Abstract
Recent studies suggest simultaneous or metachronous lesions in multiorgans characterized by elevated serum levels of IgG4 and abundant infiltration of IgG4-positive plasma cells with various degrees of fibrosis. Two Japanese research committees for IgG4-RD, one from fibrosclerosis (Okazaki team) and the other from lymph proliferation (Umehara team) supported by the “Research Program for Intractable Disease” of the Ministry of Health, Labor, and Welfare of Japan, have agreed with the unified nomenclature as “IgG4-RD” and proposed the comprehensive diagnostic criteria (CDC) for IgG4-RD. Validation of the CDC demonstrated satisfactory sensitivity for the practical use of general physicians and nonspecialists but low sensitivity in the organs to be difficult in taking biopsy specimens such as type1 autoimmune pancreatitis (IgG4-related AIP), compared with IgG4-related sialadenitis/dacryoadenitis (Mikulicz's disease) and IgG4-related kidney disease. Although the diagnostic criteria covering all IgG4-RD are hard to be established, combination with the CDC and organ-specific diagnostic criteria should improve sensitivity.
1. Introduction
Recent studies have suggested simultaneous or metachronous lesions in multiorgans characterized by elevated serum levels of IgG4 and abundant infiltration of IgG4-positive plasma cells with various degrees of fibrosis, which lead us to propose the concept of a systemic disease [1, 4, 10, 23, 24]. However, there are many synonyms suggesting a systemic disease, such as IgG4-related autoimmune disease [1], IgG4-related sclerosing disease [4], IgG4-related plasmacytic syndrome (SIPS) [23], IgG4-related multiorgan lymphoproliferative syndrome (IgG4-MOLPS) [10], and systemic IgG4-related disease, all of which may refer to the same conditions [24, 25] (Table 1). To simplify these conditions, members of two Japanese research committees for IgG4-related disease, one from view of fibrosclerosis (Chaired by Prof. Okazaki) [24] and the other from lymph proliferation (Chaired by Professor. Umehara H) [25], both of which are supported by the “Research for Intractable Disease” Program from the Ministry of Health, Labor, and Welfare of Japan, have agreed with unification of different nomenclatures as “IgG4-related disease (IgG4-RD)” and proposed the comprehensive diagnostic criteria (CDC) for IgG4-RD [15]. As it still remains unclear whether pathogenetic mechanisms in each involved organ-are same or not, the term IgG4-RD was appointed as minimally reflecting these conditions to avoid misdiagnosis of malignancy as much as possible.
Table 1.
Nomenclatures of IgG4-related conditions.
| Nomenclature | Authors | (year) |
|---|---|---|
| IgG4-related autoimmune disease | Kamisawa et al. [1] | (2003) |
| IgG4-associated multifocal systemic fibrosis | van der Vliet and Perenboom [2] | (2004) |
| IgG4-related systemic disease | Kamisawa et al. [3] | (2004) |
| IgG4-related sclerosing disease | Kamisawa et al. [4–7] | (2006) |
| Hyper-IgG4 disease | Neild et al. [8] | (2006) |
| IgG4-related disease | Zen et al. [9] | (2007) |
| Systemic IgG4 plasmacytic syndrome (SIPS) | Masaki et al. [10] | (2009) |
| IgG4-related multiorgan Lymphoproliferative syndrome (IgG4-MOLPS) | Masaki et al. [10] | (2009) |
| IgG4-associated disease | Geyer et al. [11] | (2010) |
2. The Concept of IgG4-Related Disease
The two Japanese research committees independently analyzed the clinical features and conditions of IgG4-RD and finally resulted in the following consensus with close collaboration [15, 24, 25]. (1) Patients with IgG4-RD show diffuse/focal organ enlargement, with mass-forming or nodular/thickened lesions in various organs, including the central nervous system [26], lachrymal/salivary glands [10, 23], thyroid gland [27, 28], lungs [29], pancreas [30, 31], biliary duct [32], liver [33], gastrointestinal tract [34, 35], kidneys [36], prostate gland [37], retroperitoneum [38], skin [39], lymph nodes [5, 40, 41], and artery [42, 43]. These conditions are quite similar to multifocal idiopathic fibrosclerosis (MIF) [44]. (2) These multiorgan lesions may occur synchronously or metachronously, with the prominent infiltration of lymphocytes and IgG4-positive plasmacytes with fibrosis. (3) IgG4-RD mainly affects middle-aged to elderly men except for IgG4-related dacryoadenitis/sialadenitis. Although clinical symptoms depending on involved organs are relatively mild, some patients develop serious complications such as obstructive jaundice due to hepatic, gallbladder, or pancreatic lesions; hydronephrosis due to retroperitoneal fibrosis; respiratory symptoms due to pulmonary lesions. (4) Steroid treatment is effective in many patient with IgG4-RD. However, prognosis and risk factors of recurrence still remain unclear. (5) Although the infiltration of IgG4-positive cells and increased serum concentrations of IgG4 characteristic of IgG4-RD, the severity of fibrosis is dependent on the individual organs involved. For example, storiform fibrosis and obliterative phlebitis are characteristic of pancreatic, biliary tract, and retroperitoneal lesions but are rarely observed in lachrymal/salivary glands or lymph nodes.
3. IgG4-Related Disease (IgG4-RD) as the Comprehensive Nomenclature [24, 25]
In addition to MIF, there are many synonyms, such as IgG4-related autoimmune disease [1], “IgG4-related sclerosing disease” [4], IgG4-related plasmacytic disease (SIPS) [23], and “IgG4 + sMOLPS” [10], all of which may refer to the same conditions. It has been debated which one is the most appropriate. Storiform fibrosis and obliterative phlebitis are characteristic of biliopancreatic, retroperitoneal, and renal lesions, but rarely observed in lachrymal/salivary glands and lymphnodes [24, 25]. Then, the nomenclature of “IgG4-related sclerosing disease” is mainly based on the fibrous swollen organs, whereas those of “IgG4-SIPS” and “IgG4-MOLPS” are based on lymphoplasmacytic proliferation and swollen lymph nodes without fibrosis [24, 25]. Although most patients have multiorgan lesions synchronously or metachronously, about 10–20% of the patients show a solitary organ involved without confirming other organ involvement [24, 25]. Therefore, it is unclear whether the pathogenetic mechanism is same among individual organs or not. In addition to IgG4-RD, IgG4-associate conditions such as high serum levels of IgG4 or abundant infiltration of IgG4-positive cells were reported in some patients with malignancy; pancreatic [6, 45], biliary [46] and salivary cancer [47], gastrointestinal sarcoma [48], and ocular adnexal lymphoma [49–51]. Therefore, the term “systemic” may lead us to misdiagnosis of other organ lesions showing IgG4-related conditions in cases of malignancy [51]. Based on these findings, the members of Umehara and Okazaki teams have agreed that the term “IgG4-related disease” is appointed as minimally accepting these conditions at this moment.
4. Comprehensive Diagnostic Criteria for IgG4-RD [15, 24, 25]
The patients with IgG-4-related disease show organ enlargement or nodular/hyperplastic lesions in organs in the entire body, synchronously or metachronously, due to the prominent infiltration and fibrosis of lymphocytes and plasmacytes; however, the causes of the disease are still not clear. The organs known to be affected include the central nervous system, lacrimal/salivary glands, thyroid gland, lungs, pancreas, biliary duct, liver, gastrointestinal tracts, kidneys, prostate gland, retroperitoneum, skin, arteries, and lymph nodes. Although it remains unclear whether this disease is the same as multifocal fibrosclerosis, that is a possibility. Clinical symptoms vary depending on the organ in which the lesions are located, which suggests that it is hard to establish criteria covering all patients with IgG4-RD. Therefore, specific diagnostic criteria are required for each involved organ such IgG4-related Mikulicz's disease (IgG4-related dacryoadenitis/sialadenitis [12] (Table 2), type 1 AIP (IgG4-related pancreatitis) [13] (Table 3), and IgG4-related kidney disease [14, 41] (Table 4). However, these organ-specific criteria do not cover other organs or are not familiar to general clinicians and specialists. Moreover, to avoid misdiagnosis of malignancy, all physicians have to know this emerging disease entity and can make a diagnosis of IgG4-RD. Therefore, the CDC for IgG4-RD, containing three major criteria (clinical, hematological and histopathological examinations), have been proposed for practical use of general physicians and nonspecialist [15] (Table 5). Although sensitivity of the CDC for definitive IgG4-RD is low in the organs to be difficult in taking biopsy specimens, it can detect possible cases of IgG4-RD. In the probable or possible cases, organ specific criteria should be used concurrently.
Table 2.
Diagnostic criteria for IgG4+ Mikulicz's disease [12] (approved by the Japanese Society for Sjögren's Syndrome, 2008).
| (1) Symmetrical swelling of at least 2 pairs of lachrymal, parotid, and submandibular glands continuing for more than 3 months, | |
| (2) elevated serum IgG4 (>135 mg/dL), | |
| or | |
| (3) histopathological features including lymphocyte and IgG4+ plasma cell infiltration (IgG4+ plasma cells/IgG+ plasma cells > 50%) with typical tissue fibrosis or sclerosis. | |
| Differential diagnosis is necessary from other disorders, including sarcoidosis, Castleman's disease, Wegener's granulomatosis, lymphoma, and cancer. Although the diagnostic criteria for Sjögren's syndrome (SS) may also include some patients with IgG4+ Mikulicz's disease, the clinicopathological conditions of patients with typical SS and IgG4+ Mikulicz's disease are different. |
Table 3.
International Consensus Diagnostic Criteria (ICDC) for autoimmune pancreatitis [13].
| Diagnosis | Primary basic for diagnosis | Imaging Evidence | Collateral evidence |
|---|---|---|---|
| Definitive type 1 AIP | Histology | Typical/indeterminate | Histologically confirmed LPSP (level 1 H) |
| Imaging | Typical | Any non-D level 1/level 2 | |
| Indeterminate | Two or more from level 1 (+level 2 D*) | ||
| Response to steroid | Indeterminate | Level 1 S/OOI + Rt or level 1 D + level 2 S/OOI/H + Rt | |
|
| |||
| Probable type 1 AlP | Indeterminate | Level 2 S/OOI/H + Rt | |
|
| |||
| *Level 2 D is counted as level 1 in this setting. | |||
|
| |||
| Criterion | Level 1 | Level 2 | |
|
| |||
| P | Parenchymal imaging | Typical: diffuse enlargement with delayed enhancement (sometimes associated with rim-like enhancement) | Indeterminate (including atypical†): segmental/focal enlargement with delayed enhancement |
|
| |||
| D | Ductal imaging (ERP) | Long (>1/3 length of the main pancreatic duct) or multiple strictures without marked upstream dilatation | Segmental/focal narrowing without marked upstream dilatation (duct size, <5 mm) |
|
| |||
| S | Serology | IgG4, >2× upper limit of normal value a or b | IgG4, 1-2× upper limit of normal value a or b |
| OOI | Other organ involvement | ||
| (a) Histology of extrapancreatic organs: | (a) Histology of extrapancreatic organs including endoscopic biopsies of bile duct‡: | ||
| any three of the following: | both of the following: | ||
| (1) marked lymphoplasmacytic infiltration with fibrosis and without granulocytic infiltration; | (1) marked lymphoplasmacytic infiltration without granulocytic infiltration; | ||
| (2) storiform fibrosis; | (2) abundant (>10 cells/HPF) IgG4-positive cells. | ||
| (3) obliterative phlebitis; | |||
| (4) abundant (>10 cells/HPF) IgG4-positive cells. | |||
| (b) Typical radiological evidence | (b) Physical or radiological evidence: | ||
| at least one of the following: | at least one of the following: | ||
| (1) segmental/multiple proximal (hilar/intrahepatic) or proximal and distal bile duct stricture; | (1) symmetrically enlarged salivary/lachrymal glands; | ||
| (2) retroperitoneal fibrosis; | (2) radiological evidence of renal involvement described in association with AIP. | ||
|
| |||
| H | Histology of the pancreas | LPSP (core biopsy/resection): | LPSP (core biopsy): |
| at least 3 of the following: | any 2 of the following: | ||
| (1) periductal lymphoplasmacytic infiltrate without granulocytic infiltration; | (1) periductal lymphoplasmacytic infiltrate without granulocytic infiltration; | ||
| (2) obliterative phlebitis; | (2) obliterative phlebitis; | ||
| (3) storiform fibrosis; | (3) storiform fibrosis; | ||
| (4) abundant (>10 cells HPF) IgG4-positive cells. | (4) abundant (>10 cells/HPF) IgG4-positive cells. | ||
|
| |||
| Diagnostic steroid trial | |||
| Response to steroid (Rt)* | Rapid (≤2 wk) radiologically demonstrable resolution or marked improvement in pancreatic/extrapancreatic manifestations | ||
Table 4.
Diagnostic criteria for IgG4-related kidney disease [14].
| (1) Presence of some kidney damage, as manifested by abnormal urinalysis or urine marker(s) or decreased kidney function with either elevated serum IgG or IgE or hypocomplementemia | |
| (2) Abnormal renal radiologic findings: | |
| (a) multiple low-density lesions on enhanced computed tomography; | |
| (b) diffuse kidney enlargement; | |
| (c) hypovascular solitary mass in the kidney; | |
| (d) hypertrophic lesion of the renal pelvic wall without irregularities of the renal pelvic surface. | |
| (3) Elevated serum IgG4 level (>135 mg/dL) | |
| (4) Histological findings in the kidney: | |
| (a) dense lymphoplasmacytic infiltration by >10 IgG4-positive plasma cells/high power field (HPF) and/or IgG4+/IgG+ positive plasma cells > 40%; | |
| (b) characteristic (sclero-) fibrosis surrounding nests of lymphocytes and/or plasma cells; | |
| (5) Histological findings in extrarenal organ(s): | |
| dense lymphoplasmacytic infiltration by >10 IgG4-positive plasma cells/HPF and/or IgG4/IgG-positive plasma cells > 40% | |
| Definite: (1) + (3) + (4) (a), (b) | |
| (2) + (3) + (4) (a), (b) | |
| (2) + (3) + (5) | |
| (1) + (3) + (4) (a) + (5) | |
| Probable: (1) + (4) (a), (b) | |
| (2) + (4) (a), (b) | |
| (2) + (5) | |
| (3) + (4) (a), (b) | |
| Possible: (1) + (3) | |
| (2) + (3) | |
| (1) + (4) (a) | |
| (2) + (4) (a) | |
| Appendix: | |
| (1) Clinically and histologically, the following diseases should be excluded: | |
| Wegener's granulomatosis, Churg-Strauss syndrome, and extramedullary plasmacytoma. | |
| (2) Radiologically, the following diseases should be excluded: | |
| Malignant lymphoma, urinary tract carcinomas, renal infarction, and pyelonephritis. | |
| (Rarely, Wegener's granulomatosis, sarcoidosisand metastatic carcinoma) |
Table 5.
Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011 [15].
| [Concept] | |
| IgG4-related disease (IgG4-RD) shows organ enlargement or nodular/hyperplastic lesions in various organs concurrently or metachronously, due to marked infiltration of lymphocytes and IgG4-positive plasma cells, as well as fibrosis of unknown etiology. IgG4-RD affects various organs, including the pancreas, bile duct, lacrimal gland, salivary gland, central nervous system, thyroid, lung, liver, gastrointestinal tract, kidney, prostate, retroperitoneum, arteries, lymph nodes, skin, and breast. Although many patients with IgG4-RD have lesions in several organs, either synchronously or metachronously, others show involvement of a single organ. Clinical symptoms vary depending on the affected organ, and some patients may experience serious complications, such as obstruction or compression symptoms due to organomegaly or hypertrophy and organ dysfunction caused by cellular infiltration or fibrosis. Steroid therapy is often effective. | |
|
| |
| [Comprehensive clinical diagnostic criteria for IgG4-RD, 2011] | |
| (1) Clinical examination shows characteristic diffuse/localized swelling or masses in single or multiple organs. | |
| (2) Hematological examination shows elevated serum IgG4 concentrations (≥135 mg/dL). | |
| (3) Histopathologic examination shows; | |
| (1) marked lymphocyte and plasmacyte infiltration and fibrosis | |
| (2) infiltration of IgG4-positive plasma cells: ratio of IgG4/IgG positive cells > 40% and > 10 IgG4-positive plasma cells/HPF. | |
| Definite: (1) + (2) + (3), Probable: (1) + (3), Possible: (1) + (2) | |
| However, it is important to differentiate IgG4-RD from malignant tumors of each organ (e.g. cancer, lymphoma) and similar diseases (e.g. Sjögren's syndrome, primary sclerosing cholangitis, Castleman's disease, secondary retroperitoneal fibrosis, Wegener's granulomatosis, sarcoidosis, and Churg-Strauss syndrome) by additional histopathological examination. Even when patients cannot be diagnosed using the CCD criteria, they may be diagnosed using organ-specific diagnostic criteria for IgG4RD. | |
(1) Clinical Examination —
Physical examinations and imaging on US/CT/MRI can show the characteristic diffuse/localized swelling, masses, or thickness in single or multiple organs (Figure 1).
Figure 1.
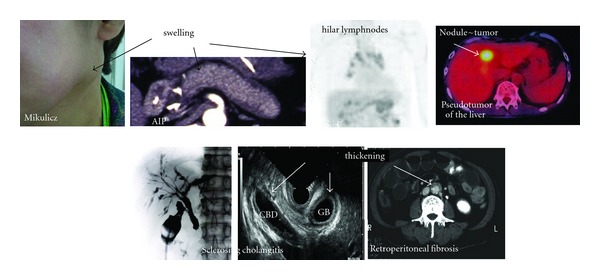
Clinical findings of IgG4-related disease. Physical examinations and imaging on US/CT/MRI can show the characteristic diffuse/localized swelling, masses, or thickness in single or multiple organs.
(2) Immunological Examination —
(a) Increase of Serum Levels of IgG4 —
The cutoff value for serum IgG4 concentration, 135 mg/dL, was based on receiver operating characteristic (ROC) curves, and its validity was confirmed in patients with autoimmune pancreatitis [7] (Table 6). In patients with single-organ involvement and serum IgG4 concentration less than 135 mg/dL, the IgG4/IgG ratio may be helpful in making a diagnosis.
However, elevated IgG4 may be also observed in other diseases (e.g., atopic dermatitis, pemphigus, asthma, and multicentric Castleman's disease), especially in about 10% of malignancy, which suggests that high serum IgG4 is not necessarily specific marker of IgG4-RD [6]. Although a high cut-off value with >270 mg/dL of IgG4 increases specificity but decreased sensitivity of IgG4-RD differing from pancreatic cancer [45]. Therefore, at present, the significance of elevated IgG4 in the pathogenesis/pathophysiology of IgG4-RD still remains unknown.
(b) Other Immunological Markers —
In addition to increased serum levels of IgG4, high serum levels of polyclonal γ-globulin, IgG, and IgE are often, and hypocomplementemia may occur [52]. As these markers are less sensitive for IgG4-RD, they are not included as a diagnostic criterion.
Table 6.
Sensitivity and specificity of serum levels of IgG4 in patients with type 1 AIP.
| Cut-off | Sensitivity | Specificity | |||
|---|---|---|---|---|---|
| mg/dL | n | Median/(range) | n | (vs cancer) | |
| Japan | 135 | ||||
| Okazaki et al. [16] | 71 | 80% 410 (3–3670) | 101 | 98% | |
| Okazaki et al. [17] | 52 | 73% 505 (43–1540) | NS | ||
| Kawa et al. [18] | 64 | 92% 618 (8–2855) | 80 | 98% | |
| Korea | 135 | ||||
| Choi et al. [19] | 30 | 73% 473 (10–1764) | 76 | 99% | |
| USA | 140 | ||||
| Ghazale et al. [20] | 45 | 76% 550 (16–2890) | 135 | 90% | |
| Raina et al. [21] | 26 | 44% (8–825) | NS | ||
| Italy | 135 | ||||
| (focal) | 55 | 66% 267 | NS | ||
| Frulloni et al. [22] | (diffuse) | 32 | 27% 78 | ||
(3) Histopathologic Examination —
Although tissue biopsies are difficult to obtain from some organs, including the pancreas, retroperitoneum and ocular cavity, histopathological examination is important.
(a) Marked Lymphocyte and Plasmacyte Infiltration and Fibrosis. —
Storiform or swirling fibrosis or obliterative phlebitis is Characteristic of IgG4-RD and may be important in its diagnosis.
(b) Infiltration of IgG4-Positive Plasma Cells —
IgG4/IgG-positive cells more than 40% [53] or 50% [12] have been reported in lymphnodes of the patients with IgG4-RD. On the other hand, more than 10 IgG4-positive plasma cells are recommended that in diagnosis of type 1 AIP [13]. Based on these findings, the CDC for IgG4-RD recommend both the ratio of IgG4/IgG-positive cells >40% and infiltration of >10 IgG4-positive plasma cells/HPF for the definitive diagnosis [15]. Eosinophilic infiltration is often observed along with infiltration of IgG4-positive cells. It is noted that reactive infiltration of IgG4-positive cells and fibrosis may be observed in various diseases and clinical conditions, such as rheumatoid synovitis, inflammatory oral and skin lesions, and around cancer. However, it is noted that some additional immune-mediated conditions with increased serum interleukin-6 (IL-6) such as multicentric Castleman's disease may show elevated serum IgG4 and/or IgG4+/IgG+ plasma cell ratios >40%.
(4) Prohibition of Facile Steroid Treatment in the CDC for IgG4-RD —
Patients with malignant lymphoma or paraneoplastic lesions can sometimes be improved by steroid administration. Therefore, steroid trials should be strictly avoided. Efforts should be made to collect tissue samples for diagnosis. However, patients having disease in organs difficult to biopsy, such as the pancreas, retroperitoneum, and pituitary, and respond to steroids may possibly have IgG4-RD. In accordance with the guidelines for treatment of autoimmune pancreatitis, patients should be started on 0.5-0.6 mg/kg/day/prednisolone. If patients do not respond to the initial steroid therapy, the diagnosis should be reviewed again.
(5) Diseases to be Excluded or Differentiated —
(a) Malignancies (e.g., Cancer, Lymphoma) —
In cases of malignancy in the involved organs, it is essential to determine whether malignant cells are present histopathologically.
(b) Similar Diseases —
Other similar benign diseases including Sjögren's syndrome, primary sclerosing cholangitis, multicentric Castleman's disease, idiopathic retroperitoneal fibrosis, Wegener's granulomatosis, sarcoidosis, and Churg-Strauss syndrome should be differentially diagnosed using the diagnostic criteria for each disease. It is noted that multicentric Castleman's disease, one of hyper IL-6 syndromes should be excluded from IgG4-RD, even if the CDC for IgG4-RD are fulfilled.
5. Sensitivity and Specificity of the CDC Criteria and Diagnostic Algorithm for IgG4-RD
The sensitivity of CDC for definitive/probable IgG4-RD is satisfactory in IgG4-related MD [12] and IgG4-related KD [14], but not in type 1 AIP [6, 13]. The major reason of low sensitivity in type 1 AIP is that enough biopsy samples of the pancreas are not easily obtained in most of these patients. In addition, endoscopic ultrasonography (EUS), guide fine needle aspiration (FNA), is available in a few of institutes in Japan, for examples only 16 of 226 (7%) board member institutes in Kink district of Japan Gastroenterological Endoscopy Society (JGES). On the other hand, the sensitivity of the CDC for possible IgG4-RD is satisfactory in type 1 AIP (Table 7). In contrast, patients with type 1 AIP could not be diagnosed by the comprehensive diagnostic criteria (0%) for definite, because biopsies could not be obtained from most of these patients. Therefore, combination of the CDC and organ-specific criteria should increase the sensitivity of diagnosis, even in the possible cases of IgG4-RD.
Table 7.
Validation of a combination of CDC and organ-specific criteria for type 1 AIP.
| Compared with pancreas cancer, the sensitivity of comprehensive criteria for definite/probable AIP was 0%, but 78% for possible AIP, and specificity was 100% in any groups. Although it is hard to take an enough size of specimen in diagnosis of AIP malignancy can be usually denied by EUS-FNA. Therefore, the CDC are enough for detecting possible AIP, but not for definite/probable AIP. | ||||
|---|---|---|---|---|
| AIP (n = 60) PaCa (n = 17) Total (n = 77) |
JPS 2006 | ICDC for type 1 AIP | CDC for IgG4-RD | |
|
| ||||
| Diagnosis of AIP | Definite AIP | Definite/probable AIP | Definite/probable AIP | Possible |
|
| ||||
| sensitivity | 70% | 97% | 0% | 78% |
| specificity | 100% | 100% | 100% | 100% |
| PPV | 100% | 100% | 0% | 100% |
| NPV | 49% | 8% | 100% | 57% |
| accuracy | 77% | 95% | 22% | 83% |
PaCa: pancreas cancer, PPV: positive predictive value, NPV: negative predictive value.
Based on these findings, a diagnostic algorithm for IgG4-RD in combination with the CDC and other organ-specific criteria has been proposed, although they have a limitation to the utility of the criteria proposed [15] (Figure 2). In patients with (a) organ enlargement, mass or nodular lesions, or organ dysfunction, performing of both (b) measurement of serum IgG4 and (c) tissue biopsy is recommended. In the cases with >135 mg/dL of IgG4, diagnostic histopathological findings of >10 IgG4 cells/HPF and an IgG4/IgG cell ratio >40 can diagnose them as definitive AIP. In possible or probable cases fulfilling criterion (a) with (b), or (c), organ-specific criteria for each disease should be applied. It is important to differentiate IgG4-RD from malignant tumors of each organ (e.g., cancer, lymphoma) and similar diseases (e.g., Sjögren's syndrome, primary sclerosing cholangitis, Castleman's disease, secondary retroperitoneal fibrosis, Wegener's granulomatosis, sarcoidosis, and Churg-Strauss syndrome) by additional histopathological examination. Future studies including other organ diseases similar to IgG4-RD are needed to establish the diagnostic efficacy of CDC.
Figure 2.
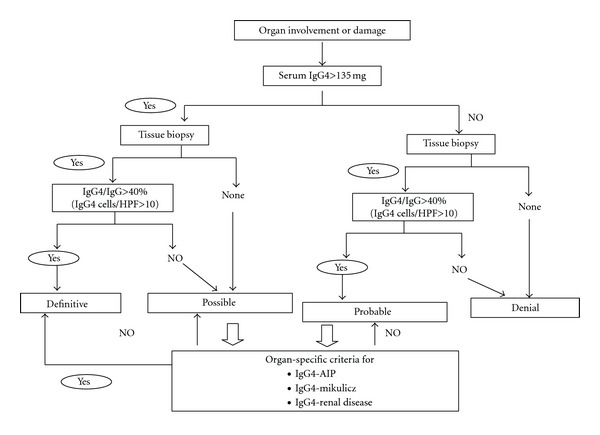
Diagnostic algorithm for IgG4-RD in Japan.
6. Conclusion
“All Japan Research Team for IgG4-RD” unified the nomenclatures as “IgG4-related disease (IgG4-RD)” and proposed the comprehensive diagnostic criteria (CDC) for IgG4-RD. The CDC for IgG4-RD was made for the practical use and for general physicians to differentiate IgG4-RD from malignancy or similar diseases as much as possible. Although sensitivity of the CDC for definitive IgG4-RD is low in the organs to be difficult in taking biopsy specimens, it can detect possible cases of IgG4-RD. In the probable or possible cases, organ-specific criteria should be used concurrently.
Authors' Contribution
K. Okazaki and H. Umehara declare that they equally contributed to this work.
Disclosure
Japanese Research Committee of IgG4-RD (The Working Group of Japanese Research Committee of IgG4-RD) is supported by the Ministry of Health, Labor, and Welfare of Japan.
Acknowledgments
This study was partially supported by: (1) a Grant-in-Aid for Scientific Research (C) of the Ministry of Culture and Science of Japan (23591017); (2) Health and Labor Sciences Research grants (K.O.) for Intractable Diseases, from the Minister of Labor and Welfare of Japan; (3) grants-in-aid from CREST Japan Science and Technology Agency.
References
- 1.Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. Journal of Gastroenterology. 2003;38(10):982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 2.van der Vliet HJ, Perenboom RM. Multiple pseudotumors in IgG4-associated multifocal systemic fibrosis. Annals of Internal Medicine. 2004;141(11):896–897. doi: 10.7326/0003-4819-141-11-200412070-00033. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Funata N, Hayashi Y. Lymphoplasmacytic sclerosing pancreatitis is a pancreatic lesion of IgG4-related systemic disease. The American Journal of Surgical Pathology. 2003;27(8):1119–1127. doi: 10.1097/01.pas.0000126634.43301.45. [DOI] [PubMed] [Google Scholar]
- 4.Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. Journal of Gastroenterology. 2006;41(7):613–625. doi: 10.1007/s00535-006-1862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamisawa T, Nakajima H, Egawa N, Funata N, Tsuruta K, Okamoto A. IgG4-related sclerosing disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis and retroperitoneal fibrosis with lymphadenopathy. Pancreatology. 2006;6(1-2):132–137. doi: 10.1159/000090033. [DOI] [PubMed] [Google Scholar]
- 6.Kamisawa T, Chen PY, Tu Y, et al. Pancreatic cancer with a high serum IgG4 concentration. World Journal of Gastroenterology. 2006;12(38):6225–6228. doi: 10.3748/wjg.v12.i38.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okazaki K, Kawa S, Kamisawa T, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. Journal of Gastroenterology. 2006;41(7):626–631. doi: 10.1007/s00535-006-1868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neild GH, Rodriguez-Justo M, Wall C, Connolly JO. Hyper-IgG4 disease: report and characterisation of a new disease. BMC Medicine. 2006;4, article 23 doi: 10.1186/1741-7015-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zen Y, Fujii T, Sato Y, Masuda S, Nakanuma Y. Pathological classification of hepatic inflammatory pseudotumor with respect to IgG4-related disease. Modern Pathology. 2007;20(8):884–894. doi: 10.1038/modpathol.3800836. [DOI] [PubMed] [Google Scholar]
- 10.Masaki Y, Dong L, Kurose N, et al. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Annals of the Rheumatic Diseases. 2009;68(8):1310–1315. doi: 10.1136/ard.2008.089169. [DOI] [PubMed] [Google Scholar]
- 11.Geyer JT, Ferry JA, Harris NL, et al. Chronic sclerosing sialadenitis (Küttner tumor) is an IgG4-associated disease. American Journal of Surgical Pathology. 2010;34(2):202–210. doi: 10.1097/PAS.0b013e3181c811ad. [DOI] [PubMed] [Google Scholar]
- 12.Masaki Y, Sugai S, Umehara H. IgG4-related diseases including Mikulicz’s disease and sclerosing pancreatitis: diagnostic insights. Journal of Rheumatology. 2010;37(7):1380–1385. doi: 10.3899/jrheum.091153. [DOI] [PubMed] [Google Scholar]
- 13.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the international association of pancreatology. Pancreas. 2011;40(3):352–358. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 14.Kawano M, Saeki T, Nakashima H, et al. Proposal for diagnostic criteria for IgG4-related kidney disease. Experimental Nephrology. 2011;15(5):615–626. doi: 10.1007/s10157-011-0521-2. [DOI] [PubMed] [Google Scholar]
- 15.Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Modern Rheumatology. 2012;22(1):21–30. doi: 10.1007/s10165-011-0571-z. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki K, Kawa S, Kamisawa T, et al. Japanese clinical guidelines for autoimmune pancreatitis. Pancreas. 2009;38(8):849–866. doi: 10.1097/MPA.0b013e3181b9ee1c. [DOI] [PubMed] [Google Scholar]
- 17.Okazaki K, Uchida K, Matsushita M, Takaoka M. How to diagnose autoimmune pancreatitis by the revised Japanese clinical criteria. Journal of Gastroenterology. 2007;42(supplement 18):32–38. doi: 10.1007/s00535-007-2049-5. [DOI] [PubMed] [Google Scholar]
- 18.Kawa S, Okazaki K, Kamisawa T, Shimosegawa T, Tanaka M. Japanese consensus guidelines for management of autoimmune pancreatitis: II. Extrapancreatic lesions, differential diagnosis. Journal of Gastroenterology. 2010;45(4):355–369. doi: 10.1007/s00535-009-0197-5. [DOI] [PubMed] [Google Scholar]
- 19.Choi EK, Kim MH, Lee TY, et al. The sensitivity and specificity of serum immunoglobulin G and immunoglobulin G4 levels in the diagnosis of autoimmune chronic pancreatitis: Korean experience. Pancreas. 2007;35(2):156–161. doi: 10.1097/MPA.0b013e318053eacc. [DOI] [PubMed] [Google Scholar]
- 20.Ghazale A, Chari ST, Smyrk TC, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. American Journal of Gastroenterology. 2007;102(8):1646–1653. doi: 10.1111/j.1572-0241.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 21.Raina A, Yadav D, Krasinskas AM, et al. Evaluation and management of autoimmune pancreatitis: experience at a large us center. American Journal of Gastroenterology. 2009;104(9):2295–2306. doi: 10.1038/ajg.2009.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frulloni L, Scattolini C, Falconi M, et al. Autoimmune pancreatitis: differences between the focal and diffuse forms in 87 patients. American Journal of Gastroenterology. 2009;104(9):2288–2294. doi: 10.1038/ajg.2009.327. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, Ohara M, Suzuki C, et al. Elevated IgG4 concentrations in serum of patients with Mikulicz’s disease. Scandinavian Journal of Rheumatology. 2004;33(6):432–433. doi: 10.1080/03009740410006439. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki K, Uchida K, Miyoshi H, Ikeura T, Takaoka M, Nishio A. Recent concept of autoimmune pancreatitis and IgG4-related disease. Clinical Reviews in Allergy and Immunology. 2010;41(2):126–138. doi: 10.1007/s12016-010-8214-2. [DOI] [PubMed] [Google Scholar]
- 25.Umehara H, Okazaki K, Masaki Y, et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Modern Rheumatology. 2012;22(1):1–14. doi: 10.1007/s10165-011-0508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimatsu A, Oki Y, Fujisawa I, Sano T. Pituitary and stalk lesions (Infundibulo-hypophysitis) associated with immunoglobulin G4-related systemic disease: an emerging clinical entity. Endocrine Journal. 2009;56(9):1033–1041. doi: 10.1507/endocrj.k09e-277. [DOI] [PubMed] [Google Scholar]
- 27.Dahlgren M, Khosroshahi A, Nielsen GP, Deshpande V, Stone JH. Riedel’s thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis Care and Research. 2010;62(9):1312–1318. doi: 10.1002/acr.20215. [DOI] [PubMed] [Google Scholar]
- 28.Kojima M, Hirokawa M, Kuma H, et al. Distribution of IgG4- and/or IgG-positive plasma cells in Hashimoto’s thyroiditis: an immunohistochemical study. Pathobiology. 2010;77(5):267–272. doi: 10.1159/000319873. [DOI] [PubMed] [Google Scholar]
- 29.Zen Y, Inoue D, Kitao A, et al. IgG4-related lung and pleural disease: a clinicopathologic study of 21 cases. American Journal of Surgical Pathology. 2009;33(12):1886–1893. doi: 10.1097/PAS.0b013e3181bd535b. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida K, Toki F, Takeuchi T, Watanabe SI, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Digestive Diseases and Sciences. 1995;40(7):1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 31.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. The New England Journal of Medicine. 2001;344(10):732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 32.Nakazawa T, Ohara H, Sano H, et al. Cholangiography can discriminate sclerosing cholangitis with autoimmune pancreatitis from primary sclerosing cholangitis. Gastrointestinal Endoscopy. 2004;60(6):937–944. doi: 10.1016/s0016-5107(04)02229-1. [DOI] [PubMed] [Google Scholar]
- 33.Eguchi S, Takatsuki M, Hidaka M, et al. De novo autoimmune hepatitis after living donor liver transplantation is unlikely to be related to immunoglobulin subtype 4-related immune disease. Journal of Gastroenterology and Hepatology. 2008;23(7, part 2):e165–e169. doi: 10.1111/j.1440-1746.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- 34.Fujita T, Ando T, Sakakibara M, Hosoda W, Goto H. Refractory gastric ulcer with abundant IgG4-positive plasma cell infiltration: a case report. World Journal of Gastroenterology. 2010;16(17):2183–2186. doi: 10.3748/wjg.v16.i17.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueno K, Watanabe T, Kawata Y, et al. IgG4-related autoimmune pancreatitis involving the colonic mucosa. European Journal of Gastroenterology and Hepatology. 2008;20(11):1118–1121. doi: 10.1097/MEG.0b013e3282f82970. [DOI] [PubMed] [Google Scholar]
- 36.Uchiyama-Tanaka Y, Mori Y, Kimura T, et al. Acute tubulointerstitial nephritis associated with autoimmune-related pancreatitis. American Journal of Kidney Diseases. 2004;43(3):e18–e25. doi: 10.1053/j.ajkd.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Nishimori I, Kohsaki T, Onishi S, et al. IgG4-related autoimmune prostatitis: two cases with or without autoimmune pancreatitis. Internal Medicine. 2007;46(24):1983–1989. doi: 10.2169/internalmedicine.46.0452. [DOI] [PubMed] [Google Scholar]
- 38.Hamano H, Kawa S, Ochi Y, et al. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. The Lancet. 2002;359(9315):1403–1404. doi: 10.1016/s0140-6736(02)08359-9. [DOI] [PubMed] [Google Scholar]
- 39.Miyagawa-Hayashino A, Matsumura Y, Kawakami F, et al. High ratio of IgG4-positive plasma cell infiltration in cutaneous plasmacytosis—is this a cutaneous manifestation of IgG4-related disease? Human Pathology. 2009;40(9):1269–1277. doi: 10.1016/j.humpath.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Cheuk W, Yuen HKL, Chu SYY, Chiu EKW, Lam LK, Chan JKC. Lymphadenopathy of IgG4-related sclerosing disease. American Journal of Surgical Pathology. 2008;32(5):671–681. doi: 10.1097/PAS.0b013e318157c068. [DOI] [PubMed] [Google Scholar]
- 41.Sato Y, Kojima M, Takata K, et al. Systemic IgG4-related lymphadenopathy: a clinical and pathologic comparison to multicentric Castleman’s disease. Modern Pathology. 2009;22(4):589–599. doi: 10.1038/modpathol.2009.17. [DOI] [PubMed] [Google Scholar]
- 42.Kasashima S, Zen Y, Kawashima A, et al. Inflammatory abdominal aortic aneurysm: close relationship to IgG4-related periaortitis. American Journal of Surgical Pathology. 2008;32(2):197–204. doi: 10.1097/PAS.0b013e3181342f0d. [DOI] [PubMed] [Google Scholar]
- 43.Stone JH, Khosroshahi A, Hilgenberg A, Spooner A, Isselbacher EM, Stone JR. IgG4-related systemic disease and lymphoplasmacytic aortitis. Arthritis and Rheumatism. 2009;60(10):3139–3145. doi: 10.1002/art.24798. [DOI] [PubMed] [Google Scholar]
- 44.Comings DE, Skubi KB, van Eyes J, Motulsky AG. Familial multifocal fibrosclerosis. Findings suggesting that retroperitoneal fibrosis, mediastinal fibrosis, sclerosing cholangitis, Riedel’s thyroiditis, and pseudotumor of the orbit may be different manifestations of a single disease. Annals of Internal Medicine. 1967;66(5):884–892. doi: 10.7326/0003-4819-66-5-884. [DOI] [PubMed] [Google Scholar]
- 45.Ghazale A, Chari ST, Smyrk TC, et al. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. American Journal of Gastroenterology. 2007;102(8):1646–1653. doi: 10.1111/j.1572-0241.2007.01264.x. [DOI] [PubMed] [Google Scholar]
- 46.Straub BK, Esposito I, Gotthardt D, et al. IgG4-associated cholangitis with cholangiocarcinoma. Virchows Archiv. 2011;458(6):761–765. doi: 10.1007/s00428-011-1073-2. [DOI] [PubMed] [Google Scholar]
- 47.Gill J, Angelo N, Yeong ML, McIvor N. Salivary duct carcinoma arising in IgG4-related autoimmune disease of the parotid gland. Human Pathology. 2009;40(6):881–886. doi: 10.1016/j.humpath.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 48.Joo M, Chang SH, Kim H, Gardner JM, Ro JY. Primary gastrointestinal clear cell sarcoma: report of 2 cases, one case associated with IgG4-related sclerosing disease, and review of literature. Annals of Diagnostic Pathology. 2009;13(1):30–35. doi: 10.1016/j.anndiagpath.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Cheuk W, Yuen HKL, Chan ACL, et al. Ocular adnexal lymphoma associated with IgG4+ chronic sclerosing dacryoadenitis: a previously undescribed complication of IgG4-related sclerosing disease. American Journal of Surgical Pathology. 2008;32(8):1159–1167. doi: 10.1097/PAS.0b013e31816148ad. [DOI] [PubMed] [Google Scholar]
- 50.Kubota T, Moritani S, Yoshino T, Nagai H, Terasaki H. Ocular adnexal marginal zone B cell lymphoma infiltrated by IgG4-positive plasma cells. Journal of Clinical Pathology. 2010;63(12):1059–1065. doi: 10.1136/jcp.2010.082156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto M, Takahashi H, Tabeya T, et al. Risk of malignancies in IgG4-related disease. doi: 10.1007/s10165-011-0520-x. Modern Rheumatology. In press. [DOI] [PubMed] [Google Scholar]
- 52.Muraki T, Hamano H, Ochi Y, et al. Autoimmune pancreatitis and complement activation system. Pancreas. 2006;32(1):16–21. doi: 10.1097/01.mpa.0000188308.75043.e4. [DOI] [PubMed] [Google Scholar]
- 53.Zen Y, Harada K, Sasaki M, et al. Distribution of IgG4- and/or IgG-positive plasma cells in Hashimoto’s thyroiditis: an immunohistochemical study. Pathobiology. 2010;77(5):267–272. doi: 10.1159/000319873. [DOI] [PubMed] [Google Scholar]


