Abstract
Fruits, such as grapes, are essential food of the Mediterranean diet. Grape extracts have potent antioxidant and chemopreventive properties in vitro. Numerous studies have examined the effects of plant extract administration on redox status at rest in animals and humans but their results are controversial. However, there are no studies comparing the in vitro and in vivo effects of plant extracts on oxidative stress using exercise as an oxidant stimulus. Thus, the aim of this study was to investigate whether a polyphenol-rich grape pomace extract of the Vitis vinifera species possesses in vitro antioxidant properties and to examine whether these properties apply in an in vivo model at rest and during exercise. Our findings indicate that the tested extract exhibits potent in vitro antioxidant properties because it scavenges the DPPH• and ABTS•+ radicals and inhibits DNA damage induced by peroxyl and hydroxyl radicals. Administration of the extract in rats generally induced oxidative stress at rest and after exercise whereas exercise performance was not affected. Our findings suggest that the grape pomace extract does not behave with the same way in vitro and in vivo.
1. Introduction
Reactive oxygen and nitrogen species are involved in physiological processes such as signal transduction [1] and adaptations during exercise [2]. However, when reactive species are excessively produced, they may cause muscle damage [3] and fatigue [4]. Strenuous exercise leads to overproduction of reactive species and consequently to oxidative stress [5–7]. A very important contributor of reactive species during exercise is the enzyme xanthine oxidase [2], which catalyzes the oxidation of hypoxanthine to xanthine to uric acid. Xanthine oxidase uses molecular oxygen as the electron acceptor during purine degradation thereby resulting in superoxide radical (O2 •−) and hydrogen peroxide (H2O2) production [8]. The role of xanthine oxidase is dual as it results not only in reactive species production but also in generation of uric acid, one of the most potent antioxidant molecules in plasma [9, 10].
Various studies have examined the antioxidant effects of plant extracts using in vitro tests. Their findings have mostly shown that grape extracts are strong free-radical scavengers in vitro [11–13]. Apart from grape extracts, it has also been observed that extracts from legumes are potent antioxidant agents in vitro [14–16]. Generally, in the vast majority of the relevant literature, extracts derived from different plants possess antioxidant properties in vitro judged by their capacity to scavenge free radicals.
In a number of studies, plant extracts possessing antioxidant properties in vitro have been administered in rodents and humans before exercise to examine whether these effects also apply in vivo. These studies mainly examined the effects of plant extracts supplementation on oxidative stress in blood and other tissues, yet the findings are controversial. More specifically, it has been demonstrated that administration of several plant extracts protected tissues from exercise induced oxidative stress [17–19]. In contrast, other studies in humans have examined the effects of plant extract administration on redox status at rest and reported antioxidant [20] or prooxidant effects [21, 22]. The effects of the vast majority of plant extracts on redox status are usually attributed to specific compounds they are consisted of. These compounds are polyphenols, which are secondary metabolites of plants. They protect plants against harmful environmental conditions and are divided in two main categories, namely, flavonoids and nonflavonoids [23].
The aforementioned data indicate that the potential antioxidant function of a plant extract in vivo cannot be safely extrapolated from in vitro tests, since they do not take into account (among others) the metabolic transformations and interactions that clearly affect the bioavailability and biological action of polyphenols. It is a common practice to use antioxidants as a way to enhance exercise performance. In order to make practical recommendations for the use of antioxidants, it is important to use both in vitro and in vivo models. Research from our laboratory has demonstrated that several grape extracts of the Vitis vinifera species possess potent antioxidant properties in vitro as they scavenge several free radicals, such as 1,1-diphenyl-2-picrylhydrazyl (DPPH•), 2,2′-Azino-bis-(3-ethyl-benzthiazoline-sulphonic acid (ABTS•+), superoxide (O2 •−), hydroxyl (OH•), and peroxyl (ROO•) radicals [24–26]. It would be interesting to examine whether the in vitro properties of a grape extract also apply to an in vivo model, particularly considering the lack of studies in which the effects of the same extract in an in vitro and an in vivo model in the context of exercise are investigated. Thus, the aim of this study was to examine whether a polyphenol-rich grape pomace extract possesses in vitro antioxidant properties and whether the in vitro properties of the extract translate to an in vivo model when the extract is administered before exhaustive aerobic exercise in rats.
2. Material and Methods
This study is divided in two parts. In the first part, a polyphenol-rich grape pomace extract was examined for its possible antioxidant properties in vitro employing several assays. In the second part, the effects of the extract on redox status in an in vivo model using exercise as an oxidant stressor were investigated.
2.1. In Vitro Experiment
2.1.1. Preparation of the Grape Pomace Extract
The grape pomace used belongs to the species Vitis vinifera and to the variety Batiki Tyrnavou (red grapes grown in Central Greece). The raw material was dried in a shady, well-ventilated place and extracted with ethanol (96%) at 50°C for 4 hours. After filtration, the solvent was evaporated under reduced pressure, and the residue (grape pomace extract) was kept at −20°C by the time of analysis for the investigation of its polyphenolic content.
2.1.2. LC-HRMS Analysis of the Extract
For the characterization of the polyphenols content of the grape pomace extract an LC-HRMS method was developed and applied. For the analysis an Accela LC system (ThermoFinnigan, San Jose, USA) consisted of an HPLC pump, a degasser, an autosampler and a PDA detector were employed. Particularly for the HRMS analysis, an Orbitrap spectrometer (ThermoFinnigan, San Jose, USA) hyphenated to the HPLC-DAD system was used. The orbital trap allowed mass resolution around 30 000 and mass measurement accuracy close to 2 ppm to be achieved. The MS system was equipped with an ESI ionization probe and the analysis was performed in positive and negative mode. A Hypersil GOLD column (Thermo Scientific) (100 × 2.1 mm, 3 μm) was used for the analysis. A fast gradient elution method was developed and applied. The mobile phase used consisted of 0.1% aqueous formic acid (solvent A) and acetonitrile (solvent B), at a flow rate of 400 μL/min, at room temperature. The elution conditions used were initial A-B (95 : 5); in 5 min A-B (90 : 10); in 10 min A-B (80 : 20); in 15 min A-B (70 : 30); in 22 min A-B (50 : 50); in 25 min A-B (40 : 60); in 35 min A-B (5 : 95), hold until 40 min, back to initial conditions in 5 min; equilibration for 10 min. The chromatograms were recorded at 220, 280, and 365 nm by monitoring spectra within a wavelength range of 190–700 nm. Mass spectra were recorded in a range from m/z 100 to 1500. The ESI source was operated at a sheath gas flow of 30 arb, auxiliary gas flow of 10 arb, ion spray voltage of 3.5 kV, and a capillary temperature of 40°C. For all the high-accurate m/z measurements, the mass tolerance was set to 5 ppm. Measurements outside that range were rejected. Identification of compounds was accomplished by comparing the retention time (Rt), UV spectrum, HRMS, spectra of the peaks in the sample to those of standard compounds (Extrasynthese, Lyon, France). Xcalibur 2.0.7 SP1 software was used for the operation and processing of the data.
2.1.3. Assessment of Extract Total Polyphenol Content
The total polyphenol content of the grape pomace extract was determined using the Folin-Ciocalteu reagent [27]. Folin-Ciocalteu reagent (0.5 mL) and distilled water (5 mL) were added to the sample (0.1 mL). It was incubated for 3 min at room temperature (RT) and was subsequently mixed with 25% w/v solution of sodium carbonate (1.4 mL) and distilled water (3 mL). Following 1 h incubation at RT in the dark, the absorbance was measured at 765 nm. Blank contained Folin-Ciocalteu reagent and distilled water without the extract. The optical density of the sample (0.1 mL) in 25% w/v solution of sodium carbonate (1.4 mL) and distilled water (8 mL) at 765 nm was also measured. The total polyphenol content was determined by a standard curve of absorbance values in correlation with standard concentrations (0, 50, 150, 250, 500 μg/mL) of gallic acid. The total polyphenol content is presented as mg of gallic acid per g of extract.
2.1.4. DPPH•Radical Scavenging Assay
Free-radical scavenging capacity of the extract was evaluated using the DPPH• radical [28]. Briefly, the reaction was carried out in 1 mL of methanol containing freshly made DPPH•(100 μM) in methanol and the extract at different concentrations (2–50 μg/mL). The contents were vigorously mixed, incubated at RT in the dark for 20 min, and the absorbance was measured at 517 nm. In each experiment, the tested sample alone in methanol was used as blank and DPPH• alone in methanol was used as control.
2.1.5. ABTS•+ Radical Scavenging Assay
ABTS•+ radical scavenging capacity of the extract was determined according to Cano et al. [29] with slight modifications. Briefly, the reaction was carried out in 1 mL of distilled water containing ABTS (1 mM), H2O2 (30 μM) and horse radish peroxidase (6 μM). The solution was vigorously mixed, incubated at RT in the dark for 45 min until ABTS•+ radical formation, and the absorbance was measured at 730 nm. Then, 10 μL of different extract concentrations (2–50 μg/mL) were added in the reaction mixture and the decrease in absorbance at 730 nm was read. In each experiment, the tested sample in distilled water containing ABTS and H2O2 was used as blank, and the ABTS•+ radical solution with H2O was used as control.
2.1.6. Calculation of % Radical Scavenging Capacity
The radical scavenging capacity (RSC) of the extract was expressed as the percentage of DPPH• or ABTS•+ elimination calculated according to the following equation:
| (1) |
Ac is the absorbance of control and As is the absorbance of the sample. In order to compare the radical scavenging efficiency of the samples, IC50 values were also calculated, expressing the concentration of the extract that scavenges DPPH• or ABTS•+ radical by 50%. All experiments were carried out in triplicate on three separate occasions.
2.1.7. Peroxyl Radical-Induced DNA Strand Scission Assay
The assay was performed using the protocol of Chang et al. [30]. ROO• were generated from thermal decomposition of 2,2′-azobis(2-amidinopropane hydrochloride, AAPH). The reaction mixture (10 μL), containing Bluescript-SK+ plasmid DNA (1 μg), the extract at different concentrations (1–100 μg/mL), and AAPH (2.5 mM) in phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4), was incubated in the dark for 45 min at 37°C. The reaction was terminated by the addition of loading buffer (3 μL, 0.25% bromophenol blue and 30% glycerol) and analyzed in 0.8% agarose gel electrophoresis at 70 V for 1 h. The gels were stained with ethidium bromide (0.5 μg/mL), distained with water, photographed by UV translumination using the Vilber Lourmat photodocumentation system (DP-001.FDC, Torcy, France), and analyzed with Gel-Pro Analyzer version 3.0 (Media Cybernetics, Silver Spring, USA).
2.1.8. Hydroxyl Radical-Induced DNA Strand Scission Assay
OH•-induced plasmid DNA relaxation assay was performed according to the method of Keum et al. [31] with slight modifications. OH• were generated from UV photolysis of H2O2. The reaction mixture (10 μL) was consisted of Bluescript-SK+ plasmid DNA (1 μg), Tris-HCl (10 mM, 1 mM EDTA), the extract at different concentrations (100–1600 μg/mL), and H2O2 (40 mM). The reaction mixture was irradiated with a 300 W UV lamp (OSRAM) for 3 min at the distance of 50 cm. The reaction was terminated by the addition of loading buffer (3 μL, 0.25% bromophenol blue and 30% glycerol) and analyzed in gel electrophoresis as described previously. Additionally, Bluescript-SK+ plasmid DNA was also treated with the extract alone at the highest concentration used (1600 μg/mL) in order to test its effects on plasmid DNA conformation.
2.1.9. Inhibition of Free-Radical-Induced DNA Damage
Induction of DNA strand breaks by ROO• and OH• was evaluated by the conversion of supercoiled Bluescript-SK+ plasmid double-stranded DNA to open circular conformation analyzed in agarose gel electrophoresis. Preventive activity of the extract was assessed by inhibition of conversion of supercoiled (unnicked) conformation to open circular (nicked). The percentage inhibition of radical-induced DNA strand cleavage by the extract was calculated using the following equation:
| (2) |
S 0 is the percentage of supercoiled conformation in the negative control sample (plasmid DNA alone), S p is the percentage of supercoiled conformation in the positive control sample (plasmid DNA with the radical initiating factor), and S is the percentage of supercoiled conformation in the sample containing plasmid DNA, the extract, and the radical initiating factor. In order to compare the efficiency of preventive capacity of the extract, IC50 value was evaluated showing the concentration needed to inhibit relaxation of supercoiled conformation induced by ROO• and OH• by 50%. All experiments were carried out in triplicate on three separate occasions. Bluescript-SK+ plasmid DNA was isolated from a large-scale bacterial culture.
2.2. In Vivo Experiment
2.2.1. Animals
Fourty adult male Wistar rats (9 weeks old, weighing 285 ± 5 g, mean ± SEM) were purchased from the Hellenic Pasteur Institute. Rats were housed under controlled environmental conditions (12-hour light/dark cycle, temperature 18–21°C, humidity 50–70%) in cages of three. Commercial rat chow and tap water were provided ad libitum. The project was reviewed and approved by the institutional review board and the appropriate state authority.
Rats were randomly divided into four experimental groups as follows: (a) saline-administered and sacrificed 1 h after administration, (b) saline-administered, exercised 1 h after administration and sacrificed immediately after exercise, (c) grape pomace extract-administered and sacrificed 1 h after administration, and (d) grape pomace extract-administered, exercised 1 h after administration and sacrificed immediately after exercise.
2.2.2. Grape Pomace Extract Administration
A single dose of 300 mg·kg−1 body weight of grape pomace extract was administered intraperitoneally 1 h before the acute swimming protocol. This was in the range of commonly administered doses of plant extracts in similar experimental protocols [19, 21, 22, 32]. The polyphenolic composition of the tested extract, which is rich in catechin, is presented in Table 1. It has been previously referred to that 1 hour is enough for polyphenols, such as catechin, administered within extracts to reach their maximal concentration in blood [23].
Table 1.
Polyphenolic composition of the Batiki Tyrnavou grape pomace extract.
| Polyphenols | Rt (min) | m/z [M±H]± |
|---|---|---|
| Flavan-3-ols | ||
| Catechin | 3.83 | 291.0863 [M+H]+
289.0707 [M−H]− |
| Epicatecin | 5.93 | 291.0863 [M+H]+
289.0707 [M−H]− |
| Epicatechin-3-gallate | 9.25 | 441.0816 [M−H]− |
| Anthocyanidins | ||
| Cyanidin | 9.25 | 609.1467 [M−H]− |
| Malvidin | 11.74 | 331.0812 [M+H]+ |
| Delphinidin | 13.55 | 301.0359 [M−H]− |
| Petunidin | 15.99 | 315.0516 [M−H]− |
| Anthocyanins | ||
| Myrtillin | 9.53 | 463.0888 [M−H]− |
| Kuromanin | 10.63 | 447.0939 [M−H]− |
| Oenin | 6.72 | 491.1201 [M−H]− |
| Peonidin-3-O-glucoside | 5.76 | 463.1235 [M+H]+ |
| Phenolic acids | ||
| Gallic acid | 0.78 | 169.0147 [M−H]− |
| Caftaric acid | 26.18 | 311.0398 [M−H]− |
| Flavonols | ||
| Quercetin | 9.56 | 301.0357 [M−H]− |
| Kaempferol | 15.79 | 285.0407 [M−H]− |
|
| ||
| TPC (total polyphenol content) | 648 (mg gallic acid/g extract) | |
2.2.3. Swimming Familiarization
Rats were allowed to acclimatize for 7 days in the animal facility before the beginning of the exercise protocol. The animals were then familiarized to swimming for a period of five days before the actual swimming protocol was implemented. During the first day of familiarization the rats remained in the water tank for 10 min free of load. The next two days the rats swam for 10 min with a load equal to 1% of their body weight adjusted at the base of their tails. The last two days the load increased to 2% of animal's body weight. Finally, the rats were rested for three days before the swimming protocol took place.
2.2.4. Swimming Protocol
Rats subjected to exercise individually swam until exhaustion in deep water tanks (diameter: 1.0 m, depth: 0.7 m) at a water temperature of 33–36°C, as previously described [5]. Constant load equal to 4% of the rats' body weight was adjusted at the base of their tail in order to achieve uninterrupted swimming. An animal was considered to have reached exhaustion when it was unable to constantly keep its nose out of the water. Swimming was selected as an exercise modality because, unlike treadmill running, it induces minor muscle damage [33]. Thus, any effects of swimming on oxidative stress are only partly attributed to muscle damage, which increases production of reactive species.
2.2.5. Blood and Tissue Collection
Rats were sacrificed by decapitation following short exposure to ether. Blood was collected in EDTA tubes and centrifuged immediately at 1,370 g for 10 min at 4°C to allow plasma isolation. The packed erythrocytes were lysed with 1 : 1 (v/v) distilled water, inverted vigorously, and centrifuged at 4,020 g for 15 min at 4°C. Tissues were quickly removed and snaped-frozen in liquid nitrogen. Plasma, erythrocyte lysate and tissues were then stored at −80°C until biochemical analysis. In preparation for tissue biochemical analysis, tissue samples were initially ground using mortar and pestle under liquid nitrogen. One part of tissue powder was then homogenized with two parts (weight/volume) of 0.01 M phosphate buffered saline pH 7.4 (138 mM NaCl, 2.7 mM KCl, and 1 mM EDTA) and a cocktail of protease inhibitors (1 μM aprotinin, 1 μg/mL leupeptin and 1 mM PMSF) was added. The homogenate was vigorously vortexed and a brief sonication treatment on ice was applied. The homogenate was then centrifuged at 12,000 g for 30 min at 4°C and the supernatant was collected.
2.2.6. Oxidative Stress Markers
Xanthine oxidase activity, TBARS concentration, protein carbonyls concentration, reduced glutathione (GSH) concentration, catalase activity, and total antioxidant capacity (TAC) were measured as previously described [5, 34]. Each assay was performed in triplicate. Blood and tissue samples were stored in multiple aliquots at −80°C and thawed only once before analysis. All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2.7. Statistics
Data of the in vitro experiment were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett's test for multiple pair-wise comparisons. Data of the in vivo experiment (oxidative stress markers) were analyzed by two-way (treatment × time) ANOVA. Pairwise comparisons were performed through Bonferroni t-test. Performance data were analyzed using independent Student's t-test. The level of statistical significance was set at P < 0.05. All results are expressed as mean ± SEM. Data were analyzed using SPSS, version 13.0 (SPSS Inc., Chicago, Ill).
3. Results
3.1. In Vitro Experiment
3.1.1. LC-HRMS Analysis of the Grape Pomace Extract
The ethanolic extract of grape pomace was analyzed using a fast HPLC-HRMS method, in positive and negative mode. Using purchased standard compounds (purity ≥ 95%), several flavonoids and especially flavan-3-ols, anthocyanins and anthocyanidins, were identified. The well-known flavan-3-ols of wine, catechin, and epicatecin were traced at Rt = 3.83 min, and Rt = 5.93 min, respectively. Both isomeric compounds were detected in positive and negative mode based on their pseudomolecular ions [M+H]+ at m/z 291.0863 and [M−H]− at m/z 289.0707, respectively. Additionally, epicatechin-3-gallate was identified at Rt = 9.25 min based on its pseudomolecular ion [M−H]− at m/z 441.0816. Moreover, the anthocyanidins cyanidin, malvidin, delphinidin, and petunidin were detected at 9.25 min, 11.74 min 13.55 min, 15.99 min based on their corresponding pseudomolecular ions [M−H]− at m/z 609.1467, [M+H]+ at m/z 331.0812, [M−H]− at m/z 301.0359, and [M−H]− at m/z 315.0516, respectively. Likewise, the anthocyanins myrtillin, kuromanin, oenin, and peonidin-3-O-glucoside were traced. In particular, Myrtillin was detected mainly in negative mode, where its pseudomolecular ion [M−H]− at m/z 463.0888 and Rt = 9.53 min was observed. At Rt = 10.63 min, kuromanin was detected through its peudomolecular ion [M−H]− at m/z 447.0939 while at Rt = 6.72 min oenin was detected through its peudomolecular ion [M−H]− at m/z 491.1201. The peonidin-3-O-glucoside was also identified based on its pseudomolecular ion [M+H]+ at m/z 463.1235 at Rt = 5.76 min.
Apart from the above-mentioned flavonoids, two phenolic acids, gallic acid (Rt = 0.78 min) and caftaric acid (Rt = 26.18 min), were also identified based on their pseudomolecular ions [M−H]− at m/z 169.0147 and [M−H]− at m/z 311.0398, respectively. Finally, the flavonols quercetin at Rt = 9.56 min and kaempferol at Rt = 15.79 min were detected and identified with the same way and in particular based on their peudomolecular ions [M−H]− at m/z 301.0357 and [M−H]− at m/z 285.0407. The last four compounds have been previously isolated and identified in our laboratory and their purity, determined by HPLC-DAD analysis is estimated from 86 to 98%. It is important to note that due to the capabilities of Orbitrap analyzer, all the m/z measurements were highly accurate, and specifically the calculated Δm for all the compounds under investigation was found from 0.5 to 3.2 ppm.
3.1.2. Total Polyphenol Content
Total polyphenol content of the extract was evaluated and found equal to 648 mg of gallic acid per g of extract.
3.1.3. Radical Scavenging Capacity of the Extract
The tested extract exerted significant capacity to scavenge the DPPH• and ABTS•+ radicals. The results are expressed as IC50 values. The lower the IC50 value, the higher the antioxidant capacity of the extract. The IC50 data for the DPPH• and ABTS•+ radicals are 25 and 5.5 mg/mL, respectively (Figure 1).
Figure 1.
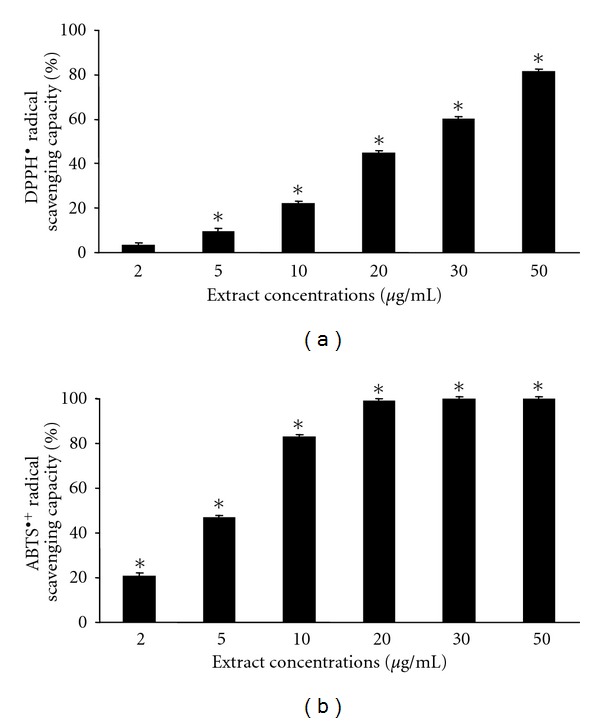
DPPH• and ABTS•+ radical scavenging capacity of the grape pomace extract. *Significantly different from the control value (P < 0.05).
3.1.4. Protective Activity of the Extract against Free Radical-Induced DNA Damage
The tested extract exhibited significant protective activity on DNA. Particularly, it inhibited DNA damage induced by ROO• and OH• radicals (Figure 2). The IC50 values for ROO• and OH• radicals are 1.5 and 500 mg/mL, respectively.
Figure 2.
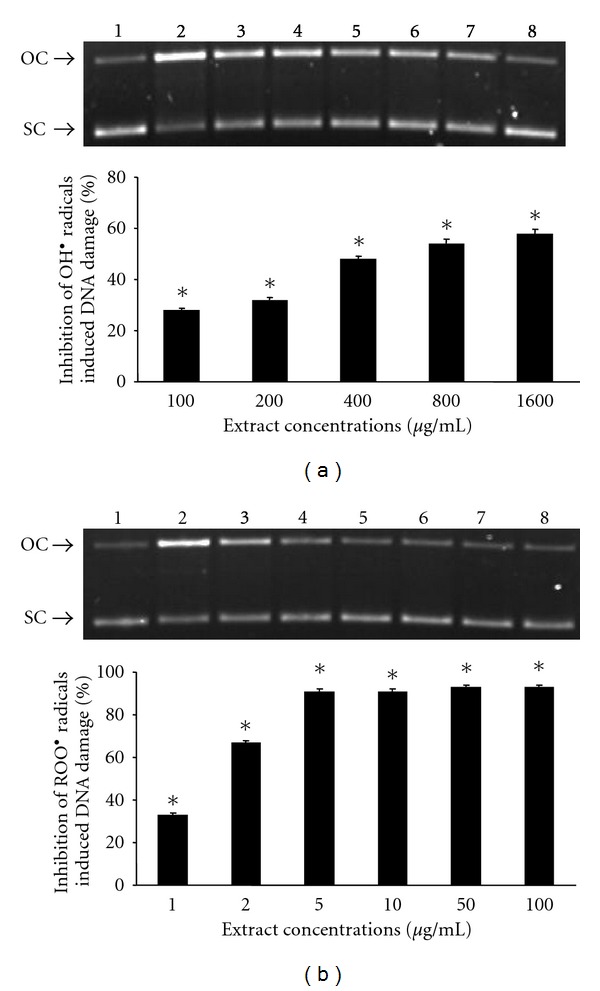
Protective activity of the grape pomace extract on DNA strand scission induced by OH• and ROO•. (a) Extract antioxidant activity against OH•. Bluescript-SK+ plasmid DNA was exposed to UV plus H2O2 (lane 2) or to UV plus H2O2 in the presence of 100 μg/mL, 200 μg/mL, 400 μg/mL, 800 μg/mL and 1600 μg/mL of the extract, respectively (lanes 3–7) or to 1600 μg/mL of the extract alone (lane 8). (b) Extract antioxidant activity against ROO•. Bluescript-SK+ plasmid DNA was exposed to ROO• alone (lane 2) or to ROO• in the presence of 1 μg/mL, 2 μg/mL, 5 μg/mL, 10 μg/mL, 50 μg/mL and 100 μg/mL of the extract, respectively (lanes 3–8). Lane 1 represents Bluescript-SK+ plasmid DNA without any treatment. *Significantly different from the control value (P < 0.05). OC: open circular conformation of the plasmid, SC: supercoiled conformation of the plasmid.
3.2. In Vivo Experiment
3.2.1. Exercise Performance
Swimming performance was measured in 20 animals of the exercised groups. Half of the rats were treated with saline and the other half with the extract. No difference in performance between the saline-treated and extract-treated groups was observed. The swimming time to exhaustion for the saline- and extract-treated animals was 46.1 ± 2.0 and 45.1 ± 1.4 min, respectively.
3.2.2. Oxidative Stress Markers
Plasma —
In xanthine oxidase (Figure 3(a)), significant main effects of treatment and time were found. In post hoc within-group comparisons, xanthine oxidase activity significantly increased after exercise in the saline-treated group only. In post hoc between-group comparisons, xanthine oxidase activity was significantly lower in the extract group compared to the saline group, both at rest and at postexercise. In TAC (Figure 3(b)), significant main effects of treatment and time were found. In the within-group comparisons, TAC increased after exercise in both saline- and extract-treated groups. In the between-group comparisons, TAC was higher in the extract group compared to the saline group at postexercise only. In protein carbonyls (Figure 3(c)) significant main effects of treatment, time, as well as interaction of treatment × time were found. In the within-group comparisons, protein carbonyl concentration increased after exercise in both saline and extract-treated groups. In the between-group comparisons, protein carbonyl concentration was higher in the extract group compared to saline group at rest only. In TBARS (Figure 3(d)) significant main effects of treatment and time were found. In the within-group comparisons, TBARS concentration increased after exercise in the saline-treated group only. In the between-group comparisons, TBARS concentration was higher in the extract-treated group compared to the saline-treated group at rest only.
Figure 3.
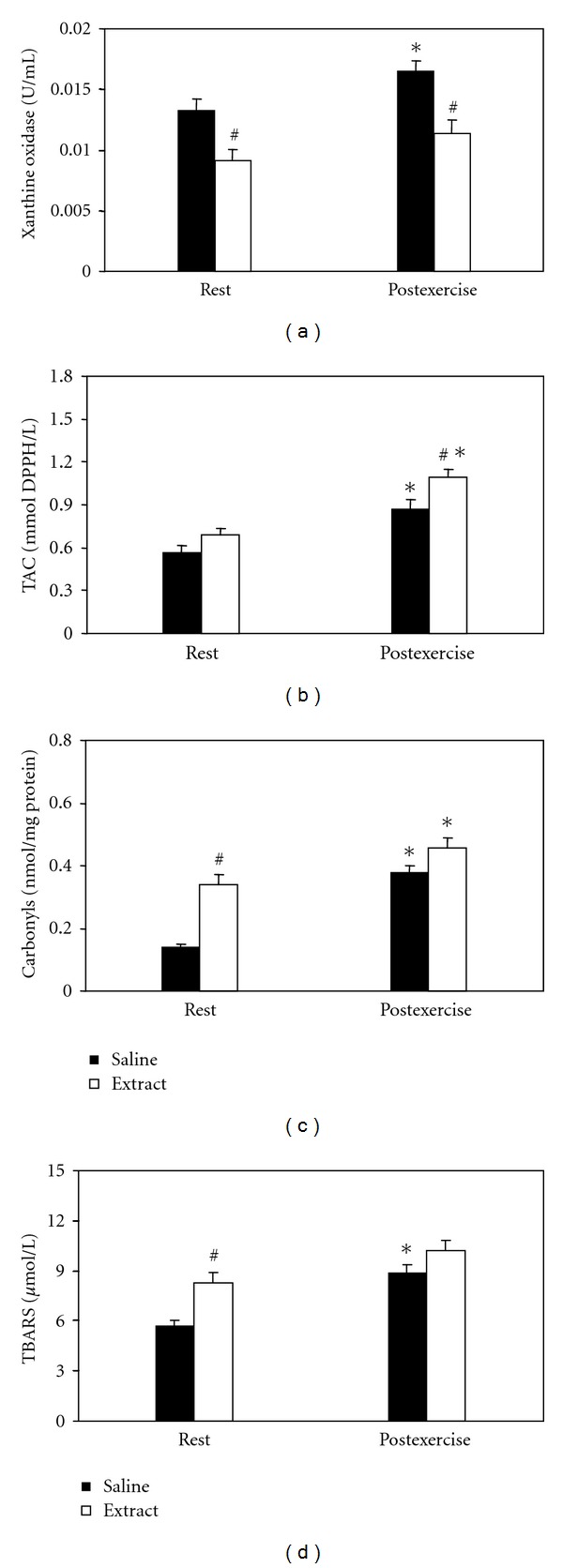
Effects of the grape pomace extract on oxidative stress markers in plasma at rest and postexercise. *Significantly different from the rest value within either the saline or the extract group (P < 0.05). #Significantly different between the saline- and the extract-treated groups at the same time point (P < 0.05).
Erythrocytes —
In protein carbonyls (Figure 4(a)), significant main effects of treatment and time were found. In the within-group comparisons, protein carbonyl concentration increased after exercise in both saline, and extract-treated groups. In the between-group comparisons, protein carbonyl concentration was higher in the extract group compared to saline group at rest only. In TBARS (Figure 4(b)), significant main effects of treatment and time were found. In the within-group comparisons, TBARS concentration increased after exercise in both saline- and extract-treated groups. In the between-group comparisons, TBARS concentration was higher in the extract group compared to the saline group at postexercise only. In GSH (Figure 4(c)) an interaction of treatment × time was found. In the within-group comparisons, GSH concentration decreased postexercise in the saline group only. In the between-group comparisons, GSH concentration was lower in the extract group compared to the saline group at postexercise only. In catalase (Figure 4(d)), neither significant main effects nor interaction were found.
Figure 4.
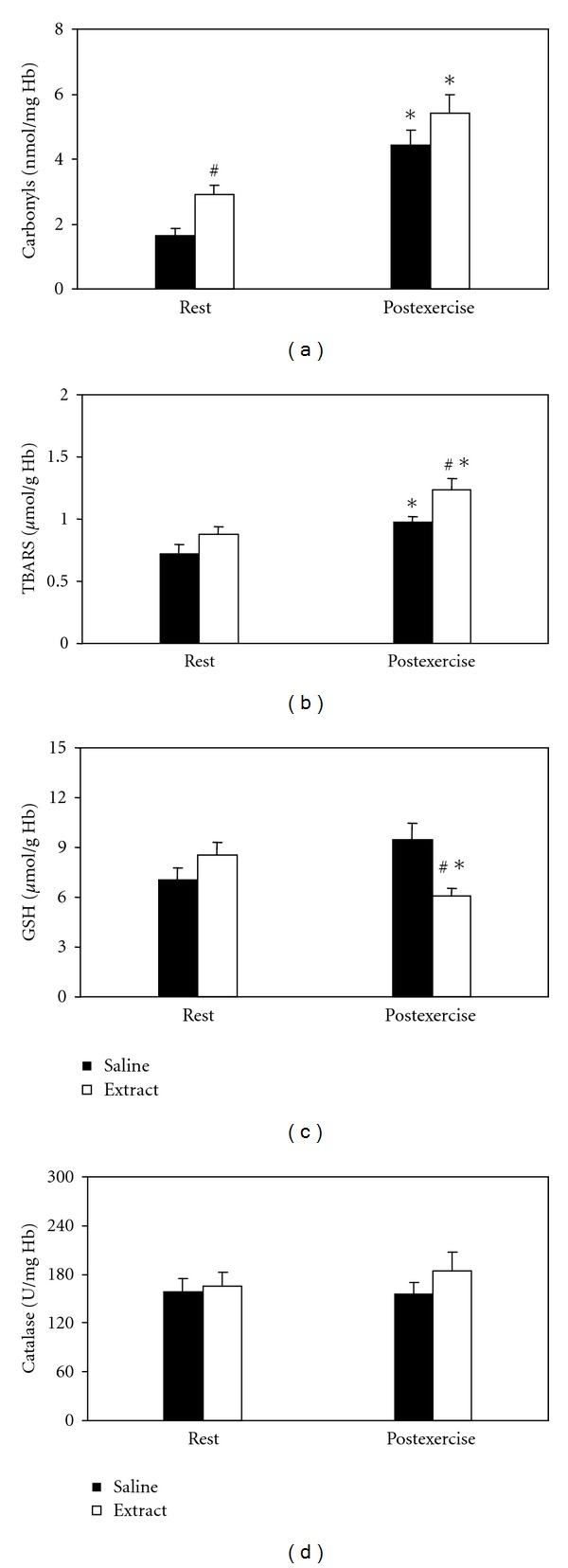
Effects of the grape pomace extract on oxidative stress markers in erythrocytes at rest and postexercise. *Significantly different from the rest value within either the saline or the extract group (P < 0.05). #Significantly different between the saline- and the extract-treated groups at the same time point (P < 0.05).
Gastrocnemius Muscle —
In xanthine oxidase (Figure 5(a)), significant main effect of time and interaction of treatment × time was found. In post hoc within-group comparisons, xanthine oxidase activity significantly decreased at postexercise in the saline-treated group only. In post hoc between-group comparisons, xanthine oxidase activity decreased in the extract-treated group at rest and increased in the same group compared to the saline-treated group at postexercise. In TAC (Figure 5(b)), protein carbonyls (Figure 5(c)) and GSH (Figure 5(e)), neither significant main effects nor interaction were found. In TBARS (Figure 5(d)), an interaction of treatment × time was found. In within-group and between-group comparisons, TBARS concentration increased at postexercise in the saline-treated group only. In catalase (Figure 5(f)), significant main effects of treatment and time were found. In within-group and between-group comparisons, catalase activity increased at postexercise in the extract-treated group only.
Figure 5.
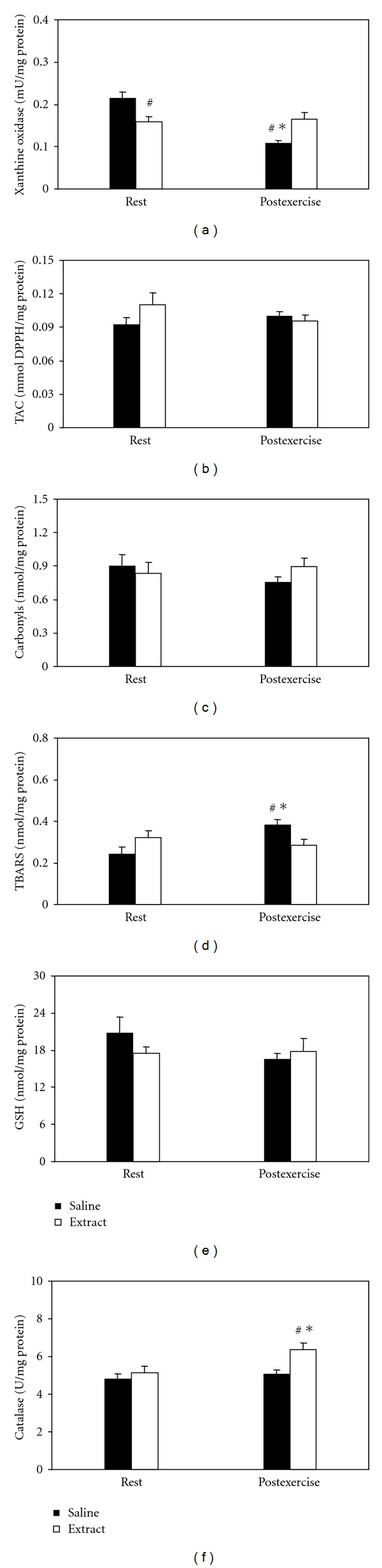
Effects of the grape pomace extract on oxidative stress markers in gastrocnemius muscle at rest and postexercise. *Significantly different from the rest value within either the saline or the extract group (P < 0.05). #Significantly different between the saline- and the extract-treated groups at the same time point (P < 0.05).
Heart —
In xanthine oxidase (Figure 6(a)), and GSH (Figure 6(e)), neither significant main effects nor interaction were found. In TAC (Figure 6(b)), main effect of treatment was found. In protein carbonyls (Figure 6(c)), main effect of time and interaction of treatment × time was found. In post hoc within-group comparisons, protein carbonyl concentration increased at postexercise in the saline-treated group only. In post hoc between-group comparisons, protein carbonyl concentration increased at rest in the extract-treated group only. In TBARS (Figure 6(d)), main effects of treatment and time were found. In within-group comparisons, TBARS concentration at postexercise increased in both saline-treated and extract-treated groups. In between-group comparisons, TBARS concentration increased in saline-treated group at rest and at postexercise. In catalase (Figure 6(f)), main effect of time was found.
Figure 6.
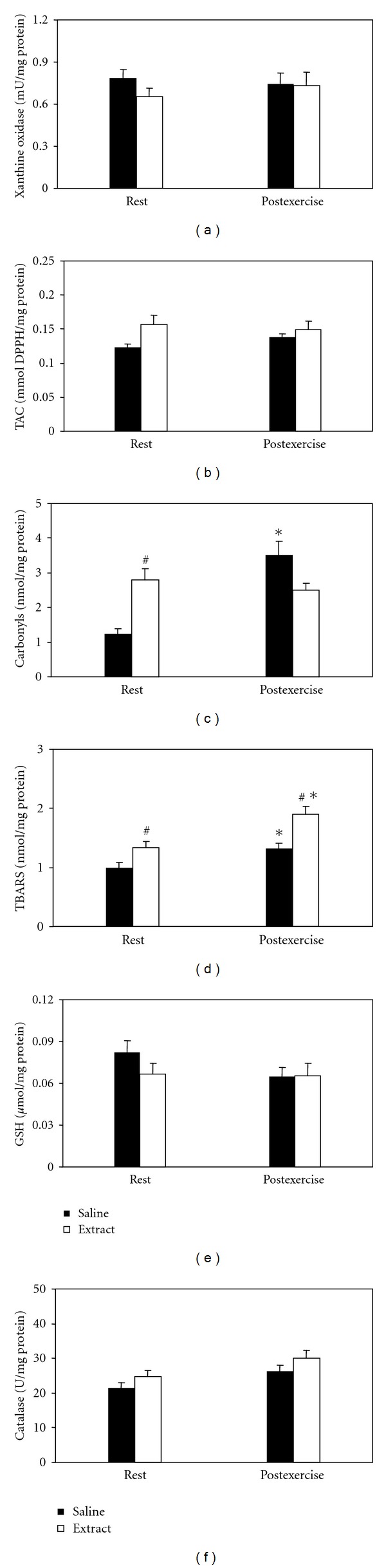
Effects of the grape pomace extract on oxidative stress markers in heart at rest and postexercise. *Significantly different from the rest value within either the saline or the extract group (P < 0.05). #Significantly different between the saline- and the extract-treated groups at the same time point (P < 0.05).
Liver —
In xanthine oxidase (Figure 7(a)), TAC (Figure 7(b)) and catalase (Figure 7(f)), neither significant main effects nor interaction were found. In protein carbonyls (Figure 7(c)), main effect of time was found. In TBARS (Figure 7(d)), main effect of treatment and interaction of treatment × time were found. In post hoc within-group comparisons, TBARS concentration at postexercise increased in extract-treated group. In post hoc between-group comparisons, TBARS concentration increased in extract-treated group compared to the saline-treated group at postexercise. In GSH (Figure 7(e)), main effects of treatment and time were found. In within-group comparisons, GSH concentration at postexercise decreased in saline-treated group. In between-group comparisons, GSH concentration decreased in extract-treated group compared to the saline-treated group at rest.
Figure 7.
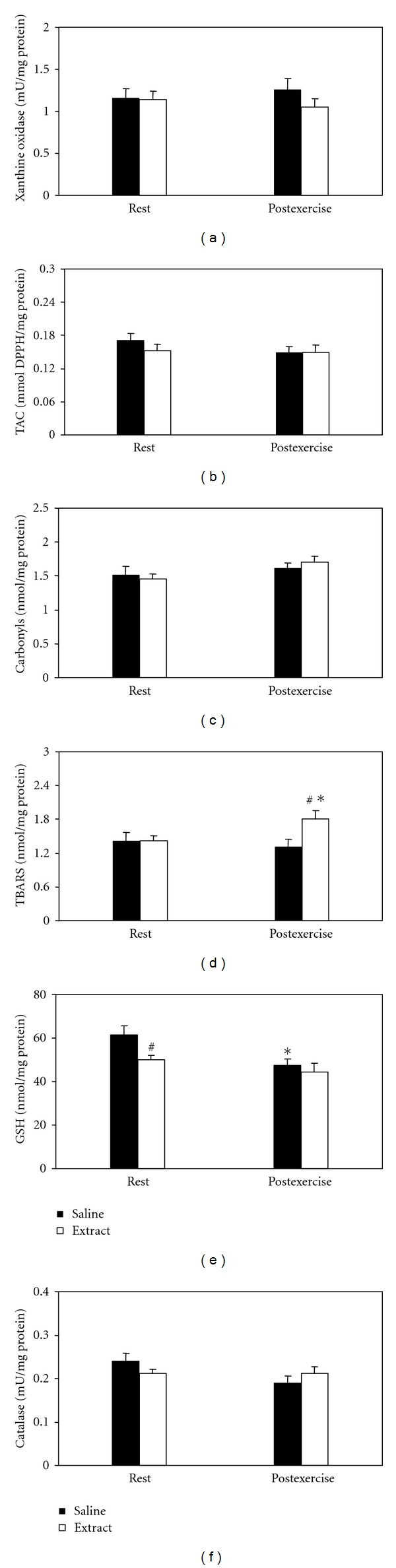
Effects of the grape pomace extract on oxidative stress markers in liver at rest and postexercise. *Significantly different from the rest value within either the saline or the extract group (P < 0.05). #Significantly different between the saline- and the extract-treated groups at the same time point (P < 0.05).
4. Discussion
Over the last decades, various plant extracts have gained a lot of interest because of their beneficial effects on human health. Vegetables and fruits are substantial part of the Mediterranean diet. Grapes, in particular, are thought to possess health-related properties. It has been established that grape consumption is related to the prevention of chronic diseases such as cardiovascular diseases [35] and cancer [36]. The biological importance of grape extracts is mainly attributed to the antioxidant properties of the polyphenolic compounds they possess [35, 37]. This is the main reason why polyphenolic compounds and plant extracts have been increasingly used as part of the diet or as nutritional supplements. Nevertheless, polyphenols may also act as prooxidants as they may induce free-radical production mainly via Fenton reaction [38, 39].
The rationale of the present study was to compare the effects of a polyphenol-rich grape pomace extract on redox status using both in vitro and in vivo models. The tested extract was initially examined for its possible antioxidant capacity. The results demonstrated that the extract has potent antioxidant and chemopreventive properties in vitro as it scavenges free radicals (DPPH• or ABTS•+) and prevents DNA damage induced by ROO• and OH• radicals. It is established that ROO• are the major factors initiating the cascade reactions of lipid peroxidation [40]. Thus, the preventive activity of the extract against the detrimental effects of ROO• on DNA in a relatively low concentration implies that it might participate in protection against lipid peroxidation. Furthermore, the extract could be considered as a chemopreventive agent as ROO• and lipid peroxidation cause mutations in DNA and are crucial for the initiation of carcinogenic process [41]. The protective effect of the extract on the DNA damage induced by OH•, despite the fact that it was observed in much higher concentration that against ROO•, is of high importance. It is known that OH• are highly reactive and can easily cause mutations in DNA [42]. Given that UV radiation is one of the main producers of OH•, it could be considered that the extract possesses preventive properties in vitro against the effects of UV radiation. These findings are in accordance with the potent in vitro antioxidant and chemopreventive properties of other grape extracts of the Vitis vinifera species [24–26].
Thereafter, the intention of this study was to examine if the in vitro antioxidant properties of the extract apply in an in vivo experimental model using exercise as an oxidant stimulus. Swimming was chosen as an experimental model because it causes limited muscle damage and the requirement for antioxidant activity is much less due to a dramatic reduction in inflammatory processes related to muscle damage and repair. The grape pomace extract was administered in rats before exhaustive swimming and generally induced oxidative stress at rest. This is evident by the increased concentration in plasma and erythrocyte protein carbonyls, plasma TBARS, heart protein carbonyls and TBARS, as well as the decreased concentration in liver GSH in the extract-treated rats compared to the saline counterparts at rest.
Exercise, as expected, enhanced one of the main pathways that contribute to free-radical production during exercise as seen by the increased activity of xanthine oxidase in plasma in saline group postexercise. Exercise alone induced oxidative stress as indicated by the increased protein carbonyl concentration in plasma, erythrocytes, and heart, the increased TBARS concentration in plasma, erythrocytes, gastrocnemius muscle, and heart, the increased TAC in plasma, and the decreased GSH concentration in liver in saline group postexercise. The effects of exercise alone on oxidative stress that are described in the present paper are in line with previous findings. Thus, it has also been found that exercise increases plasma, erythrocyte, and gastrocnemius muscle protein carbonyl concentration [2, 43–45] and lipid peroxidation in plasma, erythrocytes, and gastrocnemius muscle [43, 45, 46].
Extract administration inhibited xanthine oxidase activity in plasma postexercise. In a previous study of our research group, it has been demonstrated that the grape pomace extract used is an in vitro inhibitor of xanthine oxidase activity, and this could be a possible reason for the decrease in the activity of the enzyme [47]. It is important to mention that xanthine oxidase is one of the major contributors of reactive species during exercise. However, emphasis should also be given on the contribution of mitochondria, which are very much loaded during strenuous physical exercise because of the high energy demand. Furthermore, in some cases, extract administration in combination with exercise induced oxidative stress further than that induced by exercise alone as shown by the increased TBARS concentration in erythrocyte and liver, catalase activity in gastrocnemius, and TAC in plasma, as well as the decreased GSH in erythrocytes. The prooxidant effects of plant extracts after exercise have also been referred to in previous studies. Specifically, artichoke-leaf extract administration did not limit oxidative damage to erythrocytes in competitive rowers subjected to strenuous training [21]. On the contrary, there is evidence indicating the in vivo antioxidant activity of several plant extracts administered before exercise [48–50]. It should be mentioned that timing is a variable that may influence antioxidant recommendations. For example, the outcome may differ if the extract is administered before exercise, after exercise or studied under chronic supplementation.
To our knowledge, there are no studies comparing the in vitro and in vivo effects of a plant extract on redox status before exercise. However, there are few in vitro versus in vivo studies measuring oxidative stress markers in response to other oxidative stress stimuli such as diabetes [51, 52], exposure in xenobiotics [1, 53], or reactive oxygen species [54]. These studies have shown that several different extracts exhibit antioxidant or chemopreventive properties both in vitro and in vivo. Nevertheless, other studies demonstrated that the antioxidant in vitro activity does not always apply to in vivo models. In particular, black tea extract and its major polyphenolic antioxidant constituent, epigallocatechin gallate, protect against lipid peroxidation induced by the water-soluble radical generator AAPH in vitro. However, this is not the case when they are consumed by human subjects as they do not protect plasma from lipid peroxidation [55]. Furthermore, despite the high antioxidant capacity of individual apple polyphenols and apple extracts in vitro, ingestion of large amounts of apples and apple polyphenols by humans does not appear to result in equivalent in vivo antioxidant effects [56]. This disagreement is not surprising. Polyphenols when consumed are absorbed by the gastrointestinal tract, and their concentration in plasma does not reach concentrations higher than 1 μmol/L because of its rapid metabolism by tissues [57]. Administration of 2 g of catechin and 50 mg of gallic acid (the most abundant polyphenols in the grape pomace extract used in the present paper) resulted in 3.5 μmol/L and 1.8 μmol/L plasma concentrations, respectively [58]. The fact that the polyphenolic compounds are degraded in metabolites with smaller molecular weight is partly responsible for their different in vitro and in vivo effects on redox status [59]. Besides, polyphenols are metabolized as typical xenobiotics and such metabolism alters or decreases their antioxidant capacity [57]. These data raise serious concerns whether any potential antioxidant effects of polyphenols on redox status in vivo can be simply extrapolated from their antioxidant activities in vitro.
A main finding of the present study was that the administration of the grape pomace extract did not affect exercise performance, as indicated by the almost identical swimming time to exhaustion between the saline- and extract-treated groups. Several studies that examined the effects of antioxidant supplementation on exercise performance have reported controversial results. More specifically, exercise performance was not affected after administration of vitamin E, ascorbic acid and other antioxidants in humans and rats [60–64] or supplementation of black currant extract [20], artichoke extract [21], rhodiola rosea extract [22] in humans, or panax ginseng extract [65] in humans and selenium administration in rats [66]. On the contrary, performance was improved after administration of N-acetyl cysteine in humans [67], tocotrienols in rats [68], as well as vitamin E [69], Pseudosasa japonica leaves [70], and green-tea extract [71] in mice. Furthermore, it has been previously reported that antioxidant supplementation barely affects exercise performance by more than 10% [72]. In a previous study of our research group administration of allopurinol, a potent inhibitor of xanthine oxidase, markedly decreased performance and caused a 4-fold decrease in xanthine oxidase activity in plasma and gastrocnemius muscle [5]. As a consequence, there was an inhibition of uric acid production, one of the most potent antioxidant molecules in plasma [9, 10]. In the current study, the grape extract inhibited xanthine oxidase activity in the examined tissues by only about 30%. This differential effect of allopurinol and grape pomace extract on the reduction of xanthine oxidase activity might be a reason why performance was not affected after extract administration.
The data of the present study illustrate that the in vitro antioxidant activity of a grape pomace extract does not necessarily translate to in vivo antioxidant activity either at rest or after exercise. This finding suggests that the in vitro antioxidant activity of the particular grape pomace extract was not effective when applied to an in vivo system, at least when exercise is used as an oxidant stimulus. In the light of these findings, we suggest that the term “antioxidant” may be system-related. Therefore, the common practice of supplementing antioxidants before exercise should be examined with a more critical view and further be investigated. An alternative and also interesting suggestion is that the pro-oxidant effect of grape pomace extract might be beneficial because it triggers the antioxidant machinery of the body to respond with a more efficient way. Whatever the case it should be, the answer can bring new evidence in the oxidative stress field.
References
- 1.Ji LL. Antioxidant signaling in skeletal muscle: a brief review. Experimental Gerontology. 2007;42(7):582–593. doi: 10.1016/j.exger.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Gomez-Cabrera MC, Borrás C, Pallardo FV, Sastre J, Ji LL, Viña J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. Journal of Physiology. 2005;567(1):113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikolaidis MG, Paschalis V, Giakas G, et al. Decreased blood oxidative stress after repeated muscle-damaging exercise. Medicine and Science in Sports and Exercise. 2007;39(7):1080–1089. doi: 10.1249/mss.0b013e31804ca10c. [DOI] [PubMed] [Google Scholar]
- 4.Betters JL, Criswell DS, Shanely RA, et al. Trolox attenuates mechanical ventilation-induced diaphragmatic dysfunction and proteolysis. American Journal of Respiratory and Critical Care Medicine. 2004;170(11):1179–1184. doi: 10.1164/rccm.200407-939OC. [DOI] [PubMed] [Google Scholar]
- 5.Veskoukis AS, Nikolaidis MG, Kyparos A, et al. Effects of xanthine oxidase inhibition on oxidative stress and swimming performance in rats. Applied Physiology, Nutrition and Metabolism. 2008;33(6):1140–1154. doi: 10.1139/H08-102. [DOI] [PubMed] [Google Scholar]
- 6.Michailidis Y, Jamurtas AZ, Nikolaidis MG, et al. Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Medicine and Science in Sports and Exercise. 2007;39(7):1107–1113. doi: 10.1249/01.mss.0b013e318053e7ba. [DOI] [PubMed] [Google Scholar]
- 7.Nikolaidis MG, Jamurtas AZ, Paschalis V, et al. Exercise-induced oxidative stress in G6PD-deficient individuals. Medicine and Science in Sports and Exercise. 2006;38(8):1443–1450. doi: 10.1249/01.mss.0000228938.24658.5f. [DOI] [PubMed] [Google Scholar]
- 8.McCord JM, Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. Journal of Biological Chemistry. 1968;243(21):5753–5760. [PubMed] [Google Scholar]
- 9.Wayner DDM, Burton GW, Ingold KU, Barclay LRC, Locke SJ. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochimica Biophysica Acta. 1987;924(3):408–419. doi: 10.1016/0304-4165(87)90155-3. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B, Gutteridge J. Free Radicals in Biology and Medicine. New York, NY, USA: Oxford University Press; 2007. [Google Scholar]
- 11.Fauconneau B, Waffo-Teguo P, Huguet F, Barrier L, Decendit A, Merillon JM. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sciences. 1997;61(21):2103–2110. doi: 10.1016/s0024-3205(97)00883-7. [DOI] [PubMed] [Google Scholar]
- 12.Bagchi D, Bagchi M, Stohs SJ, et al. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology. 2000;148(2-3):187–197. doi: 10.1016/s0300-483x(00)00210-9. [DOI] [PubMed] [Google Scholar]
- 13.Murthy KNC, Singh RP, Jayaprakasha GK. Antioxidant activities of grape (Vitis vinifera) pomace extracts. Journal of Agricultural and Food Chemistry. 2002;40(21):941–947. doi: 10.1021/jf0257042. [DOI] [PubMed] [Google Scholar]
- 14.Beninger CW, Hosfield GL. Antioxidant activity of extracts, condensed tannin fractions, and pure flavonoids from Phaseolus vulgaris L. Seed coat color genotypes. Journal of Agricultural and Food Chemistry. 2003;51(27):7879–7883. doi: 10.1021/jf0304324. [DOI] [PubMed] [Google Scholar]
- 15.Manna C, Migliardi V, Golino P, et al. Oleuropein prevents oxidative myocardial injury induced by ischemia and reperfusion. Journal of Nutritional Biochemistry. 2004;15(8):461–466. doi: 10.1016/j.jnutbio.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Heimler D, Vignolini P, Dini MG, Romani A. Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. Journal of Agricultural and Food Chemistry. 2005;53(8):3053–3056. doi: 10.1021/jf049001r. [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Park KS, Chang MJ, Sung JH. Effects of Panax ginseng extract on exercise-induced oxidative stress. Journal of Sports Medicine and Physical Fitness. 2005;45(2):178–182. [PubMed] [Google Scholar]
- 18.Voces J, Alvarez AI, Vila L, Ferrando A, Cabral De Oliveira C, Prieto JG. Effects of administration of the standardized Panax ginseng extract G115 on hepatic antioxidant function after exhaustive exercise. Comparative Biochemistry and Physiology C. 1999;123(2):175–184. doi: 10.1016/s0742-8413(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 19.Voces J, Cabral de Oliveira AC, Prieto JG, et al. Ginseng administration protects skeletal msucle from oxidative stress induced by acute exercise in rats. Brazilian Journal of Medical and Biological Research. 2004;37(12):1863–1871. doi: 10.1590/s0100-879x2004001200012. [DOI] [PubMed] [Google Scholar]
- 20.Skarpanska-Stejnborn A, Basta P, Pilaczynska-Szczesniak L. The influence of supplementation with black currant (Ribes nigrum) extract on selected prooxidative-antioxidative balance in rowers. Studies in Physical Culture and Tourism. 2006;13:51–58. [Google Scholar]
- 21.Skarpañska-Stejnborn A, Pilaczynska-Szczesniak L, Basta P, Deskur-Smielecka E, Horoszkiewicz-Hassan M. The influence of supplementation with artichoke (Cynara scolymus L.) extract on selected redox parameters in rowers. International Journal of Sport Nutrition and Exercise Metabolism. 2008;18(3):313–327. doi: 10.1123/ijsnem.18.3.313. [DOI] [PubMed] [Google Scholar]
- 22.Skarpanska-Stejnborn A, Pilaczynska-Szczesniak L, Basta P, Deskur-Smielecka E. The influence of supplementation with Rhodiola rosea L. extract on Selected redox parameters in professional rowers. International Journal of Sport Nutrition and Exercise Metabolism. 2009;19(2):186–199. doi: 10.1123/ijsnem.19.2.186. [DOI] [PubMed] [Google Scholar]
- 23.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American Journal of Clinical Nutrition. 2005;81(1, supplement):230S–42S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 24.Stagos D, Kouris S, Kouretas D. Plant phenolics protect from bleomycin-induced oxidative stress and mutagenicity in Salmonella typhimurium TA102. Anticancer Research. 2004;24(2 B):743–745. [PubMed] [Google Scholar]
- 25.Stagos D, Kazantzoglou G, Magiatis P, Mitaku S, Anagnostopoulos K, Kouretas D. Effects of plant phenolics and grape extracts from Greek varieties of Vitis vinifera on Mitomycin C and topoisomerase I-induced nicking of DNA. International Journal of Molecular Medicine. 2005;15(6):1013–1022. [PubMed] [Google Scholar]
- 26.Stagos D, Spanou C, Margariti M, et al. Cytogenetic effects of grape extracts (Vitis vinifera) and polyphenols on mitomycin C-induced sister chromatid exchanges (SCEs) in human blood lymphocytes. Journal of Agricultural and Food Chemistry. 2007;55(13):5246–5252. doi: 10.1021/jf0635255. [DOI] [PubMed] [Google Scholar]
- 27.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology. 1999;299:152–178. [Google Scholar]
- 28.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technology. 1995;28(1):25–30. [Google Scholar]
- 29.Cano A, Hernández-Ruíz J, García-Cánovas F, Acosta M. An end-point method for estimation of the total antioxidant activity in plant material. Phytochemical Analysis. 1998;9(4):196–202. [Google Scholar]
- 30.Chang ST, Wu JH, Wang SY, Kang PL, Yang NS, Shyur LF. Antioxidant activity of extracts from acacia confusa bark and heartwood. Journal of Agricultural and Food Chemistry. 2001;49(7):3420–3424. doi: 10.1021/jf0100907. [DOI] [PubMed] [Google Scholar]
- 31.Keum YS, Park KK, Lee JM, et al. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Letters. 2000;150(1):41–48. doi: 10.1016/s0304-3835(99)00369-9. [DOI] [PubMed] [Google Scholar]
- 32.Ferrando A, Vila L, Voces JA, Cabral AC, Alvarez AI, Prieto JG. Effects of a standardized Panax ginseng extract on the skeletal muscle of the rat: a comparative study in animals at rest and under exercise. Planta Medica. 1999;65(3):239–244. doi: 10.1055/s-1999-14081. [DOI] [PubMed] [Google Scholar]
- 33.Komulainen J, Takala TES, Vihko V. Does increased serum creatine kinase activity reflect exercise-induced muscle damage in rats? International Journal of Sports Medicine. 1995;16(5):150–154. doi: 10.1055/s-2007-972983. [DOI] [PubMed] [Google Scholar]
- 34.Veskoukis AS, Kouretas D, Panoutsopoulos GI. Substrate specificity of guinea pig liver aldehyde oxidase and bovine milk xanthine oxidase for methyl- and nitrobenzaldehydes. European Journal of Drug Metabolism and Pharmacokinetics. 2006;31(1):11–16. doi: 10.1007/BF03190636. [DOI] [PubMed] [Google Scholar]
- 35.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. The Lancet. 1992;339(8808):1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 36.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. International Journal of Cancer. 2004;108(5):733–740. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 37.Soleas GJ, Diamandis EP, Goldberg DM. Wine as a biological fluid: history, production, and role in disease prevention. Journal of Clinical Laboratory Analysis. 1997;11(5):287–313. doi: 10.1002/(SICI)1098-2825(1997)11:5<287::AID-JCLA6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cotelle N. Role of flavonoids in oxidative stress. Current Topics in Medicinal Chemistry. 2001;1(6):569–590. doi: 10.2174/1568026013394750. [DOI] [PubMed] [Google Scholar]
- 39.Halliwell B. Dietary polyphenols: good, bad, or indifferent for your health? Cardiovascular Research. 2007;73(2):341–347. doi: 10.1016/j.cardiores.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Mylonas C, Kouretas D. Lipid peroxidation and tissue damage. In Vivo. 1999;13(3):295–309. [PubMed] [Google Scholar]
- 41.Lim P, Wuenschell GE, Holland V, et al. Peroxyl radical mediated oxidative DNA base damage: implications for lipid peroxidation induced mutagenesis. Biochemistry. 2004;43(49):15339–15348. doi: 10.1021/bi048276x. [DOI] [PubMed] [Google Scholar]
- 42.Cadet J, Delatour T, Douki T, et al. Hydroxyl radicals and DNA base damage. Mutation Research. 1999;424(1-2):9–21. doi: 10.1016/s0027-5107(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 43.Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL. Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Medicine and Science in Sports and Exercise. 2000;32(9):1576–1581. doi: 10.1097/00005768-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Stadtman ER, Levine RL. Protein oxidation. Annals of the New York Academy of Sciences. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 45.You T, Goldfarb AH, Bloomer RJ, Nguyen L, Sha X, McKenzie MJ. Oxidative stress response in normal and antioxidant supplemented rats to a downhill run: changes in blood and skeletal muscles. Canadian Journal of Applied Physiology. 2005;30(6):677–689. doi: 10.1139/h05-148. [DOI] [PubMed] [Google Scholar]
- 46.Ajmani RS, Fleg JL, Demehin AA, et al. Oxidative stress and hemorheological changes induced by acute treadmill exercise. Clinical Hemorheology and Microcirculation. 2003;28(1):29–40. [PubMed] [Google Scholar]
- 47.Spanou C, Veskoukis AS, Dimitrios S, et al. Effects of grape extracts on the in vitro activity of enzymes involved in oxidative stress regulation. In Vivo. 2011;25(4):657–662. [PubMed] [Google Scholar]
- 48.Paula FB, Gouvêa CM, Alfredo PP, Salgado I. Protective action of a hexane crude extract of Pterodon emarginatus fruits against oxidative and nitrosative stress induced by acute exercise in rats. BMC Complementary and Alternative Medicine. 2005;5, article 17 doi: 10.1186/1472-6882-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu F, Lu S, Yu F, et al. Protective effects of polysaccharide from Euphorbia kansui (Euphorbiaceae) on the swimming exercise-induced oxidative stress in mice. Canadian Journal of Physiology and Pharmacology. 2006;84(10):1071–1079. doi: 10.1139/y06-052. [DOI] [PubMed] [Google Scholar]
- 50.Niu AJ, Wu JM, Yu DH, Wang R. Protective effect of Lycium barbarum polysaccharides on oxidative damage in skeletal muscle of exhaustive exercise rats. International Journal of Biological Macromolecules. 2008;42(5):447–449. doi: 10.1016/j.ijbiomac.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Puiggròs F, Sala E, Vaque M, et al. In vivo, in vitro, and in silico studies of CU/ZN-superoxide dismutase regulation by molecules in grape seed procyanidin extract. Journal of Agricultural and Food Chemistry. 2009;57(9):3934–3942. doi: 10.1021/jf8034868. [DOI] [PubMed] [Google Scholar]
- 52.Cho EJ, Lee YA, Hye HY, Yokozawa T. Protective effects of broccoli (Brassica oleracea) against oxidative damage in vitro and in vivo . Journal of Nutritional Science and Vitaminology. 2006;52(6):437–444. doi: 10.3177/jnsv.52.437. [DOI] [PubMed] [Google Scholar]
- 53.Lee HS, Nam HW, Kyoung HK, Lee H, Jun W, Lee KW. Antioxidant effects of aqueous extract of Terminalia chebulain Vivo and in Vitro . Biological and Pharmaceutical Bulletin. 2005;28(9):1639–1644. doi: 10.1248/bpb.28.1639. [DOI] [PubMed] [Google Scholar]
- 54.Shi GF, An LJ, Jiang B, Guan S, Bao YM. Alpinia protocatechuic acid protects against oxidative damage in vitro and reduces oxidative stress in vivo . Neuroscience Letters. 2006;403(3):206–210. doi: 10.1016/j.neulet.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 55.Cherubini A, Beal MF, Frei B. Black tea increases the resistance of human plasma to lipid peroxidation in vitro, but not ex vivo . Free Radical Biology and Medicine. 1999;27(3-4):381–387. doi: 10.1016/s0891-5849(99)00064-7. [DOI] [PubMed] [Google Scholar]
- 56.Lotito SB, Frei B. Relevance of apple polyphenols as antioxidants in human plasma: contrasting in vitro and in vivo effects. Free Radical Biology and Medicine. 2004;36(2):201–211. doi: 10.1016/j.freeradbiomed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Archives of Biochemistry and Biophysics. 2008;476(2):107–112. doi: 10.1016/j.abb.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez-Panchon MS, Villano D, Troncoso AM, Garcia-Parrilla MC. Antioxidant activity of phenolic compounds: from in vitro results to in vivo evidence. Critical Reviews in Food Science and Nutrition. 2008;48(7):649–671. doi: 10.1080/10408390701761845. [DOI] [PubMed] [Google Scholar]
- 59.Shirai M, Kawai Y, Yamanishi R, Kinoshita T, Chuman H, Terao J. Effect of a conjugated quercetin metabolite, quercetin 3-glucuronide, on lipid hydroperoxide-dependent formation of reactive oxygen species in differentiated PC-12 cells. Free Radical Research. 2006;40(10):1047–1053. doi: 10.1080/10715760600794287. [DOI] [PubMed] [Google Scholar]
- 60.Lawrence JD, Bower RC, Riehl WP, Smith JL. Effects of α tocopherol acetate on the swimming endurance of trained swimmers. American Journal of Clinical Nutrition. 1975;28(3):205–208. doi: 10.1093/ajcn/28.3.205. [DOI] [PubMed] [Google Scholar]
- 61.Sumida S, Tanaka K, Kitao H, Nakadomo F. Exercise-induced lipid peroxidation and leakage of enzymes before and after vitamin E supplementation. International Journal of Biochemistry. 1989;21(8):835–838. doi: 10.1016/0020-711x(89)90280-2. [DOI] [PubMed] [Google Scholar]
- 62.Snider IP, Bazzarre TL, Murdoch SD, Goldfarb A. Effects of coenzyme athletic performance system as an ergogenic aid on endurance performance to exhaustion. International Journal of Sport Nutrition. 1992;2(3):272–286. doi: 10.1123/ijsn.2.3.272. [DOI] [PubMed] [Google Scholar]
- 63.Rokitzki L, Logemann E, Sagredos AN, Murphy M, Wetzel-Rothl W, Keul J. Lipid peroxidation and antioxidative vitamins under extreme endurance stress. Acta Physiologica Scandinavica. 1994;150(5):149–158. doi: 10.1111/j.1748-1716.1994.tb09732.x. [DOI] [PubMed] [Google Scholar]
- 64.Powers SK, Hamilton K. Antioxidants and exercise. Clinics in Sports Medicine. 1999;18(3):525–536. doi: 10.1016/s0278-5919(05)70166-6. [DOI] [PubMed] [Google Scholar]
- 65.Engels HJ, Wirth JC. No ergogenic effects of ginseng (Panax ginseng C.A. Meyer) during graded maximal aerobic exercise. Journal of the American Dietetic Association. 1997;97(10):1110–1115. doi: 10.1016/S0002-8223(97)00271-X. [DOI] [PubMed] [Google Scholar]
- 66.Lang JK, Gohil K, Packer L, Burk RF. Selenium deficiency, endurance exercise capacity, and antioxidant status in rats. Journal of Applied Physiology. 1987;63(6):2532–2535. doi: 10.1152/jappl.1987.63.6.2532. [DOI] [PubMed] [Google Scholar]
- 67.Reid MB, Stokic DS, Koch SM, Khawli FA, Leis AA. N-acetylcysteine inhibits muscle fatigue in humans. Journal of Clinical Investigation. 1994;94(6):2468–2474. doi: 10.1172/JCI117615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee SP, Mar GY, Ng LT. Effects of tocotrienol-rich fraction on exercise endurance capacity and oxidative stress in forced swimming rats. European Journal of Applied Physiology. 2009;107(5):587–595. doi: 10.1007/s00421-009-1159-6. [DOI] [PubMed] [Google Scholar]
- 69.Novelli GP, Bracciotti G, Falsini S. Spin-trappers and vitamin E prolong endurance to muscle fatigue in mice. Free Radical Biology and Medicine. 1990;8(1):9–13. doi: 10.1016/0891-5849(90)90138-9. [DOI] [PubMed] [Google Scholar]
- 70.You Y, Kim K, Heo H, et al. Stimulatory effects of Pseudosasa japonica leaves on exercise performance. Bioscience, Biotechnology and Biochemistry. 2006;70(10):2532–2535. doi: 10.1271/bbb.60137. [DOI] [PubMed] [Google Scholar]
- 71.Murase T, Haramizu S, Shimotoyodome A, Nagasawa A, Tokimitsu I. Green tea extract improves endurance capacity and increases muscle lipid oxidation in mice. American Journal of Physiology. 2005;288(3):R708–R715. doi: 10.1152/ajpregu.00693.2004. [DOI] [PubMed] [Google Scholar]
- 72.Marshall RJ, Scott KC, Hill RC, et al. Supplemental vitamin C appears to slow racing greyhounds. Journal of Nutrition. 2002;132(6):1616S–1621S. doi: 10.1093/jn/132.6.1616S. [DOI] [PubMed] [Google Scholar]


