Abstract
Objective. To evaluate whether the length of time of rupture of membranes (ROM) in optimally managed HIV-positive women on highly active antiretroviral therapy (HAART) with low viral loads (VL) is predictive of the risk of mother to child transmission (MTCT) of the human immunodeficiency virus (HIV). Study Methods. A retrospective case series of all HIV-positive women who delivered at two academic tertiary centers in Toronto, Canada from January 2000 to November 2010 was completed. Results. Two hundred and ten HIV-positive women with viral loads <1,000 copies/ml delivered during the study period. VL was undetectable (<50 copies/mL) for the majority of the women (167, 80%), and <1,000 copies/mL for all women. Mode of delivery was vaginal in 107 (51%) and cesarean in 103 (49%). The median length of time of ROM was 0.63 hours (range 0 to 77.87 hours) for the entire group and 2.56 hours (range 0 to 53.90 hours) for those who had a vaginal birth. Among women with undetectable VL, 90 (54%) had a vaginal birth and 77 (46%) had a cesarean birth. Among the women in this cohort there were no cases of MTCT of HIV. Conclusions. There was no association between duration of ROM or mode of delivery and MTCT in this cohort of 210 virally suppressed HIV-positive pregnant women.
1. Introduction
In economically developed countries, the human immunodeficiency virus (HIV) infection is now considered a chronic disease, with life expectancy approaching that of the general population [1]. Many HIV-positive women choose to pursue pregnancies [2]. Management of the HIV-positive pregnant patient should focus on both decreasing the risk of mother to child transmission (MTCT) and minimizing maternal and neonatal complications.
The Society of Obstetricians and Gynaecologists of Canada (SOGC) and American College of Obstetricians and Gynecologists (ACOG) recommend that elective cesarean section (cesarean section before labor or rupture of membranes (ROMs) be performed for delivery when viral load is detectable [3] or greater than 1000 copies/mL [4] as there is a 12-fold increased risk of MTCT [3, 5]. This is based on several studies that showed that the combination of intrapartum zidovudine (ZDV) and elective cesarean section significantly decreased vertical transmission compared to other delivery modes [6–8]. With the addition of highly active antiretroviral therapy (HAART), the risk of vertical transmission has continued to decrease [5].
ROM increases fetal exposure to maternal blood and vaginal fluids, and prolonged duration of ROM has been shown to be a significant risk factor for vertical transmission [6, 9–11]. Evidence exists that after 4 hours of ROM the risk of MTCT rises and the protective effect of a cesarean section is lost [9, 10]. However, these conclusions were based on studies in which only intrapartum monotherapy with ZDV was used and maternal VL was not known. Since the addition of HAART, subsequent research has been performed to determine if prolonged duration of ROM remains an important risk factor for vertical transmission. These studies have demonstrated that there is no increased risk of transmission with ROM longer than 4 hours and no protective effect of cesarean section [5, 12]. Given that postpartum morbidity from cesarean section is potentially higher in HIV-positive women [13], achieving a vaginal birth in this population is beneficial.
Given the paucity of literature addressing this question, the objective of this study was to review our experience and evaluate whether the effect of duration of ROM or mode of delivery on MTCT still exists among optimally managed HIV-positive women on HAART.
2. Methods
Following ethical approval, we performed a retrospective chart review of all HIV-positive women who delivered at Mount Sinai and St. Michael's Hospitals in Toronto, Canada, between January 2000 and November 2010.
Women were defined as “optimally managed” if they were taking antepartum HAART and had a VL less than 1000 copies/mL at the time of delivery. Thus, eligibility criteria included pregnant women with a predelivery diagnosis of HIV, adherence to antepartum HAART, VL less than 1000 copies/mL at the time of delivery, and delivery at either of the two sites. If a woman had had multiple deliveries during the time period, the most recent delivery data was collected. Women not on HAART or with viral loads greater than 1000 copies/mL were excluded.
Information regarding the patient's age, ethnic background, gravidity, parity, administration of intravenous ZDV, time of ROM, time of birth, mode of delivery, and intrapartum procedures (artificial rupture of membranes (AROMs), fetal scalp electrodes) were all recorded from the antenatal charts and/or from the medical records. The VL closest to the date of birth was recorded. Duration of ROM was expressed as total length of time in minutes from time of ROM to time of birth. The proportion of women receiving intravenous ZDV, with vaginal birth or cesarean section, and with any invasive procedures was determined.
All children born to HIV-positive mothers were prescribed a 6-week course of oral zidovudine and referred for follow-up care in the pediatric HIV clinic, at the Hospital for Sick Children, in Toronto. The rate of MTCT was determined in collaboration with this clinic.
3. Results
During the study period, 213 HIV-positive women delivered at the participating centers. Of these, 3 were excluded due to a viral load > 1000 copies/m—therefore the final study cohort consisted of 210 women. During the 10-year study period, the number of HIV-positive pregnant women seeking care steadily increased over time.
Demographic characteristics are reported in Table 1. The majority of the group (135, 64%) was of African descent. Eighty (38%) women gave birth to their first child. A high rate (16%) of preterm birth, defined as delivery <37 weeks gestational age, was observed in our cohort. The average gestational age at birth was 38 weeks and 2 days with a range of 24 weeks and 6 days to 41 weeks and 3 days.
Table 1.
Optimally managed HIV-positive women who delivered between January 2000 and November 2010.
| Characteristic | |
|---|---|
| Mean age | 32 years (range 16–43) |
| Race | |
| African | 135 (64%) |
| Caucasian | 23 (11%) |
| Asian | 16 (8%) |
| Caribbean | 17 (8%) |
| Other | 11 (5%) |
| Missing | 8 (4%) |
| Average gravidity | 3 |
| Average parity | 1 |
| Gestational Age >37 weeks at delivery | 177 (84%) |
Of the 210 women, 200 had a recorded VL prior to delivery. The other 10 women had a VL recorded as “less than 1000 copies/mL”. Of those 200 patients with known VL, the majority of the group (n = 167, 84%) had an undetectable VL (less than 50 copies/mL) at the time of delivery. The highest viral load in this cohort was 706 copies/mL. The majority of women (n = 179, 85%) received adequate intrapartum ZDV, defined as having received a loading dose followed by 3 hours of maintenance infusion prior to delivery.
In the entire cohort, 107 women (51%) had a vaginal birth and 103 (49%) had a cesarean birth. Among women with cesarean birth, 75 (73%) were performed electively (prior to labor) and 28 (27%) were performed in labor. Among women with undetectable VL, 90 (54%) had a vaginal birth, and 77 (46%) had a cesarean birth.
In this cohort, 46 women had AROM. Of those, 20 women had dilation between 0 and 4 cm, 20 had greater than 4 cm but less than 10 cm, and 6 were ruptured just prior to delivery. Among the 107 women with a vaginal birth, six had a vacuum-assisted vaginal delivery and one had a fetal scalp electrode placed.
The median length of time of rupture of membranes for the entire cohort was 0.63 hours (0.00–77.87). The median length of time of rupture of membranes for the vaginal birth group was 2.56 hours (0.00–53.90) and cesarean birth group was 0.02 hours (0.00–77.87) (P < 0.0001). For those women with an undetectable VL, the median length of time of rupture of membranes was 0.62 hours (0.00–77.87) and for those with a detectable VL was 0.57 hours (0.00–33.63) (P > 0.92). When removing those who were ruptured less than 0.03 hours (elective cesareans, precipitous vaginal deliveries, etc.), there were 131 patients whose membranes were ruptured for 0.05 hours or longer. Their median length of time of rupture was 3.53 hours (0.05–77.87). In total, 59 (28%) women had rupture of membranes for 4 hours or longer. For women who were less than 37 weeks at the time of delivery, the median length of time of rupture was 0.63 hours (0.00–77.87), and 0.66 hours (0.00–53.90) for those greater than 37 weeks. The median lengths of time of rupture and gestational ages are shown in Figure 1.
Figure 1.
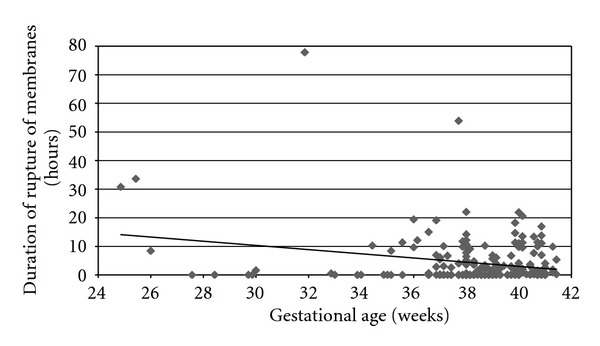
Gestational age and median lengths of time of rupture.
There were no cases of MTCT in this cohort of HIV-positive pregnant women.
4. Discussion
This study suggests that, in a group of 210 optimally managed HIV-positive women who gave birth over a 10-year period, increasing the duration of ROM did not increase the likelihood of MTCT. Since the use of HAART, little has been published specifically exploring the role of duration of ROM and MTCT in optimally managed women [11, 12].
In this cohort of women, the median length of time of ROM for the entire group was 0.63 hours with a range of 0 to 77.87 hours. A large proportion of the group was ruptured for minutes only, and when removing those women, the median increases to 3.53 hours, with a range from 0.05 hours to 77.87 hours. The median for the undetectable VL women was similar to the entire group, at 0.65 hours, which follows since the majority of the group had undetectable VL. It is clear that some women certainly had ROM longer than the previously recommended time limit of 4 hours [9, 10], but given that this recommendation is based on studies where women were not on HAART and maternal VL was not known, this recommendation may not apply to women who are optimally managed. In all of our subgroups, the range extends outside the 4-hour recommendation without increasing the association with MTCT.
Previous literature has stated that even in women with undetectable VL, elective cesarean birth reduces MTCT; however when adjusted for HAART, the effect was no longer significant [5]. In our study the median times of ROM for the vaginal (2.56 hours) and cesarean groups (0.02 hours) were statistically different, but neither group had a case of MTCT. This is consistent with previous literature that for women on HAART, mode of delivery does not influence risk of MTCT, even if the length of time of ROM increased.
The adherence rate for IV ZDV prior to delivery in this study was 85%, which is similar to rates in other studies [14]. All women who did not receive adequate IV ZDV prior to delivery either had precipitous deliveries or operative deliveries for emergency indications (cord prolapse, footling breech in labor). In the majority of cases, the ZDV was started on admission using preprinted orders, but delivery occurred before three hours of maintenance infusion could be completed.
Our cesarean birth rate was 49%, which is well above the national average of approximately 26% [15]. This may be related to several factors. First, the majority of the group was multiparous, accounting for two-thirds of the women who underwent cesarean. Many of these women chose to undergo a repeat cesarean, similar to HIV-negative women in Canada [14]. Second, some HIV-positive women choose to undergo an elective cesarean regardless of previous delivery status or VL [3]. A large proportion of women in this study originate from resource-poor countries where different strategies are employed, such as cesarean birth, to prevent MTCT [15].
In our cohort of HIV-positive women, the preterm birth rate was 16%, which is double the average preterm birth rate in Canada of 8%. This increased preterm birth rate has been observed in other studies of HIV-positive women on HAART [5, 16]. In previous work done at one of our institutions, the preterm delivery rate in a cohort of HIV-positive women was similar to the control group (in press). Possible reasons for the increased rate in the current cohort could include earlier induction of labor for abnormal liver function tests or preeclampsia, or spontaneous preterm labor. These factors could also be a contributor to the high cesarean birth rate observed in this study. More research is urgently required to further explore these relationships.
Preterm premature rupture of membranes (PPROMs) in optimally managed HIV-positive women poses a clinical dilemma for management when weighing the risk of prematurity over the risk of MTCT. Our study did not specifically address the issue of PPROM; however there was a small number of preterm patients who were ruptured for greater than the 4-hour recommendation without a case of MTCT. Overall though, the median lengths of time of rupture for both the preterm and term patients were similar. More studies with a larger number of optimally managed patients with PPROM are required to further explore this complex issue.
Traditionally, the use of invasive procedures during labor in HIV-positive women has been discouraged because of the potential for increasing MTCT [5–10]. In this study, AROM was employed for labor induction and during the active phase of labor in 46 women without increasing the likelihood of vertical transmission. One patient did have a fetal scalp electrode placed, although this is not typical practice even among optimally managed women and is not recommended.
During the study period of ten years, we observed a trend of an increasing number of deliveries. There are many possible reasons for this, including an increase in the proportion of women among HIV-positive persons, changing immigration patterns, and better long-term management of HIV resulting in better overall health and an increased desire for childbearing. Since the introduction of HAART in 1998, there has been a steady decline in antenatal monotherapy use in Ontario, with an increasing acceptance of and adherence to HAART regimens [14]. This also may have resulted in an increasing number of women who met inclusion criteria of antenatal HAART use and low VL over time. Given the number of cases from January to November 2010, the number for 2011 is projected to be similar to 2008 and 2009.
This study has several strengths. We had a large cohort of HIV-positive women managed by a small number of practitioners at only two geographic sites. This resulted in a cohort of optimally managed and virally suppressed women, with the majority having undetectable VL and all having VL less than 1,000 copies/mL. Physicians and nurses at these two sites have experience in the antenatal and intrapartum care of these women, which increases the likelihood of appropriate decision-making regarding medication use and labor management. Finally, complete follow-up of children born to women in this study allows us to evaluate MTCT rates over time. There are also some limitations. Due to the relatively small proportion of HIV-positive women delivering in Canada, our ability to examine larger cohorts of women is limited without employing a multicentre approach. The size of our cohort prevents us from determining small differences in transmission rate. To more accurately investigate the relationship of duration of ROM and MTCT of HIV in optimally managed women, a larger multicenter study would be required.
With no cases of MTCT, we were unable to evaluate patient-specific or labor and delivery characteristics predictive of transmission. However, we found no association with increasing duration of ROM or mode of delivery and MTCT. It is reassuring that, in the current era and among women with low VL, allowing length of time of ROM to increase beyond four hours does not appear to increase the risk of MTCT.
5. Conclusion
In a cohort of over 200 optimally managed HIV-positive women on HAART with low VL, there were no cases of MTCT. Increasing the duration of ROM did not increase the likelihood of MTCT. Further, MTCT was not related to mode of delivery. Allowing optimally managed women with low VL to continue in labor beyond four hours of ROM is acceptable. Additional studies with larger numbers of women are important to further evaluate the impact of duration of ROM and mode of delivery on MTCT among HIV-positive women in the current era.
References
- 1.Therapy Cohort Collaboration Antiretroviral. Life expectancy of individuals on combination antiretrovrial therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loutfy MR, Hart TA, Mohammed SS, et al. Fertility desires and intentions of HIV-positive women of reproductive age in Ontario, Canada: a cross-sectional study. PloS Nne. 2009;4(12) doi: 10.1371/journal.pone.0007925. Article ID e7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher M, Cohen H, Gruslin A, Money D, Steben M, Wong T. Mode of delivery for pregnant women infected by the human immunodeficiency virus. Journal of the Society of Obstetricians and Gynaecologists of Canada. 2001;23(4):348–350. [Google Scholar]
- 4.Committee Opinion ACOG. Scheduled cesarean delivery and the prevention of vertical transmission of HIV infection. The American College of Obstetricians and Gynecologists. 2000;(234) [Google Scholar]
- 5.Collaborative Study European. Mother-to-Child transmission of HIV infection in the era of highly active antiretroviral therapy. Clinical Infectious Diseases. 2005;40:458–465. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 6.International Perinatal HIV Group The. The mode of delivery and the risk of vertical transmission of human immunodificiency virus type 1. New England Journal of Medicine. 1999;340:977–987. doi: 10.1056/NEJM199904013401301. [DOI] [PubMed] [Google Scholar]
- 7.Mandelbrot L, Le Chenadec J, Berrebi A, et al. Perinatal HIV-1 transmission: Interaction between zidovudine prophylaxis and mode of delivery in the French perinatal cohort. Journal of the American Medical Association. 1998;280(1):55–60. doi: 10.1001/jama.280.1.55. [DOI] [PubMed] [Google Scholar]
- 8.Parazzini F, Ricci E, Di Cintio E, et al. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. Lancet. 1999;353(9158):1035–1039. doi: 10.1016/s0140-6736(98)08084-2. [DOI] [PubMed] [Google Scholar]
- 9.Minkoff H, Burns DN, Landesman S, et al. The relationship of the duration of ruptured membranes to vertical transmission of human immunodeficiency virus. American Journal of Obstetrics and Gynecology. 1995;173(2):585–589. doi: 10.1016/0002-9378(95)90286-4. [DOI] [PubMed] [Google Scholar]
- 10.Landesman S, Kalish L, Burns D, et al. Obstetrical factors and the transmission of human immunodeficiency virus type 1 from mother to child. The Women and Infants Transmission Study. New England Journal of Medicine. 1996;334(25):1617–1623. doi: 10.1056/NEJM199606203342501. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Tejedor A, Perales A, Maiques V. Duration of ruptured membranes and extended labor are risk factors for HIV transmission. International Journal of Gynecology and Obstetrics. 2003;82(1):17–23. doi: 10.1016/s0020-7292(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Tejedor A, Maiques V, Perales A, Lopez-Aldeguer J. Influence of Highly Active Antiretroviral Treatment (HAART) on risk factors for vertical HIV transmission. Acta Obstetricia et Gynecologica Scandinavica. 2009;88:882–887. doi: 10.1080/00016340903062836. [DOI] [PubMed] [Google Scholar]
- 13.Read JS, Newell MK. Efficacy and safety of cesarean delivery for prevention of mother-to-child transmission of HIV-1. Cochrane Database of Systematic Reviews. 2005;(4) doi: 10.1002/14651858.CD005479. Article ID CD005479. [DOI] [PubMed] [Google Scholar]
- 14.Gahir S, Anger GJ, Ibrahim M, Read S, Piquette-Miller M. Management of hiv positive pregnancies in ontario: Current status. Canadian Journal of Clinical Pharmacology. 2009;16(1):e68–e77. [PubMed] [Google Scholar]
- 15.Institute for Health Information Canadian. Giving Birth in Canada: Regional Trends from 2001-2002 to 2005-2006. July 2007, http://secure.cihi.ca/cihiweb/en/downloads/Childbirth_AiB_FINAL_E.pdf.
- 16.Rudin C, Spaenhauer A, Keiser O, et al. Antiretroviral therapy during pregnancy and premature birth: analysis of Swiss data. HIV Medicine. 2011;12(4):228–235. doi: 10.1111/j.1468-1293.2010.00876.x. [DOI] [PubMed] [Google Scholar]


