Abstract
Pterygota macrocarpa and Cola gigantea are African medicinal plants used in traditional medicine for the treatment of sores, skin infections, and other inflammatory conditions including pains. This study therefore aims at investigating the antimicrobial properties of ethanol leaf and stem bark extracts of P. macrocarpa and C. gigantea using the agar diffusion and the micro-dilution techniques and also determining the anti-inflammatory properties of the extracts of these plants in carrageenan-induced foot edema in seven-day old chicks. The minimum inhibitory concentration of both ethanol leaf and bark extracts of P. macrocarpa against the test organisms was from 0.125 to 2.55 mg/mL and that of C. gigantea extracts was 0.125 to 2.75 mg/mL. Extracts with concentration of 50 mg/mL were most active against the test organisms according to the agar diffusion method. All the extracts of P. macrocarpa and C. gigantea at 30, 100, and 300 mg/kg body weight except ethanol leaf extract of C. gigantea exhibited significant anti-inflammatory effects (P ≤ 0.001).
1. Introduction
The search for newer antimicrobial agents from various sources has become imperative because of the emergence of resistance strains of microorganisms against orthodox antibiotics especially difficulty to treat infections from resistant strains of bacteria [1] and also the fact that the number of scientists who are developing new antibacterial agents has dwindled, even as bacteria evolve ever more clever mechanisms of resistance to antibiotics [2]. The recent search for new antibiotics includes various sources such as the synthetic compounds, bioactive agents from aquatic microorganisms, and natural products including medicinal plants. In Africa and other developing countries, it is estimated that 70 to 80% of people rely on traditional healers and herbal practitioners for their health needs [3, 4] and medicinal plants are the main source of remedies used in this therapy. Some of these medicinal plants are used for the management of several different disease conditions such as bacterial infections, parasitic infections, skin diseases, hypertension, pains, and inflammation such as rheumatoid arthritis [5–8].
Several medicinal plants including their isolated compounds have been found to exhibit biological activities related to their traditional uses, for example, geraniin and furosin isolated from Phyllanthus mellerianus (Kuntze) Exell. have been found to possess wound healing properties ascribed to this plant as wound healing agent [9]. Cryptolepine, an alkaloid from Cryptolepine sanguinolenta, has been shown to possess antimicrobial and antiplasmodial activities which have gone to confirm its medicinal uses as anti-infective and antimalarial agent [10, 11].
Pterygota macrocarpa K. Schum. belongs to the family Sterculiaceae and is known in local Asante-Twi language as kyereye in Ghana. It is a large tree that grows in dense semideciduous forests usually distributed in West Africa from Sierra Leone to Cameroun. The soaked leaves are used to treat stomachache, pains, and disorders of digestion. Leaf decoctions are used for the treatment of gonorrhea and other urinary tract infections [12–14]. Traditionally, the bark is used in the management of haemorrhoids, dropsy, swellings, edema, gout, leprosy, and pain [15]. The seeds of P. macrocarpa have been found to contain phytate, oxalate and tannins [16].
Cola gigantea A. Chev. belongs to the family Sterculiaceae and is commonly known as giant cola and local Asante-Twi name is watapuo in Ghana. It is a large tree in dry semideciduous forests in West Africa and the West Indies. The nuts (mostly called kola) are often used to treat whooping cough, asthma, malaria, and fever. Other traditional uses include increasing the capacity for physical exertion and for enduring fatigue without food, stimulating a weak heart, and treating nervous debility, weakness, lack of emotion, nervous diarrhea, depression, despondency, brooding, anxiety, and sea sickness [12, 14, 15, 17]. Kola nut is the name of the mature fruits of the Cola species [18] and has a bitter flavour and high caffeine content [19, 20], and when the fruit is ingested, it acts as stimulants and thus creates an ecstatic and euphoric state [20]. The caffeine present acts as a bronchodilator, expanding the bronchial air passages [21]. These fruits are also chewed in communities during traditional ceremonies and also are known to reduce hunger pangs. The ethanol leaf extract of C. gigantea has been shown to be active against Candida albicans and phytochemical screening of the leaf extract indicated the presence of alkaloids, saponins, tannins, anthraquinones, and cardenolides [22]. The aim of this study is to investigate the antimicrobial and anti-inflammatory activities of ethanol stem bark and leaf extracts of P. macrocarpa and C. gigantea.
2. Materials and Methods
2.1. Plant Material and Chemicals
Stem bark and leaves of Pterygota macrocarpa and Cola gigantea were collected in July, 2007, from the Bobiri Forest Reserves of the Forestry Research Institute of Ghana (FORIG) near Kubease, Ashanti Region, Ghana, and identified and authenticated by Dr. A. Asase, Department of Botany, University of Ghana, Ghana. Unless stated otherwise, all the chemicals were purchased from Sigma (Deisenhofen, Germany).
2.2. Preparation of Extracts
The plant materials were air dried and powdered, and 200 g each of the dried powdered material of P. macrocarpa and C. gigantea was extracted, respectively, with 70% ethanol (1.5 L) using Soxhlet apparatus. The ethanol extracts obtained were evaporated to dryness under reduced pressure and kept in a dessicator. The yields of the stem and leaf extracts of P. macrocarpa were 4.2 and 12.4% w/w, respectively. And the yields of C. gigantea were 3.6 and 16.5% w/w for stem bark and leaf extracts, respectively. Various quantities of the ethanol leaf extract (CGLE) and ethanol stem bark extract (CGBE) of C. gigantea and ethanol leaf extract (PMLE) and ethanol stem bark extract (PMBE) of P. macrocarpa were dissolved in normal saline and methanol for acute anti-inflammatory and antimicrobial determinations, respectively.
2.3. Preliminary Phytochemical Screening
Phytochemical screening was conducted on both leaf and stem bark of P. macrocarpa and C. gigantea to ascertain the presence of carbohydrates, tannins, sapogenetic glycosides, flavonoids, steroids, and alkaloids [23, 24]. The tannins content was determined according to the method of Glasl [25] using pyrogallol (Merck, Darmstadt, Germany, purity 99.5%, HPLC) as reference compound.
2.4. Determination of Antimicrobial Activity
2.4.1. Agar Diffusion Method
The antimicrobial activities of the extracts (PMLE, CGLE, PMBE, and CGBE) and reference drugs (chloramphenicol and clotrimazole (Sigma, Deisenhofen, Germany)) were determined using the agar diffusion method [26]. Nutrient agar (Oxoid Limited, United Kingdom) and Sabouraud agar (Oxoid Limited, United Kingdom) media were used for both determinations of antibacterial and antifungal activities, respectively. 0.1 mL of 18 h culture of the test organisms (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, Bacillus subtilis NCTC 10073, and clinical fungal agent, Candida albicans, were used to seed nutrient agar and Sabouraud agar plates, respectively. In each of these plates, 4 equidistant wells (10 mm) were cut out using sterile cork borer and were filled with 200 μL each of the different concentrations of extracts and reference drugs and allowed to diffuse at room temperature for 1 h. The zones of inhibition were measured after 24 h incubation at 37°C (for bacteria) and after 72 h at 30°C (for fungi). The activities of the methanol (solvent) alone were also determined.
2.5. Determination of Minimum Inhibitory Concentration (MIC) Using Microdilution Technique
The MICs of the extracts (PMLE, CGLE, PMBE, and CGBE) against the test bacteria were determined using the microdilution technique as described by Eloff [27] and modified by Agyare and Koffuor [26]. Test solutions (100 mg/mL) of both extracts were prepared, test solution (25–100 μL) was serially diluted with distilled water to 100 μg/mL, and 50 μL of an 18 h old culture of one of the test bacteria grown in nutrient broth (Oxoid Limited, United Kingdom) was added to each well in the microplates. The covered microplates were incubated at 37°C for 24 h. To indicate growth, 30 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, thiazolyl blue) dissolved in distilled water was added to the microplate wells and incubated at 37°C for 30 min. C. albicans was cultivated in Sabouraud dextrose broth (Oxoid Limited, United Kingdom) and then incubated for 3 days at 30°C. The MICs of PMLE, CGLE, PMBE, and CGBE against the test fungus were determined according to the guidelines described in the National Committee for Clinical Laboratory Standards [28] for filamentous fungi. The minimum inhibitory concentration of the each extract against the test organisms was detected as the minimum concentration of extracts where there was no microbial growth, that is, nonformation of blue color after the addition MTT to the medium [29]. The previous experiment was repeated three times.
2.6. Determination of Acute Anti-Inflammatory Activity
The carrageenan-induced inflammatory model in seven-day-old chicks [30] was employed and the responsiveness of these chicks to anti-inflammatory drugs/extracts was determined.
2.7. Experimental Animals
7-days-old cockerels Gallus gallus (100–120 g) (Strain: Shaver 579) purchased from Darko Farms Company Limited, Kumasi, Ghana, were maintained in the Animal House of the Department of Pharmacology, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. The chicks were housed in stainless steel cages and fed with normal commercial poultry diet (GAFCO, Tema, Ghana), given water ad libitum, and maintained under laboratory conditions (temperature 28–30°C, relative humidity 60–70%, and normal light-dark cycle). A day before the experiment, the chicks were brought to the laboratory and habituated to experimenter handling and the apparatus to minimize the effect of stress and novelty. The 7-day-old chicks were used in experiment. All procedures and techniques used in these studies were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH, Department of Health Services publication no. 83-23, revised 1985). The protocols for the study were approved by the Departmental Ethics Committee.
2.8. Experimental Design
At the beginning of the experiment the chicks (7 days old) were randomly assigned to one of fifteen groups (n = 5). The initial foot volumes of the chicks were measured using plethysmometer (IITC Life Science Inc., CA, USA) after which 0.01 mL of 2% carrageenan was injected into the plantar of the right foot to induce inflammation. The inflammation produced was measured, the increase in foot volumes was calculated, and those with an increase between 15 and 40% were selected and put into thirteen groups of five after which they were injected intraperitoneally with either diclofenac (Sigma, purity 98% HPLC) (10, 30 and 100 mg/kg) or dexamethasone (Sigma, purity 98% HPLC) (0.25, 0.5, and 1.0 mg/kg) based on recommended effective human doses per body weight and PMLE, CGLE (30, 100, and 300 mg/kg), or PMBE and CGBE (30, 100 and 300 mg/kg) given orally based on preliminary investigation. One group did not receive any drug (control). Foot volumes were measured again at hourly interval posttreatment for 4 h. The percentage change in foot volume after induction and treatment of inflammation was calculated and recorded for analysis.
2.9. Statistical Analysis
GraphPad Prism Version 5.0 for Windows (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses. Data are presented as mean ± SEM (N = 5) and analyzed by one-way ANOVA followed by Dunnett's multiple comparison's test. P < 0.05 was considered statistically significant in all analyses. The graphs were plotted using Sigma Plot for Windows version 11.0 (Systat Software Inc., Germany).
3. Results
3.1. Preliminary Phytochemical Screening
Both the leaf and stem bark of P. macrocarpa and C. gigantea were found to contain tannins (with varying amounts), alkaloids, steroids, saponins, and carbohydrate while the leaves of the two plants contain flavonoids. The stem bark and leaves of P. macrocarpa were found to contain sapogenetic glycosides (Table 1).
Table 1.
Preliminary phytochemical screening of dried leaves and stem barks of C. gigantea (CG) and P. macrocarpa (PM). +: presence of secondary metabolite; −: absence of secondary metabolites.
| Secondary metabolites | Alkaloids | Saponins | Flavonoids | Steroids | Carbohydrates | Sapogenetic glycosides | Tannins (% w/w) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Plant material/part | |||||||
| CG leaf | + | + | + | + | + | − | 1.57 |
| CG stem bark | + | + | − | + | + | − | 1.02 |
| PM leaf | + | + | + | + | + | + | 1.45 |
| PM stem bark | + | + | − | + | + | + | 1.14 |
3.2. Antimicrobial Activity
The ethanol extracts (PMLE, PMBE, CGLE, and CGBE) were found to be active against the test bacteria (E. coli, P. aeruginosa, S. aureus, B. subtilis) with varying mean zones of inhibition and C. albicans was found to be less susceptible to the extracts. With respect to the agar diffusion method, the extracts (PMLE, CGLE, PMBE, and CGBE) with concentrations of 50 mg/mL exhibited the highest activity against the test organisms (Table 3). The minimum inhibitory concentration ranges of P. macrocarpa extracts (PMLE and PMBE) against the test organisms were from 0.125 to 2.55 mg/mL and those of C. gigantea extracts (CGLE and CGBE) were from 0.125 to 2.75 mg/mL (Table 2).
Table 3.
Antimicrobial activity of ethanol leaf extract (CGLE) and ethanol stem bark extract (CGBE) of C. gigantea and ethanol leaf extract (PMLE) and ethanol stem bark extract (PMBE) of P. macrocarpa by agar diffusion method. Activity of the methanol (solvent) used to dissolve the extracts was determined. Mean zones of inhibition (plus diameter of well) are mean (mm) of 3 independent experiments, mean ± SD, n = 4 replicates, and diameter of well/cup = 10 mm. Reference antimicrobial agents: CPC: chloramphenicol (1 mg/mL); CTZ: clotrimazole (1 mg/mL); ND: Not determined.
| Mean zones of growth inhibition (mm) | |||||
|---|---|---|---|---|---|
|
| |||||
| Extract (mg/mL) | Test organisms | ||||
| S. aureus | B. subtilis | E. coli | P. aeruginosa | C. albicans | |
| ATCC 25923 | NCTC 10073 | ATCC 25922 | ATCC 27853 | ||
| CGLE | |||||
| 10 | 19.67 ± 0.58 | 16.80 ± 0.40 | 12.20 ± 0.17 | 12.24 ± 0.13 | 12.65 ± 0.58 |
| 25 | 24.60 ± 0.53 | 19.33 ± 0.58 | 16.53 ± 0.24 | 15.50 ± 0.58 | 16.47 ± 0.48 |
| 50 | 28.33 ± 0.58 | 22.30 ± 0.42 | 19.67 ± 0.56 | 17.50 ± 0.47 | 22.13 ± 0.23 |
| CGBE | |||||
| 10 | 16.67 ± 0.58 | 13.33 ± 0.58 | 12.30 ± 0.58 | 0.0 | 13.67 ± 0.58 |
| 25 | 13.00 ± 0.00 | 17.80 ± 0.35 | 14.12 ± 0.42 | 0.0 | 18.90 ± 0.48 |
| 50 | 18.67 ± 0.58 | 19.50 ± 0.50 | 18.67 ± 0.58 | 13.47 ± 0.58 | 21.85 ± 0.23 |
| PMLE | |||||
| 10 | 0.0 | 17.80 ± 0.35 | 0.0 | 0.0 | 12.80 ± 0.20 |
| 25 | 14.60 ± 0.58 | 18.50 ± 0.50 | 19.33 ± 0.58 | 0.0 | 14.00 ± 0.00 |
| 50 | 19.67 ± 0.58 | 22.53 ± 0.12 | 21.47 ± 0.50 | 16.30 ± 0.23 | 22.33 ± 0.58 |
| PMBE | |||||
| 10 | 0.0 | 12.33 ± 0.58 | 11.33 ± 0.58 | 0.0 | 12.80 ± 0.20 |
| 25 | 14.50 ± 0.50 | 14.67 ± 0.58 | 12.67 ± 0.58 | 0.0 | 15.40 ± 0.36 |
| 50 | 17.13 ± 0.23 | 16.73 ± 0.58 | 15.83 ± 0.29 | 12.3 ± 1.20 | 18.60 ± 0.58 |
| CPC | 25.67 ± 0.38 | 31.21 ± 0.38 | 30.67 ± 0.85 | 22.33 ± 0.38 | ND |
| CTZ | ND | ND | ND | ND | 25.60 ± 0.61 |
| Methanol | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Table 2.
Minimum inhibitory concentrations (MICs) of ethanol leaf extract (CGLE) and ethanol stem bark extract (CGBE) of C. gigantea and ethanol leaf extract (PMLE) and ethanol stem bark extract (PMBE) of P. macrocarpa determined by microdilution method. The experiments were repeated three times. Reference antimicrobial agents: CPC: chloramphenicol; CTZ: clotrimazole; ND: Not determined.
| Extract/MIC (mg/mL) |
S. aureus
ATCC 25923 |
B. subtilis
NCTC 10073 |
E. coli
ATCC 25922 |
P. aeruginosa
ATCC 27853 |
C. albicans |
|---|---|---|---|---|---|
| CGLE | 0.250 | 0.125 | 0.175 | 1.55 | 1.55 |
| CGBE | 0.125 | 0.150 | 0.250 | 2.75 | 1.75 |
| PMLE | 0.150 | 0.125 | 1.250 | 1.85 | 1.75 |
| PMBE | 0.125 | 0.175 | 0.155 | 2.55 | 0.75 |
| CPC | 0.025 | 0.020 | 0.025 | 0.055 | ND |
| CTZ | ND | ND | ND | ND | 0.025 |
3.3. Anti-Inflammatory Activity
All the animals injected with carrageenan exhibited acute inflammation which manifested as increased foot volume. The control group however showed increased inflammation till the fourth hour. All the extract-treated groups except CGLE- (F 3,80 = 1.80, P = 0.1545: Figure 3) treated group exhibited significant anti-inflammatory effects (PMLE: F 3,80 = 47.52, P < 0.0001; PMBE: F 3,80 = 35.58, P < 0.001; CGBE: F 3,80 = 1.80, P = 0.1545: Figures 1, 2, and 4). Similar effects were observed after treating the animals with diclofenac (F 3,80 = 79.81, P < 0.0001) and dexamethasone (F 3,80 = 90.49, P < 0.0001) used as positive controls (Figure 5).
Figure 3.
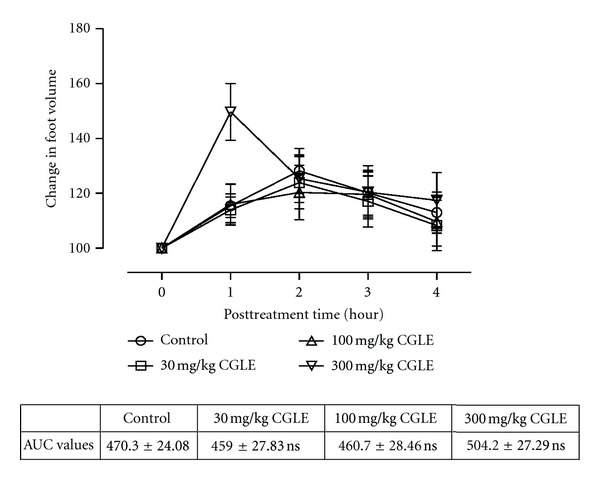
Effects of ethanol leaf extract (CGLE) of C. gigantea (30–300 mg/kg) on carrageenan-induced paw oedema. Values are expressed as mean ± SEM (N = 5), significantly different from control. *P < 0.05. Control is the untreated birds.
Figure 1.
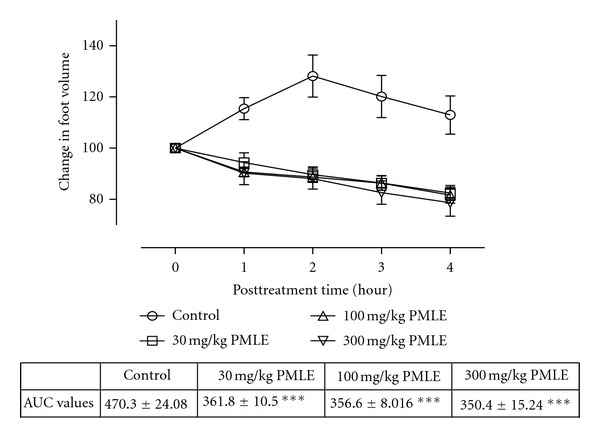
Effect of ethanol leaf extract (PMLE) of P. macrocarpa (30–300 mg/kg) on carrageenan-induced paw oedema. Values are expressed as mean ± SEM (N = 5), significantly different from control. **P < 0.05, **P < 0.01, and ***P < 0.001. Control is the untreated birds.
Figure 2.
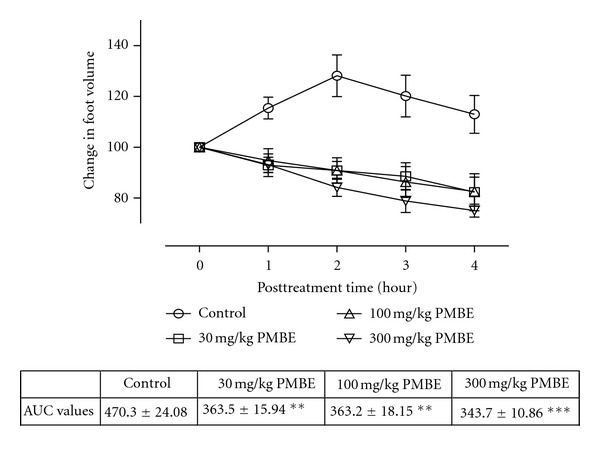
Effect of ethanol stem bark extract (PMBE) of P. macrocarpa (30–300 mg/kg) on carrageenan-induced paw oedema. Values are expressed as mean ± SEM (N = 5), significantly different from control. **P < 0.05, **P < 0.01, and ***P < 0.001. Control is the untreated birds.
Figure 4.
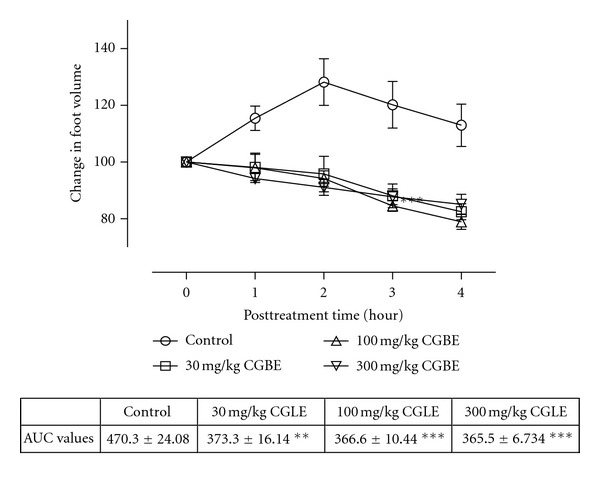
Effects of Ethanol stem bark extract (CGBE) of C. gigantea (30–300 mg/kg) on carrageenan-induced paw oedema. Values are expressed as mean ± SEM (N = 5), significantly different from control. **P < 0.05, **P < 0.01, and ***P < 0.001. Control is the untreated birds.
Figure 5.
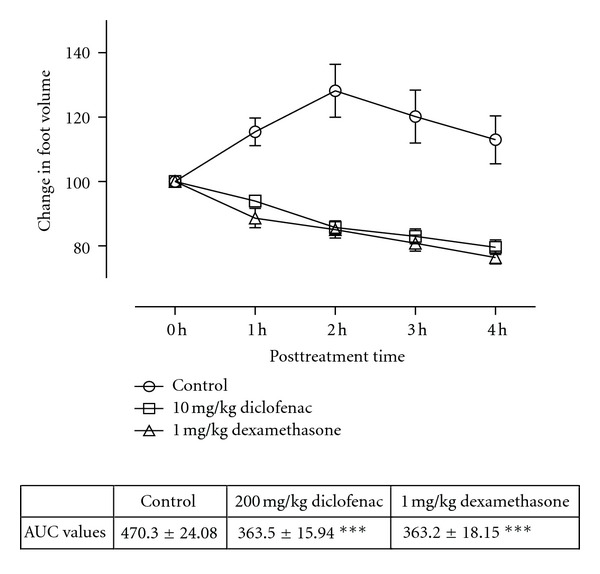
Effects of diclofenac (10 mg/kg) and dexamethasone (3 mg/kg) on carrageenan-induced paw oedema. Values are expressed as mean ± SEM (N = 5), significantly different from control. **P < 0.05, **P < 0.01, and ***P < 0.001. Control is the untreated birds.
4. Discussion
The present studies indicate the antimicrobial and anti-inflammatory properties of ethanol extracts of P. macrocarpa (PMLE and PMBE) and C. gigantea (CGLE and CGBE). These findings were similar to our previous work [27] with ethanol extract of Funtumia elastica Preuss Stapf. (Apocynaceae) which confirmed the plant as having both antimicrobial and anti-inflammatory properties. The antibacterial and antifungal activities of the P. macrocarpa are being reported for the first time. The MIC against the test bacteria is from the 0.125 to 2.55 mg/mL and the fungal agent (C. albicans) is from 0.75 to 1.75 mg/mL. With respect to the agar diffusion method, concentration of extracts from both leaves and the stem bark of P. macrocarpa less than 25 mg/mL did not exhibit activity against S. aureus and P. aeruginosa and less activity against E. coli at concentration of 25 mg/mL but the MICs for the previous test bacteria were comparable to the MICs of the C. gigantea extracts. This observation goes to support the assumption that size of inhibition halos of different extracts cannot be used for the determination of the relative antimicrobial potency since a more diffusible but less active extract could give a bigger diameter than a nondiffusible but more active extract [27, 31].
The antimicrobial activities exhibited by the ethanol extracts of leaves and stem bark of C. gigantea (CGLE and CGBE) are in line with previous antimicrobial works on the leaves of different species of Cola [22, 32, 33] where different extracts of cola were found to exhibit inhibitory activities against certain bacteria and fungi with respect to the leaf extract. The MIC range of both the leaf and stem bark extracts of C. gigantea against the test bacteria is from 0.125 to 2.75 mg/mL and the mean zones of inhibition of the different concentration (10–50 mg/mL) extracts (crude) were almost the same as those of the reference antibacterial agent, chloramphenicol at concentration of 1000 μg/mL. However, with the agar diffusion technique, the leaf extracts of C. gigantea (10 to 50 mg/mL) showed more activity against both test bacteria and fungus compared to the extracts from the stem bark. P. aeruginosa was found to be generally less susceptible to all extracts from P. macrocarpa and C. gigantean, respectively (Table 3), and this was not surprising since it has been found to be resistant to most orthodox antibiotics [34]. The antimicrobial properties may justify the use of these plants for the treatment of various bacterial and fungal infections such as gonorrhea and urethral infections and sores.
The study also establishes the anti-inflammatory activity of the ethanol extracts of the leaves and stem bark of P. macrocarpa and C. gigantean, respectively. Carrageenan-induced oedema has been commonly used as an experimental animal model for acute inflammation and is established to be biphasic. The early phase (1 to 2 hours) of the carrageenan model is chiefly mediated by serotonin, histamine, and increased synthesis of prostaglandins in the damaged tissues. The late phase is sustained by prostaglandin release and mediated by bradykinin, leukotrienes, polymorphonuclear cells, and prostaglandins produced by tissue macrophages [35]. The extracts (PMLE, PMPE, and CGBE) inhibited the inflammation induced with carrageenan in both phases indicating the ability of these extracts to inhibit the synthesis or release of inflammatory mediators such as histamine, serotonin, bradykinin, and leukotrienes.
In comparing the four extracts (PMLE, CGLE, PMBE, and CGBE), only CGLE had insignificant anti-inflammatory activity in the carrageenan-induced hind paw oedema. However the CGBE had significant activity. The reason for this observation may be due to different chemical composition of the stem bark and leaves of the same plant. Traditionally, the bark is used in the management of haemorrhoids: dropsy, swelling, edema, gout, leprosy, and pain [15], and hence these results may confirm the medicinal uses of the bark. The CGBE extract may have relatively high amounts of the bioactive constituents in relation to the leaves. Phytochemical screening of C. gigantea showed the presence of alkaloids and this confirms the findings of Sonibare et al. [22]. Members of the cola family are closely related to Theobroma family of South America which have the methylxanthine alkaloids such as caffeine, theobromine, and theophylline as secondary metabolites. Caffeine is one of the alkaloids of genus cola and it is used as an analgesic and anti-inflammatory adjuvant [36, 37].
There was no significant difference in anti-inflammatory activity between the leaf and stem bark extracts (PMLE and PMBE) of P. macrocarpa. However, the leaves are traditionally used as diuretics, antiflatulence, and a remedy for stomach, bladder, and urinary problems [15].
Powdered dried leaves and stem bark of P. macrocarpa and C. gigantea showed the presence of tannins and with different tannin contents (1.02–1.57% w/w). Amoo and Agunbiade [38] reported on the high tannin content of the seeds of P. macrocarpa. Similar results were found in the samples of leaves and bark of P. macrocarpa. Tannins form a class of polyphenolic compounds which can act as antioxidants, antiviral, antibacterial, antiparasitic, and anti-inflammatory activity [39–42]. During the inflammatory cascade, antioxidants act as scavengers for free radicals protecting cells against oxidants which are mostly reactive oxygen and nitrogen species [43–45]. The aforementioned findings may justify the traditional medicinal uses of the P. macrocarpa and C. gigantea and hence there is the need to perform bioactivity fractionation of the active extracts to isolate the compounds that may be responsible for the antimicrobial and anti-inflammatory properties.
5. Conclusion
The minimum inhibitory concentration ranges of both ethanol leaf and bark extracts of P. macrocarpa against the test organisms were from 0.125 to 2.55 mg/mL and those of C. gigantea extracts were from 0.125 to 2.75 mg/mL. Extracts (10, 25, and 50 mg/mL) of P. macrocarpa and C. gigantea exhibited antimicrobial activity with concentrations of 50 mg/mL showing the highest zones of inhibition against the test organisms. All the extracts of P. macrocarpa and C. gigantea at 30, 100, and 300 mg/kg except leaf extract of C. gigantea exhibited significant anti-inflammatory activity. The aformentioned activities may confirm the ethnobotanical uses of these two plants as antimicrobial and anti-inflammatory agents. It is recommended that the bioactive extracts should be fractionated and the active compounds responsible for the previous pharmacological properties should be isolated.
Conflict of Interests
The authors have no conflict of interest to declare.
Acknowledgments
The authors wish to express their gratitude to Mr. Thomas Ansah of the Department of Pharmacology, KNUST, Kumasi, Ghana, for his technical assistance and Miss Angela Mensah for working on the project with them in the laboratory.
References
- 1.Tenover FC. Mechanisms of antimicrobial resistance in bacteria. American Journal of Medicine. 2006;119(6):S3–S10. doi: 10.1016/j.amjmed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Krause RM. The origin of plagues: old and new. Science. 1992;257(5073):1073–1078. doi: 10.1126/science.257.5073.1073. [DOI] [PubMed] [Google Scholar]
- 3.Agyare C, Asase A, Lechtenberg M, Niehues M, Deters A, Hensel A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. Journal of Ethnopharmacology. 2009;125(3):393–403. doi: 10.1016/j.jep.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Nyika A. Ethical and regulatory issues surrounding African traditional medicine in the context of HIV/AIDS. Developing World Bioethics. 2007;7(1):25–34. doi: 10.1111/j.1471-8847.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 5.Muthu C, Ayyanar M, Raja N, Ignacimuthu S. Medicinal plants used by traditional healers in Kancheepuram District of Tamil Nadu, India. Journal of Ethnobiology and Ethnomedicine. 2006;2:p. 43. doi: 10.1186/1746-4269-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayyanar M, Ignacimuthu S. Traditional knowledge of Kani tribals in Kouthalai of Tirunelveli hills, Tamil Nadu, India. Journal of Ethnopharmacology. 2005;102(2):246–255. doi: 10.1016/j.jep.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Pei SJ. Ethnobotanical approaches of traditional medicine studies: some experiences from Asia. Pharmaceutical Biology. 2001;39:74–79. doi: 10.1076/phbi.39.s1.74.0005. [DOI] [PubMed] [Google Scholar]
- 8.Rossato SC, Leitão-Filho HDF, Begossi A. Ethnobotany of Caicaras of the Atlantic Forest coast (Brazil) Economic Botany. 1999;53(4):387–395. [Google Scholar]
- 9.Agyare C, Lechtenberg M, Deters A, Petereit F, Hensel A. Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: feraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine. 2011;18(7):617–624. doi: 10.1016/j.phymed.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Gopalan RC, Emerce E, Wright CW, Karahalil B, Karakaya AE, Anderson D. Effects of the anti-malarial compound cryptolepine and its analogues in human lymphocytes and sperm in the Comet assay. Toxicology Letters. 2011;207(3):322–325. doi: 10.1016/j.toxlet.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Sawer IK, Berry MI, Brown MW, Ford JL. The effect of cryptolepine on the morphology and survival of Escherichia coli, Candida albicans and Saccharomyces cerevisiae. Journal of Applied Bacteriology. 1995;79(3):314–321. doi: 10.1111/j.1365-2672.1995.tb03143.x. [DOI] [PubMed] [Google Scholar]
- 12.Ghana Herbal Pharmacopoiea. Accra, Ghana: The Advent Press; 2003. [Google Scholar]
- 13.Keay RWJ. Trees of Nigeria. Oxford, UK: Claredon Press; 1989. [Google Scholar]
- 14.Irvine FR. Woody Plants of Ghana. London, UK: Oxford University Press; 1961. [Google Scholar]
- 15.Burkill HM. The Useful Plants of West Tropical Africa. 2nd edition. Vol. 3. London, UK: Royal Kew Botanical Gardens; 1995. [Google Scholar]
- 16.Amoo IA, Agunbiade FO. Some nutrient and anti-nutrient components of Pterygota macrocarpa seed flour. Pacific Journal of Science and Technology. 2009;10(2):949–955. [Google Scholar]
- 17.Odugbemi T. Outlines and Pictures of Medicinal Plants from Nigeria. Vol. 10. Lagos, Nigeria: University of Lagos Press; 2006. [Google Scholar]
- 18.Duke JA. Handbook of Nuts. Boca Raton, Fla, USA: CRC Press; 2001. [Google Scholar]
- 19.Blades M. Functional foods or neutraceuticals. Nutrition and Food Science. 2000;30(2):73–75. [Google Scholar]
- 20.Benjamin LT, Rogers AM, Rosenbaum A. Coca-Cola, caffeine, and mental deficiency: Harry Hollingworth and the Chattanooga trial of 1911. Journal of the History of the Behavioral Sciences. 1991;27(1):42–55. doi: 10.1002/1520-6696(199101)27:1<42::aid-jhbs2300270105>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Jayeola OC. Preliminary studies on the use of kola nuts (Cola nitida) for soft drink production. Journal of Food Technology in Africa. 2001;6(1):25–26. [Google Scholar]
- 22.Sonibare MA, Soladoye MO, Esan OO, Sonibare OO. Phytochemical and antimicrobial studies of four species of Cola Schott & Endl. (Sterculiaceae) African Journal of Traditional, Complementary and Alternative Medicines. 2009;6(4):518–525. doi: 10.4314/ajtcam.v6i4.57182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner H, Bladt S. Plant Drug Analysis: A Thin Layer Chromatography. 2nd edition. New York, NY, USA: Springer Verlag; 1996. [Google Scholar]
- 24.Harborne JB. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. 3rd edition. London, UK: Chapman and Hall; 1998. [Google Scholar]
- 25.Glasl H. On the photometry in drug-standardization. Deutsche Apotheker Zeitung. 1983;123(42):1979–1987. [Google Scholar]
- 26.Agyare C, Koffuor GA. Antimicrobial and anti-inflammatory properties of Fumtumia elastica . doi: 10.3109/13880209.2012.738330. Pharmaceutical Biology. In press. [DOI] [PubMed] [Google Scholar]
- 27.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica. 1998;64(8):711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. NCCLS Document. M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi: proposed standard. [Google Scholar]
- 29.Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnology Annual Review. 2005;11:127–152. doi: 10.1016/S1387-2656(05)11004-7. [DOI] [PubMed] [Google Scholar]
- 30.Roach JT, Sufka KJ. Characterization of the chick carrageenan response. Brain Research. 2003;994(2):216–225. doi: 10.1016/j.brainres.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 31.Cos P, Vlietinck AJ, Berghe DV, Maes L. Anti-infective potential of natural products: how to develop a stronger in vitro “proof-of-concept”. Journal of Ethnopharmacology. 2006;106(3):290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Reid KA, Jäger AK, Light ME, Mulholland DA, Van Staden J. Phytochemical and pharmacological screening of Sterculiaceae species and isolation of antibacterial compounds. Journal of Ethnopharmacology. 2005;97(2):285–291. doi: 10.1016/j.jep.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Adeniyi BA, Groves MJ, Gangadharam PRJ. In vitro anti-mycobacterial activities of three species of Cola plant extracts (Sterculiaceae) Phytotherapy Research. 2004;18(5):414–418. doi: 10.1002/ptr.1468. [DOI] [PubMed] [Google Scholar]
- 34.Lambert PA. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa . Journal of the Royal Society of Medicine. 2002;95(supplement 41):22–26. [PMC free article] [PubMed] [Google Scholar]
- 35.Brito ARMS, Antonio MA. Oral anti-inflammatory and anti-ulcerogenic activities of a hydroalcoholic extract and partitioned fractions of Turnera ulmifolia (Turneraceae) Journal of Ethnopharmacology. 1998;61(3):215–228. doi: 10.1016/s0378-8741(98)00049-x. [DOI] [PubMed] [Google Scholar]
- 36.Laska EM, Sunshine A, Mueller F, Elvers WB, Siegel C, Rubin A. Caffeine as an analgesic adjuvant. Journal of the American Medical Association. 2011;306(21):2293–2404. [Google Scholar]
- 37.Migliardi JR, Armellino JJ, Friedman M, Gillings DB, Beaver WT. Caffeine as an analgesic adjuvant in tension headache. Clinical Pharmacology and Therapeutics. 1994;56(5):576–586. doi: 10.1038/clpt.1994.179. [DOI] [PubMed] [Google Scholar]
- 38.Amoo IA, Agunbiade FO. Some nutrients and anti-nutrients components of Pterygota macrocarpa seed flour. Electronic Journal of Environmental, Agricultural and Food Chemistry. 2010;9(2):293–300. [Google Scholar]
- 39.Lü L, Liu SW, Jiang SB, Wu SG. Tannin inhibits HIV-1 entry by targeting gp41. Acta Pharmacologica Sinica. 2004;25(2):213–218. [PubMed] [Google Scholar]
- 40.Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K. Antibacterial action of several tannins against Staphylococcus aureus . Journal of Antimicrobial Chemotherapy. 2001;48(4):487–491. doi: 10.1093/jac/48.4.487. [DOI] [PubMed] [Google Scholar]
- 41.Kolodziej H, Kiderlen AF. Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitised RAW 264.7 cells. Phytochemistry. 2005;66(17):2056–2071. doi: 10.1016/j.phytochem.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Souza SMC, Aquino LC, Milach AC, Jr, Bandeira MA, Nobre ME, Viana GS. Anti-inflammatory and antiulcer properties of tannins from Myracrodruon urundeuva Allemão (Anacardiaceae) in rodents. Phytotherapy Research. 2006;21(3):220–225. doi: 10.1002/ptr.2011. [DOI] [PubMed] [Google Scholar]
- 43.Conner EM, Grisham MB. Inflammation, free radicals and antioxidants. Nutrition. 1996;12(4):274–277. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 44.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochemical Journal. 1996;313(1):17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chapple ILC. Reactive oxygen species and antioxidants in inflammatory diseases. Journal of Clinical Periodontology. 1997;24(5):287–296. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]


