Abstract
Desulfurobacterium thermolithotrophum L'Haridon et al. 1998 is the type species of the genus Desulfurobacterium which belongs to the family Desulfurobacteriaceae. The species is of interest because it represents the first thermophilic bacterium that can act as a primary producer in the temperature range of 45-75 °C (optimum 70°C) and is incapable of growing under microaerophilic conditions. Strain BSAT preferentially synthesizes high-melting-point fatty acids (C18 and C20) which is hypothesized to be a strategy to ensure the functionality of the membrane at high growth temperatures. This is the second completed genome sequence of a member of the family Desulfurobacteriaceae and the first sequence from the genus Desulfurobacterium. The 1,541,968 bp long genome harbors 1,543 protein-coding and 51 RNA genes and is a part of the Genomic Encyclopedia of Bacteria and Archaea project.
Keywords: anaerobic, thermophilic, neutrophilic, obligately chemolithoautotrophic, Gram-negative, marine, sulfur-reducing, Desulfurobacteriaceae, GEBA
Introduction
Strain BSAT (= DSM 11699) is the type strain of the species Desulfurobacterium thermolithotrophum, which is the type species of its genus Desulfurobacterium [1], that currently consists of three validly named species [19]. The genus name is derived from the Latin words 'de' meaning 'from', 'sulfur', and 'bacterium' meaning 'a stick, staff', yielding the Neo-Latin word 'Desulfurobacterium' meaning 'sulfur-reducing rod-shaped bacterium' [1]. The species epithet is derived from the latinized Greek word 'thermê' meaning 'heat', the latinized Greek word 'lithos' meaning 'stone' and the latinized Greek word 'trophos' meaning 'feeder, rearer, one who feeds', yielding the Neo-Latin word 'thermolithotrophum' meaning 'referring to its thermophilic way of life and lithotrophic metabolism' [1,2]. Strain BSAT was collected from the Snake Pit vent field on the mid Atlantic Ridge with the help of the submersible Nautile at a depth of 3,500 m [1]. Although it shares most features with other members of the Aquificales, it is distinct in its inability to grow under microaerophilic conditions [1]. Strain BSAT was the first non-hyperthermophilic primary producer isolated from deep-sea vents [1]. Here we present a summary classification and a set of features for D. thermolithotrophum strain BSAT, together with the description of the complete genomic sequencing and annotation.
Classification and features
A representative genomic 16S rRNA sequence of D. thermolithotrophum BSAT was compared using NCBI BLAST [3,4] under default settings (e.g. considering only the high-scoring segment pairs (HSPs) from the best 250 hits) with the most recent release of the Greengenes database [5] and the relative frequencies of taxa and keywords (reduced to their stem [6] were determined, weighted by BLAST scores. The most frequently occurring genera were Desulfurobacterium (30.3%), Thermoanaerobacter (18.8%), Thermovibrio (14.2%), Balnearium (11.0%) and Persephonella (4.1%) (80 hits in total). Regarding the two hits to sequences from members of the species, the average identity within HSPs was 98.9%, whereas the average coverage by HSPs was 92.8%. Regarding the single hit to sequences from other members of the genus, the average identity within HSPs was 98.6%, whereas the average coverage by HSPs was 64.4%. Among all other species, the one yielding the highest score was “Desulfurobacterium crinifex” (AJ507320), which corresponded to an identity of 98.6% and HSP coverage of 64.4%. (Note that the Greengenes database uses the INSDC (= EMBL/NCBI/DDBJ) annotation, which is not an authoritative source for nomenclature or classification.) The highest-scoring environmental sequence was AF068800 ('hydrothermal vent clone VC2.1Bac24'), which showed an identity of 99.7% and an HSP coverage of 92.7%. The most frequently occurring keywords within the labels of all environmental samples which yielded hits were 'hydrotherm' (5.4%), 'vent' (4.9%), 'microbi' (3.6%), 'water' (2.9%) and 'deep' (2.0%) (167 hits in total). The most frequently occurring keyword within the labels of those environmental samples which yielded hits of a higher score than the highest scoring species was 'hydrotherm, vent' (50.0%) (1 hit in total).
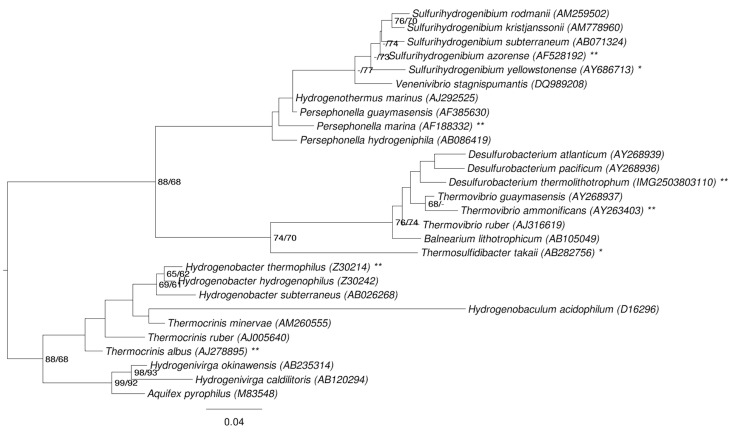
Figure 1 shows the phylogenetic neighborhood of D. thermolithotrophum BSAT in a 16S rRNA based tree. The sequences of the two identical 16S rRNA gene copies in the genome differ by two nucleotides from the previously published 16S rRNA sequence (AJ001049).
Figure 1.
Phylogenetic tree highlighting the position of D. thermolithotrophum relative to the type strains of the other species within the order Aquificales. The tree was inferred from 1,422 aligned characters [7,8] of the 16S rRNA gene sequence under the maximum likelihood (ML) criterion [9]. Rooting was done initially using the midpoint method [10] and then checked for its agreement with the current classification (Table 1). The branches are scaled in terms of the expected number of substitutions per site. Numbers adjacent to the branches are support values from 1,000 ML bootstrap replicates [11] (left) and from 1,000 maximum parsimony bootstrap replicates [12] (right) if larger than 60%. Lineages with type strain genome sequencing projects registered in GOLD [13] are labeled with one asterisk, those also listed as 'Complete and Published' with two asterisks (referenced in [14-17] and CP002444).
Table 1. Classification and general D. thermolithotrophum BSAT in accordance with the MIGS recommendations [18] and the NamesforLife database [19].
| MIGS ID | Property | Term | Evidence code |
|---|---|---|---|
| Current classification | Domain Bacteria | TAS [20] | |
| Phylum ‘Aquificae’ | TAS [22] | ||
| Class Aquificae | TAS [23,24] | ||
| Order Aquificales | TAS [23,25,26] | ||
| Family Desulfurobacteriaceae | TAS [25] | ||
| Genus Desulfurobacterium | TAS [1,25,27] | ||
| Species | Desulfurobacterium thermolithotrophum | TAS [1] | |
| MIGS-7 | Strain | BSAT | TAS [1] |
| MIGS-12 | Reference for biomaterial | DSM 11699 | TAS [1] |
| Gram stain | negative | TAS [1] | |
| Cell shape | rod-shaped | TAS [1] | |
| Motility | motile | TAS [1] | |
| Sporulation | non-sporulating | TAS [1] | |
| Temperature range | 40-75°C | TAS [1] | |
| Optimum temperature | 70°C | TAS [1] | |
| Salinity | 15 to 70 g per l, optimum at 35 g | TAS [1] | |
| MIGS-22 | Oxygen requirement | strictly anaerobic | TAS [1] |
| Carbon source | CO2 | NAS | |
| Energy metabolism | chemolitoautotrophic, sulfur reduction | TAS [1] | |
| MIGS-6 | Habitat | marine | TAS [1] |
| MIGS-15 | Biotic relationship | free-living | TAS [1] |
| Biosafety level | 1 | TAS [28] | |
| MIGS-19 | Trophic level | level 1 primary producer | TAS [1] |
| MIGS-23.1 | Isolation | deep-sea hydrothermal vent chimney | TAS [1] |
| MIGS-4 | Geographic location | Snake Pit vent field, Mid-Atlantic Ridge | TAS [1] |
| MIGS-5 | Sample collection time | November/December 1995 | TAS [1,29] |
| MIGS-4.1 | Latitude | 23.36 | TAS [1,29] |
| MIGS-4.2 | Longitude | -44.93 | TAS [1,29] |
| MIGS-4.3 | Depth | 3,500 m | TAS [1,29] |
| MIGS-4.4 | Altitude | -3,500 m | TAS [1] |
Evidence codes - TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). These evidence codes are from of the Gene Ontology project [30].
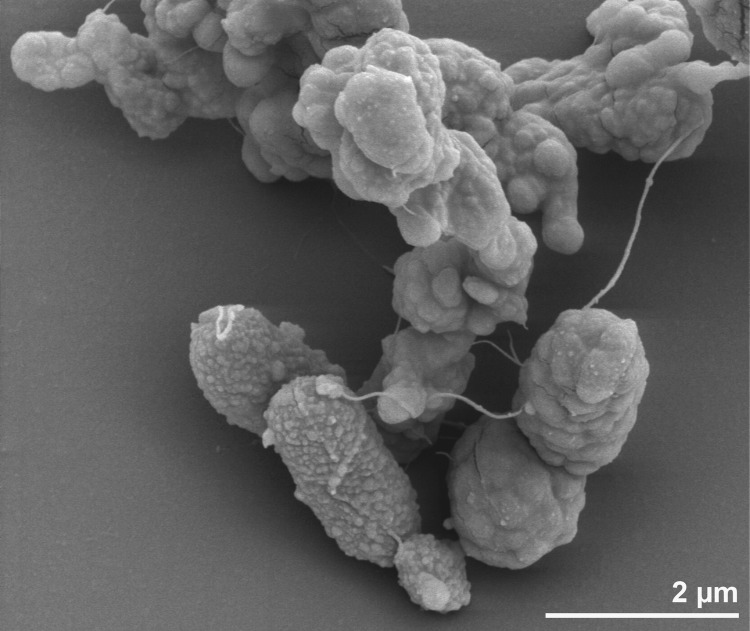
The cells of strain BSAT are small rods, about 1-2 µm long and 0.4-0-5 µm wide and occur singly or in pairs (Figure 2) [1]. Cells stain Gram-negative and are motile via three polar flagella; spores are not produced [1]. Strain BSAT grows between 40 and 75°C with an optimum around 70°C, while no growth is detected at 37 or 80°C after 48 h incubation [1]. Growth occurs between pH 4.4 and 8, with an optimum around pH 6.25. No growth is detected at pH 3.7 or 8.5 after 48h incubation at 70°C [1]. Growth is observed in sea salts at concentrations ranging from 15 to 70g/l, with an optimum of approximately 35g/l (corresponding to 23 g NaCl/l [1]). No growth was observed in sea salts at concentrations of 10 and 80 g/l after 48 h incubation at 70°C [1]. Under optimal growth conditions (temperature, pH and NaCl), the doubling time of strain BSAT is around 135 min [1]. Strain BSAT is a strictly anaerobic chemolithotrophic organism that uses sulfur as an electron acceptor in the presence of H+ for growth [1]. It utilizes thiosulfate, sulfite and polysulfides as alternative electron acceptors with H2 as an electron donor. Cysteine, nitrate or nitrite are not utilized and growth on sulfur, thiosulfate, polysulfides or sulfite was accompanied by exponential H2S production [1]. No growth was observed on acetate, formate, methanol, monomethylamine and yeast extract with N2-CO2 or H2 atmosphere in the presence or absence of sulfur [1]. Nitrate, tryptone and yeast extract were used as nitrogen sources [1]. Growth of strain BSAT was inhibited by chloramphenicol, penicillin G and rifampicin at 100 µg/ml but not by streptomycin when added before incubation at the optimum temperature [1].
Figure 2.
Scanning electron micrograph of D. thermolithotrophum BSAT
Chemotaxonomy
The total lipid content of strain BSAT is about 6% of the total dry weight and is characterized by the presence of aminophospholipids and a phospholipid at about 66%, Rf 0.7 and 30%, R f 0.5, respectively, as well as minor compounds [1]. Gas chromatographic analysis of fatty acid components of both compounds revealed the presence of saturated and monounsaturated acyl chains [1]. The phosphoinositol contains C16:0 (15%), C18:1 (41%) identified as methyl-oleate, and C18:0 (44%) identified as stearate. The phosphoamino-positive compounds contained C16:0 (14%), C18:1 (43%), C18:0 (31%) and C20:0 (12%), as well as minor compounds [1].
Genome sequencing and annotation
Genome project history
This organism was selected for sequencing on the basis of its phylogenetic position [31], and is part of the Genomic Encyclopedia of Bacteria and Archaea project [32]. The genome project is deposited in the Genomes On Line Database [13] and the complete genome sequence is deposited in GenBank. Sequencing, finishing and annotation were performed by the DOE Joint Genome Institute (JGI). A summary of the project information is shown in Table 2.
Table 2. Genome sequencing project information.
| MIGS ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | Finished |
| MIGS-28 | Libraries used | Four genomic libraries: one 454 pyrosequence standard library, two 454 PE library (10.5 kb insert size), one Illumina library |
| MIGS-29 | Sequencing platforms | Illumina GAii, 454 GS FLX Titanium |
| MIGS-31.2 | Sequencing coverage | 282.0 × Illumina; 40.0 x pyrosequence |
| MIGS-30 | Assemblers | Newbler version 2.3, p Velvet version 0.7.63, phrap version SPS - 4.24 |
| MIGS-32 | Gene calling method | Prodigal 1.4, GenePRIMP |
| INSDC ID | CP002543 | |
| Genbank Date of Release | March 2, 2011 | |
| GOLD ID | Gc01671 | |
| NCBI project ID | 51497 | |
| Database: IMG-GEBA | 2503754020 | |
| MIGS-13 | Source material identifier | DSM 11699 |
| Project relevance | Tree of Life, GEBA |
Growth conditions and DNA isolation
D. thermolithotrophum strain BSAT, DSM 11699, was grown anaerobically in DSMZ medium 829 (Desulfurobacterium medium) [34] at 70°C. DNA was isolated from 0.5-1 g of cell paste using Qiagen Genomic 500 DNA Kit (Qiagen 10262) following the standard protocol as recommended by the manufacturer without modifications. DNA is available through the DNA Bank Network [35].
Genome sequencing and assembly
The genome was sequenced using a combination of Illumina and 454 sequencing platforms. All general aspects of library construction and sequencing can be found at the JGI website [36]. Pyrosequencing reads were assembled using the Newbler assembler (Roche). The initial Newbler assembly consisting of 96 contigs in one scaffold was converted into a phrap [37] assembly by making fake reads from the consensus, to collect the read pairs in the 454 paired end library. Illumina GAii sequencing data (45.0 Mb) was assembled with Velvet [38] and the consensus sequences were shredded into 1.5 kb overlapped fake reads and assembled together with the 454 data. The 454 draft assembly was based on 192.1 Mb 454 draft data and all of the 454 paired end data. Newbler parameters are -consed -a 50 -l 350 -g -m -ml 20. The Phred/Phrap/Consed software package [37] was used for sequence assembly and quality assessment in the subsequent finishing process. After the shotgun stage, reads were assembled with parallel phrap (High Performance Software, LLC). Possible mis-assemblies were corrected with gapResolution [36], Dupfinisher [39], or sequencing cloned bridging PCR fragments with subcloning. Gaps between contigs were closed by editing in Consed, by PCR and by Bubble PCR primer walks (J.-F. Chang, unpublished). A total of 101 additional reactions were necessary to close gaps and to raise the quality of the finished sequence. Illumina reads were also used to correct potential base errors and increase consensus quality using a software Polisher developed at JGI [40]. The error rate of the completed genome sequence is less than 1 in 100,000. Together, the combination of the Illumina and 454 sequencing platforms provided 322.0 × coverage of the genome. The final assembly contained 126,482 pyrosequence and 12,545,740 Illumina reads.
Genome annotation
Genes were identified using Prodigal [41] as part of the Oak Ridge National Laboratory genome annotation pipeline, followed by a round of manual curation using the JGI GenePRIMP pipeline [42]. The predicted CDSs were translated and used to search the National Center for Biotechnology Information (NCBI) nonredundant database, UniProt, TIGR-Fam, Pfam, PRIAM, KEGG, COG, and InterPro databases. Additional gene prediction analysis and functional annotation was performed within the Integrated Microbial Genomes - Expert Review (IMG-ER) platform [33].
Genome properties
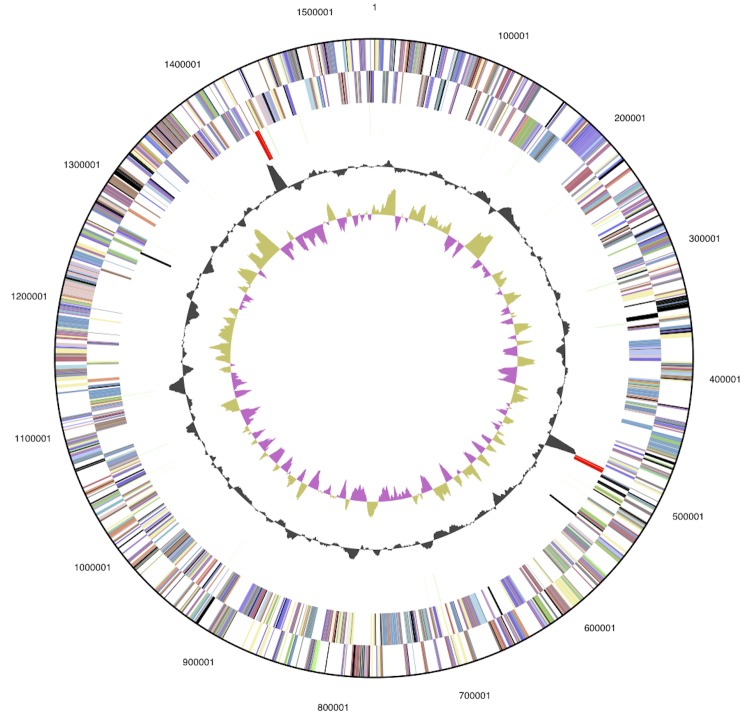
The genome consists of one circular chromosome with a total length of 1,541,968 bp and a G+C content of 35.0% (Table 3 and Figure 3). Of the 1,594 genes predicted, 1,543 were protein-coding genes, and 51 RNAs; 34 pseudogenes were also identified. The majority of the protein-coding genes (75.5%) were assigned a putative function while the remaining ones were annotated as hypothetical proteins. The distribution of genes into COGs functional categories is presented in Table 4.
Table 3. Genome Statistics.
| Attribute | Value | % of Total |
|---|---|---|
| Genome size (bp) | 1,541,968 | 100.00% |
| DNA coding region (bp) | 1,448,295 | 93.93% |
| DNA G+C content (bp) | 538,896 | 34.95% |
| Number of replicons | 1 | |
| Extrachromosomal elements | 0 | |
| Total genes | 1,594 | 100.00% |
| RNA genes | 51 | 3.20% |
| rRNA operons | 2 | |
| tRNA genes | 43 | 2.70% |
| Protein-coding genes | 1,543 | 96.80% |
| Pseudo genes | 34 | 2.13% |
| Genes with function prediction | 1,204 | 75.53% |
| Genes in paralog clusters | 600 | 37.64% |
| Genes assigned to COGs | 1,330 | 83.44% |
| Genes assigned Pfam domains | 1,327 | 83.25% |
| Genes with signal peptides | 394 | 24.72% |
| Genes with transmembrane helices | 322 | 20.20% |
| CRISPR repeats | 1 |
Figure 3.
Graphical circular map of the genome. From bottom to top: Genes on forward strand (color by COG categories), Genes on reverse strand (color by COG categories), RNA genes (tRNAs green, rRNAs red, other RNAs black), GC content, GC skew.
Table 4. Number of genes associated with the general COG functional categories.
| Code | value | %age | Description |
|---|---|---|---|
| J | 142 | 9.7 | Translation, ribosomal structure and biogenesis |
| A | 0 | 0.0 | RNA processing and modification |
| K | 47 | 3.2 | Transcription |
| L | 126 | 8.6 | Replication, recombination and repair |
| B | 1 | 0.1 | Chromatin structure and dynamics |
| D | 20 | 1.4 | Cell cycle control, cell division, chromosome partitioning |
| Y | 0 | 0.0 | Nuclear structure |
| V | 13 | 0.9 | Defense mechanisms |
| T | 52 | 3.6 | Signal transduction mechanisms |
| M | 110 | 7.5 | Cell wall/membrane/envelope biogenesis |
| N | 67 | 4.6 | Cell motility |
| Z | 0 | 0.0 | Cytoskeleton |
| W | 0 | 0.0 | Extracellular structures |
| U | 74 | 5.1 | Intracellular trafficking, secretion, and vesicular transport |
| O | 55 | 3.8 | Posttranslational modification, protein turnover, chaperones |
| C | 113 | 7.7 | Energy production and conversion |
| G | 42 | 2.9 | Carbohydrate transport and metabolism |
| E | 108 | 7.4 | Amino acid transport and metabolism |
| F | 57 | 3.9 | Nucleotide transport and metabolism |
| H | 88 | 6.0 | Coenzyme transport and metabolism |
| I | 38 | 2.6 | Lipid transport and metabolism |
| P | 57 | 3.9 | Inorganic ion transport and metabolism |
| Q | 13 | 0.9 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 140 | 9.6 | General function prediction only |
| S | 97 | 6.6 | Function unknown |
| - | 264 | 16.6 | Not in COGs |
Acknowledgements
We would like to gratefully acknowledge the help of Thomas Hader (University of Regensburg) for growing D. thermolithotrophum cultures. This work was performed under the auspices of the US Department of Energy's Office of Science, Biological and Environmental Research Program, and by the University of California, Lawrence Berkeley National Laboratory under contract No. DE-AC02-05CH11231, Lawrence Livermore National Laboratory under Contract No. DE-AC52-07NA27344, and Los Alamos National Laboratory under contract No. DE-AC02-06NA25396, UT-Battelle and Oak Ridge National Laboratory under contract DE-AC05-00OR22725, as well as German Research Foundation (DFG) INST 599/1-2.
References
- 1.L'Haridon S, Cilia V, Messner P, Raguénès G, Gambacorta A, Sleytr UB, Prieur D, Jeanthon C. Desulfurobacterium thermolithotrophum gen. nov., sp. nov., a novel autotrophic, sulphur-reducing bacterium isolated from a deep-sea hydrothermal vent. Int J Syst Bacteriol 1998; 48:701-711 10.1099/00207713-48-3-701 [DOI] [PubMed] [Google Scholar]
- 2.List of Changes in Taxonomic Opinion N° 2. Int J Syst Evol Microbiol 2005; 55:1403-1404 [DOI] [PubMed] [Google Scholar]
- 3.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990; 215:403-410 [DOI] [PubMed] [Google Scholar]
- 4.Korf I, Yandell M, Bedell J. BLAST, O'Reilly, Sebastopol, 2003. [Google Scholar]
- 5.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl Environ Microbiol 2006; 72:5069-5072 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter MF. An algorithm for suffix stripping Program: electronic library and information systems 1980; 14:130-137.
- 7.Lee C, Grasso C, Sharlow MF. Multiple sequence alignment using partial order graphs. Bioinformatics 2002; 18:452-464 10.1093/bioinformatics/18.3.452 [DOI] [PubMed] [Google Scholar]
- 8.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 2000; 17:540-552 [DOI] [PubMed] [Google Scholar]
- 9.Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web-servers. Syst Biol 2008; 57:758-771 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- 10.Hess PN, De Moraes Russo CA. An empirical test of the midpoint rooting method. Biol J Linn Soc Lond 2007; 92:669-674 10.1111/j.1095-8312.2007.00864.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pattengale ND, Alipour M, Bininda-Emonds OR, Moret BM, Stamatakis A. How many bootstrap replicatesa necessary? J Comput Biol 2010; 17:337-354 10.1007/978-3-642-02008-7_13 [DOI] [PubMed] [Google Scholar]
- 12.Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4.0 b10. Sinauer Associates, Sunderland, 2002. [Google Scholar]
- 13.Liolios K, Chen IM, Mavromatis K, Tavernarakis N, Hugenholtz P, Markowitz VM, Kyrpides NC. The Genomes On Line Database (GOLD) in 2009: status of genomic and metagenomic projects and their associated metadata. Nucleic Acids Res 2010; 38:D346-D354 10.1093/nar/gkp848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reysenbach AL, Hamamura N, Podar M, Griffiths E, Ferreira S, Hochstein R, Heidelberg J, Johnson J, Mead D, Pohorille A, et al. Complete and draft genome sequences of six members of the Aquificales. J Bacteriol 2009; 191:1992-1993 10.1128/JB.01645-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeytun A, Sikorski J, Nolan M, Lapidus A, Lucas S, Han J, Tice H, Cheng JF, Tapia R, Goodwin L, et al. Complete genome sequence of Hydrogenobacter thermophilus type strain (TK-6T). Stand Genomic Sci 2011; 4:131-143 10.4056/sigs.1463589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arai H, Kanbe H, Ishii M, Igarashi Y. Complete genome sequence of the thermophilic, obligately chemolithoautotrophic hydrogen-oxidizing bacterium Hydrogenobacter thermophilus TK-6. J Bacteriol 2010; 192:2651-2652 10.1128/JB.00158-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirth R, Sikorski J, Brambilla E, Misra M, Lapidus A, Copeland A, Nolan M, Lucas S, Chen F, Tice H, et al. Complete genome sequence of Thermocrinis albus type strain (CDC 1076T). Stand Genomic Sci 2010; 2:194-202 10.4056/sigs.761490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, Tatusova T, Thomson N, Allen MJ, Angiuoli SV, et al. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol 2008; 26:541-547 10.1038/nbt1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrity G. NamesforLife. BrowserTool takes expertise out of the database and puts it right in the browser. Microbiol Today 2010; 37:9 [Google Scholar]
- 20.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 1990; 87:4576-4579 10.1073/pnas.87.12.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrity GM, Holt JG. The Road Map to the Manual. In: Garrity GM, Boone DR, Castenholz RW (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 1, Springer, New York, 2001, p. 119-169. [Google Scholar]
- 22.Reysenbach AL. Phylum BI. Aquificae. In: Garrity GM, Boone DR, Castenholz RW (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 1, Springer, New York, 2001, p. 359-367. [Google Scholar]
- 23.List Editor Validation List no. 85. Validation of publication of new names and new combinations previously effectively published outside the IJSEM. Int J Syst Evol Microbiol 2002; 52:685-690 10.1099/ijs.0.02358-0 [DOI] [PubMed] [Google Scholar]
- 24.Reysenbach AL. Class I. Aquificae class. nov. In: Garrity GM, Boone DR, Castenholz RW (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 1, Springer, New York, 2001, p. 359. [Google Scholar]
- 25.L'Haridon S, Reysenbach AL, Tindall BJ, Schönheit P, Banta A, Johnsen U, Schumann P, Gambacorta A, Stackebrandt E, Jeanthon C. Desulfurobacterium atlanticum sp. nov., Desulfurobacterium pacificum sp. nov. and Thermovibrio guaymasensis sp. nov., three thermophilic members of the Desulfurobacteriaceae fam. nov., a deep branching lineage within the Bacteria. Int J Syst Evol Microbiol 2006; 56:2843-2852 10.1099/ijs.0.63994-0 [DOI] [PubMed] [Google Scholar]
- 26.Reysenbach AL. Order I. Aquificales ord. nov. In: Garrity GM, Boone DR, Castenholz RW (eds), Bergey's Manual of Systematic Bacteriology, Second Edition, Volume 1, Springer, New York, 2001, p. 359. [Google Scholar]
- 27.Alain K, Rolland S, Crassous P, Lesongeur F, Zbinden M, le Gall C, Godfroy A, Page A, Juniper SK, Cambon-Bonavita MA, et al. Desulfurobacterium crinifex sp. nov., a novel thermophilic, pinkish-streamer forming, chemolithoauto-trophic bacterium isolated from a Juan de Fuca Ridge hydrothermal vent and amendment of the genus Desulfurobacterium. Extremophiles 2003; 7:361-370 10.1007/s00792-003-0329-4 [DOI] [PubMed] [Google Scholar]
- 28.BAuA. 2010, Classification of Bacteria and Archaea in risk groups. http://www.baua.de TRBA 466, p. 77.
- 29.Harmsen HJM, Prieur D, Jeanthon C. Distribution of microorganisms in deep-sea hydrothermal vent chimneys investigated by whole-cell hybridization and enrichments of thermophilic subpopulations. Appl Environ Microbiol 1997; 63:2876-2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene Ontology: tool for the unification of biology. Nat Genet 2000; 25:25-29 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klenk HP, Göker M. En route to a genome-based classification of Archaea and Bacteria? Syst Appl Microbiol 2010; 33:175-182 10.1016/j.syapm.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 32.Wu D, Hugenholtz P, Mavromatis K, Pukall R, Dalin E, Ivanova NN, Kunin V, Goodwin L, Wu M, Tindall BJ, et al. A phylogeny-driven genomic encyclopaedia of Bacteria and Archaea. Nature 2009; 462:1056-1060 10.1038/nature08656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Markowitz VM, Ivanova NN, Chen IMA, Chu K, Kyrpides NC. IMG ER: a system for microbial genome annotation expert review and curation. Bioinformatics 2009; 25:2271-2278 10.1093/bioinformatics/btp393 [DOI] [PubMed] [Google Scholar]
- 34.List of growth media used at DSMZ: http//www.dsmz.de/catalogues/catalogue-microorganisms/culture-technology/list-of-media-for-microorganisms.html
- 35.Gemeinholzer B, Dröge G, Zetzsche H, Haszprunar G, Klenk HP, Güntsch A, Berendsohn WG, Wägele JW. The DNA Bank Network: the start from a German initiative. Biopreserv Biobank 2011; 9:51-55 10.1089/bio.2010.0029 [DOI] [PubMed] [Google Scholar]
- 36.JGI website. http://www.jgi.doe.gov/
- 37.The Phred/Phrap/Consed software package. http://www.phrap.com
- 38.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res 2008; 18:821-829 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han C, Chain P. Finishing repeat regions automatically with Dupfinisher. In: Proceeding of the 2006 international conference on bioinformatics & computational biology. Arabnia HR, Valafar H (eds), CSREA Press. June 26-29, 2006: 141-146. [Google Scholar]
- 40.Lapidus A, LaButti K, Foster B, Lowry S, Trong S, Goltsman E. POLISHER: An effective tool for using ultra short reads in microbial genome assembly and finishing. AGBT, Marco Island, FL, 2008 [Google Scholar]
- 41.Hyatt D, Chen GL, LoCascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 2010; 11:119 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pati A, Ivanova NN, Mikhailova N, Ovchinnikova G, Hooper SD, Lykidis A, Kyrpides NC. GenePRIMP: a gene prediction improvement pipeline for prokaryotic genomes. Nat Methods 2010; 7:455-457 10.1038/nmeth.1457 [DOI] [PubMed] [Google Scholar]