Abstract
Doxorubicin, an anthracycline antibiotic commonly used as a chemotherapeutic agent for breast cancer, is well known to cause cardiotoxicity. We report the case of an active, otherwise healthy 57-year-old breast cancer survivor who, 17 years after chemotherapy, presented with symptoms of overt heart failure. She had no cardiac risk factors, and neither laboratory nor imaging findings suggested myocarditis or dilated cardiomyopathy. Echocardiographic findings and differential diagnosis led us to attribute her condition to late doxorubicin-induced cardiomyopathy. By virtue of tapered medical therapy, her left ventricular ejection fraction improved from 0.20 to 0.55 in 8 months, and she was asymptomatic after 1 year. The reversibility of left ventricular dysfunction in our patient and the very late appearance of cardiotoxicity secondary to doxorubicin therapy raise questions about the pathogenesis and prevalence of late doxorubicin-induced cardiomyopathy and how to improve outcomes in patients who present with related symptoms of heart failure.
Key words: Adult; cardiomyopathies/chemically induced; cardiovascular diseases/therapy; doxorubicin/adverse effects; heart failure/chemically induced; risk factors; ventricular function, left/drug effects
Early-onset cardiomyopathy induced by doxorubicin (DOX) occurs during the year after the completion of chemotherapy. Late-onset DOX-induced cardiomyopathy, which occurs later than a year after chemotherapy has been completed, has been reported chiefly in pediatric populations.1 The late-onset cardiomyopathy presents in approximately 1.6% to 5% of patients,2 most of whom have normal cardiac examination results before and just after chemotherapy. Early detection of chemotherapy-induced cardiotoxicity is crucial in order to prevent irreversible cardiac dysfunction, which occurs in more than one third of patients.3 Here, we present the case of a woman in whom DOX-induced cardiomyopathy occurred 17 years after chemotherapy.
Case Report
In January 2009, a 57-year-old woman emergently presented with acute shortness of breath. She had a 1-month history of progressive shortness of breath and a gradual decrease in exercise capacity secondary to mild dyspnea. She reported no additional symptoms. At age 40, she had been diagnosed with a stage IIA, T1bN1, left-sided breast cancer. Initial treatment had included a lumpectomy and axillary node dissection. She subsequently underwent 4 cycles of DOX therapy (75 mg/m2), followed by 8 cycles of cyclophosphamide, methotrexate, and 5-fluorouracil. Multigated acquisition scans before and after chemotherapy showed normal cardiac function. After chemotherapy, she underwent left whole-breast radiation with an axillary boost. Because the tumor had been estrogen receptor-positive, her subsequent medical regimen consisted only of anti-estrogen therapy. She took tamoxifen for 5 years, and, ever since, the aromatase inhibitor letrozole. In the 17 years after chemotherapy, she had been active and in relatively good health.
In addition to her other symptoms, she now presented with tachycardia, tachypnea, and hypertension. She had marked jugular venous distention, an S3, pulmonary rales, and trace peripheral edema. Initial laboratory values were within normal limits except for an elevated level of N-terminal pro-brain natriuretic peptide (>2,000 pg/mL). Results of investigation into the new-onset cardiomyopathy included normal cardiac enzyme levels, an electrocardiogram (ECG) that revealed no ischemic changes, and a coronary angiogram of normal appearance. The ECG showed sinus tachycardia with frequent premature ventricular complexes, left-axis deviation, left atrial enlargement, and low-voltage QRS complexes with nonspecific ST changes (Fig. 1). A 2-dimensional echocardiogram revealed a left ventricular ejection fraction (LVEF) of 0.20, severe diffuse left ventricular (LV) hypokinesis, and a mildly dilated left atrium.
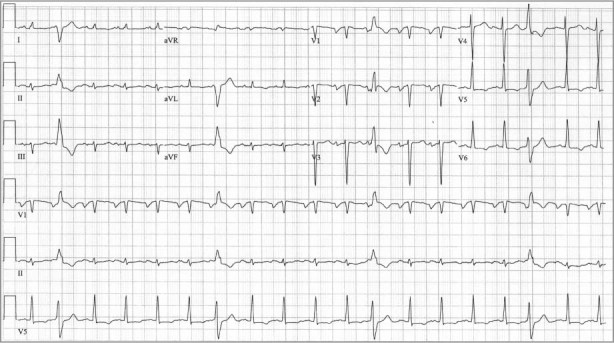
Fig. 1 Electrocardiogram shows sinus tachycardia with frequent premature ventricular complexes, left atrial enlargement, left-axis deviation, and low-voltage QRS complexes with nonspecific ST changes.
To better define the cause of the LV systolic dysfunction, cardiovascular magnetic resonance (CMR) was performed. It confirmed the LVEF of 0.20. The T2-weighted sequence showed slow flow secondary to LV dysfunction, and no myocardial edema (Fig. 2A). Late gadolinium enhancement disclosed diffuse myocardial thinning and no scarring (Fig. 2B).
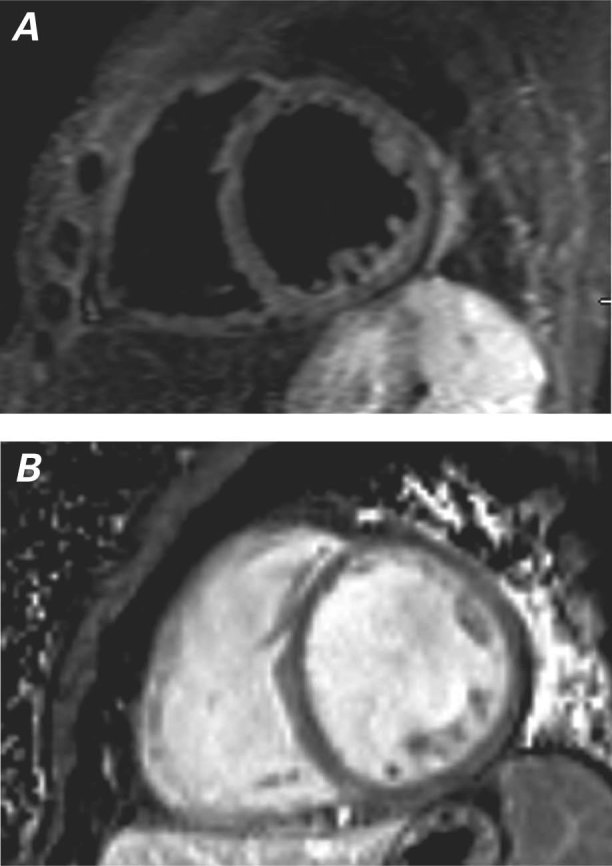
Fig. 2 Cardiovascular magnetic resonance (short-axis view). A) T2-weighted sequence shows slow flow secondary to left ventricular dysfunction and no myocardial edema. B) Late gadolinium enhancement shows diffuse myocardial thinning and no scarring.
The patient was treated medically. Her symptoms progressively improved during therapy, which consisted of a β-blocker, an angiotensin-converting enzyme inhibitor, digoxin, and a diuretic. The therapy was slowly tapered, and her LVEF increased from 0.20 to 0.55 during an 8-month period. All medications except for low-dose metoprolol were discontinued after 1 year, and she remained asymptomatic.
Discussion
Certain findings and the absence of other findings led us to conclude that late DOX cardiotoxicity was the most probable cause of our patient's cardiomyopathy. The ECG findings were consistent with changes previously reported during acute and subacute DOX-induced cardiomyopathy.4 In addition, the diffuse myocardial thinning without scarring could have indicated cellular changes that have been reported in DOX-induced cardiomyopathy.2
The Differential Diagnosis in our Patient
Dilated Cardiomyopathy or Myocarditis. The results of our initial investigations did not support a diagnosis of dilated cardiomyopathy or myocarditis. The laboratory results included normal levels of cardiac enzymes and a normal white blood cell count, with no eosinophilia. Viral serology was negative. Coronary angiography revealed no significant obstructive coronary artery disease. Imaging showed no edema or LV dilation (54 mm at the papillary muscle on CMR). Despite the absence of cardiac risk factors, an early stage of dilated cardiomyopathy cannot be entirely ruled out.
The diagnosis of myocarditis was very unlikely, because the patient's clinical presentation was insidious and she had no myocarditis-related symptoms. Fulminant myocarditis presents with echocardiographic findings of a thickened LV wall and normal LV size, and acute myocarditis presents with marked LV chamber dilation and normal LV wall thickness. Our patient's echocardiographic findings were inconsistent with these changes.5 There was also no pericardial effusion or regional wall-motion abnormality that mimicked myocardial infarction.
Subclinical Factors. None of the several hypotheses for the possible mechanisms of late DOX-induced cardiomyopathy have been proved. One intriguing theory is the “multiple-hit” phenomenon,6 whereby DOX-based adjuvant therapy causes subclinical LV dysfunction that would render the patient increasingly susceptible to progressive dysfunction related to aging and other disease processes. However, our patient had normal LV size, normal cardiac biomarker levels, and no cardiac risk factors, yet she presented with overt symptoms of heart failure 17 years after chemotherapy.
Effects of Combination Chemotherapy. A synergistic effect of the combination chemotherapy, dominated by the long-term effects of DOX, would be difficult to measure in our patient's case. Cardiac dysfunction associated with cyclophosphamide, methotrexate, and 5-fluoruracil therapy has an acute clinical presentation; in contrast, the presentation associated with DOX is subclinical and late.7,8 Our patient had no symptoms during or just after her chemotherapy. The other agents and the radiation therapy could have contributed to early subclinical myocardial damage; however, their independent role in the development of her cardiomyopathy is uncertain.
Doxorubicin Cardiotoxicity
Doxorubicin cardiotoxicity is cumulative and typically occurs at an average total dose of 500 mg/m2; however, studies9 have shown that cardiotoxicity can occur at a cumulative dose of as low as 300 mg/m2. In a recent study on the early detection and prediction of cardiotoxicity,10 21% of patients developed chemotherapy-related cardiotoxicity after DOX doses of less than 300 mg/m2.
Late DOX cardiotoxicity that manifests itself as heart failure in adults is a rare entity, and its pathogenesis is speculative. Accordingly, clinical responses to treatment—and the outcomes thereof—are variable. The clinical manifestation of LV dysfunction secondary to DOX therapy also varies, from irreversibility to complete recovery or from subclinical to overt heart-failure symptoms. Doxorubicin cardiotoxicity reversibility has occurred in almost two thirds of cases, but more than one third of DOX-treated patients have shown no improvement in cardiac function. We found 3 reports of symptomatic heart failure late after DOX therapy (at 2.5, 6, and 7 yr, respectively).11-13 One patient presented with advanced heart failure and died soon thereafter.11 The other 2 patients each presented with moderate-to-severe symptoms and progressed to a well-compensated state.12,13
The exact factors responsible for the irreversibility of DOX-induced LV dysfunction are unknown. The absence of cardiac risk factors and the presence of a normal LVEF before and immediately after chemotherapy might have affected our patient's recovery of LV function upon early medical management.
Sudden cardiac death has been associated with anthracycline use and has been reported as late as 15 years after therapy.14 Sudden cardiac death is more prevalent in patients with LVEFs lower than 0.35, which is a Class 1 indication for the placement of an implantable cardioverter-defibrillator (ICD). The indication for an ICD in cardiotoxicity-related systolic dysfunction and the appropriate time interval for its placement after the onset of symptoms are both unknown. Furthermore, there are no guidelines in this specific subset of the patient population, since the reversibility of LV dysfunction is unpredictable. Because functional capacity does not necessarily correlate with LVEF, our active and apparently healthy patient might have had undetected, depressed LVEF for years before the development of heart failure and thereby have been at higher risk of sudden cardiac death even before the diagnosis of heart failure was established. Therefore, DOX-induced cardiomyopathy might warrant even closer follow-up than other causes of systolic dysfunction.
Our patient's late-onset DOX-induced cardiomyopathy was reversed through medical management. No strict guidelines have been developed by the American College of Cardiology and American Heart Association in regard to early detection, treatment options, or monitoring of cardiac function in patients who are undergoing chemotherapy. The goal of breast cancer therapy with anthracyclines is to treat the underlying malignancy without adversely affecting cardiac function. Early detection and close monitoring are essential in DOX use, given the chronicity of DOX-induced cardiomyopathy's presentation and the potentially irreversible myocardial damage that can result. To prevent morbidity and death in breast cancer survivors who have undergone DOX chemotherapy, early predictors of heart failure and sudden cardiac death need to be defined.
Footnotes
Address for reprints: Erick Avelar, MD, FACC, Director, Noninvasive Cardiac Imaging Program, University of Connecticut Health Center, 263 Farmington Ave., Farmington, CT 06030, E-mail: eavelar@uchc.edu
References
- 1.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart 2008; 94(4):525–33. [DOI] [PubMed]
- 2.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 2009;53(24):2231–47. [DOI] [PubMed]
- 3.Aiken MJ, Suhag V, Garcia CA, Acio E, Moreau S, Priebat DA, et al. Doxorubicin-induced cardiac toxicity and cardiac rest gated blood pool imaging. Clin Nucl Med 2009;34(11): 762–7. [DOI] [PubMed]
- 4.Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology 2010;115(2):155–62. [DOI] [PMC free article] [PubMed]
- 5.Blauwet LA, Cooper LT. Myocarditis. Prog Cardiovasc Dis 2010;52(4):274–88. [DOI] [PMC free article] [PubMed]
- 6.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol 2007;50(15):1435–41. [DOI] [PubMed]
- 7.Curigliano G, Mayer EL, Burstein HJ, Winer EP, Goldhirsch A. Cardiac toxicity from systemic cancer therapy: a comprehensive review. Prog Cardiovasc Dis 2010;53(2):94–104. [DOI] [PubMed]
- 8.Perez-Verdia A, Angulo F, Hardwicke FL, Nugent KM. Acute cardiac toxicity associated with high-dose intravenous methotrexate therapy: case report and review of the literature. Pharmacotherapy 2005;25(9):1271–6. [DOI] [PubMed]
- 9.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 2003;97(11):2869–79. [DOI] [PubMed]
- 10.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011;107(9):1375–80. [DOI] [PMC free article] [PubMed]
- 11.Gottlieb SL, Edmiston WA Jr, Haywood LJ. Late, late doxorubicin cardiotoxicity. Chest 1980;78(6):880–2. [DOI] [PubMed]
- 12.Lishner M, Elis A, Ravid M. Late doxorubicin cardiotoxicity. Anticancer Drugs 1992;3(4):367–9. [DOI] [PubMed]
- 13.Freter CE, Lee TC, Billingham ME, Chak L, Bristow MR. Doxorubicin cardiac toxicity manifesting seven years after treatment. Case report and review. Am J Med 1986;80(3): 483–5. [DOI] [PubMed]
- 14.Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med 1996;125(1):47–58. [DOI] [PubMed]


