Abstract
Epithelial and endothelial tubes form the basic structure of many organs and tissues in the fruit fly Drosophila melanogaster, the nematode Caenorhabditis elegans, zebrafish and mammals. Comparison of how tubes form during development defines several pathways that generate a single unbranched tube or dichotomously branching tubular networks. The formation of tubes can be induced directly by intrinsic signals within epithelial primordia or by inductive signaling between adjacent epithelia and the mesenchyme. Both processes are hierarchically controlled by master transcriptional regulators, growth factors and their receptors, directed cell migration and cellular reorganization, which is controlled by changes in the cytoskeleton and protein trafficking. This review provides a summary of these pathways based upon articles published in the Tube Morphogenesis Series in Trends in Cell Biology.
During the past year, Trends in Cell Biology has published a series of articles on tube morphogenesis in epithelial and endothelial cells; several broad reviews on this topic have also been published elsewhere during this time [1–3]. This series has been a celebration. A celebration of nature’s extraordinary diversity in the shape and organization of tubes, from an excretory cell of length 1 mm that runs the length of the body of C. elegans to the branching tree of > 1 million nephrons in a mammalian kidney, where each branch comprises a tube formed from hundreds of cells. It has also been a celebration of the breadth of scientific disciplines, including developmental biology, genetics, biochemistry and molecular biology, brought together to examine tube formation, and of different experimental systems, including Drosophila, C. elegans, zebrafish and mammals, used to examine how tubes are formed (Figure 1).
Figure 1.
Distribution of epithelial tubes. (a) Immunostained image of the developing tracheal system in a stage-16 embryo of Drosophila. Micrograph by Stefan Luschnig/Mark Krasnow. (b) Immunostained image of the developing airways of the lung in a day-14 mouse embryo. Micrograph by Ross Metzger/Mark Krasnow.
Tubes form the basic structure of many organs, including lung and trachea, kidney, the mammary gland, the vascular system and the gastrointestinal and urinary–genital tracts (Figure 1). They usually comprise a single layer of cells (or, in the excretory cell of C. elegans, one cell), often surrounded by additional layers of cells that form barriers to separate the ‘outside’ and ‘inside’ compartments of the organism and regulate vectorial transport of ions, gases, liquids and solutes between these compartments. The formation of tubes involves interactions between different cell types and various environmental cues that result in tubular structures containing one or more cell types with potentially different functions. Defining how tubes form is not only important for understanding the fundamental aspects of development, but also for gaining insights into the etiology of diseases and potentially their treatment by organ regeneration.
This article is a reflection on some of the major points made in the Tube Morphogenesis Series, including the different ways that tubes are formed, what we currently know about their regulation and some of the open questions that remain. This is not meant to be an exhaustive comparison, rather one person’s opportunity to exploit the juxtaposition of the reviews collected in this series. In so doing, only the reviews are referenced, and the reader is encouraged to look up each review and the original references therein for complete information and citations.
Formation of a tube – so many possibilities
Single-cell and multi-cell tubes
With one exception, the reviews in this series described how multicellular tubes are formed. The exception is an extraordinary organ in the nematode C. elegans, termed the excretory cell, which regulates osmolarity; it is extraordinary because an entire organ is formed from a single cell. As described by Buechner [4], the excretory cell is shaped like a letter ‘H’ and comprises two hollow canals, one on each side of the animal, which are connected by a bridge to a duct, or pore, and the ‘outside’. The lumens of the canals are surrounded by the continuous apical membrane of the cell, without discontinuities formed by lateral membranes of interconnected cells characteristic of multicellular tubes. Each canal is formed by coordinate dorso-lateral extension of the apical and basal membranes of the cell to a length of 300 μm (~10 times the width of a normal epithelial cell in tissue culture) during the first larval stage and eventually to 1 mm, the length of the adult worm.
Multicellular tubes form by one of two basic processes, both of which include changes in the cell shape, as well as the division of cells, the intercalation and expansion of cells and the directed migration of groups of cells. One process of forming a simple unbranched tube involves localized invagination or internalization of a small group of cells to form a hollow tube. As discussed by Andrew and colleagues [5], one example is the development of the salivary gland of Drosophila. The gland forms from ~100 cells located in epithelial primordia either side of the ventral midline. Cells elongate along the anterior–posterior axis, thicken and then, in an ordered process starting with a ‘tip’ cell followed by surrounding cells, internalize and move inwards to form a hollow tube.
A second process of tube formation involves the migration of individual cells from an epithelial structure and their subsequent rearrangement to form a hollow tube, as exemplified by the formation of vascular systems in vivo [6–8] or tubules derived from renal epithelial cells grown in vitro [9,10]. In this case, migrating cells remain attached to each other to form a chain and eventually a cord of cells, from which a central lumen space contiguous along the length of the cord is generated to form a tube.
Increasing tube complexity by branching
Increased complexity in tube organization is generated by branching to form a tubular network. Branching can occur by the fusion of different tubes into a network. Samakovlis and colleagues [11] used tracheal development in Drosophila as a simple experimental system to analyze this process (Figure 1a) [3,11]. Individual tracheal tubes are initiated from 20 identical tracheal metamers and subsequently become interconnected by branched outgrowth and fusion, all without additional cell division. Primary growth of tubes occurs in a predetermined orientation that requires the extension and growth of lamellipodia towards migratory cues, coordinate movements of the nucleus and the cell body and the enlargement of the apical surface to accommodate the growing lumenal space. There are four types of branches, each with a fixed number of cells and characteristic dimension, requiring substantial changes in cell–cell contacts and cell shape, which result in the repositioning of cells relative to each other and their fusion to form an interconnected branched tracheal network (Figure 1a).
A branched tubular network can also be formed by a stereotypic pattern of dichotomous branching of tubes that is characteristic of the development of lung (Figure 1b) [12], mammary gland [13] and kidney [14,15] in vertebrates. An additional complexity in these systems is the requirement for instructive interactions between different cell types (often epithelia and mesenchyme) to define the fate of cells, and the outgrowth and differentiation of tubes. As reviewed by Chuang and McMahon [12], during lung development two primary buds, which will give rise to the right and left lobes of the lung, are derived from endodermal primordia and form an initial tube lined with a monolayer of epithelial cells and surrounded by mesoderm. Stereotypic branching of these tubes initially gives rise to five secondary buds that branch again, and then those branches continue to bifurcate (Figure 1b) [12]. Bissell and colleagues [13] described how precursors of epithelial tubes in the mammary gland are formed from epithelial placodes that grow out as a double-layered tube comprising a layer of epithelial cells surrounded by a layer of myoepithelium; primary branches are formed from proliferating and advancing end-buds and are the site for the outgrowth of secondary and tertiary buds, which grow by bifurcation. Dressler [15] and Drummond [14] each discussed how, during kidney development, the intermediate mesoderm commits to a nephrogenic fate and forms an organ primordia from which cells outgrow and eventually form the pronephric duct (collecting system). The meta-nephric mesenchyme, adjacent to the mesoderm, undergoes conversion to epithelial cells, which aggregate, form a lumenal space, expand and undergo multiple rounds of dichotomous branching and patterning to give rise to >1 million nephron tubules in the mammalian kidney [14–16].
Regulating tube formation
The descriptions of how single-cell tubes and multicellular branched and unbranched tubes are formed raise many questions about how these complex processes are initiated and subsequently controlled, and whether mechanisms regulating cell rearrangements during tube formation are evolutionarily conserved. Three levels of regulation appear to be involved in the formation of tubes: master regulation of gene transcription, localized expression of growth factors and their receptors involved in the conversion of cells (e.g. from mesenchyme to epithelia) and in directed cell migration/tube outgrowth, and expression of a broad range of downstream effectors that regulate the dynamics of membrane and cytoskeletal leading to cell rearrangements and polarized organization (Figure 2).
Figure 2.
Hierarchical regulation of tube formation. Master transcription-factor regulators specify cell fate during the formation of epithelial tubes [5,14,15]. Localized intercellular signaling by growth factors and their receptors on adjacent tissues (epithelium and mesenchyme) further localize and induce outgrowth of cells to form tubes [6,9–13,15], which are then directed by chemoattractant cues and cell migration [4,5,10,11,13]. Intracellular downstream effectors of master transcriptional regulators and growth factor receptors rearrange cells necessary for the formation and elongation of tubes by altering the cytoskeleton, protein distributions, and cell adhesion to the extracellular matrix and other cells [4,5,7–9,13,14].
Master regulators
Analysis of tube formation in several systems has shown that decisions on the fate of cells and their initial movements are controlled by master regulators and are genetically hard-wired (Figure 2). As discussed in detail by Andrew and colleagues [5], analysis of tube formation during the development of the salivary gland of Drosophila has identified a complex of three transcription factors (Scr, exd, hth) that control tube formation. This complex appears to specify the fate of the salivary gland because a loss of function of any of these transcription factors blocks the formation of the salivary gland. Although the fate of the primordia of the salivary gland, from which the tube will emerge (see above), is determined by this complex of transcription factors, additional transcription factors (tsh and Abd-B) expressed in the ectoderm restrict the number of cells within the ectoderm that will form the primordia of salivary gland. Andrew and colleagues [5] pointed out that further restriction of the fate of primordia to either salivary-duct cells or secretory cells is determined by factors that work as both local activators and boundary repressors (Figure 3). For example, epidermal growth factor (EGF) induces the formation of ducts but represses the formation of secretory cells, and fkh ensures the survival of secretory cells but also represses duct-specific gene expression. Two additional transcription factors, hairy and hkb, regulate proper internalization of cells, and their downstream targets, klar and crb, appear to regulate vesicle trafficking and determine the identity of apical membranes, respectively [5].
Figure 3.
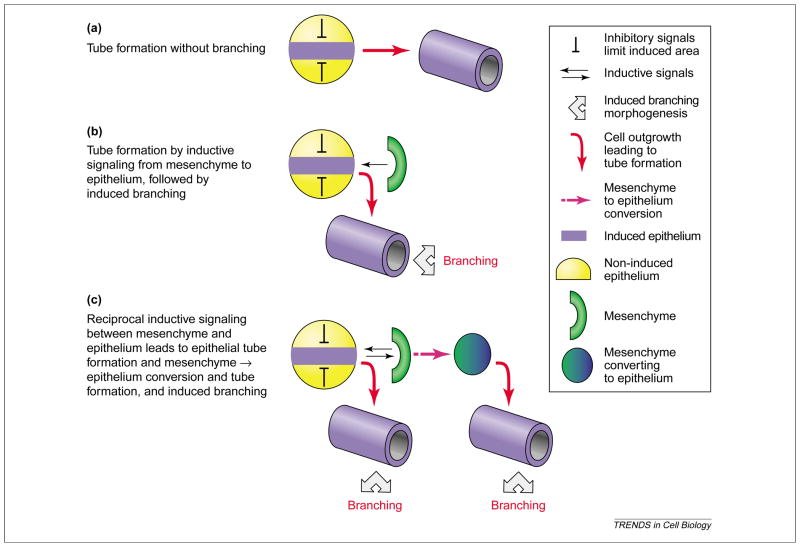
Schematic representation of different pathways leading to the formation of tubes. (a) A small group of cells in an epithelial primordium is induced by intrinsic signals to invaginate and form a tube. The size of the induced group of cells is limited by inhibitory signals from the surrounding epithelium. Tube formation occurs without branching (e.g. in the salivary gland of Drosophila). (b) Local signaling by growth factors from mesenchyme induces invagination of epithelial cells and formation of tubes. The size of the induced group of cells is limited by inhibitory signals from the surrounding epithelium and the mesenchyme (not shown). Stereotypic dichotomous branching of the tube occurs subsequently to form a tubular network (e.g. during the development of mammalian lung). (c) Reciprocal local signaling between adjacent epithelium and mesenchyme induces outgrowths of the epithelium leading to the formation of tubes and subsequent branching. Induction of mesenchyme results in conversion to epithelium from which cell outgrowths from another series of branching tubes (e.g. during kidney development).
Studies of kidney development in vertebrates [14,15] also point to the importance of both master transcription factors in determining cell fate within the intermediate mesoderm and other factors in maintaining the differentiated state. As noted by Dressler [15], expression of master regulatory transcription factors (wt1, pax2, sim1) locally confines pronephric cell fate within the intermediate mesoderm to specific cell lineages. Similar to the secretory gland primordia in Drosophila, these genes activate a small group of cells to a specific fate and concomitantly suppress that fate in the surrounding cells, thereby forming boundaries between different segments of the nephron. Drummond pointed out that additional transcription factors, emx2 and sall1, specify the pattern of tube outgrowth and subsequent rounds of dichotomous branching [14] (see below).
Localized growth factors, directional cues and receptors
Several examples were presented in the series that illustrate how the localized secretion of growth factors from one group of cells in the immediate vicinity of cells expressing the receptors for these growth factors determines the fate of cells and direct outgrowth of tubes (Figure 2). Samakovlis and colleagues [11] discussed that, during the development of trachea in Drosophila placodes, cells destined to form different branches of the tracheal network are restricted to nonoverlapping patterns by localized expression of specific growth/signaling factors, such as DPP/transforming growth factor-β (TGFβ), Wg (Wnt) and EGF/EGF activator rhomboid, which in turn regulate expression of transcription factors specific for different types of secondary/tertiary branches. Subsequently, another growth factor, fibroblast growth factor [FGF (bnl)], produced by cell patches around each tracheal sack, and its receptor [FGF-R (btl)] promote invagination and migration of branches. Further directed migration of branches is specified by migration cues slit and its receptor robo, and loss of function in either results in diminished outgrowth of primary branches (Figure 2) [11].
Signaling between different cell types, usually of epithelial and mesenchymal origin, plays a crucial role in decisions concerning the cell fate, tube formation and differentiation in the development of vertebrate lung, kidney and mammary gland (Figures 2 and 3). During lung development, members of the Hedgehog, FGF, TGFβ and Wnt families and their receptors are key players that signal between mesenchyme and epithelium to induce the outgrowth of epithelial cells, which form the initial lung buds. Chuang and McMahon [12] discussed the regulatory pathways thought to be controlled by Shh expressed in the lung epithelium and its receptor Ptch1 expressed in the mesenchyme, and by Fgf10 expressed in mesenchyme and its receptor Fgfr expressed in the epithelium. Bmp4, a member of the TGFβ family, seems to antagonize Fgf10 signaling to confine the outgrowth of lung buds to localized positions in the primordia [12]. Shh also plays an important role in the development of the vascular system in zebrafish by defining the fate of arterial endothelial cells, but in this case the growth factor downstream of Shh is vascular endothelial growth factor (VEGF) [6].
Hepatocyte growth factor (HGF), EGF and FGF-1 also play roles in branching morphogenesis in the mammary gland [13]. Bissell and colleagues proposed that these growth factors contribute to the branching of tubes by acting as proliferative signals when an appropriate morphogenetic signal is present to induce structural changes in tube cells. Epimorphin appears to play such a morphogenetic role, because tube branching induced by different growth factors can be inhibited with function-blocking antibodies to epimorphin. In the developing mammary gland, epimorphin is expressed in the stroma and mesenchyme surrounding epithelial tube outgrowths [13].
During the development of mammalian kidney, interactions between the epithelium (ureteric bud) and mesenchyme are important for specifying bud outgrowth from the epithelium to form the collecting (duct) system and for the conversion of the mesenchyme to epithelium to form the nephron tubule (Figure 3). Dressler described in detail how the outgrowth of tubes from the epithelium requires the growth factor–receptor complex of glial-cell-line-derived neurotrophic factor (GDNF) and its receptor RET tyrosine kinase, and an additional protein GFPα1 that is required for RET activation; mutations in this signaling complex block the outgrowth of tubes from the epithelium (ureteric bud) [15]. Similar to events in the developing lung [12], Bmp4 expression in the epithelium limits the extent of GDNF–RET signaling so that bud outgrowth is restricted to a small region of the epithelium. The subsequent branching of tubes is regulated by members of the Fgf and TGFβ (Bmp) families [15]. Rosario and Birchmeier [10] discussed the roles of another growth factor–receptor signaling pathway comprising HGF and its receptor Met, which has been implicated in stimulating the formation and outgrowth of epithelial tubes [9,10]; in addition, downstream targets of this signaling pathway have been described in detail (see below). However, the loss of function of HGF or Met does not cause kidney abnormalities showing that the HGF–Met pathway works with, or is redundant to, other growth-factor signaling pathways [10]. Little is understood about mesenchyme to epithelium conversion that gives rise to the nephron tubule. Several signals have been implicated in the induction of epithelial tubes including Wnt4, LIF and FGF2 [15]; in Wnt4−/− mice, the mesenchyme aggregates but does not form polarized epithelial tubes. However, it remains unclear how these signals lead to the formation of epithelial cells and tubes.
Another class of genes termed the polycystins appear to play an important role in kidney development [16]. Boletta and Germino [16] discussed how inactivation mutations or deletions of pkd1 and pkd2 result in the formation of large renal cysts at the developmental stage when tubules undergo elongation and maturation. Mutations in these genes are found in the relatively common (1 in 500–1000 incidence) human disease, polycystic kidney [16]. Although the functions of PKD1 and PKD2 are not understood, Boletta and Germino [16] discussed recent studies indicating that the two proteins interact, that PKD2 has properties of a nonselective cation channel and that PKD1 has an extracellular domain characteristic of some cell-adhesion molecules. PKD proteins have been localized to the primary cilium in renal tubule cells. Although the significance of this localization is not fully understood, Boletta and Germino [16] noted that fluid-flow-induced bending of the cilium induces transient inward calcium currents that might regulate the rate of ion/solute reabsorption or cell differentiation. Interestingly, PKD1 has a regulatory role in several signaling pathways such as the activation of the transcription factor AP-1, modulation of the Wnt pathway and activation of the JAK2–STAT1 pathway, which can lead to the suppression of growth [16].
Forming tubes: downstream effectors of master regulators and growth-factor signaling
Once induced by signaling events, how do epithelial tubes elongate, migrate and become functionally polarized with a hollow, fluid-filled lumen? Tube elongation and migration require changes in the dynamics of membrane and cytoskeleton (Figures 2 and 3). Morphological studies of tube outgrowth and extension reveal that tip cells extend filopodia and lamellipodia that form long narrow protrusions into the surrounding extracellular environment [4,5,11]. Although many pathways involving growth-factor receptors participate in the induction of tube elongation and migration (see above), in most cases little is known about their downstream effector pathways. As noted by Rosario and Birchmeier [10] and by Mostov and colleagues [9], an exception is HGF-Met signaling in which HGF binding to its receptor met leads to the recruitment of a complex of signaling proteins to Met, which in turn regulates the dissociation of adherens junctions and stimulates cell motility, proliferation and morphogenesis.
Changes in the surrounding extracellular matrix (ECM) are important to permit cell migration during branching morphogenesis. Buechner [4] described genetic studies in the simplest system for cell extension, the excretory cell in C. elegans, that revealed that cell outgrowth is reduced by mutations in the receptors of basement-membrane (ECM), such as pat-3/β1-integrin, proteins of the basement membrane, such as laminins and perlecan, migration cues, such as netrin and its receptor, and proteins involved in intracellular integrin signaling and changes to the cytoskeletal organization [4]. Similarly, mutations in integrins inhibit proper extension of lamellipodia and cell migration in developing salivary gland tubes in Drosophila [5]. Alterations in the composition of the ECM have been studied during the development of the mammary gland [13] and the vascular system [7]. As noted above, epimorphin plays an important role in tube branching in the mammary gland. As discussed by Bissell and colleagues [13], downstream effectors induced by epimorphin include members of the matrix metalloproteinase (MMP) family, which remodel ECM by selective proteolysis of ECM proteins. The expression of MMPs is required for side branching and leads to remodeling of the stroma to allow and support tube invasion through the surrounding ECM [13]. In the developing vascular system, endothelial cells form tubes (see above) and then recruit and become surrounded by vascular smooth muscle cells that produce and organize a complex trilayered ECM [7,8]. Li and colleagues [7] described that this ECM shows signaling activity necessary for the migration of endothelial cells, provides mechanical support during development and maintains the competence of the tubular network under hemodynamic pressure, as also discussed by Keshet and colleagues [8]. Abnormalities in vascular ECM components lead to severe defects in function, including increased cell proliferation, expression of MMPs, degradation of vessel wall [loss of fibrillin (Marfan syndrome)], dilation and rupture of arteries (loss of collagens I/III) and obstructive vascular disorders (loss of elastin) [7].
Tube formation requires not only directed outgrowth and migration but also the re-shaping of cells to form a closed monolayer, which surrounds a hollow fluid-filled lumen, and the generation of distinct cell-membrane surfaces for vectorial transport of ions, solutes and liquids between the lumenal and extracellular spaces. The changes in the cell shape must be coordinated as cells form tubular outgrowths, migrate as multicell units and then, in some systems, bifurcate to form branches (Figure 3). In the developing salivary gland of Drosophila, the initial internalization of cells from the placodes involves the constriction of the apical membrane, resulting in transition from a columnar to pyramidal cell shape. During this time, the apical membrane increases 4–6 times in diameter and long microvilli are formed; during subsequent maturation of the lumenal space, the diameter of apical membranes and the length of microvilli decrease [5]. Mechanisms involved in these changes to the cell shape are poorly understood, although Andrew and colleagues [5] noted that unknown downstream targets of transcription factor Fkh are required for the changes in the cell shape and apical constriction, as downstream targets (klar, crb) of Hairy/Hkb are involved in determining the identity of apical membranes (see above). In the excretory cell of C. elegans, several groups of genes are involved in the formation of lumen [4]. Buechner described how loss-of-function mutations in one group, Exc, results in the formation of a series of fluid-filled cysts of varying diameter along the length of the cell, rather than one continuous narrow lumen. Little is known about the nature of these abnormalities [4]; however, it appears that the regulation of the actin cytoskeleton is important for the formation of lumen. One gene that affects lumen diameter is sma-1. Sma-1 encodes a cytoskeletal protein βH-spectrin that might regulate lumenal dimension by anchoring the apical-membrane-associated actin cytoskeleton; another gene, exc-5, encodes a guanine exchange factor that might activate Cdc42, a small GTPase that regulates actin assembly and distribution [4].
Cell–cell adhesion also regulates the organization of cells, especially in the context of the formation of apical and basolateral membrane domains [9]. In the kidney of developing zebrafish, Drummond [14] noted that the conversion of mesenchyme to epithelia requires expression of cadherin-17, and that the loss of function of this cadherin results in the loss of cell–cell adhesion and mislocalization of the basolateral membrane protein Na+/K+–ATPase to the apical membrane. During the development of tubules in mammalian kidney, there are sequential changes in the type of cadherins expressed, beginning with cadherin-11, then cadherin-6 and lastly E-cadherin; the functional significance of these transitions is not understood [15]. As discussed by Mostov and colleagues [9], studies of renal cyst formation in vitro have shown that many cell types have the ability to form three-dimensional cysts within ECM gels and, in doing so, they establish three distinct cell surfaces: apical, lateral and basal. Cyst formation requires cadherin-mediated cell–cell adhesion, and orientation of the apical and basolateral surfaces depends on proper cell–ECM interactions [9]. Mechanisms involved in the sorting and delivery of proteins to apical and basolateral membranes have been studied extensively in open monolayers of polarized epithelial cells in culture; however, little is understood about mechanisms for inducing and controlling protein distribution in complex three-dimensional tubes [9].
Concluding remarks – closure, but many openings remain
Tube formation involves several levels of regulation: master regulation of gene transcription, localized expression of growth factors and their receptors and downstream regulation of membrane and cytoskeleton dynamics. The review articles in Tube Morphogenesis Series in Trends in Cell Biology [4–16] reveal many potential parallels in the molecular controls that define decisions on cell fate and cue morphogenetic movements during tube formation, cellular rearrangements and instructional cell–cell/cell–ECM interactions involved in the formation of tubes. Many gaps remain, however, and it is not yet possible to construct a complete picture of tube formation in one system, from master transcriptional regulators and induction of cell differentiation to mechanisms of cell outgrowth and dynamics of migration.
The experimental systems reviewed in this series provide different insights into the process and regulation of tube formation. Each experimental system has its advantages, such as genetic epistasis to identify master regulators and downstream signaling pathways, live cell microscopy to examine cell movements and organization and purification of protein complexes to construct signaling pathways and readouts of downstream effectors. Further crossover between experimental approaches in these systems should advance our understanding of tube formation. Drosophila, long renowned as a suitable organism for genetic analysis, is increasingly being used to examine cell dynamics during tube formation [5,11]. Zebrafish are not only suitable for genetic analysis [14], but also for live imaging of complex tube formation, for example, in the vascular system, because of the transparency of their embryos [6]. Analysis of complex tube organization during the development of mammalian lung, kidney and mammary gland has been amenable to genetic analysis by gene knockout [12,15,16], and cocultures of epithelium and mesenchyme derived from organ primordia provide an approach to examine inductive cues and cellular rearrangements in vitro [13,15]. Recent advances in the use of siRNA to knockdown protein levels in cells should increase the pace of studying gene/protein functions in these isolated in vitro systems. Remarkably, cyst and tube formation can be mimicked in vitro by established cell lines [9,10]. Although analysis of inductive cues might not be particularly informative, these structures provide a very useful and easily manipulated system to investigate mechanisms underlying cell outgrowth, migration and interactions, in addition to the regulation of protein distributions to functionally distinct plasma membrane domains.
As shown in the review articles of this series, these are very exciting times in biology for a broad understanding of how complex structures are constructed, organized and function, especially when different developmental organisms and experimental approaches are used to address common problems. Epithelial and endothelial tubes are two of the most basic three-dimensional structures formed during development. They are also, relative to many other organ structures, simple in design and evolutionarily conserved. Future studies built on the work described in this series will probably lead to a comprehensive understanding of tube morphogenesis.
Acknowledgments
I am very grateful to Stefan Luschnig, Ross Metzger and Mark Krasnow from Stanford University for the images in Figure 1.
References
- 1.Hogan BLM, Kolodziej PA. Organogensis: molecular mechanisms of tubulogenesis. Nat Rev Genet. 2002;3:513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- 2.Affolter M, et al. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev Cell. 2003;4:11–18. doi: 10.1016/s1534-5807(02)00410-0. [DOI] [PubMed] [Google Scholar]
- 3.Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- 4.Buechner M. Tubes and the single C. elegans excretory cell. Trends Cell Biol. 2002;12:479–484. doi: 10.1016/s0962-8924(02)02364-4. [DOI] [PubMed] [Google Scholar]
- 5.Abrams EW, et al. Constructing an organ: the Drosophila salivary gland as a model of tube formation. Trends Cell Biol. 2003;13:247–254. doi: 10.1016/s0962-8924(03)00055-2. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein BM. Vascular cell biology in vivo: a new piscine paradigm. Trends Cell Biol. 2002;12:439–445. doi: 10.1016/s0962-8924(02)02358-9. [DOI] [PubMed] [Google Scholar]
- 7.Brooke BS, et al. Extracellular matrix in vascular morphogenesis and disease: structure versus signal. Trends Cell Biol. 2003;13:51–56. doi: 10.1016/s0962-8924(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 8.Dor Y, et al. Making vascular networks in the adult: branching morphogenesis without a roadmap. Trends Cell Biol. 2003;13:131–136. doi: 10.1016/s0962-8924(03)00022-9. [DOI] [PubMed] [Google Scholar]
- 9.Zegers MMP, et al. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 2003;13:169–176. doi: 10.1016/s0962-8924(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 10.Rosario M, Birchmeier W. How to make tubes: signaling by the Met receptor tyrosine kinase. Trends Cell Biol. 2003;13:328–335. doi: 10.1016/s0962-8924(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 11.Uv A, et al. Drosophila tracheal morphogenesis: intricate cellular solutions to basic plumbing problems. Trends Cell Biol. 2003;13:301–309. doi: 10.1016/s0962-8924(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 12.Chuang PT, McMahon AP. Branching morphogenesis of the lung: new molecular insights into an old problem. Trends Cell Biol. 2003;13:86–91. doi: 10.1016/s0962-8924(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 13.Radisky DC, et al. Delivering the message: epimorphin and mammary epithelial morphogenesis. Trends Cell Biol. 2003;13:426–434. doi: 10.1016/s0962-8924(03)00146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond I. Making a zebrafish kidney: a tail of two tubes. Trends Cell Biol. 2003;13:357–365. doi: 10.1016/s0962-8924(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 15.Dressler GR. Tubulogenesis in the developing mammalian kidney. Trends Cell Biol. 2002;12:390–395. doi: 10.1016/s0962-8924(02)02334-6. [DOI] [PubMed] [Google Scholar]
- 16.Bolleta A, Germino GG. Role of polycystins in renal tubulogenesis. Trends Cell Biol. 2003;13:484–492. doi: 10.1016/s0962-8924(03)00169-7. [DOI] [PubMed] [Google Scholar]