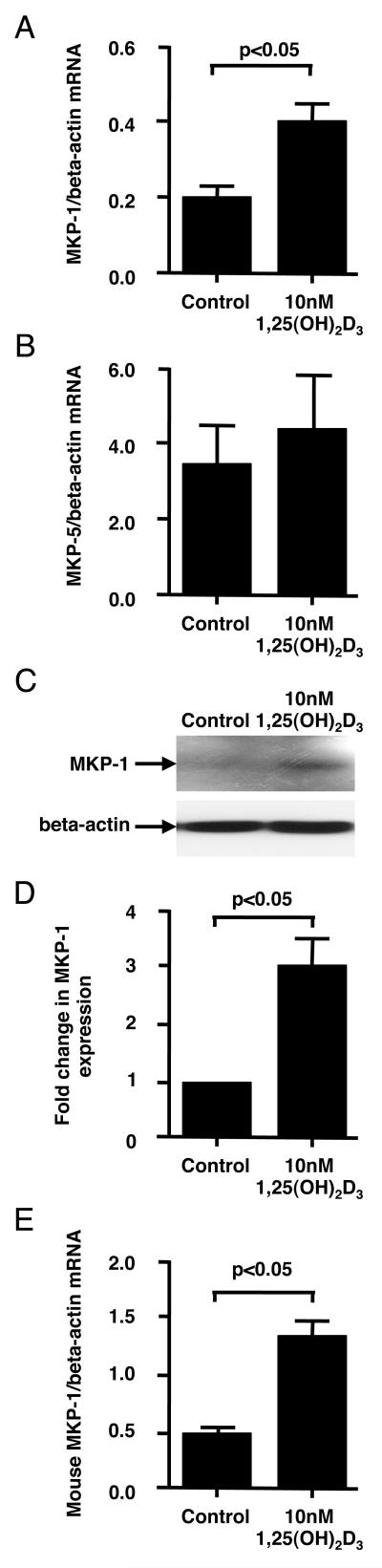
MKP-1 plays a critical role in switching off p38 signaling and cytokine production in monocytes/macrophages after the inflammatory stimuli (18). Since vitamin D pretreatment significantly inhibited LPS-induced p38 phosphorylation, we examined whether this process was mediated via MKP-1 or other phosphatases. Pretreatment of human PBMC with 10 nM 1,25(OH)2D3 for 24 h resulted in significant increases in MKP-1 mRNA (2.5±0.1 fold, p<0.05) (Fig. 3A) and protein (3.1±0.4 fold, p<0.05) (Fig. 3C, 3D) expressions by the adherent PBMC fraction, which consisted mainly of monocytes. The expression of MKP-5, another phosphatase that has been reported to dephosphorylate p38, was not changed in adherent PBMC fraction after 24h of culture with 1,25(OH)2D3 (Fig. 3B).
Figure 3. Vitamin D induces MKP-1 by human and mouse monocytes/macrophages.
Human PBMC were cultured in hormone-free medium containing 10 nM 1,25(OH)2D3 or vehicle control for 24 h. Adherent PBMC fraction was collected. (A, B) Human MKP-1 and MKP-5 mRNA levels were tested by real-time PCR and normalized to beta-actin mRNA levels (n=6 experiments). (C) Human MKP-1 protein levels were tested by Western blot. (D) Fold changes in the densitometry of human MKP-1 to beta-actin expression from Western blot are provided (n=4 experiments). (E) Murine BMM cells were cultured in DMEM for 18 h, and then treated with 10 nM 1,25(OH)2D3 or vehicle control for 6 h. mRNA levels of murine MKP-1 were tested and normalized to beta-actin mRNA (n=3 experiments). All values represent mean±SEM.
To examine the mechanism of MKP-1 upregulation by vitamin D, we used murine bone marrow-derived macrophage cultures. Similarly to human monocytes, when murine bone marrow derived macrophages from wild type mice (C57BL6×129) were preincubated with 10 nM 1,25(OH)2D3, a significant increase in MKP-1 mRNA expression was observed. The maximum increase in MKP-1 mRNA expression was found after 6 h of treatment with vitamin D (2.6 ± 0.2 fold increase, n=3, p<0.01) (Fig. 3E).
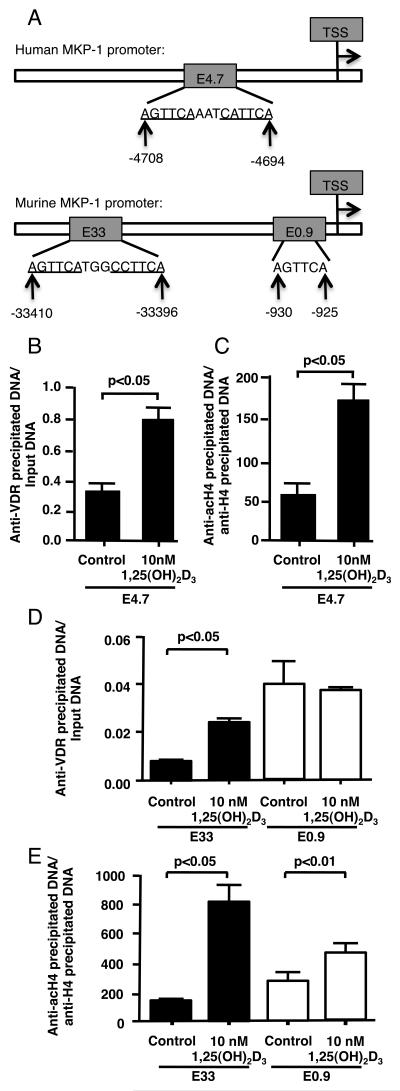
We examined the presence of potential VDRE sites in the human and murine MKP-1 promoter. A VDRE (labeled E4.7) sequence AGTTCAAATCATTCA was located at −4708 to −4694 from the transcriptional start site of human MKP-1 gene. A VDRE (labeled E33) sequence AGTTCATGGCCTTCA was located at −33410 to −33396 from the transcriptional start site of murine MKP-1 gene. Several additional half VDRE sites were also found at murine MKP-1 promoter, with the closest one with a sequence AGTTCA (labeled E0.9) located at −930 to −925 from the transcriptional start site (the scheme of the potential VDR binding sites in the human and murine MKP-1 promoter is shown in Fig. 4A). VDR binding and histone H4 acetylation at the E4.7, E33 and E0.9 sites of the human and murine MKP-1 promoters were tested by ChIP assays. We found that treatment of adherent PBMC with 10 nM 1,25(OH)2D3 for 24 h enhanced VDR binding and histone H4 acetylation by 2.4±0.5 fold (p<0.05) and 3.0±0.8 (p<0.05) fold, respectively, at the E4.7 site of the human MKP-1 promoter (Fig. 4B, 4C). We found that treatment of murine bone marrow-derived macrophages with 10 nM 1,25(OH)2D3 for 6 h enhanced VDR binding and histone H4 acetylation by 3.7±0.4 fold (p<0.05) and 6.26±0.04 (p<0.05) fold, respectively, at the E33 site of the murine MKP-1 promoter (Fig. 4D, 4E). No increase in VDR binding and only a slight yet significant (p<0.01) increase in histone H4 acetylation were observed at the E0.9 site (Fig. 4D, 4E). These data suggest that vitamin D bound VDR interacts with the VDRE upstream of MKP-1 gene, thus, potentially influencing MKP-1 transcription.
Figure 4. Vitamin D regulates MKP-1 expression.
(A) Schematic representation of the potential VDRE in human and murine MKP-1 promoter. TSS – transcriptional start site. (B, C). The recruitment of VDR to E4.7 of human MKP-1 promoter and histone H4 acetylation at this site as determined by ChIP assay after 24 h of treatment of human adherent PBMC with vitamin D. (D, E) The recruitment of VDR to E33 and E0.9 VDRE sites of the murine MKP-1 promoter and histone H4 acetylation at these sites as determined by ChIP assay after 6 h of treatment of murine BMM with vitamin D. The quantity of anti-VDR antibody precipitated DNA was normalized to Input DNA, anti-acetylated histone H4 antibody precipitated DNA was normalized to anti-histone H4 antibody precipitated DNA. Values represent mean ± SEM (n=3 experiments).
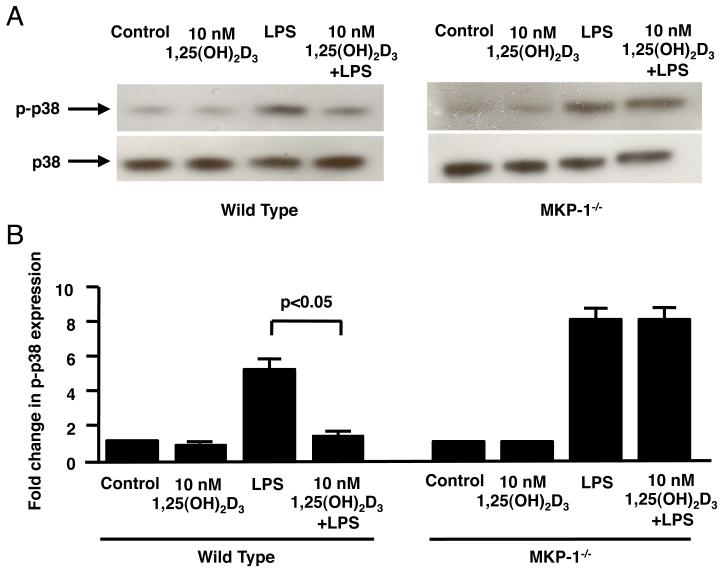
Vitamin D inhibits LPS-induced p38 phosphorylation in macrophages derived from the bone marrow of the wild type but not the MKP-1−/− mice
Next, we tested the ability of vitamin D to inhibit LPS-induced p38 phosphorylation in macrophages derived from bone marrow of the wild type and MKP-1−/− mice (29). Ten minutes of treatment with LPS resulted in a significant increase in p38 phosphorylation by 4.8±0.2 fold (n=3, p<0.05) and 8.2±0.2 fold (n=3, p<0.01) in wild type and MKP-1−/− macrophages, respectively (Fig. 5A, 5B). As shown by Western blot, pretreatment with 10 nM 1,25(OH)2D3 significantly inhibited LPS-induced p38 MAP kinase activation by a mean of 93% (n=3, p<0.05) in bone marrow-derived macrophage from wild type mice (Fig. 5B); but this inhibition was abolished in bone marrow-derived macrophage from MKP1−/− mice (Fig. 5B).
Figure 5. Vitamin D inhibits LPS-induced p38 phosphorylation in bone marrow macrophages from wild type but not MKP-1−/− mice.
(A) BMMs from wild-type and MKP1−/− mice were cultured in DMEM containing 10 nM 1,25(OH)2D3 or vehicle control for 24 h, followed by 10 ng/ml LPS stimulation for 10 min. Whole cell extracts were prepared, and blotted against phosphorylated p38 and total p38. (B) Fold changes in the densitometry of phosphorylated p38 normalized to total p38 are provided. Values represent mean±SEM (n=3 experiments).
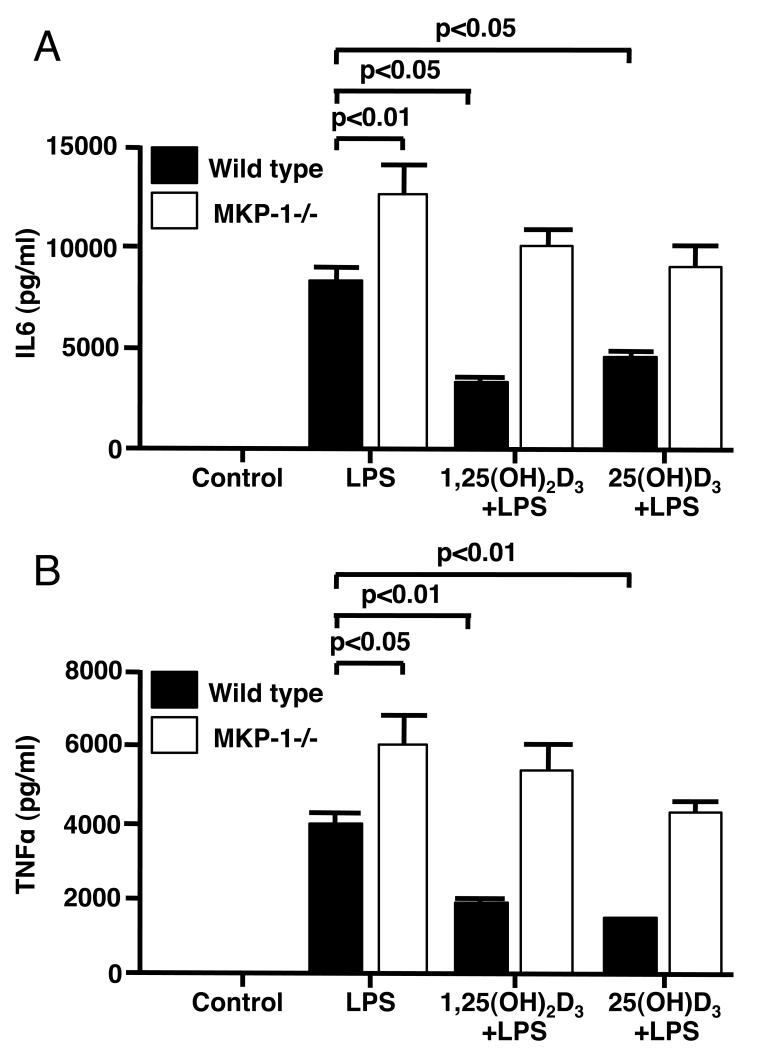
Inhibition of LPS-induced production of IL6 and TNF-α in bone marrow macrophages from wild type mice by vitamin D was significantly compromised in MKP-1−/− mice
To examine whether inhibition of LPS-induced p38 activation by vitamin D in mouse macrophages influenced cytokine production, macrophages derived from the bone marrow of wild type and MKP-1−/− mice were preincubated with either 10 nM 1,25(OH)2D3 or 75 nM (30ng/ml) 25(OH)D3 for 24 h followed by stimulation with 10 ng/ml of LPS for 24 h. Significantly higher levels of IL-6 and TNF-α were observed in culture supernatants of the LPS-treated BMM from MKP-1 −/− mice as compared to wild type mice (p<0.01 and p<0.05 respectively). The amounts of LPS-induced IL-6 protein in culture supernatants were inhibited 67% and 61% by 1,25(OH)2D3 and 25(OH)D3 respectively in wild type macrophages. LPS-induced IL-6 production was inhibited only to 18% and 33%, respectively in MKP-1−/− macrophages (Fig. 6A). Similarly we observed that 1,25(OH)2D3 and 25(OH)D3 inhibited LPS-induced TNF-α protein in culture supernatants by 48% and 57%, respectively in wild type macrophages, while the inhibition of LPS-induced production of these cytokines was only 16% and 29%, respectively, in MKP-1−/− macrophages (Fig. 6B).
Figure 6. Inhibition of LPS-induced production of IL-6 and TNF-α in bone marrow macrophages from wild type mice by vitamin D was significantly compromised in MKP-1−/− mice.
BMMs from wild-type and MKP1−/− mice were cultured in DMEM containing 10 nM 1,25(OH)2D3 or 75nM (30ng/ml) 25(OH)D3 for 24 h, followed by 10 ng/ml LPS stimulation for 24 h. IL-6 (A) and TNF-α (B) protein levels in the culture supernatants were detected by ELISA. Values represent mean±SEM (n=3 experiments).
DISCUSSION
In this study, we examined the effects of vitamin D at physiologic concentrations on LPS-stimulated inflammatory responses in monocytes/macrophages. We found that both 1,25(OH)2D3, an active form of vitamin D, and 25(OH)D3, that requires conversion by monocytes into an active form(23), dose-dependently inhibited p38 phosphorylation and cytokines, IL-6 and TNF-α, production in LPS-stimulated human monocytes. Upon vitamin D treatment, the expression of MKP-1 phosphatase mRNA and protein was significantly upregulated in both human monocytes and murine bone marrow-derived macrophages. We demonstrated that vitamin D treatment increased VDR binding to a putative VDRE both in human and murine MKP-1 promoter and enhanced histone H4 acetylation near this VDRE site. Utilizing macrophages derived from MKP-1−/− mice, we further demonstrated that vitamin D was no longer able to suppress LPS-induced p38 activation followed by the compromised ability to inhibit LPS-induced IL-6 and TNF-α production in the absence of MKP-1. Our current study therefore identified the upregulation of MKP-1 by physiologic concentrations of vitamin D as a novel pathway by which vitamin D inhibits LPS-induced p38 activation and cytokine production in monocytes/macrophages.
In recent years, vitamin D deficiency in humans has received significant attention (1, 24). According to the recent brief of the National Center for the Health Statistics in 2001-2006, 32% of the US population had serum 25(OH)D3 levels <20ng/ml; 8% of the US population had serum 25(OH)D3 levels <12ng/ml(30). Aside from its classical role as a modulator of calcium metabolism and bone health, vitamin D has been shown to have potent anti-inflammatory effects and consequently has been considered for adjunctive therapy for numerous chronic diseases including asthma, arthritis and prostate cancer (4, 31, 32). A variety of pro- and anti-inflammatory effects for the vitamin D had been reported (33, 34). It has been shown that vitamin D can directly induce the production of the important antimicrobial peptides, cathelicidin and human beta defensin 4, by human monocytes/macrophages and epithelial cells (23, 35). Anti-proliferative and proapoptotic activity has been shown in vitamin D-treated tumor cells due to the induction of cyclin-dependent kinase inhibitors p21Waf/Cip1 and p27Kip1, inhibition of c-Myc and anti-apoptotic Bcl-2 (32). Vitamin D had been demonstrated to suppress prostaglandin pathways in the tumor cell lines via inhibition of COX-2 production and stimulation of 15-PGDH production by the cells (32). Vitamin D has also been shown to interfere with NFκB activation and signaling by increasing the expression of IκBα in the cells, thus interfering with the nuclear translocation of the activated NFκB subunits (36). It has also been reported that vitamin D can influence dendritic cells maturation and function (37). Several studies have highlighted the capacity of vitamin D to modulate the population and function of FoxP3+ and IL-10-producing T-regulatory cells (37).
The current study found that human monocytes are capable of responding to treatment with two different forms of vitamin D: 1,25(OH)2D3 and 25(OH)D3. 25(OH)D3 is converted into a functionally active form, 1,25(OH)2D3, by the enzyme 25-hydroxyvitamin D3-1α-hydroxylase (CYP27b1); a process that primarily occurs in kidneys (38, 39). However, it has been shown that monocytes, macrophages, and dendritic cells also express CYP27b1 (35, 40). Therefore 1,25(OH)2D3 can be produced locally and exert immunomodulatory effects (41). We demonstrated that 15 ng/ml of the 25(OH)D3 (a concentration amount considered in this study vitamin D deficiency (24)) did not suppress LPS-induced IL-6 and TNF-α production in human monocytes. We found that 25(OH)D3 at 30 ng/ml and higher, i.e. levels considered vitamin D sufficient in humans, significantly inhibited IL-6 and TNF-α production induced by LPS. Furthermore, the degree of suppression of IL-6 and TNF-α production by 30 ng/ml of 25(OH)D3 was comparable to the effects achieved by 0.04 ng/ml (0.1 nM) of the active form of the vitamin D. These data suggest that human monocytes have the ability to convert 25(OH)D3 to an active form. Further, our study demonstrates that serum concentrations of 25(OH)D3 does matter for the optimal anti-inflammatory response of human monocytes, since the amount of the available circulating 25(OH)D3 influences local tissue concentrations of the active vitamin D (23, 35). These data support the idea that in order to achieve optimal anti-inflammatory effects by vitamin D it is important to maintain serum vitamin D levels above 30 ng/ml in the physiologic range (23, 42).
Dual-specificity phosphatases are a heterogeneous group of protein phosphatases that can dephosphorylate both phosphotyrosine and phosphothreonine residues within the same substrate. MKP-1 is the best-studied member of this family and is characterized for its role in the regulation of MAP kinase signaling cascades (43, 44). In this study, we demonstrated that vitamin D can upregulate MKP-1 expression by monocytes/macrophages and utilize this pathway for the inhibition of the LPS-induced p38 phosphorylation. In this study we focused on the early stages of monocyte activation by LPS to assess the contribution of vitamin D-regulated phosphatases on LPS-induced MAPK activation. We did not extend this assessment to 30 min post LPS stimulation of avoid the effects of LPS-induced MKP-1 (18). Another member of the dual specificity phosphatase family, MKP-5, was recently shown to be induced by vitamin D treatment in normal prostate epithelial cells, resulting in inhibition of p38 activation in these cells (45). Vitamin D did not alter MKP-5 expression in human monocytes in our study.
Recently it has been reported that vitamin D treatment down-regulates TLR2, TLR4 expression by human monocytes and up-regulates CD14 expression by these cells (46). The authors concluded that down-regulation of TLR expression can substantially reduce LPS and LTA-mediated MAP kinase activation. MKP-1 expression under these conditions was not studied. Importantly, in this study 100nM of the active vitamin D was used. In our study, 10 nM of the active vitamin D was the highest dose used and we did not observe changes in TLR4 protein expression by human monocytes in response to 24 h of pretreatment with 10 nM of active form of the vitamin D (data not shown).
Vitamin D has been reported to regulate a variety of human and mouse genes through VDR-mediated VDRE binding (47, 48). Although VDREs have been well studied in genes like CYP24A1 (48, 49), no VDRE has been discovered in MKP-1 gene to date. In this study, we identified a putative VDRE site approximately 4.7 kb upstream of transcriptional start site in the human MKP-1 promoter and a putative VDRE site approximately 33 kb upstream of transcriptional start site in the distal murine MKP-1 promoter. We confirmed VDR binding to these two sites using ChIP assays. The far upstream location of the putative VDRE in murine MKP-1 promoter is not surprising as genome-wide mapping of VDREs have indicated that VDREs are predominantly located in introns and intergenic intervals (7). Following the binding of VDR to VDRE, co-activators, co-repressors or mediator proteins may be recruited to the site depending on the effects of vitamin D on a specific gene transcription. Since vitamin D treatment significantly induces MKP-1 mRNA expression, it is likely that VDR binding to the MKP-1 promoter induces recruitment of the co-activators and initiates histone acetylation, leading to transcriptional activation (10). Our ChIP assays confirmed significantly enhanced histone H4 acetylation near the putative VDRE sites (E4.7 and E33) in human and in the distal murine MKP-1 promoter, respectively, after vitamin D treatment.
Selective inhibitors for MKP-1 are currently unavailable and inhibition of MKP-1 expression is difficult to accomplish via siRNA in primary monocytes/macrophages. For these reasons, we explored the role of MKP-1 in vitamin D-mediated anti-inflammatory effects in monocytes by utilizing bone marrow-derived macrophages from MKP-1−/− mice and wild type mice. Our data suggests that vitamin D regulation of MKP-1 is one of the essential pathways that inhibits LPS-induced cytokine production by monocytes/macrophages. We demonstrated that vitamin D-mediated suppression of the LPS-induced p38 phosphorylation was abolished and the inhibition of LPS-induced production of IL6 and TNF-α was significantly reduced in macrophages derived from MKP-1−/− mice. The fact that MKP-1 knockout did not completely abolish vitamin D inhibition effect on LPS-induced cytokine production indicates that vitamin D engages additional pathways aside from MKP-1 to regulate LPS-induced proinflammatory cytokine production. This may include but not be limited to transrepression of NFkB-mediated responses by VDR as reported before (33, 36, 46). It was recently reported that LPS controls MKP-1 in activated monocytes via upregulation of the miR101 (50). We did not find changes in miR101 expression in vitamin D pretreated human monocytes (unpublished observation).
In summary, our study provides several novel discoveries: first, physiologic levels of vitamin D can modulate inflammatory activities, as 30-50 ng/ml of the 25(OH)D3 is sufficient to inhibit LPS-induced p38 activation and cytokine production in human monocytes (Fig. 1, 2). Secondly, the study identified the upregulation of MKP-1 by vitamin D as a novel mechanism by which vitamin D inhibits LPS-induced p38 activation and cytokine production in monocytes/macrophages. Finally, a putative VDR-binding site was identified in the distal murine MKP-1 promoter and human MKP-1 promoter. Our current studies suggest that patients with chronic inflammatory diseases that are vitamin D deficient (<20ng/ml) may benefit from oral supplementation of vitamin D to get their serum vitamin D level above 30 ng/ml.
ACKNOWLEDGEMENTS
We thank Maureen Sandoval and Shih-Yun Lyman for their help in preparation of this manuscript.
The authors thank Bristol-Myers Squibb that developed MKP-1−/− mice.
This work was supported in part by NIH grants AI070140, HL68628, HL37260, AI57798, and AI68956. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, National Heart, Lung, and Blood Institute or the National Institutes of Health.
LIST OF ABBREVIATIONS
- BMM
bone marrow-derived macrophages
- LPS
lipopolysaccharide
- MKP-1
mitogen-activated protein kinase phosphatase-1
- PBMC
peripheral blood mononuclear cells
- TLR4
Toll-like receptor 4
- VDR
vitamin D receptor
- VDRE
Vitamin D Response Element
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller J, Gallo RL. Vitamin D and innate immunity. Dermatol Ther. 2010;23:13–22. doi: 10.1111/j.1529-8019.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- 4.Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9:941–955. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 5.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582S–586S. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 6.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, Burrell A, Handunnetthi L, Handel AE, Disanto G, Orton SM, Watson CT, Morahan JM, Giovannoni G, Ponting CP, Ebers GC, Knight JC. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352–1360. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlberg C. The vitamin D(3) receptor in the context of the nuclear receptor superfamily: The central role of the retinoid X receptor. Endocrine. 1996;4:91–105. doi: 10.1007/BF02782754. [DOI] [PubMed] [Google Scholar]
- 9.Quack M, Carlberg C. Ligand-triggered stabilization of vitamin D receptor/retinoid X receptor heterodimer conformations on DR4-type response elements. J Mol Biol. 2000;296:743–756. doi: 10.1006/jmbi.2000.3499. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Shevde NK, Pike JW. 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J Bone Miner Res. 2005;20:305–317. doi: 10.1359/JBMR.041112. [DOI] [PubMed] [Google Scholar]
- 11.Opal SM. The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sepsis. Int J Med Microbiol. 2007;297:365–377. doi: 10.1016/j.ijmm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 13.Kracht M, Saklatvala J. Transcriptional and post-transcriptional control of gene expression in inflammation. Cytokine. 2002;20:91–106. doi: 10.1006/cyto.2002.0895. [DOI] [PubMed] [Google Scholar]
- 14.Bhavsar P, Hew M, Khorasani N, Torrego A, Barnes PJ, Adcock I, Chung KF. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax. 2008;63:784–790. doi: 10.1136/thx.2007.090027. [DOI] [PubMed] [Google Scholar]
- 15.Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 16.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 17.Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, Saklatvala J, Clark AR. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203:1883–1889. doi: 10.1084/jem.20060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169:6408–6416. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- 19.Dorfman K, Carrasco D, Gruda M, Ryan C, Lira SA, Bravo R. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene. 1996;13:925–931. [PubMed] [Google Scholar]
- 20.Riches DW, Underwood GA. Expression of interferon-beta during the triggering phase of macrophage cytocidal activation. Evidence for an autocrine/paracrine role in the regulation of this state. J Biol Chem. 1991;266:24785–24792. [PubMed] [Google Scholar]
- 21.Winston BW, Chan ED, Johnson GL, Riches DW. Activation of p38mapk, MKK3, and MKK4 by TNF-alpha in mouse bone marrow-derived macrophages. J Immunol. 1997;159:4491–4497. [PubMed] [Google Scholar]
- 22.Zhang Y, Leung DY, Nordeen SK, Goleva E. Estrogen inhibits glucocorticoid action via protein phosphatase 5 (PP5)-mediated glucocorticoid receptor dephosphorylation. J Biol Chem. 2009;284:24542–24552. doi: 10.1074/jbc.M109.021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hewison M. Antibacterial effects of vitamin D. Nat Rev Endocrinol. 2011;7:337–345. doi: 10.1038/nrendo.2010.226. [DOI] [PubMed] [Google Scholar]
- 24.Dietary Reference Intakes for Calcium and Vitamin D. Institute of Medicine of the National Academies. 2010:1–4. [PubMed] [Google Scholar]
- 25.Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci. 2008;99:836–842. doi: 10.1111/j.1349-7006.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thieringer R, Fenyk-Melody JE, Le Grand CB, Shelton BA, Detmers PA, Somers EP, Carbin L, Moller DE, Wright SD, Berger J. Activation of peroxisome proliferator-activated receptor gamma does not inhibit IL-6 or TNF-alpha responses of macrophages to lipopolysaccharide in vitro or in vivo. J Immunol. 2000;164:1046–1054. doi: 10.4049/jimmunol.164.2.1046. [DOI] [PubMed] [Google Scholar]
- 27.Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90:2312–2316. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154–1159. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Meng X, Kuhlman JR, Nelin LD, Nicol KK, English BK, Liu Y. Knockout of Mkp-1 enhances the host inflammatory responses to gram-positive bacteria. J Immunol. 2007;178:5312–5320. doi: 10.4049/jimmunol.178.8.5312. [DOI] [PubMed] [Google Scholar]
- 30.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001-2006. NCHS Data Brief. 2011:1–8. [PubMed] [Google Scholar]
- 31.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125:995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnan AV, Feldman D. Molecular pathways mediating the anti-inflammatory effects of calcitriol: implications for prostate cancer chemoprevention and treatment. Endocr Relat Cancer. 2010;17:R19–38. doi: 10.1677/ERC-09-0139. [DOI] [PubMed] [Google Scholar]
- 33.Wu S, Sun J. Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discov Med. 2011;11:325–335. [PMC free article] [PubMed] [Google Scholar]
- 34.Guillot X, Semerano L, Saidenberg-Kermanac’h N, Falgarone G, Boissier MC. Vitamin D and inflammation. Joint Bone Spine. 2010;77:552–557. doi: 10.1016/j.jbspin.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 36.Stio M, Martinesi M, Bruni S, Treves C, Mathieu C, Verstuyf A, d’Albasio G, Bagnoli S, Bonanomi AG. The Vitamin D analogue TX 527 blocks NF-kappaB activation in peripheral blood mononuclear cells of patients with Crohn’s disease. J Steroid Biochem Mol Biol. 2007;103:51–60. doi: 10.1016/j.jsbmb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep. 2011;11:29–36. doi: 10.1007/s11882-010-0161-8. [DOI] [PubMed] [Google Scholar]
- 38.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 40.Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, Kilby MD, Moss PA, Chakraverty R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–5390. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 41.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 42.Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010;21:375–384. doi: 10.1016/j.tem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases--regulating the immune response. Nat Rev Immunol. 2007;7:202–212. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- 44.Patterson KI, Brummer T, O’Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- 45.Nonn L, Peng L, Feldman D, Peehl DM. Inhibition of p38 by vitamin D reduces interleukin-6 production in normal prostate cells via mitogen-activated protein kinase phosphatase 5: implications for prostate cancer prevention by vitamin D. Cancer Res. 2006;66:4516–4524. doi: 10.1158/0008-5472.CAN-05-3796. [DOI] [PubMed] [Google Scholar]
- 46.Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, Zugel U, Steinmeyer A, Pollak A, Roth E, Boltz-Nitulescu G, Spittler A. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361–370. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 47.Sepulveda VA, Weigel NL, Falzon M. Prostate cancer cell type-specific involvement of the VDR and RXR in regulation of the human PTHrP gene via a negative VDRE. Steroids. 2006;71:102–115. doi: 10.1016/j.steroids.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Meyer MB, Goetsch PD, Pike JW. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha,25-dihydroxyvitamin D3. J Biol Chem. 2010;285:15599–15610. doi: 10.1074/jbc.M110.119958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaisanen S, Dunlop TW, Sinkkonen L, Frank C, Carlberg C. Spatio-temporal activation of chromatin on the human CYP24 gene promoter in the presence of alpha,25-Dihydroxyvitamin D3. J Mol Biol. 2005;350:65–77. doi: 10.1016/j.jmb.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 50.Zhu QY, Liu Q, Chen JX, Lan K, Ge BX. MicroRNA-101 targets MAPK phosphatase-1 to regulate the activation of MAPKs in macrophages. J Immunol. 2010;185:7435–7442. doi: 10.4049/jimmunol.1000798. [DOI] [PubMed] [Google Scholar]