Abstract
Interactions between CXCL12 and its receptors CXCR4 or CXCR7 are involved in tumor growth and metastasis in various types of human cancer. However, CXCL12 expression and its role in lung cancer are not fully elucidated. Here we examined the expression of CXCL12 in 54 lung cancer cell lines consisting of 23 small cell lung cancers (SCLCs) and 31 non-small cell lung cancers (NSCLCs). CXCL12 was overexpressed in lung cancer cell lines compared to non-malignant human bronchial epithelial cell lines (N = 6). CXCL12 expression was positively but weakly correlated with the expression of CXCR4 or CXCR7. We also examined CXCL12 expression in 89 NSCLC specimens and found that CXCL12 expression was significantly higher in tumor specimens from female patients, non-smokers and adenocarcinoma patients. Small interfering RNAs targeting CXCL12 inhibited cellular proliferation, colony formation and migration of CXCL12-overexpressing lung cancer cells; however, this inhibition did not occur in lung cancer cells that lacked CXCL12. Furthermore, the anti-CXCL12 neutralizing antibody mediated inhibitory effects in three lung cancer cell lines that overexpressed CXCL12, but not in two CXCL12 non-expressing lung cancer cell lines nor two non-malignant bronchial epithelial cell lines. The present study demonstrates that: CXCL12 is concomitantly overexpressed with CXCR4 or CXCR7 in lung cancers; CXCL12 is highly expressed in NSCLCs from females, non-smokers and adenocarcinoma patients; and disruption of CXCL12 inhibits the growth and migration of lung cancer cells. Our findings indicate that CXCL12 is required for tumor growth and provide a rationale for the anti-CXCL12 treatment strategy in lung cancer.
Keywords: CXCL12, CXCR4, CXCR7, overexpression, lung cancer
Lung cancer is the leading cause of cancer-related death in the U.S. (1) and worldwide. Lung cancer is divided into two major histological types: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). Despite improvements in therapy, most patients with lung cancer will die, in most cases from metastatic disease (2). Accordingly, there is an urgent need for the identification of novel therapeutic targets for lung cancer.
Chemokines, which are structurally-related, small (8-14kDa) polypeptide signaling molecules, bind to and activate seven-transmembrane G-protein-coupled chemokine receptors (3). Chemokines are expressed by many tumor types and play an important role in the autocrine or paracrine regulation of tumor growth and metastasis (4). Chemokine (C-X-C motif) ligand 12 (CXCL12)/stromal cell-derived factor 1 (SDF-1), a 10-kDa secreted protein, is a homeostatic chemokine that signals through chemokine (C-X-C motif) receptor 4 (CXCR4), a G protein-coupled receptor, which in turn plays a role in hematopoiesis, development, and organization of the immune system (5). Interaction between CXCL12 and CXCR4 is involved in cell proliferation, migration, adhesion and angiogenesis in human cancers of the breast, lung, ovary and pancreas and neuroblastoma, hepatic cell carcinoma and malignant mesothelioma (6-15). Recently, CXCR7/RDC1 was the second receptor for CXCL12 (16) to be identified, and it may function in regulating tumor growth (17-18). However, the expression of CXCL12 and its role in the development of lung cancer have not been fully elucidated.
In the present study, we demonstrated that CXCL12 was overexpressed in lung cancer cell lines as compared to non-malignant bronchial epithelial cells and the expression was positively but weakly correlated with the expression of CXCR4 or CXCR7, suggesting the concomitant expression of CXCL12 with CXCR4 or CXCR7. A clinicopathological analysis of the CXCL12 expression in primary NSCLC tumor specimens revealed that CXCL12 was highly expressed in NSCLCs from female patients, non-smokers and adenocarcinoma patients. Disruption of CXCL12 mediated by small interfering RNAs (siRNAs) or an anti-CXCL12 neutralizing antibody inhibited the in vitro growth and migration of CXCL12-overexpressing lung cancer cells. These results indicate that CXCL12 is required for tumor growth and that the anti-CXCL12 treatment strategy could be effective for lung cancer.
MATERIALS AND METHODS
Cell lines and specimens
We used 23 SCLC cell lines (NCI-H187, -H209, -H345, -H378, -H524, -H526, -H740, -H865, -H889, -HI045, -HI092, -HI184, -H1238, -H1339, -H1607, -H1618, -H1672, -H1963, -H2141, -H2171, -H2227, HCC33 and N417) and 31 NSCLC cell lines (NCI-H23, -H157, -H322, -H358, -H441, -H460, -H520, -H661, -H838, -H1264, -H1299, -H1395, -H1437, -HI648, -HI666, -H1792, -H1819, -H2009, -H2077, -H2087, -H2122, -H2126, -H3255, HCC15, HCC44, HCC78, HCC95, HCC193, HCC515, HCC827 and A427) that were obtained from ATCC or the Hamon Center collection (University of Texas Southwestern Medical Center) (19). Normal human bronchial epithelial cells (NHBE), small-airway epithelial (SAEC) cells and immortalized human bronchial epithelial cells (BEAS-2B, HBEC1, HBEC3 and HBEC4) were used as non-tumor lung controls. NHBE and SAEC were obtained from Clonetics (San Diego, CA), and BEAS-2B was obtained from ATCC. We previously generated the HBEC1, HBEC3, and HBEC4 cell lines (20). Cancer cells were cultured in RPMI 1640 with 5% fetal bovine serum, and human bronchial epithelial cells were cultured in Keratinocyte-SFM (Invitrogen, Carlsbad, CA) medium containing 25 μg/mL bovine pituitary extract (Invitrogen) and 5 ng/mL epidermal growth factor (Invitrogen).
Tumors were obtained from 89 patients (45 men and 44 women) with primary NSCLC cancer who underwent surgery between July 2003 and May 2008 at the Gunma University School of Medicine Hospital (Gunma, Japan). A history of cigarette smoking was obtained from patient interviews, and non-smokers were defined as patients who had smoked <100 cigarettes during their lifetime. Of 89 patients, 48 were smokers and 41 were non-smokers. Tumors were histologically classified as adenocarcinomas (N = 77) or squamous cell carcinomas (N = 12), according to the criteria of the World Health Organization. We classified the postsurgical pathologic stage as stage I in 57 tumors (IA, 38; IB, 19), stage II in 11 tumors (IIA, 6; IIB, 5) and stage III/IV in 21 tumors (IIIA, 16; IIIB, 4; IV, 1), according to the current tumor-node-metastasis classification. Normal lung specimens (N = 8) obtained from 8 patients were used as normal controls. The study protocol was approved by the institutional review board. The specimens were immediately frozen after collection and kept at −80°C until mRNA extraction was performed.
Quantitative real-time RT-PCR
The expression of the CXCL12, CXCR4 and CXCR7 genes was examined by quantitative realtime RT-PCR as previously described (21). Briefly, total RNA was extracted by using the RNeasy mini kit (Qiagen, Valencia, CA), and cDNA was synthesized by using 2 μg of total RNA with the SuperScript II First-Strand Synthesis using oligo (dT) primer system (Invitrogen) according to the manufacturer’s instructions. Primers and probes for CXCL12 (Assay ID: Hs00171022_ml), CXCR4 (Assay ID: Hs00237052_ml) and CXCR7 (Assay ID: Hs00604567_ml) were purchased from Applied Biosystems (Tokyo, Japan). For the quantitative analysis, the TBP gene was used as an internal reference gene to normalize input cDNA as described (21). PCR was performed in a reaction volume of 20 μl, including 2 μl of cDNA by using the Gene Amp 7700 Sequence Detection System and software (Applied Biosystems). The comparative Ct method was used to compute relative expression values.
Immunohistochemical analysis
Tumor specimens were stained with an anti-CXCL12 mouse monoclonal antibody (MAB 350 R&D systems Inc, MN, USA) using 4 μm sections cut from paraffin blocks. Sections were incubated with the primary antibody targeting CXCL12 overnight at 4°C. After washing with PBS, the sections were incubated with the secondary antibody, a biotinylated anti-mouse antibody from the Vecstain® ABC kit (Vector Laboratories, CA, USA), and color was developed according to the manufacturer’s instructions.
Preparation and transfection of synthetic small interfering RNA (siRNA)
Two siRNAs that target different sites on CXCL12 mRNA were purchased from Dharmacon Inc (Lafayette, CO). A siRNA against Tax (the human leukemia virus gene) was used as a negative control (22). siRNAs were transfected into cells by using Oligofectamine transfection reagent (Invitrogen), according to a previously described method (22). After 72 h, the cells were harvested for further analysis.
MTT assay
Cell viability was measured by using the 3-(4,5 dimethylthiazol-2yl)-2,5-diphenyl-tetrazolium bromide (MTT) Cell Growth Assay Kit (Chemicon International, Temecula, CA), according to the manufacturer’s protocol. Twenty-four hours after transfection with siRNAs or treatment with antibody, trypan blue-negative viable cells were re-plated and cultured in 96-well plates in replicates of 8. After 72 h, cells were then incubated with 0.5 mg/ml MTT for 4 h at 37°C. After MTT withdrawal, the resulting blue formazan cristae were solubilized, and absorbance was read at 570/630 nm with a microtiter plate reader. For the CXCL12 neutralizing assay, cells were treated with 10 μg/ml of the anti-human CXCL12/SDF-1 antibody (R&D Systems, Minneapolis, MN) or 10 μg/ml of the IgGl isotype control antibody (R&D Systems), and the MTT assay was performed after 72 h.
Colony formation assay
The in vitro growth characteristics were tested by a colony formation assay (22). Briefly, after 48 h of siRNA transfection or treatment with antibodies, cells were harvested, and 1000 trypan blue-negative viable cells were re-plated in each well of 6-well plates. The cells were cultured in RPMI 1640 supplemented with 5% serum, and surviving colonies were counted 14 days later after staining with methylene blue.
Migration assay
Cell migration was measured in a modified Boyden chamber, as previously described (23). Briefly, polycarbonate filters with 8 μm pores (Neuroprobe, Gaithersburg, MD) were coated with 100 μ/ml of collagen (Elastin Products Company, Owensville, MO) in 0.5 M acetic acid for 16 h. The coated filter was then placed on a 12-blind-well chemotaxis chamber (Neuroprobe), and cells (105 cells in 100 μl per well) were loaded into the upper wells. The cells were incubated for 15 min before being loaded. After incubation at 37°C in 5% CO2 for 4 h, the filter was disassembled. The upper side of the filter was then scraped free of cells. The cells on the lower side of the filter were fixed with methanol and stained with a Diff-Quick staining kit (International Reagent, Kobe, Japan). The number of cells that migrated to the lower side of the filter was counted.
Statistical Analysis
An unpaired t test with Welch’s correction was used for comparison between two groups, and ANOVA with Bonferroni’s post-hoc test was used for comparison between ≥3 groups. The correlation was analyzed by using the non-parametric Spearman’s rank test. In NSCLC tumor specimens, the Mann-Whitney test was used for comparison between two groups. Progression-free survival time was defined as the time from tumor resection to the first observation of tumor recurrence, and overall survival time was defined as the time to death from any cause. Progression-free survival and overall survival were analyzed by the Kaplan-Meier method and were compared between groups by the log-rank test. Statistical analysis was performed by using the GraphPad Prism version 5.0 software program for Windows (GraphPad Software, San Diego, CA). Probability values less than 0.05 were considered statistically significant.
RESULTS
Expression of CXCL12, CXCR4 and CXCR7 in lung cancer cell lines
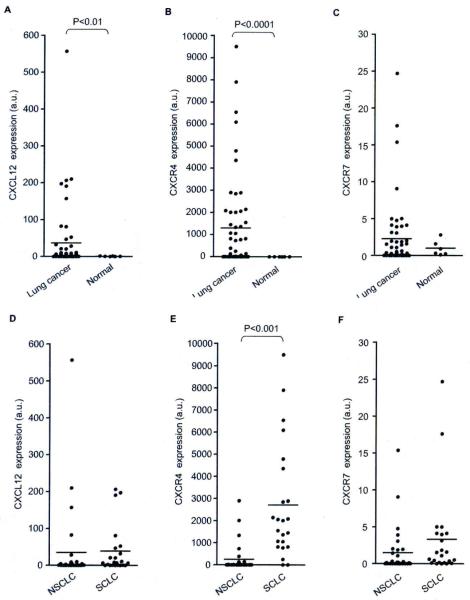
The expression of CXCL12, CXCR4 and CXCR7 mRNA in 54 lung cancer cell lines (23 SCLCs and 31 NSCLCs) was examined by quantitative real-time RT-PCR. The expression levels of CXCL12 (Fig. 1A) and CXCR4 (Fig. 1B) were significantly higher in lung cancer cells than in non-malignant bronchial epithelial cells (P<0.01 for CXCL12, and P<0.000l for CXCR4). In 41% of lung cancer cell lines (22 of 54 lines), CXCR7 expression levels were higher than the mean levels of the non-tumor controls, however, the difference was not statistically significant (Fig. 1C). The CXCR4 expression level was significantly higher in SCLCs than NSCLCs (Fig. 1E; P<0.001), whereas there was no significant difference in the expression of CXCL12 (Fig. 1D) or CXCR7 (Fig. 1F) between SCLCs and NSCLCs. Thus, CXCL12, CXCR4 and CXCR7 were overexpressed in lung cancer cell lines, and CXCR4 expression was relatively abundant in SCLCs as compared to NSCLCs.
Fig. 1.
Comparison of the mRNA levels of the (A) CXCL12, (B) CXCR4 and (C) CXCR7 genes relative to the mRNA levels of the TBP gene between lung cancer cell lines and non-malignant human bronchial epithelial cell lines, as measured by quantitative real-time RT-PCR. The expressions of (D) CXCL12, (E) CXCR4 and (F) CXCR7 were also compared between SCLC lines and NSCLC lines. Lines indicate the means of CXCL12, CXCR4 and CXCR7 expression.
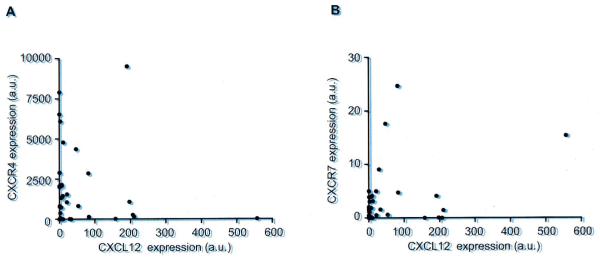
We next analyzed the correlation between CXCL12 and CXCR4 or CXCR7 expression. CXCL12 expression was significantly but weakly correlated with the expression of CXCR4 (Fig. 2A; r = 0.3002, P<0.05) and CXCR7 (Fig. 2B; r = 0.3333, P<0.05), suggesting that CXCL12 was concomitantly expressed with its receptors CXCR4 or CXCR7. On the other hand, there was no statistically significant correlation between the expression of CXCR4 and CXCR7 (data not shown).
Fig. 2.
Correlation between the expression of CXCL12 and expression of (A) CXCR4 or (B) CXCR7 in lung cancer cell lines. CXCL12 expression was significantly correlated with the expression of CXCR4 (r = 0.3002; P<0.05) or CXCR7 (r = 0.3333; P<0.05).
Clinicopathological significance of CXCL12 expression in NSCLC
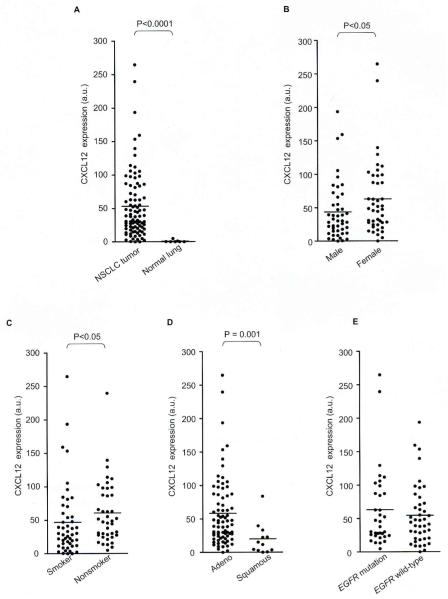
Since clinicopathological significance of CXCL12 remains unknown, we analyzed the relationship between CXCL12 expression and clinicopathological features in 89 NSCLC tumor specimens. The CXCL12 expression levels were significantly higher in the tumor specimens than in the normal lung specimens (Fig. 3A; P<0.000l).
Fig. 3.
Comparisons of CXCL12 expression between (A) NSCLC tumor specimens and normal lung specimens, (B) specimens from men and women, (C) and specimens from smokers and non-smokers, (D) adenocarcinomas and squamous cell carcinomas, and (E) adenocarcinoma specimens with and without EGFR mutations. Lines indicate the means of CXCL12 expression.
The CXCL12 expression level was significantly higher in the tumor specimens from female patients (P<0.05; Fig. 3B), non-smokers (P<0.05; Fig. 3C) and patients with adenocarcinoma histology (P = 0.001; Fig. 3D), whereas there was no significant difference in the CXCL12 expression levels when groups were classified according to patient age or pathological stage (data not shown). Since EGFR mutations were frequently found in NSCLCs arising from female patients, non-smokers and adenocarcinoma patients (24), in whom CXCL12 was highly expressed, we also assessed whether CXCL12 was highly expressed in EGFR mutation-positive tumors. In general, there are few squamous cell carcinomas of the lung which have EGFR mutations, so that it would seem to be histologically biased if the 89 patients were divided into groups with or without EGFR. Therefore, we compared the CXCL12 expression between the specimens with EGFR mutations (N = 34) and those with wild-type EGFR (N = 43) within the adenocarcinoma population. There was a tendency for higher CXCL12 expression in adenocarcinomas with EGFR mutations, although the difference did not reach statistically significant (Fig. 3E).
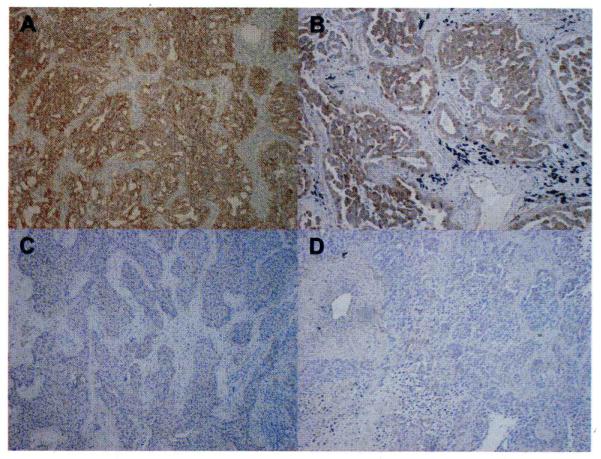
We verified whether expression of CXCL12 mRNA in tumor specimens reflects on CXCL12 protein expression. CXCL12 was strongly expressed in the cytoplasm of the tumor cells which express high levels of CXCL12 mRNA; the expressions of CXCL12 mRNA are 105.5 a.u. and 153.6 a.u. for the tumors in Fig. 4A and 4B, respectively, while fibroblasts surrounding the tumors showed very weak CXCL12 expression. In contrast, CXCL12 protein expressions were undetectable or very weak in NSCLC specimens (Fig. 4C and 4D), in which the expressions of CXCL12 mRNA are 0.0 a.u. and 0.8 a.u. in Fig. 4C and 4D, respectively.
Fig. 4.
Representative immunohistochemical staining of CXCL12 protein in NSCLC specimens. High and low CXCL12 expression in NSCLC is shown (×200). CXCL12 was expressed in the cytoplasm of tumor cells that express a high level of CXCL12 mRNA; CXCL12 mRNA expressions were (A) 105.5 a.u. and (B) 153.6 a.u., respectively. In contrast, CXCL12 protein was undetectable in tumor cells of NSCLC specimens where CXCL12 mRNA expressions were undetectable or very low; CXCL12 mRNA expressions were (C) 0.0 a.u. and (D) 0.8 a.u., respectively.
To assess the prognostic significance of CXCL12 expression in NSCLC, we analyzed the difference in disease-free survival and overall survival between stage I/II NSCLC patients (N = 68) with high CXCL12 expression and those with low CXCL12 expression. The patients were divided into two groups based on the median level of CXCL12 mRNA expression. There were no significant differences in survival between these groups. Thus, it is unlikely that CXCL 12 expression would have significant impact on survival for NSCLC patients.
siRNA-mediated silencing of CXCL12 expression inhibited the in vitro cell growth and migration of lung cancer cells
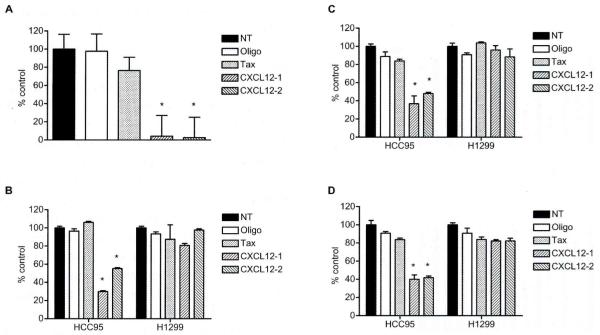
To assess the therapeutic significance of CXCL12 in lung cancer, we examined the effect of small interfering RNAs (siRNAs)-mediated CXCL12 gene silencing on the in vitro growth of CXCL12-overexpressing lung cancer cell lines. We used the HCC95 cell line, since this line expressed the highest level of CXCL12, which was 557-fold higher than the average of CXCL12 levels in non-malignant bronchial epithelial cells (N = 6). Two siRNAs targeting different sites of CXCL12 mRNA were used to verify that the effect of siRNA-mediated knockdown of CXCL12 expression is specific. CXCL12 siRNAs were transfected into HCC95 cells, and the effect on gene silencing was monitored by real-time RT-PCR. siRNAs against CXCL12 led to marked reductions of CXCL12 mRNA expression at 72 h post-transfection, as compared to untreated cells (Fig. 5A, p<0.001); both siRNAs gave similar results. On the other hand, treatment with Oligofectamine or Tax siRNA did not significantly affect the CXCL12 expression levels (Fig. 5A).
Fig. 5.
A) siRNAs against CXCL12 markedly down-regulated the expression of CXCL12 mRNA in HCC95 cells, which expressed the highest levels of CXCL12 among all lung cancer cell lines in this study. NT: treatment with medium alone; Oligo: treatment with Oligofectamine reagent alone. HCC95 cells were transfected with 100 nM siRNAs against either CXCL12 (CXCL12-1 and CXCL12-2) or Tax. After 72 h, cells were harvested and quantitative real-time RT-PCR was performed. siRNA-mediated CXCL12 knockdown inhibited cellular proliferation and migration as evaluated by (B) MTT assay, (C) colony formation assay and (D) migration assay in HCC95 cells but not in HI299 cells, in which CXCL12 was undetectable. The MTT assay was performed in replicates of 8 at 4 days after transfection of siRNAs. Columns represent the mean ± SD (bars). Cells were also replated for the colony formation assay in liquid culture at 48 h post-transfection. and surviving colonies were stained with methylene blue 14 days later. Columns represent the mean ±SD (bars) obtained from three independent experiments. For the migration assay, 105 cells/well of the 12-blind-well chemotaxis chamber were loaded after 48 h of siRNA transfection. Columns represent, the mean ± SD (bars) of three independent experiments. Treatment with medium alone was set at 100%. *, P<0.001.
The effect of CXCL12 knockdown on cellular proliferation of HCC95 cells was then examined by an MTT assay. Knockdown of CXCL12 expression resulted in the significant inhibition of cellular proliferation in HCC95 cells (P<0.001) but not in the HI299 cells that lacked CXCL12 expression, whereas the treatment with Oligofectamine or Tax siRNA did not affect the cellular proliferation in any cell lines (Fig. 5B). Furthermore, siRNA-mediated knockdown of CXCL12 significantly inhibited colony formation in HCC95 cells but not in HI299 cells (P<0.001; Fig. 5C).
We also examined the effect of siRNA-mediated CXCL12 knockdown on cell migration in HCC95 cells. CXCL12 knockdown resulted in a significant attenuation in the cell migration of HCC95 cells (P<0.001) but not H1299 cells, whereas the treatment with Oligofectamine or Tax siRNA did not affect cell migration (Fig. 5D). These results indicate that CXCL12 expression is required for cell growth and migration in lung cancer cells which have high expression levels of CXCL12.
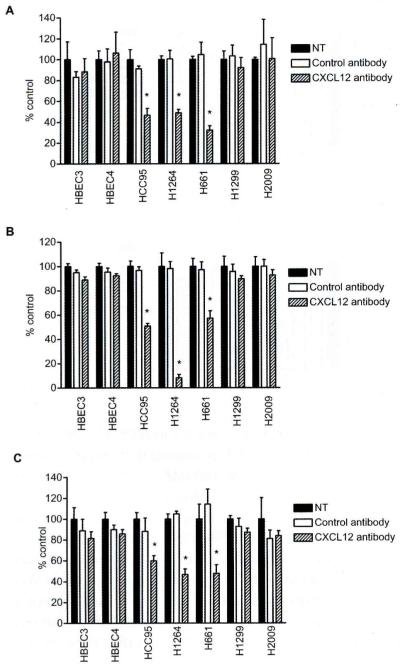
Neutralization of CXCL12 by an anti-CXCL12 antibody led to inhibition of growth and migration of CXCL12-overexpressing lung cancer cells
To further evaluate the inhibitory effects of CXCL12 silencing on cell growth and migration by a different approach, we tested whether treatment with an anti-CXCL12 neutralizing antibody inhibited cell growth and migration in HCC95, HI264 and H661 cell lines; these three cell lines expressed CXCL12 at the highest levels among all lung cancer cell lines used in this study. The treatment with an anti-CXCL12 antibody but not a control antibody significantly inhibited cellular proliferation in all cell lines (P<0.00l for all lines; Fig. 6A), but not in HI299 and H2009 lung cancer cell lines, where CXCL12 expression was undetectable, nor HBEC3 and HBEC4 non-malignant bronchial epithelial cell lines (Fig. 6A). Similarly, the treatment with an anti-CXCL12 antibody significantly inhibited colony formation in the three CXCL12-overexpressing lines (P<0.00l for all lines; Fig. 6B) but not in the CXCL12 non-expressing cancer cell lines nor the non-malignant cell lines (Fig. 6B). The effect of the anti-CXCL12 antibody on cell migration was further examined. Treatment with the CXCL12 antibody but not with the control antibody resulted in significant inhibition of cell migration in three CXCL12-overexpressing cancer cell lines (P<0.001 for all lines; Fig. 6C). Conversely, the inhibitory effect of the anti-CXCL12 antibody was not observed for the CXCL12-nonexpressing cancer cell lines and the non-malignant cell lines (Fig. 6C). Thus, we demonstrate that neutralization of CXCL12 by the anti-CXCL12 antibody is effective for inhibition of cell growth and migration in lung cancer cells that overexpress CXCL12.
Fig. 6.
A) An anti-CXCL12 neutralizing antibody inhibited cellular proliferation and migration in CXCL12-overexpressing lung cancer cell lines (HCC95, H1264 and H661) but not in non-malignant bronchial epithelial cell lines (HBEC3 and HBEC4) and lung cancer cell lines (H1299 and H2009) lacking CXCL12 expression. Cells were treated with the CXCL12 antibody (10 μg/ml) or the IgGl control antibody (10 μg/ml) for 72 h and cellular proliferation was evaluated by MTT assay. B) As for the colony formation assay, cells were treated with the CXCL12 antibody (10 μg/ml) or the IgGl antibody (10 μg/ml) in liquid culture, and surviving colonies were stained with methylene blue 14 days later. Columns represent the mean ± SD (bars) obtained from four independent experiments. C) The migration assay was performed after 48 h of treatment with the CXCL12 antibody (10 μg/ml) or the IgGl antibody (10 μg/ml). Columns represent the mean ± SD (bars) of four independent experiments. Nontreatment was set at 100%. *, P<0.001.
DISCUSSION
Since few studies have evaluated the expression of CXCL12 in lung cancers, we first examined the expression of CXCL12 and its receptors CXCR4 and CXCR7 in a large number of lung cancer cell lines. We found that CXCL12 was overexpressed in lung cancer cell lines, and the expression was positively but weakly correlated with the expression of CXCR4 or CXCR7. CXCL12 and CXCR4 chemokines have an autocrine role related to the growth and metastasis of human cancers (25-26). A newly identified CXCR7 receptor also promotes tumor development and metastasis through the interaction with CXCL12 (17, 27-28). The present results indicate co-expression of CXCL12-CXCR4/CXCR7 in lung cancer cells, suggesting that autocrine systems of CXCL12-CXCR4/CXCR7 may exist in lung cancer cells. In addition, the lack of correlation between the expression of CXCR4 and CXCR7 suggest that CXCL12 may differentially interact with CXCR4 or CXCR7, depending on the cellular context. Previous studies have found that SCLC cells express high levels of CXCR4, which mediates cellular proliferation, migration and adhesion in the tumors (29-31). Together with our result that CXCR4 was abundantly expressed in SCLCs as compared to NSCLCs, it is likely that the CXCL12-CXCR4 interaction could be more important for the development of SCLC.
Recent studies demonstrated that NSCLC tumors expressed CXCL12 and that the CXCL12 expression was correlated with poor prognosis in adenocarcinoma patients (32-33). In our analysis of CXCL12 expression in NSCLC tumor specimens, CXCL12 expression was higher in NSCLCs from female patients, non-smokers and adenocarcinoma patients. Therefore, CXCL12 seems to be implicated in tumor progression in NSCLC patients with these clinical characteristics. Of note, a recent study showed that aberrant methylation with the subsequent loss of CXCL12 expression occurred more frequently in male patients than in female patients (32), suggesting that there are different roles for CXCL12 in NSCLC arising from male patients versus female patients.
EGFR mutations in the tyrosine kinase (TK) domain, which are associated with a favorable response to EGFR TK inhibitors, are frequently found in NSCLC patients who are females or non-smokers or who have adenocarcinomas (24). In this study, we observed a tendency for higher expression of CXCL12 in NSCLC tumors with EGFR mutations compared to the tumors with wild-type EGFR. Phillips et al. reported that activation of EGFR by EGF stimulation increased CXCR4 expression (34). In addition, a recent study described that CXCL12-induced cellular proliferation was blocked by an EGFR tyrosine kinase inhibitor in ovarian cancer cells (35). More recently, EGFR mutations were found more frequently in NSCLC patients with higher CXCR7 expression compared to those with lower CXCR7 expression (28). These findings suggest the involvement of EGFR with the CXCL12-CXCR4/CXCR7 pathways. Since the number of tumor specimens analyzed in our study is relatively small, further studies with larger populations are needed to elucidate the possible role of oncogenic EGFR in the CXCL12-CXCR4/CXCR7 pathways.
The findings of CXCL12 overexpression in lung cancer prompted us to assess whether anti-CXCL12 treatment strategy could be effective for lung cancer. This idea is supported by the fact that neutralization of CXCL12 by an anti-CXCL12 antibody suppressed the metastasis of lung tumors in SCID mice (12). We herein demonstrated that the disruption of CXCL12 by siRNAs or an anti-CXCL12 neutralizing antibody led to inhibition of the in vitro growth and migration of lung cancer cells. Su et al illustrated the therapeutic significance of the CXCL12-CXCR4 chemokine pathways in lung cancer by using an anti-CXCR4 antibody to suppress tumor growth and metastasis in mouse models (36). Together with these reports, our findings indicate that targeting CXCL12 has therapeutic potential for lung cancers. Considering the recent studies describing that CXCR7, a second CXCL12 receptor, promotes lung tumor growth (17) and is expressed in NSCLC patients with a poor prognosis (28), the anti-CXCL12 treatment strategy could also be effective for lung cancer where CXCL12-CXCR7 pathways are oncogenically active.
In conclusion, we demonstrated that CXCL12 was concomitantly overexpressed with CXCR4 or CXCR7 in lung cancers. CXCL12 was expressed at higher levels in NSCLCs from female patients, non-smokers and patients with adenocarcinomas. The blockage of CXCL12 function by siRNAs or anti-CXCL12 neutralizing antibody inhibits the in vitro growth and migration of lung cancer cells. Although further in vivo studies are needed to elucidate the effectiveness of the anti-CXCL12 strategy, our findings provide an important rationale for targeting CXCL12-CXCR4/CXCR7 pathways for lung cancer.
ACKNOWLEDGEMENTS
We thank Dr. Adi F. Gazdar of the University of Texas Southwestern Medical Center for providing all lung cancer cell lines and immortalized human bronchial epithelial cells. We also thank Dr. Michael Peyton of the University of Texas Southwestern Medical Center and Drs. Shuichi Okada, Yoshio Tomizawa, Mitsuyoshi Utsugi, Kyoichi Kaira, Noriko Yanagitani, Tadayoshi Kawata, Hironobu Iijima, Masako Saito and Kunio Dobashi of the Gunma University Graduate School of Medicine for providing technical support and critical comments for this study. We also thank Dr Minato Nakazawa, associate professor of the Department of Public Health, Gunma University Graduate School of Medicine for critical advice on the statistical analysis. This work was supported in part by Grant 18790532 (to NS) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and Lung Cancer SPORE (P50CA70907), DOD PROSPECT, and Longenbaugh Foundation Grants (to JDM).
Footnotes
Declaration: None of the authors have financial or personal relationships with other people or organizations that could inappropriately influence their research.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1:49–52. doi: 10.1016/s1535-6108(02)00027-2. [DOI] [PubMed] [Google Scholar]
- 3.Murphy PM. Chemokine receptors: structure, function and role in microbial pathogenesis. Cytokine Growth Factor Rev. 1996;7:47–64. doi: 10.1016/1359-6101(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 5.Kryczek I, Wei S, Keller E, Liu R, Zou W. Stroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesis. Am J Physiol Cell Physiol. 2007;292:C987–95. doi: 10.1152/ajpcell.00406.2006. [DOI] [PubMed] [Google Scholar]
- 6.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 7.Geminder H, Sagi-Assif O, Goldberg L, et al. A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J Immunol. 2001;167:4747–57. doi: 10.4049/jimmunol.167.8.4747. [DOI] [PubMed] [Google Scholar]
- 8.Kryczek I, Lange A, Mottram P, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–72. [PubMed] [Google Scholar]
- 9.Mochizuki H, Matsubara A, Teishima J, et al. Interaction of ligand-receptor system between stromal-cell-derived factor-1 and CXC chemokine receptor 4 in human prostate cancer: a possible predictor of metastasis. Biochem Biophys Res Commun. 2004;320:656–63. doi: 10.1016/j.bbrc.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Mori T, Doi R, Koizumi M, et al. CXCR4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer. Mol Cancer Ther. 2004;3:29–37. [PubMed] [Google Scholar]
- 11.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 12.Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Strieter RM. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med. 2003;167:1676–86. doi: 10.1164/rccm.200301-071OC. [DOI] [PubMed] [Google Scholar]
- 13.Sutton A, Friand V, Brule-Donneger S. Stromal cell-derived factor-1/chemokine (C-X-C motif) ligand 12 stimulates human hepatoma cell growth, migration, and invasion. Mol Cancer Res. 2007;5:21–33. doi: 10.1158/1541-7786.MCR-06-0103. [DOI] [PubMed] [Google Scholar]
- 14.Tang CH, Tan TW, Fu WM, Yang RS. Involvement of matrix metalloproteinase-9 in stromal cell-derived factor-1/CXCR4 pathway of lung cancer metastasis. Carcinogenesis. 2008;29:35–43. doi: 10.1093/carcin/bgm220. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu Y, Dobashi K, Imai H, et al. CXCR4+FOXP3+CD25+ lymphocytes accumulate in CXCL12-expressing malignant pleural mesothelioma. Int J Immunopathol Pharmacol. 2009;22:43–51. doi: 10.1177/039463200902200106. [DOI] [PubMed] [Google Scholar]
- 16.Balabanian K, Lagane B, Infantino S, et al. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–6. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 17.Miao Z, Luker KE, Summers BC, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci USA. 2007;104:15735–40. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Shiozawa Y, Wang J, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–94. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 19.Phelps RM, Johnson BE, Ihde DC, et al. NCI-Navy Medical Oncology Branch cell line data base. J Cell Biochem Suppl. 1996;24:32–91. doi: 10.1002/jcb.240630505. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez RD, Sheridan S, Girard L, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–34. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M, Sunaga N, Shames DS, Toyooka S, Gazdar AF, Minna JD. RNA interference-mediated knockdown of DNA methyltransferase 1 leads to promoter demethylation and gene re-expression in human lung and breast cancer cells. Cancer Res. 2004;64:3137–43. doi: 10.1158/0008-5472.can-03-3046. [DOI] [PubMed] [Google Scholar]
- 22.Sunaga N, Miyajima K, Suzuki M, et al. Different roles for caveolin-1 in the development of non-small cell lung cancer versus small cell lung cancer. Cancer Res. 2004;64:4277–85. doi: 10.1158/0008-5472.CAN-03-3941. [DOI] [PubMed] [Google Scholar]
- 23.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–4. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 24.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–46. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 25.Barbieri F, Bajetto A, Stumm R, et al. Overexpression of stromal cell-derived factor 1 and its receptor CXCR4 induces autocrine/paracrine cell proliferation in human pituitary adenomas. Clin Cancer Res. 2008;14:5022–32. doi: 10.1158/1078-0432.CCR-07-4717. [DOI] [PubMed] [Google Scholar]
- 26.Uchida D, Onoue T, Tomizuka Y, et al. Involvement of an autocrine stromal cell derived factor-1/CXCR4 system on the distant metastasis of human oral squamous cell carcinoma. Mol Cancer Res. 2007;5:685–94. doi: 10.1158/1541-7786.MCR-06-0368. [DOI] [PubMed] [Google Scholar]
- 27.Meijer J, Ogink J, Roos E. Effect of the chemokine receptor CXCR7 on proliferation of carcinoma cells in vitro and in vivo. Br J Cancer. 2008;99:1493–501. doi: 10.1038/sj.bjc.6604727. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Iwakiri S, Mino N, Takahashi T, et al. Higher expression of chemokine receptor CXCR7 is linked to early and metastatic recurrence in pathological stage I nonsmall cell lung cancer. Cancer. 2009;115:2580–93. doi: 10.1002/cncr.24281. [DOI] [PubMed] [Google Scholar]
- 29.Kijima T, Maulik G, Ma PC, et al. Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 2002;62:6304–11. [PubMed] [Google Scholar]
- 30.Burger M, Glodek A, Hartmann T, et al. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003;22:8093–101. doi: 10.1038/sj.onc.1207097. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M. CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene. 2005;24:4462–71. doi: 10.1038/sj.onc.1208621. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki M, Mohamed S, Nakajima T, et al. Aberrant methylation of CXCL12 in non-small cell lung cancer is associated with an unfavorable prognosis. Int J Oncol. 2008;33:113–9. [PubMed] [Google Scholar]
- 33.Wald O, Izhar U, Amir G, et al. CD4+CXCR4highCD69+ T cells accumulate in lung adenocarcinoma. J Immunol. 2006;177:6983–90. doi: 10.4049/jimmunol.177.10.6983. [DOI] [PubMed] [Google Scholar]
- 34.Phillips RJ, Mestas J, Gharaee-Kermani M, et al. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1 alpha. J Biol Chem. 2005;280:22473–81. doi: 10.1074/jbc.M500963200. [DOI] [PubMed] [Google Scholar]
- 35.Porcile C, Bajetto A, Barbieri F, et al. Stromal cell-derived factor-1 alpha (SDF-lalpha/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivation. Exp Cell Res. 2005;308:241–53. doi: 10.1016/j.yexcr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 36.Su L, Zhang J, Xu H, et al. Differential expression of CXCR4 is associated with the metastatic potential of human non-small cell lung cancer cells. Clin Cancer Res. 2005;11:8273–80. doi: 10.1158/1078-0432.CCR-05-0537. [DOI] [PubMed] [Google Scholar]