Abstract
Finding the source of a fungal infection and selecting the most appropriate treatment for candidemia is often challenging for physicians, especially when the patient has a complex medical history. We describe the case of a 48-year-old woman who had persistent candidemia after undergoing explantation of a left ventricular assist device. The source of the infection was found to be a right atrial thrombus. The mass was removed, and the patient underwent aggressive treatment with micafungin. Removal of the right atrial mass, followed by potent antifungal treatment, resulted in a successful recovery.
Key words: Candida albicans; fungemia/drug therapy/surgery; infection, fungal; left ventricular assist device; sepsis/etiology; thrombus, right atrial
Infected right atrial thrombus is an uncommon manifestation of systemic fungal infection and is usually related to the presence of an indwelling catheter in the right atrium. We describe the case of a patient who developed a right atrial thrombus infected with Candida albicans after she underwent explantation of a left ventricular assist device (LVAD).
Case Report
A 48-year-old woman required an LVAD (HeartMate II® Left Ventricular Assist System, Thoratec Corporation; Pleasanton, Calif) for nonischemic cardiomyopathy related to doxorubicin therapy for breast cancer. After 20 months, the device was explanted because of excessive hemolysis. The patient tolerated the explantation procedure well but had persistent leukocytosis and sepsis, which further compromised her cardiac status. Blood cultures revealed candidemia, and the patient developed a high-pitched murmur in her right parasternal area. A 2-dimensional echocardiogram showed a large right atrial mass that originated from the superior vena cava (Fig. 1).
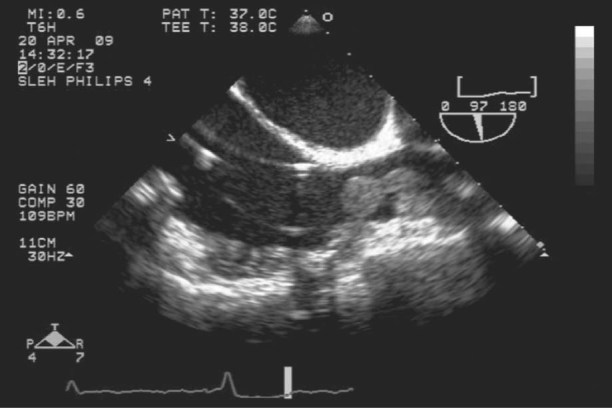
Fig. 1 Two-dimensional echocardiogram shows a large right atrial mass that originates from the superior vena cava.
The patient was taken to the operating room for removal of the mass. General anesthesia was induced, and intraoperative transesophageal echocardiography (TEE) was performed to further evaluate and monitor the heart. A right anterior thoracotomy was performed, and the patient's right groin was prepared for femoral arterial and venous cannulation for institution of cardiopulmonary bypass (CPB). To avoid dislodging the mass, we refrained from cannulating the right atrium. For the same reason, a femoral venous guidewire was advanced under TEE guidance. Despite meticulous cannulation, withdrawal of the guidewire for advancement of the venous cannula revealed a 2 × 3 × 2-cm ball-shaped mass on the tip of the guidewire (Fig. 2). After starting CPB, we opened the right atrium and performed vigorous suction of superior vena caval return blood to facilitate exposure. The rest of the thrombus originating from the superior vena cava was removed. The right atrial wall and tricuspid valve were normal (without vegetations), so the right atrium was closed.
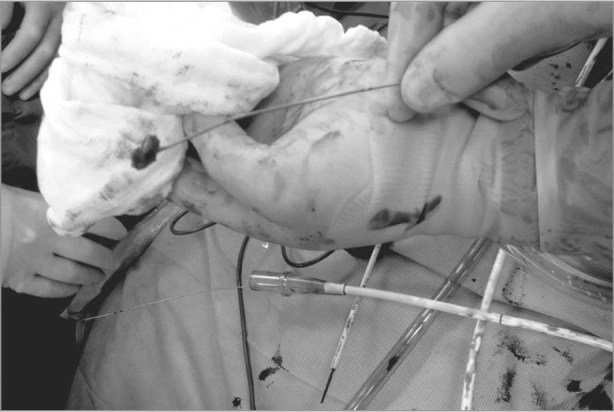
Fig. 2 Withdrawal of the femoral venous guidewire reveals a 2 × 3 × 2-cm ball-shaped mass on the tip of the guidewire.
Histopathologic examination of the thrombus showed fibrinous débris, scattered inflammatory cells, and numerous hyphae consistent with the presence of Candida species. Culture yielded a heavy growth of C. albicans. Micafungin (total dose, 2.8 g) was administered, and the patient was discharged from the hospital in good condition after completing a full 6-week course of intravenous antifungal therapy. Because the patient had iliac deep vein thrombosis, she was sent home on a regimen of warfarin for pulmonary embolus prophylaxis. The patient had 2 follow-up echocardiograms during her in-hospital recovery and another 1 week after discharge. She then underwent monthly follow-up echocardiography in the heart failure clinic as part of our standard follow-up protocol for patients who have undergone LVAD explantation.
Discussion
Right atrial thrombi can vary in their clinical presentation. They may be suspected because of difficulty with infusion or withdrawal of blood from a central venous catheter, or because of superior vena cava syndrome, a pulmonary embolism, tricuspid insufficiency, right-sided heart failure, sudden onset of a cardiac murmur, or syncopal episodes.1-4 A high degree of suspicion regarding the existence of such thrombi is necessary for early diagnosis. When present, right atrial thrombi can easily be detected by means of 2-dimensional echocardiography. In this case, the patient did not have an intravascular catheter or a remaining port in place for intravenous chemotherapy. While she was recovering in the intensive care unit after LVAD explantation, we performed daily routine physical examinations with auscultation; signs of systemic sepsis led us to diagnose the thrombus with the aid of TEE.
In contrast, diagnosis of candidemia is difficult. The results of blood cultures are negative in approximately 50% of cases; in many of these cases, the cultures do not become positive until very late in the course of the infection. The only clinical sign of a thrombotic infection is often fever or sepsis of unknown origin.5 As in right-sided endocarditis related to the presence of a central venous catheter, the most frequently encountered pathogens in central venous or right atrial septic thrombosis are Staphylococcus and Candida species.2,6 Systemic candidiasis usually occurs in patients with one or more predisposing factors, such as an immunodeficient state. Because of immunosuppression, cancer patients have a high incidence of fungal infections. Our patient had not had any chemotherapy in recent years, but she had had chronic heart failure, which was treated with LVAD therapy. Her immediate post-LVAD and post-CPB status might have adversely affected her immune system. During surgery and the early postoperative recovery period, central lines were inserted through her jugular vein. The infected thrombus probably formed while the temporary central lines were in place. Despite the later removal of these catheters, the infected thrombus remained inside the superior vena cava, resulting in persistent infection.
Although the available data are limited,7 combined medical and surgical therapy generally appears to be the preferred treatment for candidal endocarditis. In most cases involving an infected right atrial thrombus, aggressive treatment is mandatory: withdrawal of the catheter, surgical excision of the thrombus, and prolonged antifungal therapy.8 The literature contains a few reports of intracardiac masses in pediatric patients who had fungal septicemia and embolic phenomena; these cases were successfully treated without surgery.9 Our patient required immediate intervention because of her compromised hemodynamic status.
In conclusion, we have presented a highly unusual case of infected right atrial thrombosis that developed after explantation of a HeartMate II LVAD. Simple daily routine examinations alerted us to this sequela. Removal of the fungal mass, followed by aggressive antifungal treatment with micafungin, resulted in a successful recovery.
Footnotes
Address for reprints: O.H. Frazier, MD, Texas Heart Institute, MC 2-114A, P.O. Box 20345, Houston, TX 77225-0345, E-mail: lschwenke@texasheart.org
References
- 1.Pliam MB, McGough EC, Nixon GW, Ruttenberg HD. Right atrial ball-valve thrombus: a complication of central venous alimentation in an infant. Diagnosis and successful surgical management of a case. J Thorac Cardiovasc Surg 1979; 78(4):579–82. [PubMed]
- 2.Sadiq HF, Devaskar S, Keenan WJ, Weber TR. Broviac catheterization in low birth weight infants: incidence and treatment of associated complications. Crit Care Med 1987;15(1):47–50. [DOI] [PubMed]
- 3.Wolfe BM, Ryder MA, Nishikawa RA, Halsted CH, Schmidt BF. Complications of parenteral nutrition. Am J Surg 1986; 152(1):93–9. [DOI] [PubMed]
- 4.Barbeito A, Bar-Yosef S, Lowe JE, Atkins BZ, Mark JB. Unusual cause of superior vena cava syndrome diagnosed with transesophageal echocardiography. Can J Anaesth 2008;55 (11):774–8. [DOI] [PubMed]
- 5.Edwards JE Jr, Filler SG. Current strategies for treating invasive candidiasis: emphasis on infections in nonneutropenic patients. Clin Infect Dis 1992;14 Suppl 1:S106–13. [DOI] [PubMed]
- 6.Heinemann M, Frank G, Oldhafer KJ, Schmoll E. Infected intravenous port device causing tricuspid valve regurgitation. Ann Thorac Surg 1991;51(5):827–8. [DOI] [PubMed]
- 7.Pierrotti LC, Baddour LM. Fungal endocarditis, 1995–2000. Chest 2002;122(1):302–10. [DOI] [PubMed]
- 8.Haddad W, Idowu J, Georgeson K, Bailey L, Doroshow R, Pickham N. Septic atrial thrombosis. A potentially lethal complication of Broviac catheters in infants. Am J Dis Child 1986;140(8):778–80. [DOI] [PubMed]
- 9.Horner SM, Bell JA, Swanton RH. Infected right atrial thrombus–an important but rare complication of central venous lines. Eur Heart J 1993;14(1):138–40. [DOI] [PubMed]


