Abstract
Phytochemicals have received much recent attention in cancer prevention through simultaneous targeting multiple pathways in the disease progression. Here we determined that wolfberry phytochemicals was chemopreventive on the leukemic Jurkat cell. The water soluble wolfberry fractions (i.e., wolfberry phytochemicals) were enriched in carbohydrates (73.4 ± 4.5 % (w/w)), polyphenolics (1555 ± 112 mg quercetin equivalent/100 g freeze dry powder, including 213 mg rutin/100 g freeze dry powder), and had enhanced antioxidant activity (7771 ± 207 μM Trolox equivalent/100 g freeze dry powder). Wolfberry phytochemicals, but not purified wolfberry polysaccharide fractions, inhibited Jurkat cell proliferation, induced cycle arrest at the G2/M phase in a dose dependent manner starting at 1 mg/ml for 48 h. Wolfberry phytochemicals eliminated cellular reactive oxygen species, declined expression of endoplasmic reticulum (ER) stress biomarkers, including glucose regulated protein 78, inositol-requiring protein 1(IRE1), activating transcription factor 6 (ATF6), protein kinase RNA-like ER kinase (PERK), and c/EBP-homologous protein, and induced activation of AMP activated protein kinase, stabilization of β-catenin, and inhibition of NFκB, and AKT activity. Simultaneous siRNA knockdown of ATF6, IRE1 and PERK caused inhibition of cell proliferation and induction of apoptosis. Data suggested that ER stress and multiple survival/apoptosis signaling pathways were modulated by wolfberry phytochemicals during the apoptotic progression. Consumption of wolfberry could be an efficacious dietary strategy for preventing leukemia.
Keywords: AMPK, Apoptosis, Cell cycle, Endoplasmic reticulum stress, Leukemia, Reactive oxygen species, Rutin, Wolfberry
Introduction
Epidemiological studies suggest that consumption of some specific fruits, vegetables, and/or whole grains reduces risk of cancer. In the past two decades, a number of bioactive food compounds with anti-cancer activities have been isolated and characterized. Disappointedly, clinical trials do not demonstrate very promising cancer reduction by individual isolated compound [1–3]. This opens up a new direction towards the development of novel anti-cancer strategies through targeting multiple signaling pathways by cumulative and synergistic interaction of the bioactive phytochemical natural mixture but not the individual compound [4–6].
Wolfberry (Lycium barbarum L., Chinese name Goji berry) is a fruit type of food consumed for years in China and Eastern Asia. It was exported to Western countries in the last century. Fresh wolfberry fruits are bright orange-red, oblong shaped. They can be purchased fresh or a dried fruit, drink, and/or a wine. From a nutrient perspective, wolfberry contains large amounts of diester forms of lutein and zeaxanthin. In addition, it has large amount of polysaccharides and polyphenolics [7,8], and contains small molecules such as betaine, cerebroside, various vitamins, and zinc [9]. According to traditional Chinese medicine literature, wolfberry can nourish liver and kidney, help re-balance of the “Yin” and “Yan”. (i.e., energy homeostasis), boost immune system, and improve vision. However, the molecular mechanisms of how the bioactive constituents of wolfberry exert their influence on cancer prevention are not well understood.
Reactive oxygen species (ROS) are multifaceted regulators essential for cell survival/death. ROS are mainly generated in mitochondria, and are also produced in endoplasmic reticulum (ER) [10]. ROS levels in cancer cells are always elevated. A line of evidence demonstrates that phytochemical regulation on ROS controls cancer cell proliferation and death [11].
Polysaccharide fractions of wolfberry have been documented to prevent cancer cell proliferation, including gastric cancer cells [12], colon cancer cells [12], and prostate cancer cells [14]. Wolfberry polysaccharides inhibit the growth and induce apoptosis of prostate cancer PC-3 and DU-145 cells in culture, and inhibit the growth of PC-3 tumor in mice [13]. The inhibition appears through cell cycle arrest at the G0/G1 phase in colon cancer SW480 and Caco-2 cells [14]. However, the chemopreventive effect on blood cancer, such as leukemia, is largely unknown.
ER is the place of folding and secreting of newly synthesized proteins. Accumulation of unfolded and misfolded proteins in the ER triggers the cellular unfolded protein response (UPR). Persistent or prolonged UPR leads to ER stress and subsequent cell apoptosis [15,16]. The chaperone protein glucose regulated protein 78 (Grp78) acts as an ER stress sensor. In unstressed cells, GRP78 binds to the ER stress transducer proteins inositol-requiring protein-1 (IRE1), activating transcription factor 6 (ATF6), and/or protein kinase RNA-like ER kinase (PERK). When the ER stress occurs, expression levels of IRE1, ATF6 and PERK proteins are increased. GRP78 dissociates from all three transducers, which triggers activation of three transducer-mediated signaling pathways [17]. C/EBP-homologous protein (CHOP) is induced by all three ER stress transducer signaling pathways. In many cases, CHOP functions to mediate ER stress-induced apoptosis [18]. In addition, there is evidence that the ER stress signaling crosstalks with multiple signaling pathways involving the progression of both cell growth and death, including Wnt, nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), phosphoinositide 3-kinase (PI3K)-Akt, mitogen-activated protein (MAP) kinase (MAPK), and Forkhead signaling [15,16,19–21].
Targeting ER stress has been recently proposed in cancer prevention. The most well documented approach involves overloading the ER stress so the cancer cells are unable to cope, which leads to cell death [22]. Compared to normal cells, the expression of ATF6, IRE1, PERK, is elevated in various cancer cells, including leukemia [15, 16, 23]. Most recently Misra et al reported that dysfunction of GRP78 significantly inhibited proliferation of prostate cancer cells 1-LN and DU-145, by down-regulating IRE-1α, PERK, and ATF6α-dependent ER stress signaling [24], suggesting that reduction of protein expression of ER stress biomarkers could be an alternative strategy in cancer prevention.
T-cell acute lymphoblastic leukemia (T-ALL) is one of the most aggressive cancers. The survival rates of the patients with T-ALL have been improved distinctly. However, the clinical outcome is still poor due to its frequent relapses and drug resistance [23]. In the current project, using Jurkat cells as a T-ALL in vitro model, we demonstrated that water soluble wolfberry fractions, not purified polysaccharides, were chemopreventive on leukemia Jurkat cells. Wolfberry phytochemicals induced alteration of protein expression involving ER stress and multiple signaling pathways. Targeting multiple proteins related to ER stress and cancer cell growth might be a novel approach to the development of complementary dietary regimens for prevention of cancer, such as leukemia.
Materials and Methods
Preparation of water soluble wolfberry phytochemicals and polysaccharides
Dry wolfberry fruits were purchased from a local Chinese grocery store. Eight hundred grams of wolfberry fruits were blended into a powder. The powder was mixed with 4.5 volume of de-ionized water and boiled for half an hour. The mixture was filtered with 4 layers of cheese cloth. The aqueous phase was harvested and subjected to further centrifugation at 1500 × g for 30 min at 4 °C (Thermo Scientific Sorvall RC-5, Waltham, MA). The supernatants were freeze dried, which yielded about 275 gram. The freeze dried powder, (i.e., wolfberry phytochemicals), was reconstituted in phosphate-buffered saline (PBS) for further usage.
A water soluble wolfberry polysaccharide fraction 1 was isolated and purified by ion exchanged column as described [25]. Fraction 1 was freeze-dried and later reconstituted in PBS for cell proliferation assay.
Antioxidant activity by FRAP assay
The ferric reducing power of wolfberry phytochemicals was determined as described previously [26]. The working FRAP reagent was prepared by mixing 10 volumes of 300 mM acetate buffer [pH 3.6], with 1 volume of 10 mM TPTZ (2,4,6-tri(2-pyridyl)-s-triazine) in 40 mM hydrochloric acid and with 1 volume of 20 mM ferric chloride, and pre-warmed at 37°C. Freeze-dried wolfberry phytochemical powders were dissolved in Chelex treated deionized water at concentration of 5mg/ml. Thirty μl of the wolfberry phytochemical solution was mixed with 270 μl freshly prepared FRAP reagent, and incubated at 37 °C for 30 min. In the end, the absorbance of the samples was measured at 550nm. Trolox was used as a standard. The FRAP value was calculated against a Trolox standard calibration curve.
Determination of total carbohydrate
The total carbohydrate of wolfberry phytochemicals was determined by the anthrone method [27]. Briefly, wolfberry phytochemical freeze-dried powders were dissolved in anthrone solution to achieve the solution at 2 g/l anthrone in 75% H2SO4. The mixture was boiled at 100 °C for 15 min. After the samples were cooled down, the absorbance was read at 578 nm. Glucose was used as a standard.
Measurement of total polyphenolics
The amount of total polyphenolics in wolfberry phytochemicals was determined according to the Folin-Ciocalteu method [28]. Ten μl wolfberry phytochemical solution (at 1 mg/ml in deionized water) was solution mixed with 50 μl Folin-Ciocalteau’s reagent and 50 μl Na2CO3 (20%, w/v), then incubated for 2 h at room temperature. After that the absorbance of samples was measured at 760 nm. Results were expressed as mg of quercetin equivalent per 100 gram of freeze-dried wolfberry phytochemical powders.
Profiling wolfberry carotenoids and flavonoids by high-performance liquid chromatography
The amount of carotenoids and flavonoids in wolfberry phytochemicals was determined by high-performance liquid chromatography (HPLC) by the Craft Technologies, Inc (Wilson, NC, USA). Rutin content was expressed as mg/100 g freeze dry powder.
Cell culture and BrdU cell proliferation assay
Jurkat cells (ATCC, Manassas, VA) were cultured in RMPI-1640 medium supplemented with 10% fetal bovine serum and 50 μg/ml gentamicin, 0.05 unit/ml penicillin, 50 μg/ml streptomycin, at 37 °C in an atmosphere of 95% air and 5% CO2. For cell proliferation assay, Jurkat cells were seeded at 4x104cells/well in 96-well flat bottom plates and incubated overnight. Cells were then treated with wolfberry phytochemicals and/or purified polysaccharides for 48 h. After treatment, 10 μM 5-bromo-2′-deoxyuridine (BrdU) was added to the plates and cells were incubated for 4 hours at 37 °C in an atmosphere of 95% air and 5% CO2. The cells together with media and BrdU were then transferred to round bottom plates, and subsequently subjected to centrifugation at 300 g for 10 minutes to harvest cells. Then cells were fixed and labeled with BrdU detection antibodies according to the manufacturer’s instruction (Cell Signaling, Danvers, MA). Finally, the reaction solution with cells was transferred back to new flat bottom plates for absorbance reading at 450 nm. Data was normalized by the protein concentrations from the parallel studies, and further compared with the untreated Jurkat cells (the PBS group) and graphed. Per siRNA knockdown experiments, 24 hours post siRNA transfection, cells were subjected to BrdU proliferation assay.
Detection of cellular ROS
Cellular ROS level was detected as previous described by a fluorimetric assay using carboxy-H2DCFDA (C400) as the probe (Invitrogen, Carlsbad, CA) [29]. C400 working concentration was 25 μM. The fluorescence was quantified using a microplate reader with a fluorescence excitation of 485 nm and emission of 538 nm. Relative fluorescence intensity was normalized by the protein concentrations from the parallel studies, and further compared with the untreated Jurkat cells (the PBS group) and graphed.
Simultaneous knockdown of endogenous PERK, IRE1 and ATF6
Endogenous PERK, IRE1 and ATF6 were simultaneously knocked down by small interfering RNA (siRNA). The targeting sequences: PERK, 5′-CAC AAA CTG TAT AAC GGT TTA-3′; IRE1, 5′-CAG CAC GGA CGT CAA GTT TGA-3′; and ATF6, 5′-CAG CAA CCA ATT TAT CAG TTT A-3′. The equal amounts of three siRNA (final concentration: 12.5 nM for each siRNA) were co- transfected into cells at 4×105cells/ml using HiPerFect siRNA transfection reagent according to the instructions as provided (Qiagen, Valencia, CA, USA). Prior to experimentation, the cells were left for 24 hours to knockdown target genes. siRNA knockdown was monitored by Western blot. The vehicle (transfection reagent only) and negative control siRNA (AllStars Negative Control siRNA, Qiagen, catalog # 1027281) were used as negative controls.
Cell cycle arrest analysis by flow cytometry
Jurkat cells were seeded in 6-well culture plates. After wolfberry phytochemical treatments for 48 h, cells were fixed in 70% ice cold ethanol and stored overnight at 4°C. Fixed cells were washed with ice cold PBS for 3 times and further resuspended in 20μg/ml propidium iodide solution with 1 μg/ml RNase in PBS. Flow cytometry analysis was performed by a BD FACSCalibur flow cytometer E4400 (Franklin Lakes, NJ), with an excitation at 488 nm and an emission at 630 nm.
Cell apoptosis by annexin V assay and poly ADP ribose polymerase (PARP) cleavage assay
Jurkat cells were seeded in 6-well culture plates. After treatments for 48 h, cells were collected by centrifugation, stained with Annexin V-FITC and propidium idodide, and then subjected to flow cytometry immediately according to manufacturer’s instruction (Biovision, Mountain View, CA). Cell apoptosis was also determined by cleavage assay of poly ADP ribose polymerase (PARP) using anti-cleaved PARP antibody (Cell Signaling, Danvers, MA).
Western blot
Alteration of protein expression and phosphorylation was determined as described with modification [29]. Briefly, cells were collected and lysed on ice with cell lysis buffer followed by brief sonication. The cell lysis buffer contained 20 mM Tris–HCl, pH 7.5, 0.5 mM EDTA, 0.5 mM EGTA, 0.25% Triton X-100, 0.1% protease and phosphatase inhibitor cocktails (Sigma, St Louis, MO). After centrifugation at 10,000 × g for 15 min, the supernatants were used as whole cell extracts for Western blotting. Amounts of protein loaded per lane were 50μg. Antibodies against IRE1 and ATF6 were purchased from Abcam (Cambridge, MA). All other primary and secondary antibodies were purchased from Cell Signaling (Danvers, MA). Immunoreactive bands were detected by chemiluminescence (ECL; Thermo Scientific Pierce, Rockford, IL) and visualized by the FluorChem 8800 advanced image system (Alpha Innotech, San Leandro, CA). Total pixels of each protein band were used for graphing and statistic analysis.
Statistical analysis
The results were analyzed by one-way ANOVA with Dunnett’s multiple comparison using SAS 9.1 and/or student t-test. All experiments represented at least triplicates. Representative images of Western blot were shown. The level of significance (*) was considered at p ≤ 0.05. All data are mean ± S.D.
Results
Wolfberry phytochemicals are enriched in carbohydrates and polyphenolics, and have enhanced antioxidant activity
Decoction is one of traditional methods to harvest bioactive food compounds [7,8]. We used boiling water decoction to extract wolfberry phytochemicals. We yielded 275 g freeze dried water soluble wolfberry fractions (i.e. wolfberry phytochemicals), from 800 g dry wolfberry fruits, the yield rate was 34.3 % (w/w). Nutritional composition results showed that the isolated wolfberry phytochemicals contained carbohydrates at 73.4 ± 4.5 % (w/w), and polyphenolics at 1555 ± 112 mg quercetin equivalent/100 g freeze dry powder. HPLC analysis results showed that the wolfberry phytochemicals contained 213 mg rutin/100 g freeze dry powder, and non-detectable carotenoids (data not shown). The antioxidant activity of wolfberry phytochemicals was 7771 ± 207 μM Trolox equivalent/100 g freeze dry powder.
Wolfberry phytochemicals eliminate cellular reactive oxygen species (ROS) and attenuate endoplasmic reticulum stress
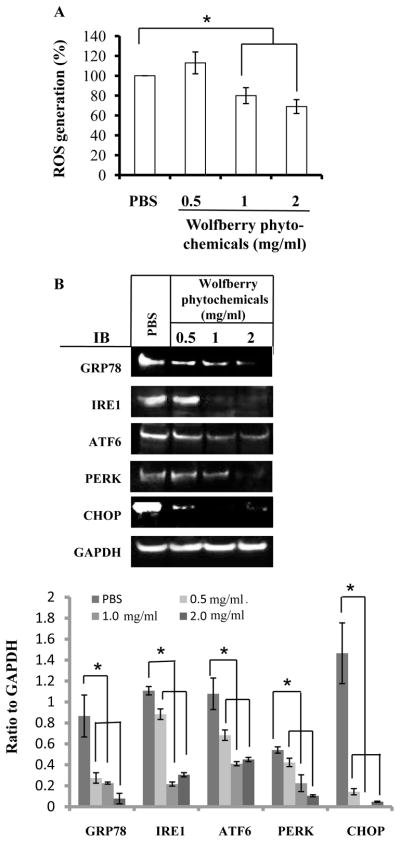
The ROS levels were determined in Jurkat cells treated with 0.5, 1, and/or 2 mg/ml wolfberry phytochemicals for 48 h. The results in Figure 1A showed that application of wolfberry phytochemicals eliminated cellular ROS levels in a dose dependent manner.
Figure 1. Elimination of ROS and attenuation of endoplasmic reticulum (ER) stress by wolfberry phytochemicals in leukemia Jurkat cells.
Cells were treated with 0.5, 1, and 2 mg/ml wolfberry phytochemicals for 48 h. Phosphate buffered saline (PBS) treatment as a control. The cellular ROS levels were determined (A). The whole cell lysates were subjected to Western blot (B). Representative images were shown. Total pixels of each protein band were used for graphing and statistical analysis, based on at least three independent Western blot experiment results. *: significance at p ≤ 0.05. GAPDH as a loading control. ROS, reactive oxygen species; Grp78, glucose regulated protein 78; IRE1, inositol-requiring protein-1; ATF6, activating transcription factor -6; PERK, protein kinase RNA-like ER kinase; CHOP, C/EBP-homologous protein; IB, immunoblot; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
As shown in Figure 1B, protein expression levels of GRP78, IRE1, ATF6, and PERK were significantly decreased in dose-dependent manners in the wolfberry treated Jurkat cells, CHOP expression, an indicator of activation of ER stress, was significantly decreased, indicating wolfberry phytochemical attenuation of ER stress in the leukemia Jurkat cells.
Wolfberry phytochemicals disrupt multiple signaling pathways: Wnt, PI3K-AKT, and NFκB
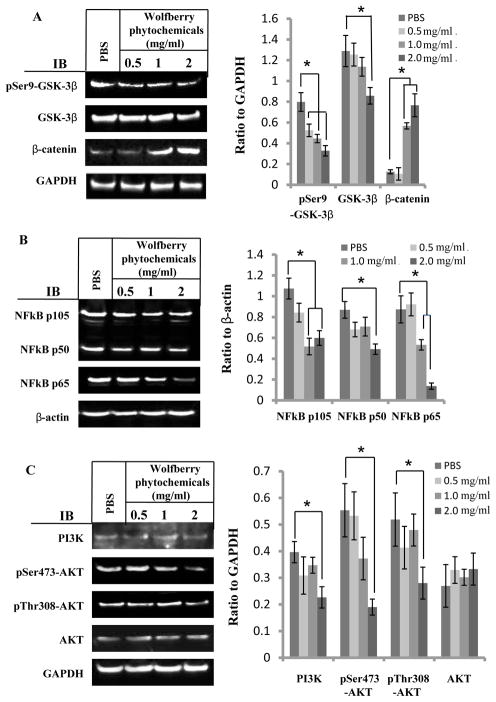
The results in Figure 2A demonstrated that the expression of β-catenin protein was increased, while the phosphorylation of glycogen synthease kinase 3 β (GSK-3β) on Ser9 was decreased dose-dependently by wolfberry treatments, indicating that wolfberry phytochemicals activated Wnt signaling by activation of GSK-3β and accumulation and stabilization of β-catenin.
Figure 2. Alteration of multi-signaling pathways in Jurkat cells by wolfberry phytochemicals.
Cells were treated with wolfberry phytochemicals for 48 h. PBS was used as a control. Alteration of cell survival/apoptosis signaling pathways was determined by Western blotting. A. Wnt signaling: Inhibition of GSK-3β phosphorylation on Ser9 and accumulation of β-catenin. B. NFκB pathway: Inhibition of NFκB p65. C. PI3K/AKT pathway: Inhibition of AKT phosphorylation on Ser473 and Thr308. Total pixels of each protein band were used for graphing and statistic analysis, based on at least three independent Western blot experiment results. GAPDH and/or β-actin, loading controls. *: significance at p ≤ 0.05. pSer9-GSK-3β, glycogen synthase kinase-3 β phosphorylation on Serine 9; Ser9-GSK-3β, glycogen synthase kinase-3 β; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K, phosphoinositide 3-kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PBS, phosphate-buffered saline.
NFκB is a protein complex which has been linked to cancer [30, 31]. ER stress crosstalks NFκB pathway [31]. Western blot results in Figure 2B showed that wolfberry phytochemicals inhibited the expression of NFκB p65, NFκB p50 and NFκB p105 significantly, in a dose dependent manner.
Alteration of PI3K-AKT signaling was shown in Figure 2C. Total protein level of PI3K was decreased in the treatment at 2.0 mg/ml. Yet, AKT protein levels were not changed. However, AKT phosphorylation on Ser473 and Thr308 was inhibited significantly by the wolfberry treatment at 2.0 mg/ml, indicating inactivation of AKT by wolfberry phytochemicals.
Wolfberry phytochemicals activate AMP-activated protein kinase
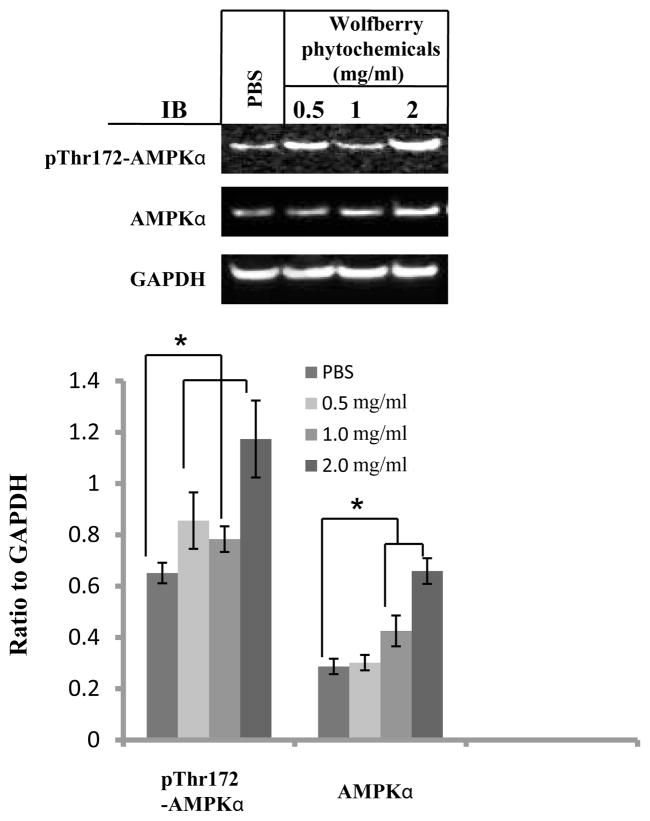
Simultaneous regulation on cell survival/apoptosis signaling pathways suggests that wolfberry phytochemicals would unhinge the regulation of cellular energy homeostasis (AMP:ATP) which are controlled by AMP-activated protein kinase (AMPK). Western blot results were shown in Figure 3. Both AMPKα and phospho-Thr172-AMPKα proteins were elevated significantly in the wolfberry phytochemical treated Jurkat cells, indicating that wolfberry phytochemicals activated AMPK.
Figure 3. Wolfberry phytochemicals activate AMPK.
Jurkat cells were treated with wolfberry phytochemicals for 48 h as indicated. PBS was used as a control. AMPKα, and AMPK α phosphorylation on Thr172 were determined by Western blot. Total pixels of each protein band were used for graphing and statistic analysis, based on at least three independent Western blot experiment results. *: significance at p ≤ 0.05. GAPDH as a loading control. AMPK, AMP-activate protein kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PBS, phosphate-buffered saline.
Wolfberry phytochemicals, not purified polysaccharides, inhibit Jurkat cell proliferation
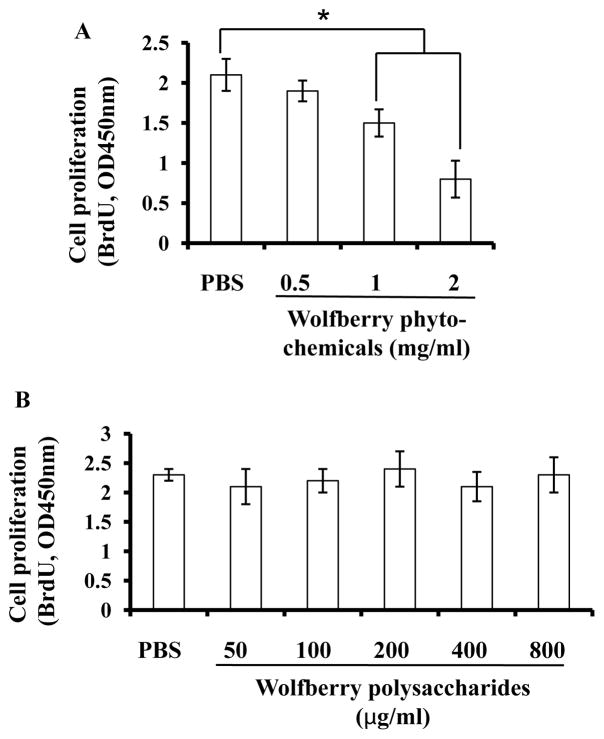
As shown in Figure 4A, the Jurkat cell proliferation rates were decreased in a dose dependent manner after treatments of wolfberry phytochemicals. In this study, over 70 % of isolated wolfberry phytochemicals were carbohydrates, the large partial could be polysaccharides [13]. Application of purified wolfberry polysaccharides up to 800 μg/ml for 48h did not inhibit proliferation of Jurkat cells shown in Figure 4B.
Figure 4. Growth inhibition of Jurkat cells by wolfberry phytochemicals, but not the purified polysacchardies.
Jurkat cells were treated with wolfberry phytochemicals (A) or purified wolfberry polysaccharides (B) for 48 h as indicated. Cell proliferation was measured as described. PBS ( phosphate-buffered saline) was used as a control. *, p ≤ 0.05.
Wolfberry phytochemicals induce cell cycle arrest at the G2/M phase and apoptosis
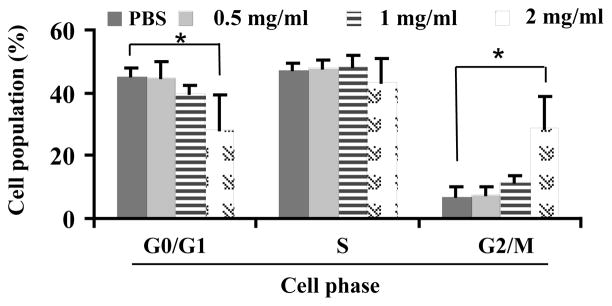
Flow cytometry results showed that treatments of wolfberry phytochemicals did not cause any change of the percentage of cell populations at the S phase. Cell populations were decreased at the G0/G1 phase and increased at the G2/M phase, in a dose dependent manner. The significant changes were observed when wolfberry phytochemicals was at 2 mg/ml (Figure 5).
Figure 5. Cell cycle arrest at G2/M phase in Jurkat cells by wolfberry phytochemicals.
Jurkat cells were treated with PBS (phosphate-buffered saline) or wolfberry phytochemicals for 48 h as indicated. The cell cycle distribution was measured as described. Cell cycle arrest at G2/M phase was dose dependently. *, p ≤ 0.05.
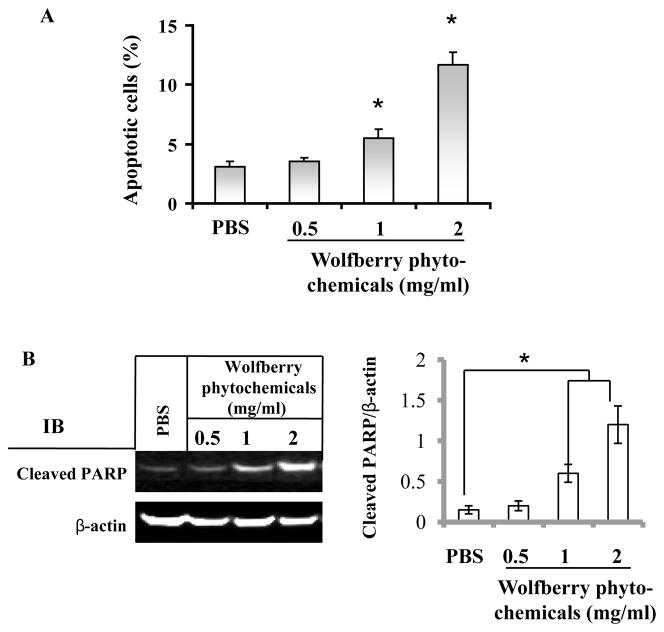
Jurkat cell apoptosis was determined by annexin V staining/flow cytometry and cleavage of PARP. Compared to untreated cells, the percentage of apoptotic cells was increased dose dependently in the wolfberry phytochemical treated cells (Figure 6A). Similarly, wolfberry phytochemicals induced PARP cleavage in a dose dependent manner (Figure 6B), suggesting apoptosis occurred in wolfberry treated Jurkat cells.
Figure 6. Jurkat cell apoptosis induced by wolfberry phytochemicals.
Wolfberry pytochemicals and/or PBS (vehicle) treated cells were subjected to apoptosis analysis by Annexin V assay (A) and cleavage of PARP by Western blot (B) as described. The percentage of apoptotic cells was increased dose-dependently. PBS, phosphate-buffered saline. *, p ≤ 0.05.
Simultaneous knockdown of ER stress biomarkers induces PARP cleavage and inhibits cell proliferation
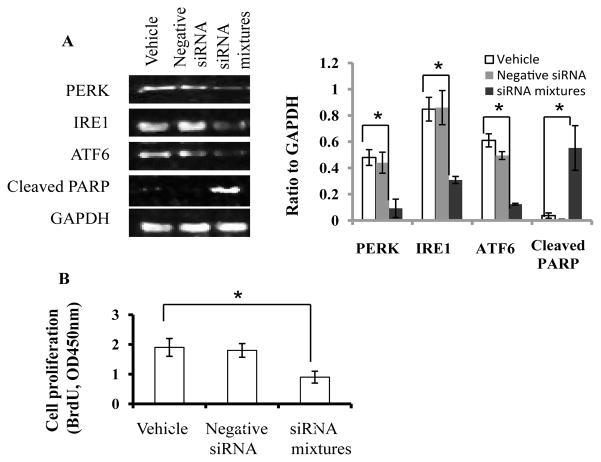
Finally we performed siRNA co-transfection to knockdown PERK, IRE1 and ATF6 simultaneously (Figure 7A). Western blot results clearly revealed that knockdown of three proteins was significant, which led to cleavage of PARP and inhibition of Jurkat cell proliferation (Figure 7A and B). Whereas ROS level was not significantly altered (data not shown).
Figure 7. Simultaneous knockdown of ER stress biomarkers induces PARP cleavage and inhibits cell proliferation.
siRNA of PERK, IRE1 and ATF6 was co-transfected into Jurkat cells to knockdown the proteins. Expression of PERK, IRE1, ATF6, and cleaved PARP was determined by Western blot. Total pixels of each protein band were used for graphing and statistic analysis, based on at least three independent Western blot experiment results (A). Cell proliferation in the vehicle and transfected cells was analyzed as described in the Materials and Methods section (B). Vehicle, transfection reagent only; siRNA mixtures, siRNA mixtures of PERK, IRE1, and ATF6. IRE1, inositol-requiring protein-1; ATF6, activating transcription factor -6; PERK, protein kinase RNA-like ER kinase; PARP, poly ADP ribose polymerase. *, p ≤ 0.05.
Discussion
In this study we demonstrated that a natural mixture of wolfberry phytochemicals induced leukemia Jurkat cell apoptosis through elimination of cellular ROS. Previous reports showed that purified resveratrol and quercetin induce Jurkat cell cycle arrest at about 20 to 100 μM [32, 33]. Compared to those reports, the doses used in the current study were fairly low. Most abundant fractions in our harvested wolfberry extracts were carbohydrates (over 73 %). Working concentration of total polyphenolics in the culture media was at the range of 7.8 – 31.1μg quercetin equivalent/ml (calculated based on total polyphenolics contents in the freeze-dried powder of wolfberry phytochemicals). The rutin doses were at the range of 1.07–4.26 μg/ml.
Wolfberry phytochemicals had inhibitory effects on cancer cell proliferation, including leukemia Jurkat cells (Figure 4A), colon cancer SW480 cells and canine lymphoma F1B cells (data not shown). However, there was no growth inhibition observed in the normal cells treated with same doses of wolfberry phytochemicals (data not shown), suggesting its specificity on anti-cancer cell proliferation. Purified polysaccharides, however, did not inhibit cell proliferation in Jurkat cells in culture (Figure 4B). The results are not consistent with the previous observation in other cancer cells, including colon and prostate cancer cells [12–14]. This may be due to difference of cancer cell culture models. The results suggested that other components or synergistic interaction of wolfberry phytochemicals might play roles in inhibition of Jurkat cell proliferation.
Unlike normal cells, most cancer cells grow and proliferate under constitutive oxidative stress condition (e.g. elevated ROS levels), which results in cellular unfolded protein response (UPR) and ER stress [34]. Phytochemicals, such as polyphenolics, have been demonstrated to induce overloading of ER stress and subsequent apoptosis in cancer cells [33–39]. Phytochemical prevention of blood cancer, such as leukemia, has not been well documented [40]. To our knowledge, this is the first report that phytochemical-eliminated ROS leaded to subsequent apoptosis through down-regulation of ER stress biomarkers in leukemia cells. High antioxidant activity of wolfberry phytochemicals scavenged reactive oxygen species, which resulted in diminishing cellular oxidative status and disrupting of cellular stress homeostasis that cancer cells live on.
A number of publications reported that targeting ER stress could be an attractive strategy for cancer prevention. It has been well documented that overloading ER stress induces cancer cell apoptosis, including leukemia Jurkat cells, though cancer cells naturally live on elevated ER stress. However, a couple of reports addressed that inhibition of ER stress biomarkers causes cancer cell death [41,24]. For instance, down-regulation of IRE-1α, PERK, and ATF6α-dependent ER stress signaling by the antibody against the COOH-terminal domain of the GRP78 protein significantly caused inhibition of growth of 1-LN and DU-145 prostate cancer cells [24]. Under tunicamycin-induced ER stress condition, GRP78 levels were declined by phytochemicals including genistein, epigallocatechin gallate, and salicylic acid [37,39,42]. CHOP has a dual role to protect from and promote cell death [43]. In this study, wolfberry phytochemicals and/or siRNA knockdown caused down-regulation of ER stress biomarkers, and inhibition of CHOP expression in the progression of Jurkat cell apoptosis, suggesting that precise control of protein levels of ER stress biomarkers is critical to cancer cell survival. Disruption of the balance by either triggering ER stress [15,16, 22, 23] or down-regulation of ER stress biomarkers (this study) may lead to leukemia Jurkat cell apoptosis.
β-catenin, a proapoptotic protein, plays a key role in the Wnt signaling pathway. GSK-3β destabilizes β-catenin by phosphorylating it at Ser33, Ser37 and Thr41 [44]. Mutations in these phosphorylation sites, which result in the stabilization of β-catenin protein levels, have been found in many tumor cell lines [45]. ER stress-induced dissociation of GRP78 from Wnt leads to downregulation of stabilized β-catenin and consequent cell proliferation [21]. Wolfberry phytochemicals induced accumulation and stabilization of β-catenin through inactivation of GSK-3β, which in turn leads to activation of cellular Wnt signaling and consequently Jurkat cell apoptosis. On the other hand, GSK-3β is also a critical downstream target of the PI3K/AKT cell survival pathway whose activity can be inhibited by AKT-mediated phosphorylation at Ser9 of GSK-3β [46].
Activation and overexpression of NFκB were found in many tumors [30]. Expression of NFκB, including NFκB p105, p65 and p50, declined dose dependently in wolfberry treated Jurkat cells, indicating that NFκB was one of the molecular targets for induction of apoptosis by wolfberry phytochemicals.
Control of AMPK is important in cancer prevention. AMPK negatively affects mTOR/AKT through phosphorylation of raptor, which leads to inhibition of CDC2 and activation of apoptosis [37]. Wolfberry phytochemical activation of AMPK might be related to inhibition of CDC2 activity leading to cell cycle arrest in human leukemia Jurkat cells, which was consistent with previous reports in other cancer cell culture models [47].
Taken together, wolfberry phytochemicals, a natural mixture of polysaccharides, polyphenolics, and other unknown compounds, dually functioned as an antioxidant, through eliminating cellular ROS, and a modulator of cell survival/death signaling pathways, through targeting ER stress and related pathways in AMPK, β-catenin, NFκB, and PI3K-AKT signaling, which lead to Jurkat cell apoptosis. Targeting multiple signaling pathways by a mixture of wolfberry phytochemicals would be considered as a novel strategy in the development of therapeutic regimens for prevention of cancer, such as leukemia. This may also help overcome the complexity of drug resistance.
Acknowledgments
The authors are very grateful for the wolfberry polysaccharides, a gift from Dr. Xiang-dong Gao, China Pharmaceutical University, Nanjing, China. This work was supported by the Kansas State University Johnson Cancer Center Innovative Research Award and the KSU NIH NCRR Grant P20-RR-017686 (to D. L.). Contribution # 12-104-J from the Kansas Agricultural Experiment Station.
Abbreviations
- GRP78
Glucose Regulated Protein 78
- IRE1
Inositol-Requiring Protein 1
- ATF6
Activating Transcription Factor 6
- PERK
Protein Kinase RNA-Like ER Kinase
- CHOP
C/EBP-Homologous Protein
- AMPK
AMP activated Protein Kinase
- UPR
Unfolded Protein Response
- NFκB
Nuclear Factor Kappa-light-chain-enhancer of activated B cells
- PI3K
Phosphoinositide 3-Kinase
- T-ALL
T-cell Acute Lymphoblastic Leukemia
- ROS
Reactive Oxygen Species
- GSK-3β
Glycogen Synthease Kinase 3 β
- PARP
Poly ADP Ribose Polymerase
- PBS
Phosphate Buffered Saline
- GAPDH
Glyceraldehyde 3-Phosphate Dehydrogenase
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The authors have declared no conflict of interest.
References
- 1.Gann PH. Randomized trials of antioxidant supplementation for cancer prevention. JAMA. 2009;301:102–103. doi: 10.1001/jama.2008.863. [DOI] [PubMed] [Google Scholar]
- 2.Kaefer CM, Milner JA. The role of herbs and spices in cancer prevention. J Nutr Biochem. 2008;19:347–361. doi: 10.1016/j.jnutbio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarkar FH, Li Y, Wang Z, Kong D. Cellular signaling perturbation by natural products. Cell Signal. 2009;21:1541–1547. doi: 10.1016/j.cellsig.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Kok TM, van Breda SG, Manson MM. Mechanisms of combined action of different chemopreventive dietary compounds: a review. Eur J Nutr. 2008;47:51–59. doi: 10.1007/s00394-008-2006-y. [DOI] [PubMed] [Google Scholar]
- 5.Pan MH, Ghai G, Ho CT. Food bioactives, apoptosis, and cancer. Mol Nutr Food Res. 2008;52:43–52. doi: 10.1002/mnfr.200700380. [DOI] [PubMed] [Google Scholar]
- 6.Ding H, Tauzin S, Hoessli DC. Phytochemicals as modulators of neoplastic phenotypes. Pathobiology. 2009;76:55–63. doi: 10.1159/000201674. [DOI] [PubMed] [Google Scholar]
- 7.Wang CC, Chang SC, Inbaraj BS, Chen BH. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010;120:184–192. [Google Scholar]
- 8.Inbaraj BS, Lu H, Kao TH, Chen BH. Simultaneous determination of phenolic acids and flavonoids in Lycium barbarum Linnaeus by HPLC-DAD-ESI-MS. J Pharm Biomed Anal. 2010;51:549–556. doi: 10.1016/j.jpba.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Chang RC, So KF. Use of anti-aging herbal medicine, Lycium barbarum, against aging-associated diseases. What do we know so far? Cell Mol Neurobiol. 2008;28:643–652. doi: 10.1007/s10571-007-9181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18:567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Miao Y, Xiao B, Jiang Z, Guo Y, Mao F, et al. Growth inhibition and cell-cycle arrest of human gastric cancer cells by Lycium barbarum polysaccharide. Med Oncol. 2010;27:785–790. doi: 10.1007/s12032-009-9286-9. [DOI] [PubMed] [Google Scholar]
- 13.Luo Q, Li Z, Yan J, Zhu F, Xu RJ, et al. Lycium barbarum polysaccharides induce apoptosis in human prostate cancer cells and inhibits prostate cancer growth in a xenograft mouse model of human prostate cancer. J Med Food. 2009;12:695–703. doi: 10.1089/jmf.2008.1232. [DOI] [PubMed] [Google Scholar]
- 14.Mao F, Xiao B, Jiang Z, Zhao J, Huang X, et al. Anticancer effect of Lycium barbarum polysaccharides on colon cancer cells involves G0/G1 phase arrest. Med Oncol. 2011;28:121–126. doi: 10.1007/s12032-009-9415-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2294. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Ni M, Lee B, Barron E, Hinton DR, et al. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460–1471. doi: 10.1038/cdd.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misra UK, Deedwania R, Pizzo SV. Activation and cross-talk between Akt, NF-kappaB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J Biol Chem. 2006;281:13694–13707. doi: 10.1074/jbc.M511694200. [DOI] [PubMed] [Google Scholar]
- 20.Guan L, Han B, Li Z, Hua F, Huang F, et al. Sodium selenite induces apoptosis by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction in human acute promyelocytic leukemia NB4 cells. Apoptosis. 2009;14:218–225. doi: 10.1007/s10495-008-0295-5. [DOI] [PubMed] [Google Scholar]
- 21.Verras M, Papandreou I, Lim AL, Denko NC. Tumor hypoxia blocks Wnt processing and secretion through the induction of endoplasmic reticulum stress. Mol Cell Biol. 2008;28:7212–7224. doi: 10.1128/MCB.00947-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao WL. Targeted therapy in T-cell malignancies: dysregulation of the cellular signaling pathways. Leukemia. 2010;24:13–21. doi: 10.1038/leu.2009.223. [DOI] [PubMed] [Google Scholar]
- 23.Ni M, Zhou H, Wey S, Baumeister P, Lee AS. Regulation of PERK signaling and leukemic cell survival by a novel cytosolic isoform of the UPR regulator GRP78/BiP. PLoS One. 2009;4:e6868. doi: 10.1371/journal.pone.0006868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misra UK, Pizzo SV. Modulation of the unfolded protein response in prostate cancer cells by antibody-directed against the carboxyl-terminal domain of GRP78. Apoptosis. 2010;15:173–182. doi: 10.1007/s10495-009-0430-y. [DOI] [PubMed] [Google Scholar]
- 25.Zou S, Zhang X, Yao W, Niu Y, Gao X. Structure characterization and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Carbohydrate Polymers. 2010;80:1161–1167. [Google Scholar]
- 26.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 27.Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954;57:508–514. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peri C, Pompei C. An assay of different phenolic fractions in wines. Am J Enol Vitic. 1971;22:55–58. [Google Scholar]
- 29.Tang L, Zhang Y, Jiang Y, Willard L, Ortiz E, et al. Dietary wolfberry ameliorates retinal structure abnormalities in db/db mice at the early stage of diabetes. Exp Biol Med (Maywood) 2011;236:1051–1063. doi: 10.1258/ebm.2011.010400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ralhan R, Pandey MK, Aggarwal BB. Nuclear factor-kappa B links carcinogenic and chemopreventive agents. Front Biosci. 2009;1:45–60. doi: 10.2741/S6. [DOI] [PubMed] [Google Scholar]
- 31.Kim YS, Young MR, Bobe G, Colburn NH, Milner JA. Bioactive food components, inflammatory targets, and cancer prevention. Cancer Prev Res. 2009;2:200–208. doi: 10.1158/1940-6207.CAPR-08-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin JN, Lin VC, Rau KM, Shieh PC, Kuo DH, et al. Resveratrol modulates tumor cell proliferation and protein translation via SIRT1-dependent AMPK activation. J Agric Food Chem. 2010;58:1584–1592. doi: 10.1021/jf9035782. [DOI] [PubMed] [Google Scholar]
- 33.Singh RP, Agarwal R. Natural flavonoidstargeting deregulated cell cycle progression in cancer cells. Curr Drug Targets. 2006;7:345–354. doi: 10.2174/138945006776055004. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Du Y, Le W, Wang K, Kieffer N, et al. Redox control of the survival of healthy and diseased cells. Antioxid Redox Signal. 2011;15:2867–2908. doi: 10.1089/ars.2010.3685. [DOI] [PubMed] [Google Scholar]
- 35.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, et al. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 37.Zhou Y, Lee AS. Mechanism for the suppression of the mammalian stress response by genistein, an anticancer phytoestrogen from soy. J Natl Cancer Inst. 1998;90:381–388. doi: 10.1093/jnci/90.5.381. [DOI] [PubMed] [Google Scholar]
- 38.Hsu YL, Chen CY, Hou MF, Tsai EM, Jong YJ, et al. 6-Dehydrogingerdione, an active constituent of dietary ginger, induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human breast cancer cells. Mol Nutr Food Res. 2010;54:1307–1317. doi: 10.1002/mnfr.200900125. [DOI] [PubMed] [Google Scholar]
- 39.Ermakova SP, Kang BS, Choi BY, Choi HS, Schuster TF, et al. (−)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–9269. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- 40.Jun DY, Kim JS, Park HS, Han CR, Fang Z, et al. Apoptogenic activity of auraptene of Zanthoxylum schinifolium toward human acute leukemia Jurkat T cells is associated with ER stress-mediated caspase-8 activation that stimulates mitochondria-dependent or -independent caspase cascade. Carcinogenesis. 2007;28:1303–1313. doi: 10.1093/carcin/bgm028. [DOI] [PubMed] [Google Scholar]
- 41.Zu K, Bihani T, Lin A, Park YM, Mori K, et al. Enhanced selenium effect on growth arrest by BiP/GRP78 knockdown in p53-null human prostate cancer cells. Oncogene. 2006;25:546–554. doi: 10.1038/sj.onc.1209071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng WG, Ruan KH, Du M, Saunders MA, Wu KK. Aspirin and salicylate bind to immunoglobulin heavy chain binding protein (BiP) and inhibit its ATPase activity in human fibroblasts. FASEB J. 2001;15:2463–2470. doi: 10.1096/fj.01-0259com. [DOI] [PubMed] [Google Scholar]
- 43.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 44.Yost C, Torres M, Miller JR, Huang E, Kimelman D, et al. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 45.Mori PJ, Sparks AB, Korinek V, Barker N, Clevers H, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 46.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 47.Gwinn DM, Asara JM, Shaw RJ. Raptor is phosphorylated by cdc2 during mitosis. PLoS One. 2010;5:e9197. doi: 10.1371/journal.pone.0009197. [DOI] [PMC free article] [PubMed] [Google Scholar]