Abstract
Coronary ostial stenosis is a rare but potentially fatal sequela of aortic surgery. The clinical presentation can include acute coronary syndromes, ventricular arrhythmias, congestive heart failure, or sudden death. Herein, we present what we believe is the first reported case of asymptomatic iatrogenic left main coronary ostial stenosis. The patient was an active 34-year-old man who had undergone a modified Bentall procedure and was asymptomatic thereafter. Seven months after that operation, exercise stress testing showed electrocardiographic signs of asymptomatic myocardial ischemia at high workload, and coronary angiography revealed severe nonatherosclerotic left main ostial stenosis. Percutaneous coronary intervention and stenting of the unprotected left main stenosis was successful, and patency at 8 months was apparent on coronary angiography.
The conventional treatment for coronary ostial stenosis, coronary artery bypass grafting, carries a high risk of perioperative infarction, morbidity, and death. We found that percutaneous coronary intervention with stenting yielded positive short- and long-term results and may provide an alternative to cardiac surgery in these high-risk patients. We recommend that physicians evaluate even asymptomatic patients for left main coronary ostial stenosis after aortic surgery so that early diagnosis and treatment can avert severe clinical manifestations.
Key words: Aortic diseases/surgery, awareness, coronary angiography/methods, coronary disease/radiography, coronary stenosis/diagnosis/etiology/therapy, exercise/physiology, iatrogenic disease, percutaneous coronary intervention, postoperative complications/therapy, treatment outcome
Coronary ostial stenosis is a potentially fatal sequela of aortic valve or aortic root replacement. Although this stenosis is rare, with a reported prevalence of 0.3% to 5% after aortic valve replacement,1-3 it can lead to catastrophic consequences. Iatrogenic coronary artery lesions can also develop in asymptomatic patients. Here, we discuss what we believe is the first case of asymptomatic ischemia caused by iatrogenic coronary ostial stenosis, in an active man who had undergone a modified Bentall procedure for acute aortic dissection.
Case Report
In October 2008, a 34-year-old man with acute type A aortic dissection and acute aortic regurgitation underwent emergency aortic root replacement. He had emergently presented at another hospital with a sharp pain in the anterior portion of the chest. His coronary risk factors included dyslipidemia and status as a former smoker. He had no history of trauma and no features of Marfan syndrome; however, his father had been diagnosed with an ascending aortic aneurysm. At the other hospital, multidetector computed tomography (MDCT) with contrast medium revealed dissection of the ascending aorta, aortic root, and aortic arch.
Aortic surgery was performed through a median sternotomy. After the institution of cardiopulmonary bypass between the right axillary artery and right atrium, cardioplegic arrest was achieved by means of intermittent anterograde administration of Custodiol® Histidine-Tryptophan-Ketoglutarate-HTK cardioplegic solution (Essential Pharmaceuticals, LLC; Newtown, Pa). Soft, flexible CalMed self-inflating balloon-tipped coronary artery perfusion cannulae (California Medical Laboratories; Costa Mesa, Calif) were placed in the coronary ostia at a flow rate of 250 mL/min (2,000 mL in 8 min).
Upon visual inspection, the dissection was seen to involve the ascending aorta and aortic arch, with an initial cleft in the aortic bulb. The aortic valve cusps were prolapsing but intact. A circumferential intimal tear was present at the level of the valve annulus. There was no involvement of the coronary ostia.
The coronary ostia were isolated in accordance with the modified button technique. The aortic valve and ascending aorta were replaced with a 23/26-mm mechanical valved tube-graft (Sorin Biomedica S.p.A.; Milan, Italy). To prevent bleeding, the coronary buttons were reinforced with a circumferential Teflon patch. The coronary ostia were reimplanted with a continuous Prolene® 6-0 suture (Ethicon, a Johnson & Johnson company; Somerville, NJ). To consolidate the aortic wall, a gelatin-resorcin-formol (GRF) glue was applied to all of the anastomoses.
Because the aortic arch was involved in the dissection and the distal intimal tear was present in the descending aorta just under the origin of the left subclavian artery, the aortic arch was replaced with a 26-mm Hemashield® Dacron tubular linear conduit (Boston Scientific Corporation; Natick, Mass), and a No. 4 morphometric Djumbodis® Dissection System bare-stent endoprosthesis (Saint Côme Chirurgie; Marseilles, France) was placed in the descending aorta. Surgery was completed uneventfully, and the patient was discharged from the hospital 9 days later on prescribed warfarin, β-blocker, and angiotensin-converting enzyme inhibitor therapy. Control of the patient's blood pressure was excellent, and the international normalized ratio of 2.64 was within therapeutic range. The patient was asymptomatic and undertook a personalized rehabilitation program, which caused him no angina or dyspnea.
For careful evaluation of the results of the aortic surgery and the status of the remaining aorta, MDCT with contrast medium was used.4 Two thoracic MDCT examinations, one in November 2008 and one in April 2009, showed the stability of the residual distal dissection flap under the origin of the subclavian artery that had been implanted with the Hemashield conduit.
In May 2009, the patient underwent an ergonometric stress test on a bicycle (protocols, 20 W × 1') as part of a cardiovascular rehabilitation evaluation.5,6 The test results yielded strong electrocardiographic signs of asymptomatic myocardial ischemia at high workload (140 W): diffuse ST-segment downsloping (0.4 mV at 80 ms after the J point) and T-wave inversion (Fig. 1). These values returned to baseline levels 5 min after exercise. Coronary angiography revealed severe nonatherosclerotic left main ostial stenosis (70%), a normal right coronary ostium, and no other lesions in the coronary tree (Fig. 2).
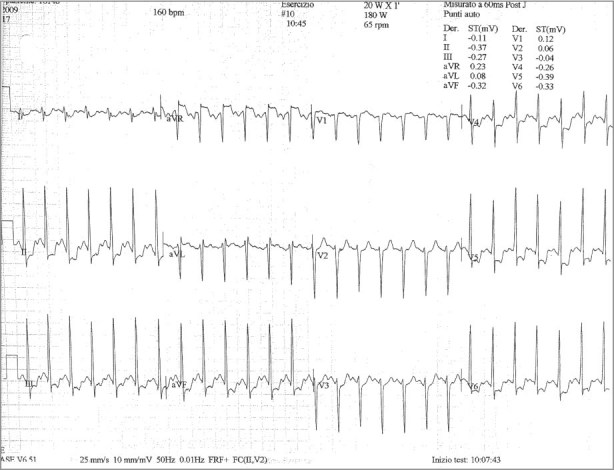
Fig. 1 Electrocardiogram during stress testing shows diffuse ST-segment downsloping and T-wave inversion at high workload.
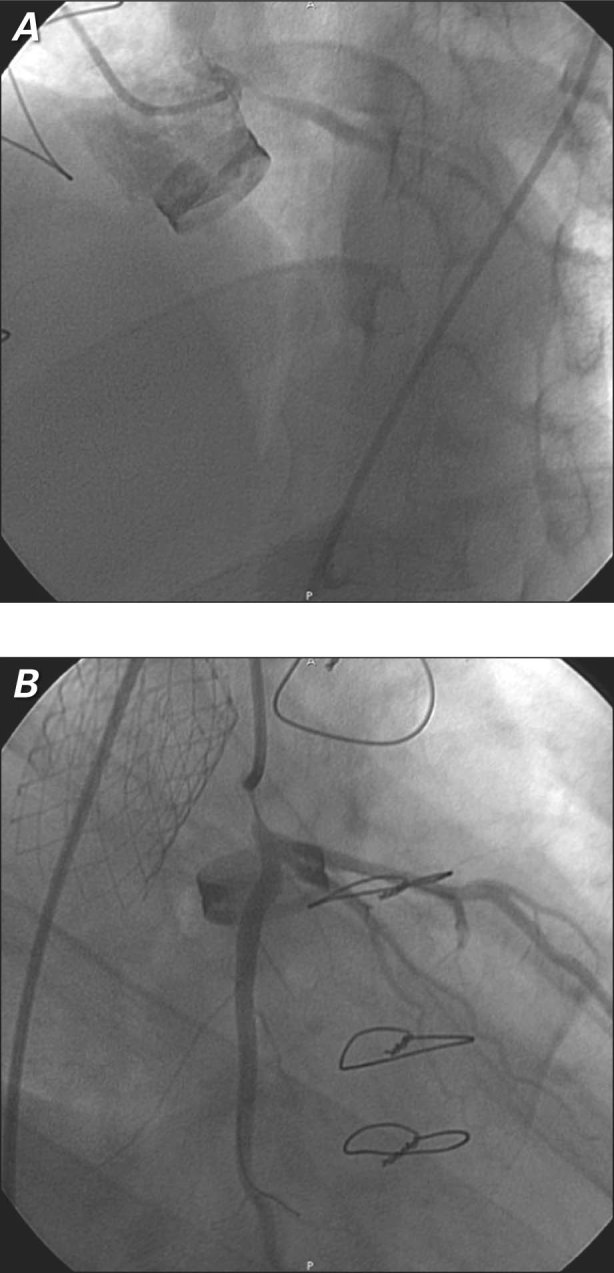
Fig. 2 Coronary angiograms after a Bentall procedure show severe nonatherosclerotic left main stenosis in A) right and B) left anterior views.
On the basis of our experience and results reported in similar cases, we decided to perform unprotected left main stenting.2,7 Unfractionated heparin was administered in order to achieve an activated coagulation time between 250 and 300 s. Two 0.014-in Hi-Torque® Floppy II guidewires (Abbott Vascular, part of Abbott Laboratories; Abbott Park, Ill) were advanced into the left anterior descending coronary artery and the left circumflex branch through a 6F JL4 guiding catheter (Cordis Corporation, a Johnson & Johnson company; Miami Lakes, Fla). We predilated the left main stenosis with a 3 × 15-mm Maverick® 2 Monorail™ balloon catheter (Boston Scientific) that was inflated to a pressure of 14 atm for 10 s. We then placed a 4 × 13-mm PRO-Kinetic Energy® coronary bare-metal stent (Biotronik AG; Bülach, Switzerland) that was inflated to a pressure of 16 atm for 15 s (Fig. 3A), and we performed postdilation with use of a 4.5 × 12-mm Quantum™ Maverick® Balloon Catheter (Boston Scientific) at high pressure. This yielded Thrombolysis in Myocardial Infarction grade 3 flow (Fig. 3B). The patient was discharged from the hospital on dual antiplatelet and warfarin therapy. In January 2010, repeat coronary angiography showed patency of the previously implanted stent. In December 2010, the patient was asymptomatic and had experienced no major or minor cardiac events.
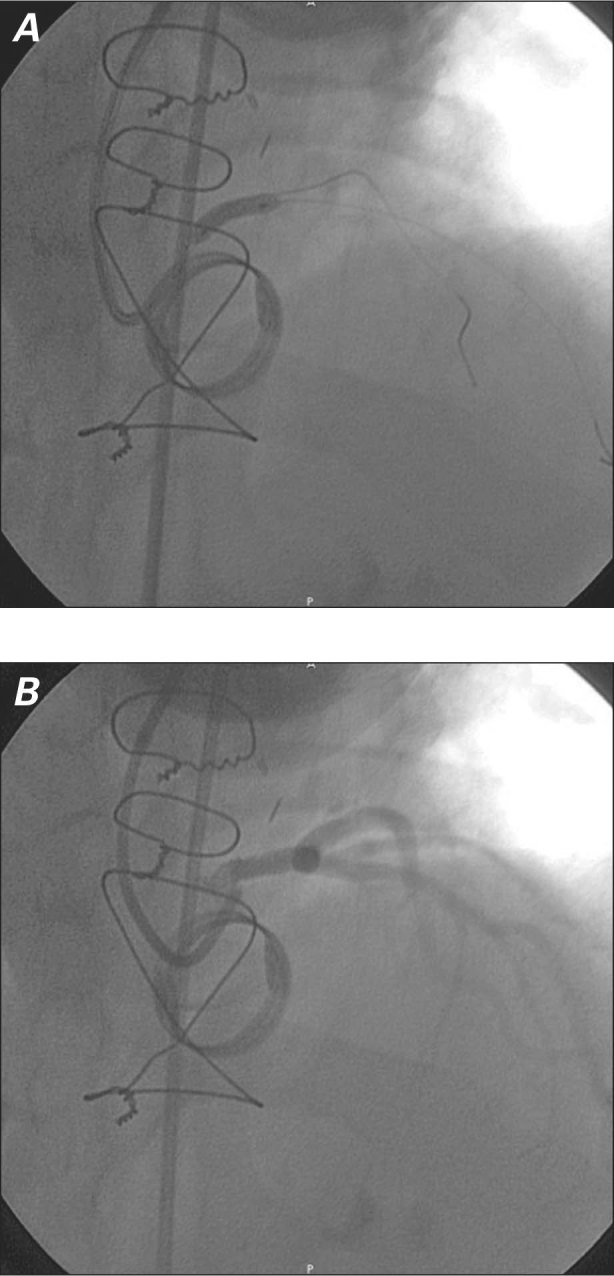
Fig. 3 Percutaneous coronary intervention in iatrogenic unprotected left main stenosis after 8 months. Coronary angiograms (right anterior view) show A) placement of a 4 × 13-mm bare-metal stent, and B) resultant Thrombolysis in Myocardial Infarction grade 3 flow.
Discussion
Previously, it was standard practice to perform routine coronary angiography after percutaneous coronary intervention (PCI) to the unprotected left main coronary artery, in view of concerns about severe consequences if restenosis were to occur. However, this recommendation was removed from the American College of Cardiology/American Heart Association/Society for Cardiovascular and Angiographic Interventions guidelines in 2009, because it was thought to be difficult to predict angiographically which patients could experience stent thrombosis, in addition to the unnecessary risks to the patient posed by angiography after left main stenting. Therefore, evaluation with coronary angiography is not currently recommended unless specific procedural features cause concern about stent durability—or, as in our patient, left main iatrogenic lesions.
Initially reported by Roberts and Morrow in 1967,8 iatrogenic coronary stenosis is a possible (albeit rare) sequela of cardiac surgery, including aortic valve and aortic root replacement. Because most descriptions of iatrogenic left main stenosis are found in case reports or small series, it is difficult to estimate the true prevalence of the condition. It has been detected in 0.3% to 5% of patients who have undergone aortic valve replacement.1-3 The frequency is probably underestimated, because some undiagnosed cases of sudden death after aortic valve replacement might be attributable to iatrogenic coronary ostial stenosis.9
Iatrogenic stenosis can present clinically as severe angina, ventricular arrhythmias, congestive heart failure, or sudden death. These manifestations are usually observed within the first 6 postprocedural months, but they may occur as late as 30 months postoperatively. In several case reports that describe this sequela, authors have postulated a variety of mechanisms underlying these lesions and have compared the surgical and percutaneous approaches to repair.2,7,10,11 From a pathologic standpoint, this kind of coronary ostial stenosis occurs consequent to a fibrous, inflammatory reaction, with intimal fibrous thickening in the absence of atherosclerotic lesions.
Anterograde cardioplegic administration can cause mechanical injury to the coronary ostia, by either direct trauma (iatrogenic damage during cannulation, or prolonged contact of the perfusion catheter's tip with the coronary intima) or indirect trauma (infusion pressure of the cardioplegic solution). Similarly, patients with prosthetic aortic valves can develop coronary ostial stenosis consequent to turbulent flow in the aortic root.12
Individual predisposition may be a factor in the pathogenesis of coronary ostial stenosis. Winkelmann and colleagues13 found a correlation between apolipoprotein-E genotype 4 and left main ostial stenosis after aortic valve replacement. Moreover, Tsukiji and associates14 reported an immunologic reaction to a heterograft as a potential mechanism of coronary ostial stenosis. Various mechanisms underlie the possible extrinsic compression of the coronary ostia: the mobilization of coronary vessels during the Bentall procedure (with or without imperfect suturing of the coronary buttons) can stretch and twist the vessels, and GRF glue used to treat coronary-button hemorrhaging can give rise to an intensive fibrous reaction that results in extrinsic compression.7
Another possible explanation is spontaneous type A aortic dissection with retrograde propagation into the coronary ostia. Although our patient had acute aortic dissection at surgery, there was no evidence of retrograde dissection of the coronary ostia; however, this complication usually occurs along with perioperative myocardial ischemia or infarction. In the differential diagnosis, we excluded iatrogenic dissection of the coronary ostia related to cardioplegia, because if this had been the case, it would have been difficult to wean the patient from extracorporeal circulation. The asymptomatic and late presentation of our patient's stenosis suggests to us that the coronary ostial narrowing was due to progressive shrinkage from an inflammatory response to the GRF glue.
Methods for the diagnosis of coronary ostial stenosis include selective coronary angiography and MDCT. Routine MDCT with evaluation of the coronary tree is a promising and noninvasive imaging technique that is effective in early detection and in evaluating the degree of stenosis with high sensitivity. The coronary MDCT enables accurate viewing of the left main system through multiplanar reconstructions, as well as density evaluation of plaque, and virtual histology. Authors have reported that this imaging method reveals both coronary ostial narrowing and residual or late dissection of the aorta.15,16
After an acute event, particularly in a relatively young and active man, special attention should be paid to formulating an appropriate rehabilitation program. Exercise on a bicycle with an ergonometric apparatus can provide insights into a patient's functional capacity, training intensity, and quality of life.5,6,17 Our patient's case affirms that exercise testing can play a key role in the diagnosis and evaluation of coronary ostial stenosis and its effects.
Because a large amount of myocardium is jeopardized by coronary ostial stenosis (especially left main stenosis), revascularization is required. At present, coronary artery bypass grafting (CABG) is the treatment of choice for unprotected left main disease, according to American Heart Association and European Society of Cardiology guidelines.18,19 However, technical and clinical difficulties accompany repeat surgery soon after the original operation, and CABG can result in high rates of perioperative infarction, morbidity, and death.20 Percutaneous coronary intervention may be feasible, safe, and effective in these postoperative patients as an alternative to CABG.2,7,11,21–23
The choice of the type of stent should always depend upon the patient's characteristics and the nature of the lesion. Drug-eluting-stent implantation would require dual antiplatelet therapy for 12 months. This possibility should be considered in a patient who already needs lifelong anticoagulation therapy, or in patients with a history of aortic dissection. From an anatomic standpoint, vessel diameter and the location of the disease are important considerations when choosing the appropriate type of stent. A large vessel diameter, together with an ostial location of the disease, led us to select a bare-metal stent for our patient. In our experience, PCI with stenting in unprotected left main disease is safe and effective, both immediately and in the long term, as an alternative to CABG.
Conclusion
Although left main ostial stenosis after aortic valve and root replacement has been described for over 40 years, it remains a challenge that requires immediate diagnosis and treatment in order to increase the patient's chances of survival.
To our knowledge, this is the first report of asymptomatic iatrogenic left main stenosis after aortic surgery. The stenosis developed in an active, relatively young patient. We recommend that physicians, cardiologists, and cardiac surgeons keep the possibility of this sequela in mind, and we believe that great care is required in the monitoring and management of these patients. Early coronary MDCT or early stress testing in the first postoperative year can reveal even asymptomatic left main disease and avert most of the severe clinical consequences. The case of our patient also shows the acute and long-term effectiveness of PCI as treatment for iatrogenic left main ostial stenosis after a Bentall procedure. Patients undergoing PCI in an unprotected left main coronary artery are at risk for adverse events and should be monitored carefully. Early evaluation with coronary angiography after PCI should be considered in order to confirm the long-term results of the PCI.
Footnotes
Address for reprints: Chiara Bernelli, MD, Cardiology Unit, DIMI–Department of Internal Medicine, Viale Benedetto XV/6, 16132 Genova, Italy, E-mail: chiarabernelli@yahoo.it
References
- 1.Pande AK, Gosselin G. Iatrogenic left main coronary artery stenosis. J Invasive Cardiol 1995;7(6):183–7. [PubMed]
- 2.Cola C, Yuste VM, Sabate M. Left main coronary artery stenosis following surgical valve replacement: changing valvular into ischemic heart disease. J Invasive Cardiol 2009;21(1):E9–11. [PubMed]
- 3.An Y, Tamita K, Furukawa Y. Iatrogenic coronary artery stenosis at the ostium of left anterior descending artery after aortic valve replacement: a case report with imaging and histological findings. J Invasive Cardiol 2010;22(12):E206–8. [PubMed]
- 4.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine [published erratum appears in Circulation 2010; 122(4):e410]. Circulation 2010;121(13):e266–369. [DOI] [PubMed]
- 5.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) [published erratum appears in J Am Coll Cardiol 2006;48(8):1731]. J Am Coll Cardiol 2002;40(8):1531–40. [DOI] [PubMed]
- 6.Guilmet D, Bonnet N, Saal JP, Le Houerou D, Ghorayeb G. Long term survival with the Bentall button operation in 150 patients [in French]. Arch Mal Coeur Vaiss 2004;97(2):83–91. [PubMed]
- 7.Balbi M, Olivotti L, Scarano F, Bertero G, Passerone G, Brunelli C, Barsotti A. Percutaneous treatment of left main coronary stenosis as a late complication of Bentall operation for acute aortic dissection. Catheter Cardiovasc Interv 2004; 62(3):343–5. [DOI] [PubMed]
- 8.Roberts WC, Morrow AG. Anatomic studies of hearts containing caged-ball prosthetic valves. Johns Hopkins Med J 1967;121(4):271–95. [PubMed]
- 9.Hadjimiltiades S, Harokopos N, Papadopoulos C, Gourassas I, Spanos P, Louridas G. Left main coronary artery stenosis after aortic valve replacement. Hellenic J Cardiol 2005;46(4): 306–9. [PubMed]
- 10.Pillai JB, Pillay TM, Ahmad J. Coronary ostial stenosis after aortic valve replacement, revisited. Ann Thorac Surg 2004;78 (6):2169–71. [DOI] [PubMed]
- 11.Rath S, Goor DA, Har-Zahav Y, Buttler A, Ziskind Z. Coronary ostial stenosis after aortic valve replacement without coronary cannulation. Am J Cardiol 1988;61(13):1156–7. [DOI] [PubMed]
- 12.Force TL, Raabe DS Jr, Coffin LH, DeMeules JD. Coronary ostial stenosis following aortic valve replacement without continuous coronary perfusion. J Thorac Cardiovasc Surg 1980;80(4):637–41. [PubMed]
- 13.Winkelmann BR, Ihnken K, Beyersdorf F, Eckel L, Skupin M, Marz W, et al. Left main coronary artery stenosis after aortic valve replacement: genetic disposition for accelerated arteriosclerosis after injury of the intact human coronary artery? Coron Artery Dis 1993;4(7):659–67. [DOI] [PubMed]
- 14.Tsukiji M, Akasaka T, Wada N, Okahashi N, Kume T, Yoshitani H, et al. Bilateral coronary ostial stenosis after aortic valve replacement with Freestyle stentless bioprosthesis: a case report [in Japanese]. J Cardiol 2004;44(5):207–13. [PubMed]
- 15.Shenoda M, Barack BM, Toggart E, Chang DS. Use of coronary computed tomography angiography to detect coronary ostial stenosis after Bentall procedure. J Cardiovasc Comput Tomogr 2009;3(5):340–3. [DOI] [PubMed]
- 16.Funada A, Mizuno S, Ohsato K, Murakami T, Moriuchi I, Misawa K, et al. Three cases of iatrogenic coronary ostial stenosis after aortic valve replacement. Circ J 2006;70(10):1312–7. [DOI] [PubMed]
- 17.Piepoli MF, Corra U, Benzer W, Bjarnason-Wehrens B, Dendale P, Gaita D, et al. Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil 2010;17(1):1–17. [DOI] [PubMed]
- 18.Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS), European Association for Percutaneous Cardiovascular Interventions (EAPCI), Wijns W, Kolh P, Danchin N, Di Mario C, et al. Guidelines on myocardial revascularization. Eur Heart J 2010;31(20): 2501–55.20802248
- 19.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58(24):e44–122. [DOI] [PubMed]
- 20.Chavanon O, Carrier M, Cartier R, Hebert Y, Pellerin M, Perrault LP. Early reoperation for iatrogenic left main stenosis after aortic valve replacement: a perilous situation. Cardiovasc Surg 2002;10(3):256–63. [DOI] [PubMed]
- 21.Franck H, Weber K, Frese W, Pieper MJ. Stent-supported angioplasty of an ostial left main stenosis following replacement of the ascending aorta with reimplantation of the coronary arteries. J Invasive Cardiol 1999;11(9):571–4. [PubMed]
- 22.Worthley MI, Burgess J, Traboulsi M. Bilateral coronary ostial stenoses post-Bentall procedure: management options in the DES era. J Invasive Cardiol 2005;17(12):680–2. [PubMed]
- 23.Marti V, Auge JM, Garcia Picart J, Guiteras P, Ballester M, Obrador D. Percutaneous transluminal coronary angioplasty as alternative treatment to coronary artery bypass surgery in iatrogenic stenosis of the left main coronary artery. J Interv Cardiol 1995;8(3):229–31. [DOI] [PubMed]


