Abstract
We present a case of hoarseness in a 68-year-old man with a post-traumatic saccular aortic arch aneurysm, effort dyspnea, and dysphonia. Oropharyngeal examination and flexible nasal endoscopy revealed left vocal fold palsy, with the left fold lying in the paramedian position. On account of these and other findings, we made the diagnosis of Ortner's syndrome. We treated the patient surgically by endoluminal repair with a Dacron patch. The postoperative course was uneventful. No additional procedure was necessary on the vocal folds, since he showed prompt postoperative speech improvement.
We believe that an accurate evaluation should be made before switching a patient to endovascular treatment. Our case shows that careful preoperative planning, coupled with the most recent cerebral protection techniques, can enable a safe and straightforward surgical solution to a complex anatomic problem.
Key words: Aortic aneurysm, thoracic/complications/radiography; hoarseness/etiology/radiography; nerve compression syndromes/etiology; recurrent laryngeal nerve/physiopathology; vocal cord paralysis/etiology/therapy
Cardiovocal syndrome, better known as Ortner's syndrome, is a rare clinical entity first described by Ortner in 1897.1 We present a case of Ortner's syndrome in a patient with a post-traumatic saccular aortic arch aneurysm, which we treated surgically by endoluminal Dacron patch exclusion.
Case Report
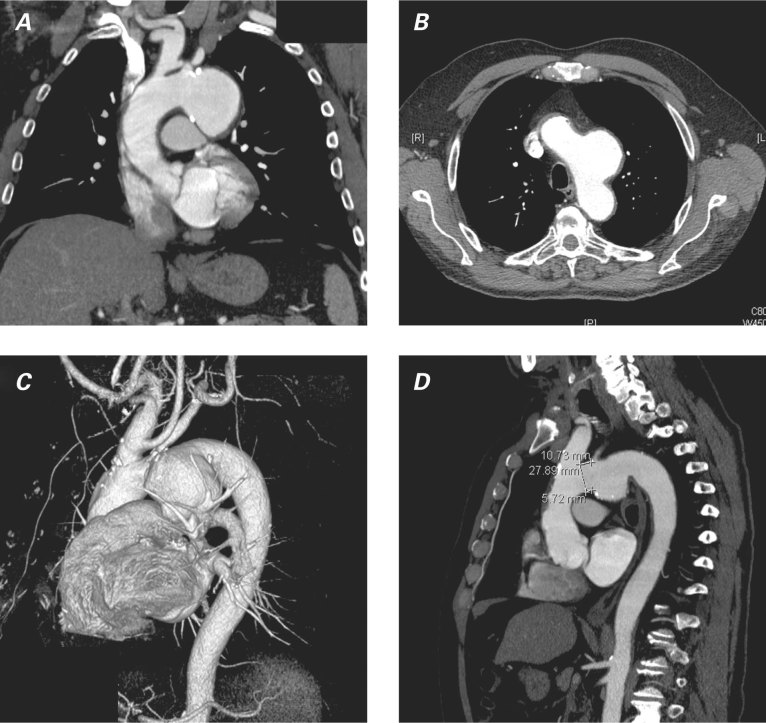
In July 2009, a 68-year-old man was referred to our institution for the investigation of effort dyspnea (New York Heart Association functional class II) and increasing dysphonia, both of 3 months' duration. He had a history of thoracic trauma due to an automobile accident 2 years earlier. Physical examination revealed normal blood pressure, absence of cardiac murmurs, and no obvious head or neck lymphadenopathy. Chest radiography showed a widened mediastinum, so a thoracic aortic aneurysm (TAA) was suspected. Therefore, the patient underwent a thoracic computed tomographic (CT) scan, which revealed a 55 × 58 × 60-mm saccular TAA arising from the aortic arch concavity and compressing the left recurrent laryngeal nerve (RLN), with non-uniform areas of calcification within the wall (Fig. 1). The left carotid artery was seen to arise from an ectatic brachiocephalic trunk (bovine trunk). There was no other mass or significant lymphadenopathy within the neck or chest. Oropharyngeal examination and flexible nasal endoscopy revealed left vocal fold palsy, with the left fold lying in the paramedian position. On account of these findings, we made the diagnosis of Ortner's syndrome. Preoperative angiography confirmed the CT scan result and ruled out significant coronary disease. In the expectation of excluding the aneurysm via an endovascular approach, we performed angio-magnetic resonance imaging of the brachiocephalic vessels and cerebral circulation (Fig. 2).
Fig. 1 Preoperative computed tomograms show the thoracic aortic aneurysm in these views: A) coronal, B) axial, C) 3-dimensional volume-rendered reconstruction, and D) sagittal.
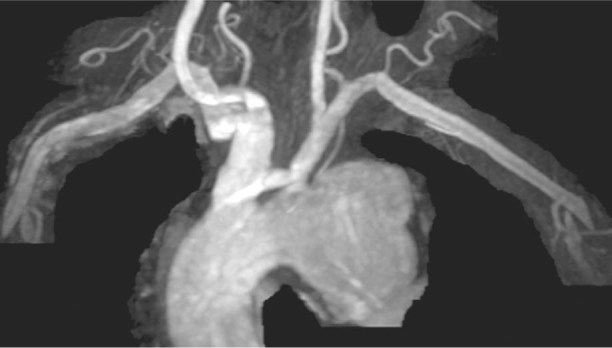
Fig. 2 Preoperative angio-magnetic resonance image shows the thoracic aortic aneurysm.
Due to the shortness of the aneurysm's proximal neck, we deemed the lesion unsuitable for an endovascular approach. After median sternotomy, we instituted cardiopulmonary bypass by a 10-mm side-graft anastomosis to the right axillary artery and a 2-stage venous cannula to the right atrium, and we induced 25 °C hypothermic circulatory arrest and antegrade selective Kazui cerebral perfusion. To repair the endoluminal aneurysm, we sutured a 25 × 20-mm Dacron patch through a longitudinal aortotomy at the level of the aneurysm. Cardiopulmonary bypass and circulatory arrest times were 181 and 46 minutes, respectively.
After an uneventful postoperative course characterized by prompt vocal improvement, the patient was discharged from the hospital on the 7th postoperative day. At the 1-month follow-up appointment, he was asymptomatic (with almost complete vocal rehabilitation). Fiberoptic laryngoscopy showed normal vocal cord movements, and a follow-up thoracic CT scan showed complete repair of the aneurysm (Fig. 3).
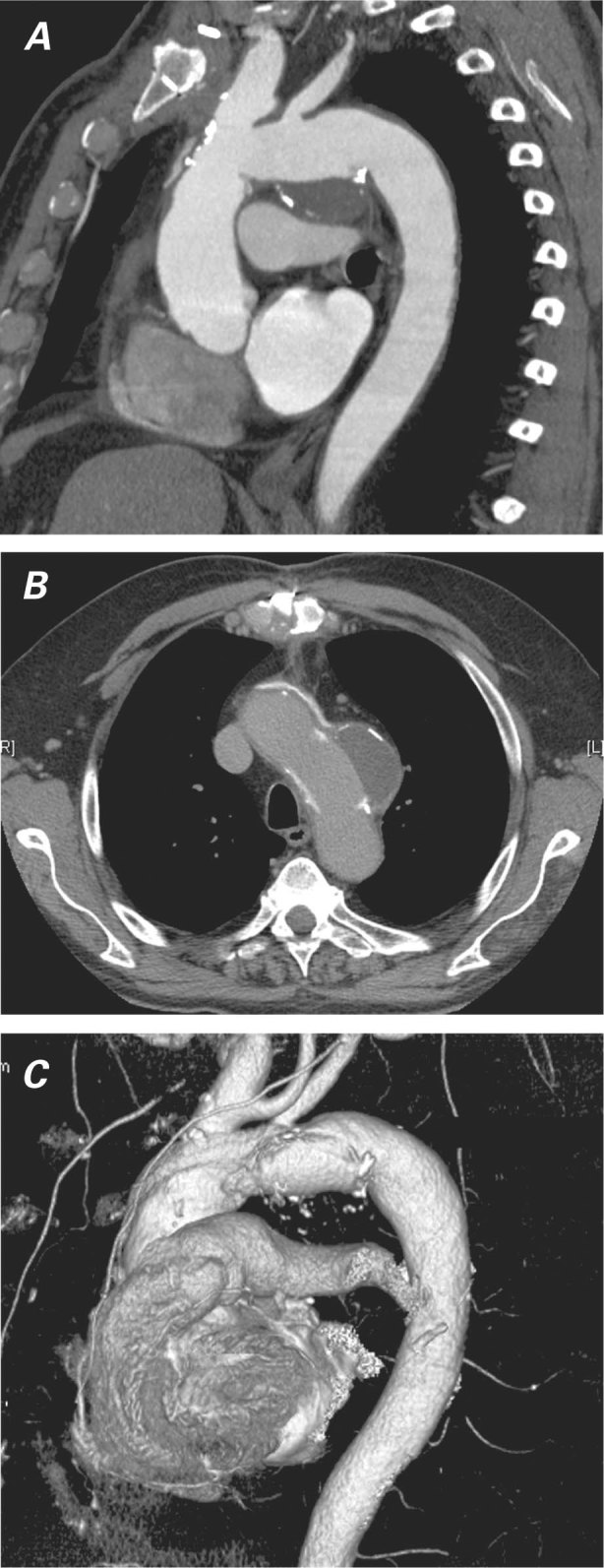
Fig. 3 Follow-up thoracic computed tomograms show the thoracic aortic aneurysm repaired in A) maximum-intensity-projection oblique sagittal view, B) axial view, and C) 3-dimensional volume-rendered reconstruction view.
Discussion
Cardiovocal syndrome, better known as Ortner's syndrome, is a rare clinical entity first described in 1897 by Norbert Ortner,1 in a patient with left atrial enlargement due to compression of the RLN by mitral valve stenosis. The lengthy course of the RLN, especially around the aortic arch, makes that nerve particularly vulnerable to compression, traction, and erosion by enlarged or displaced cardiac chambers or dilated arteries. Ortner's syndrome has been associated with several acquired and congenital cardiovascular diseases2: it presents as the first clinical manifestation in 5% of TAA and pseudoaneurysm cases, regardless of their cause, and it has been described in cases of dissecting aneurysm as well.3
Provided that the nerve is not further damaged, RLN palsy is usually reversible once the primary disease has been treated. However, complete recovery can depend on the degree, duration, and primary cause of nerve injury,4,5 with mechanical or electrical causes yielding a better prognosis than ischemic ones. This is why patients often experience hoarseness that persists late after surgery.
Once the more common causes of RLN palsy—such as malignancies, iatrogenic injuries, and metabolic, toxic, or neurologic causes5,6—have been excluded, Ortner's syndrome should be suspected, particularly in patients who have predisposing factors for cardiovascular disease. Although considerable progress has been made in operative and postoperative care, to the effect that better overall results have been achieved, TAA surgery continues to be accompanied by high rates of death and major postoperative complications.
The recent advent of aortic stents has changed dramatically the treatment of aortic disease. However, several anatomic limiting factors, including aortic or iliac tortuosity and occlusive disease, the aneurysm's proximity to the brachiocephalic vessels, and the proximal and distal aortic fixation sites, can preclude a completely successful endovascular approach or necessitate hybrid surgical debranching. In our patient, extremely large aneurysmal dimensions, symptoms of dysphonia, and the risk of aspiration pneumonia prompted our decision to perform surgery. The calcifications within the aortic wall were not uniform, and there were thin areas of fibrous aneurysm. Under these circumstances, we could not rule out rupture of the aneurysm. Due to the aneurysm's short proximal neck (5.7 mm) and to the fact that the left carotid artery originated near the left brachiocephalic trunk, we considered the aneurysm unsuitable for stented graft implantation and too risky for a complete ring resection and replacement of the diseased aorta, without a surgical debranching procedure. Furthermore, we were concerned about endotension or leakage after endovascular aneurysm exclusion, which could have prevented recovery from the laryngeal nerve palsy. Therefore, we preferred a surgical approach involving direct antegrade cerebral protection in accordance with Kazui's technique7 and circulatory arrest, in preparation for patch repair of the aneurysm.
Endovascular treatment appeals to both patients and clinicians, and modern medicine seems ineluctably drawn in that direction. Yet we believe that an accurate evaluation should be made before switching a patient to endovascular treatment. Our case shows that careful preoperative planning, coupled with the most recent cerebral protection techniques, can enable a safe and straightforward surgical solution to a complex anatomic problem.
Footnotes
Address for reprints: Marco L.S. Matteucci, MD, Department of Cardiac Surgery, Ospedali Riuniti di Ancona, Via Conca 71, 60020 Ancona, Italy, E-mail: sacha-m@libero.it
References
- 1.Ortner N. Recurrenslahmung bei mitralstenose. Wien Klin Wochenschr 1897;10:753–5.
- 2.Thirlwall AS. Ortner's syndrome: a centenary review of unilateral recurrent laryngeal nerve palsy secondary to cardiothoracic disease. J Laryngol Otol 1997;111(9):869–71. [DOI] [PubMed]
- 3.Khan IA, Wattanasauwan N, Ansari AW. Painless aortic dissection presenting as hoarseness of voice: cardiovocal syndrome: Ortner's syndrome. Am J Emerg Med 1999;17(4): 361–3. [DOI] [PubMed]
- 4.Chan P, Lee CP, Ko JT, Hung JS. Cardiovocal (Ortner's) syndrome left recurrent laryngeal nerve palsy associated with cardiovascular disease. Eur J Med 1992;1(8):492–5. [PubMed]
- 5.Mulpuru SK, Vasavada BC, Punukollu GK, Patel AG. Cardiovocal syndrome: a systematic review. Heart Lung Circ 2008;17(1):1–4. [DOI] [PubMed]
- 6.Ramadan HH, Wax MK, Avery S. Outcome and changing cause of unilateral vocal cord paralysis. Otolaryngol Head Neck Surg 1998;118(2):199–202. [DOI] [PubMed]
- 7.Kazui T, Inoue N, Yamada O, Komatsu S. Selective cerebral perfusion during operation for aneurysms of the aortic arch: a reassessment. Ann Thorac Surg 1992;53(1):109–14. [DOI] [PubMed]