Abstract
Deep sternal wound infection remains one of the most serious complications in patients who undergo median sternotomy for coronary artery bypass surgery.
We describe our experience in treating 6 consecutive patients with our treatment protocol that combines aggressive débridement, broad-spectrum antibiotics, negative-pressure wound therapy, omentoplasty with laparoscopically harvested omentum, and the use of bilateral pectoral muscle advancement flaps.
The number of débridements needed in order to attain clinically clean wounds and negative cultures varied between 1 and 10, with a median of 5. The length of stay after omentoplasty and bilateral pectoral muscle advancement flap placement varied between 11 and 22 days. One of the 6 patients developed a small wound dehiscence that was treated conservatively. No bleeding related to vacuum-assisted closure therapy was identified. Three patients had pneumonia. Two of the 3 patients had an episode of acute renal failure. The 30-day mortality rate was zero, although 1 patient died in the hospital 43 days after the reconstructive surgery, of multiple-organ failure due to pneumonia that was induced by end-stage pulmonary fibrosis. No patient died between hospital discharge and the most recent follow-up date (4–12 mo). Late local follow-up results, both functional and aesthetic, were good.
We conclude that negative-pressure wound therapy—in combination with omentoplasty using laparoscopically harvested omentum and with the use of bilateral pectoral advancement flaps—is a valuable technique in the treatment of deep sternal wound infection because it produces good functional and aesthetic results.
Key words: Coronary artery bypass grafting, debridement, deep sternal wound infection, laparoscopy, mediastinitis, negative-pressure wound therapy, omentoplasty, surgical flaps, surgical wound infection/mortality/surgery
Since the implementation of perioperative preventive measures, the incidence of deep sternal wound infection after median sternotomy has not been higher than 2% in most centers.1-5 Nevertheless, such infection is one of the most serious complications in patients undergoing cardiac surgery, because it dramatically increases rates of both morbidity and mortality.6,7 Post-sternotomy mediastinitis after coronary artery bypass grafting (CABG) is also indicative of a poor prognosis for long-term survival.6,7
Therefore, treatment should be aggressive and requires the removal of all necrotic tissues and total resection of the infected sternum. Various approaches to reconstruction after sternal débridement have been reported.1-3,8–10 We describe our experience with a combination of negative-pressure wound therapy, laparoscopic omentoplasty, and bilateral pectoral muscle flaps for deep sternal wound infections.
Patients and Methods
This study was conducted in the Center for Heart and Vascular Diseases of the UZ Brussel (Universitair Ziekenhuis Brussel) from May 2008 through June 2010. Patients who underwent CABG through a median sternotomy and developed a deep sternal wound infection were included. After the study was begun, the diagnosis of deep sternal wound infection was defined using the Centers for Disease Control (CDC) criteria for surgical-site infections (SSI).11 In addition, all infections were classified in accordance with the El Oakley-Wright classification system.3
Standard perioperative antiseptic measures were taken and a prophylactic antibiotic agent (cefazoline) was administered.
Treatment Protocol
On the basis of our experience,10,11 the treatment protocol for deep sternal wound infections in our center consists of 3 surgical steps, together with the use of broad-spectrum antibiotics:
Early, aggressive, and serial débridement. All necrotic tissue is removed, until the wound becomes clinically clean and wound cultures become (and remain) negative.
Topical negative-pressure wound therapy, which is, by standard, applied using a commercially available V.A.C.® Therapy system (KCI Concepts, Inc.; San Antonio, Texas). To minimize possible complications, like bleeding and right ventricular rupture, we have routinely placed Mepitel® silicone dressings (Mölnlycke Health Care; Gothenburg, Sweden) under the polyurethane foam and the adhesive dressing. A continuous negative pressure of −125 mmHg is applied.
Surgical reconstruction of the defect, which is done with a laparoscopically harvested omental flap, together with bilateral pectoral muscle advancement flaps.
Surgical Technique
Omentoplasty
After the creation of a pneumoperitoneum by insufflating carbon dioxide in the abdomen, 4 trocars were placed: one 10-mm umbilical trocar, one 10-mm trocar in each lumbar region, and one 5-mm trocar in the epigastric region. A 10-mm, 30° camera was used. First, the gastrocolic ligament was divided until the omental bursa was reached, using an UltraCision® harmonic scalpel. Then the greater omentum was dissected from the transverse colon. The omentum was divided together with the left gastroepiploic artery, starting at the smaller splenic vessels and close to the greater curvature of the stomach. The omentum was progressively divided from the greater curvature. Thus, a large omental flap, vascularized by the dominant right gastroepiploic artery, was created. Then, the flap was guided to the sternal defect through a substernal, transdiaphragmatic opening.
Bilateral Pectoral Muscle Advancement Flaps
Subsequently, both pectoralis major muscles were fully mobilized after division of their costal insertion; they were advanced and sutured together without tension on the midline above the omental flap. The subcutaneous tissue and skin were closed. Closed suction drains were left in the pectoralis pockets, in the lower part of the mediastinum, and below the omental flap.
Antibiotics were continued intravenously for 2 to 4 weeks after omentomyoplasty.
Results
Patients
A series of 6 patients with consecutive deep sternal wound infection was identified and treated following our protocol (see above). Three cases were classified as El Oakley-Wright class IIIa mediastinitis, 1 as class IIIb, and 2 as class IVb. Preoperative characteristics and risk factors can be found in Table I. All patients were men. The EuroSCORE-predicted mortality rate varied between 1.49% and 18.72%. No patient had preoperative renal insufficiency, nor was any patient receiving immunosuppressive therapy.
TABLE I. Preoperative Characteristics of the 6 Patients
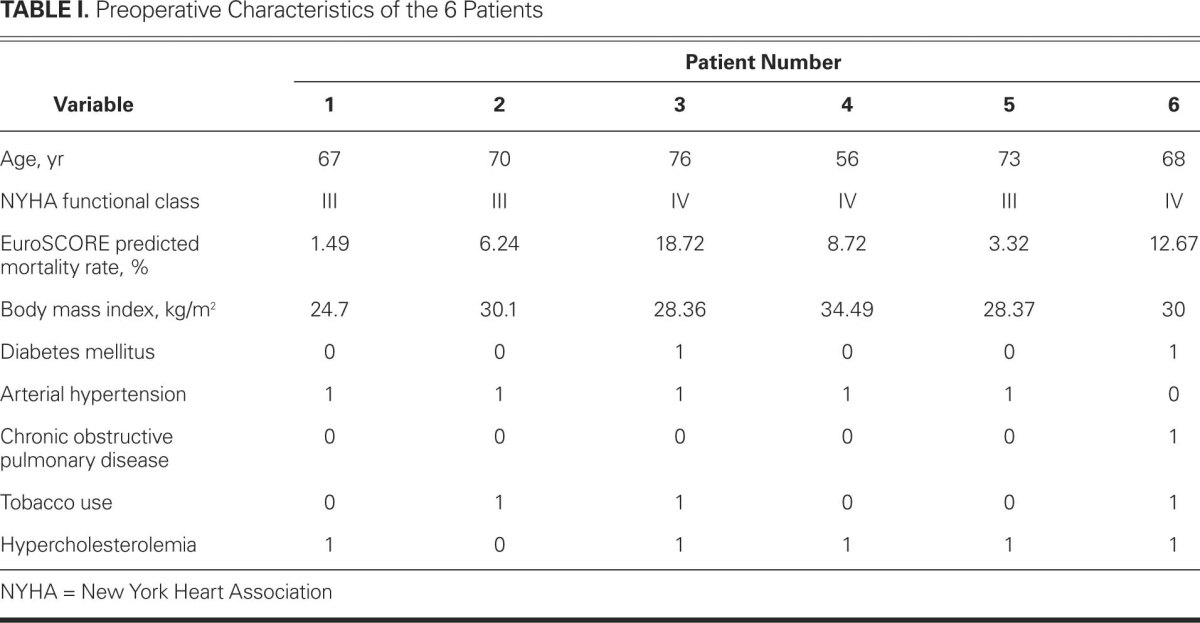
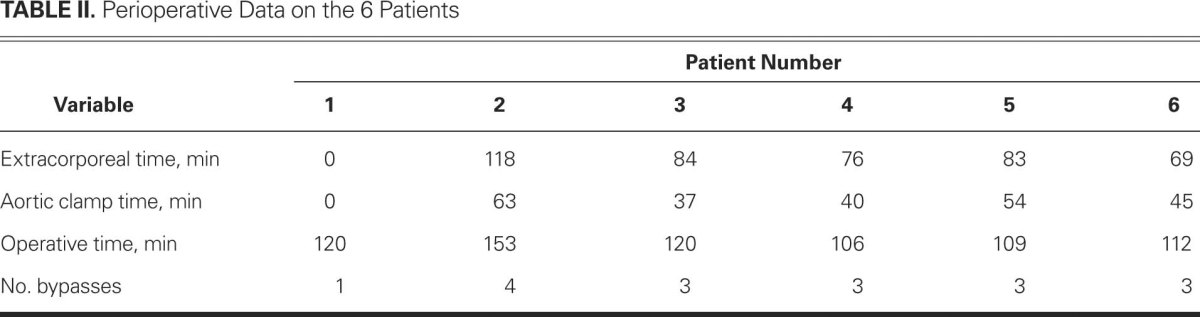
All patients underwent isolated CABG. One patient's operation was off-pump. Three of the 6 patients were diagnosed with left main stem stenosis and underwent the operation in an emergency setting. No patient underwent bilateral harvesting of the internal thoracic arteries. Blood loss varied between 250 and 1000 mL. Four of the 6 patients needed postoperative transfusion of packed red blood cells. Table II shows the extracorporeal time and other preoperatively collected data.
TABLE II. Perioperative Data on the 6 Patients
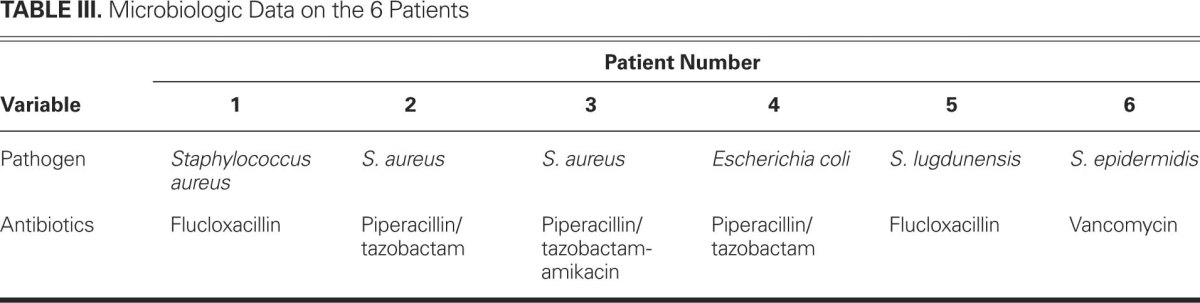
The pathogens were mainly staphylococci (5 of 6 patients). The microbiologic data and the target-directed antibiotic therapy can be found in Table III.
TABLE III. Microbiologic Data on the 6 Patients
The time intervals, the number of débridements, and the early and late outcomes can be found in Table IV.
TABLE IV. Outcomes in the 6 Patients

Morbidity and Death
Early complications were divided into local and systemic. One patient developed a small wound dehiscence that was treated conservatively. No bleeding related to vacuum-assisted closure therapy was identified. No patient developed abdominal sepsis. Four of the 6 patients developed systemic complications. Of the 3 patients who developed pneumonia, 2 had an episode of acute renal failure, which in 1 patient required temporary renal replacement therapy. The 30-day mortality rate was zero, although 1 patient died in the hospital 43 days after reconstructive surgery, of multiple-organ failure due to pneumonia that was induced by end-stage pulmonary fibrosis. No patient died between hospital discharge and the most recent follow-up date. Follow-up ranged between 4 and 28 months (average follow-up time, 13.5 mo). Late local follow-up results, both functional and aesthetic, were good. The patient who experienced small-wound dehiscence in the hospital was found, at his latest follow-up clinical examination, to have a small epigastric hernia.
Discussion
Even today, the treatment of deep sternal wound infections remains a challenge. In the past, the initial treatment existed of surgical revision, surgical rewiring, and secondary healing.12 Then Shumacker and Mandelbaum13 introduced the concept of continuous irrigation systems. Both methods had enormous mortality rates (up to 45%). Jurkiewicz and colleagues1 first described the treatment of infected median sternotomy with muscle flaps. This introduction of vascularized soft-tissue flaps reduced mortality rates. Therefore, many types of flaps have been proposed. All of them have had disadvantages, such as lack of volume or inadequate defect coverage (for example, pectoral muscle flaps) or substantial donor-site morbidity (for example, rectus abdominis muscle).14-16
The omentum has been shown to be a good flap, because of its resistance to infections. It is well vascularized, has a large number of immunologically active cells, and absorbs wound secretions.8
However, omentoplasty has been reserved as the ultimate treatment strategy for deep sternal wound infection,8 due to the high morbiditity associated with opening the abdominal cavity, which can lead to contamination and spreading of the infection. Laparotomy has also been known to harm respiratory function and to generate substantial pain. Recent advances in endoscopy have helped surgeons to find techniques to harvest the omentum laparoscopically. This has helped to minimize donor-site morbidity. The first endoscopic harvesting of the omentum was described in 1993 by Saltz and colleagues.17 The use of endoscopically harvested omental flaps for deep sternal wound infection has been reported before.17-21 Yet, data on the combination of topical negative-pressure therapy and bilateral pectoral muscle flaps are very limited.22,23
Recently, the use of topical negative-pressure therapy or vacuum-assisted closure was introduced in wound-healing management.24,25 It has many possible advantages, like absorption of wound exudates, stimulation of granulation-tissue formation, increase of blood flow in adjacent tissues, approximation of wound edges, and chest-wall stabilization. Some authors have suggested an overall survival benefit when negative-pressure therapy is compared with conventional treatment for deep sternal wound infections.26,27 For this reason, we implemented this method in our treatment protocol.
A previous study that used aggressive primary treatment and omental and muscle flaps showed relatively good outcomes in stable patients, yet outcomes were questionable in hemodynamically compromised patients.10 In these cases, topical negative therapy might also be a good bridge between initial débridement and definitive reconstructive surgery.
Bleeding complications and right ventricular rupture remain a concern when applying topical negative-pressure therapy in cases of deep sternal wound infection. Recently, as shown by Sjögren and associates,28 this risk seems to be outweighed by the benefit of superior infection control. Sjögren's results are supported by our results, because none of our patients had a right ventricular rupture or a major bleeding complication.
Whether the left or right gastroepiploic artery should be used to pedicle the omental flap remains a matter of debate.15 The right gastroepiploic artery was used in our series, because it is anatomically the largest. Although the left gastroepiploic is closer to the sternal defect, in our experience the omentum had sufficient bulk to cover the sternal defect without the need of further lengthening.
Another issue of discussion is the way the omental flap has to be guided to the thoracic cavity. We chose the transdiaphragmatic route, because some authors have said that it reduces operative time and trauma without increasing morbidity.15 Nevertheless, 1 of our 6 patients developed an epigastric hernia.
As mentioned by other investigators, the bilateral pectoral muscle flaps are often inadequate to fill the sternal defect. Desinsertion, rotatation, or denervation of the flaps can also cause functional deficits. The use of advancement flaps sutured over the midline might minimize these potential problems.9
In our experience, the use of the bilateral pectoral muscle advancement flaps over the omentoplasty resulted in excellent early and late results. The aesthetic result was especially pleasing.
Conclusion
Early detection and aggressive débridement, together with the use of broad-spectrum antibiotics, remain the main steps in controlling deep sternal wound infections. Vacuum-assisted closure therapy has proved to reduce mortality rates in patients with deep sternal wound infections.
We conclude that negative-pressure wound therapy—in combination with omentoplasty using laparoscopically harvested omentum and with the use of bilateral pectoral advancement flaps—is a valuable technique in the treatment of deep sternal wound infection because it produces good functional and aesthetic results. It eliminates the possible disadvantages of both omentoplasty and bilateral pectoral advancement, without adding significant morbidity.
Footnotes
Address for reprints: Kristof De Brabandere, MD, UZ Brussel, Laarbeeklaan 101, 1090 Brussels, Belgium, E-mail: kristof.de.brabandere@gmail.com
References
- 1.Jurkiewicz MJ, Bostwick J 3rd, Hester TR, Bishop JB, Craver J. Infected median sternotomy wound. Successful treatment by muscle flaps. Ann Surg 1980;191(6):738–44. [DOI] [PMC free article] [PubMed]
- 2.Merrill WH, Akhter SA, Wolf RK, Schneeberger EW, Flege JB Jr. Simplified treatment of postoperative mediastinitis. Ann Thorac Surg 2004;78(2):608–12. [DOI] [PubMed]
- 3.El Oakley RM, Wright JE. Postoperative mediastinitis: classification and management. Ann Thorac Surg 1996;61(3): 1030–6. [DOI] [PubMed]
- 4.Baskett RJ, MacDougall CE, Ross DB. Is mediastinitis a preventable complication? A 10-year review. Ann Thorac Surg 1999;67(2):462–5. [DOI] [PubMed]
- 5.Gualis J, Florez S, Tamayo E, Alvarez FJ, Castrodeza J, Castano M. Risk factors for mediastinitis and endocarditis after cardiac surgery. Asian Cardiovasc Thorac Ann 2009;17(6): 612–6. [DOI] [PubMed]
- 6.Ariyaratnam P, Bland M, Loubani M. Risk factors and mortality associated with deep sternal wound infections following coronary bypass surgery with or without concomitant procedures in a UK population: a basis for a new risk model? Interact Cardiovasc Thorac Surg 2010;11(5):543–6. [DOI] [PubMed]
- 7.Risnes I, Abdelnoor M, Almdahl SM, Svennevig JL. Mediastinitis after coronary artery bypass grafting risk factors and long-term survival. Ann Thorac Surg 2010;89(5):1502–9. [DOI] [PubMed]
- 8.Athanassiadi K, Theakos N, Benakis G, Kakaris S, Skottis I. Omental transposition: the final solution for major sternal wound infection. Asian Cardiovasc Thorac Ann 2007;15(3): 200–3. [DOI] [PubMed]
- 9.Tomos P, Lachanas E, Michail PO, Kostakis A. Alternative bi-pectoral muscle flaps for postoperative sternotomy mediastinitis. Ann Thorac Surg 2006;81(2):754–5. [DOI] [PubMed]
- 10.Schroeyers P, Wellens F, Degrieck I, De Geest R, Van Praet F, Vermeulen Y, Vanermen H. Aggressive primary treatment for poststernotomy acute mediastinitis: our experience with omental- and muscle flaps surgery. Eur J Cardiothorac Surg 2001;20(4):743–6. [DOI] [PubMed]
- 11.Wouters R, Wellens F, Vanermen H, De Geest R, Degrieck I, De Meerleer F. Sternitis and mediastinitis after coronary artery bypass grafting. Analysis of risk factors. Tex Heart Inst J 1994;21(3):183–8. [PMC free article] [PubMed]
- 12.Sarr MG, Gott VL, Townsend TR. Mediastinal infection after cardiac surgery. Ann Thorac Surg 1984;38(4):415–23. [DOI] [PubMed]
- 13.Shumacker HB Jr, Mandelbaum I. Continuous antibiotic irrigation in the treatment of infection. Arch Surg 1963;86:384–7. [DOI] [PubMed]
- 14.Netscher DT, Eladoumikdachi F, McHugh PM, Thornby J, Soltero E. Sternal wound debridement and muscle flap reconstruction: functional implications. Ann Plast Surg 2003;51 (2):115–25. [DOI] [PubMed]
- 15.van Wingerden JJ, Coret ME, van Nieuwenhoven CA, Totte ER. The laparoscopically harvested omental flap for deep sternal wound infection. Eur J Cardiothorac Surg 2010;37(1):87–92. [DOI] [PubMed]
- 16.Ringelman PR, Vander Kolk CA, Cameron D, Baumgartner WA, Manson PN. Long-term results of flap reconstruction in median sternotomy wound infections. Plast Reconstr Surg 1994;93(6):1208–16. [PubMed]
- 17.Saltz R, Stowers R, Smith M, Gadacz TR. Laparoscopically harvested omental free flap to cover a large soft tissue defect. Ann Surg 1993;217(5):542–67. [DOI] [PMC free article] [PubMed]
- 18.Acarturk TO, Swartz WM, Luketich J, Quinlin RF, Edington H. Laparoscopically harvested omental flap for chest wall and intrathoracic reconstruction. Ann Plast Surg 2004;53(3):210–6. [DOI] [PubMed]
- 19.Puma F, Fedeli C, Ottavi P, Porcaro G, Battista Fonsi G, Pardini A, Daddi G. Laparoscopic omental flap for the treatment of major sternal wound infection after cardiac surgery. J Thorac Cardiovasc Surg 2003;126(6):1998–2002. [DOI] [PubMed]
- 20.Milano CA, Georgiade G, Muhlbaier LH, Smith PK, Wolfe WG. Comparison of omental and pectoralis flaps for poststernotomy mediastinitis. Ann Thorac Surg 1999;67(2):377–81. [DOI] [PubMed]
- 21.Tebala GD, Ciani R, Fonsi GB, Hadjiamiri H, Barone P, Di Pietrantonio P, Zumbo A. Laparoscopic harvest of an omental flap to reconstruct an infected sternotomy wound. J Laparoendosc Adv Surg Tech A 2006;16(2):141–5. [DOI] [PubMed]
- 22.Ennker IC, Pietrowski D, Vohringer L, Kojcici B, Albert A, Vogt PM, Ennker J. Surgical debridement, vacuum therapy and pectoralis plasty in poststernotomy mediastinitis. J Plast Reconstr Aesthet Surg 2009;62(11):1479–83. [DOI] [PubMed]
- 23.Eyileten Z, Akar AR, Eryilmaz S, Sirlak M, Yazicioglu L, Durdu S, et al. Vacuum-assisted closure and bilateral pectoralis muscle flaps for different stages of mediastinitis after cardiac surgery. Surg Today 2009;39(11):947–54. [DOI] [PubMed]
- 24.Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 1997;38(6):553–62. [DOI] [PubMed]
- 25.Obdeijn MC, de Lange MY, Lichtendahl DH, de Boer WJ. Vacuum-assisted closure in the treatment of poststernotomy mediastinitis. Ann Thorac Surg 1999;68(6):2358–60. [DOI] [PubMed]
- 26.Petzina R, Hoffmann J, Navasardyan A, Malmsjo M, Stamm C, Unbehaun A, Hetzer R. Negative pressure wound therapy for post-sternotomy mediastinitis reduces mortality rate and sternal re-infection rate compared to conventional treatment. Eur J Cardiothorac Surg 2010;38(1):110–3. [DOI] [PubMed]
- 27.Sjogren J, Gustafsson R, Nilsson J, Malmsjo M, Ingemansson R. Clinical outcome after poststernotomy mediastinitis: vacuum-assisted closure versus conventional treatment. Ann Thorac Surg 2005;79(6):2049–55. [DOI] [PubMed]
- 28.Sjogren J, Gustafsson R, Nilsson J, Lindstedt S, Nozohoor S, Ingemansson R. Negative-pressure wound therapy following cardiac surgery: bleeding complications and 30-day mortality in 176 patients with deep sternal wound infection. Interact Cardiovasc Thorac Surg 2011;12(2):117–20. [DOI] [PubMed]


