Abstract
Specialized cells that form barriers such as those of the intestine adopt a distinct asymmetry. One particular protein may be a prime mover in bringing about such cellular organization.
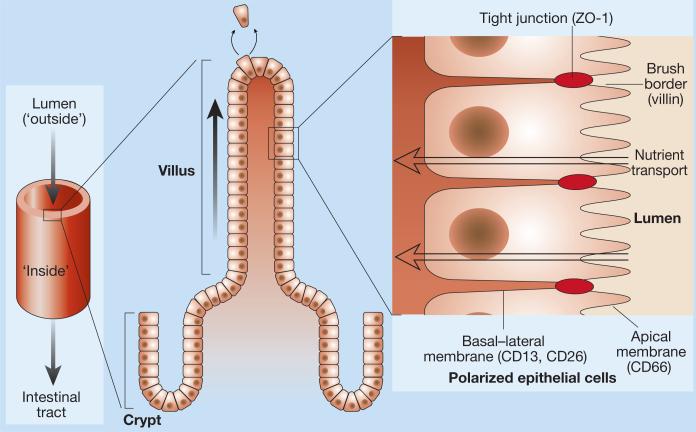
The intestine is a hollow tube lined by finger-like projections (villi) which, in turn, are covered by a sheet of highly organized cells. As in other exposed surfaces such as the skin, these specialized epithelial cells are born at the bottom of the layer and move up to replace cells that are constantly being sloughed off the top. During this time, the epithelial cells adhere tightly to each other within the sheet to provide a protective barrier between the ‘outside’ and ‘inside’ environments.
But the upper and lower surfaces of the sheet have different surroundings and carry out different functions. For example, the upper surface is involved in taking up nutrients from the food that passes through the intestine,whereas the lower surface transports these nutrients into the blood supply (Fig. 1). The corresponding cellular regions, the apical and basal–lateral domains, also contain different proteins. Knowledge of how this asymmetry, or polarization, is established is only just emerging. As they report in Cell, however, Baas et al.1 have taken investigation further into what may be a major player in promoting cell organization in this context,a protein called LKB1.
Figure 1.
Structure of the intestine and epithelial polarity. The tube-like intestine is lined by thousands of villi. Each villus contains a crypt region in which new epithelial cells are born. These epithelial cells move upwards to replace cells that have been sloughed off. The epithelial cells in the villi are arranged in a highly ordered manner. On their apical side, which faces the lumen, is a brush border of microvilli, which contains villin, and apical proteins such as CD66 are present. Neighbouring cells adhere tightly to each other to form a barrier, through proteins such as ZO-1. Proteins such as CD13 and CD26 are present on the basal–lateral domain, underneath the lateral junctions between the cells.
LKB1 has already attracted our attention (reviewed in ref. 2). A tumour suppressor, its absence is responsible for Peutz–Jeghers syndrome, a rare human disease characterized by benign overgrowths of cells in the gastrointestinal tract3. Mice that express only one copy of LKB1 also develop growths similar to those in human Peutz–Jeghers syndrome4. But why might loss of LKB1 function cause tumour growth? Normal cells have a limited lifespan, but cells lacking LKB1 grow continuously in culture until LKB1 is re-introduced4. LKB1 also regulates cell death (apoptosis), and growths from patients with Peutz–Jeghers syndrome have a low number of apoptotic cells5. LKB1 may be part of the well-characterized pathway of another tumour-suppressor gene, p53, which controls cell growth and apoptosis5.
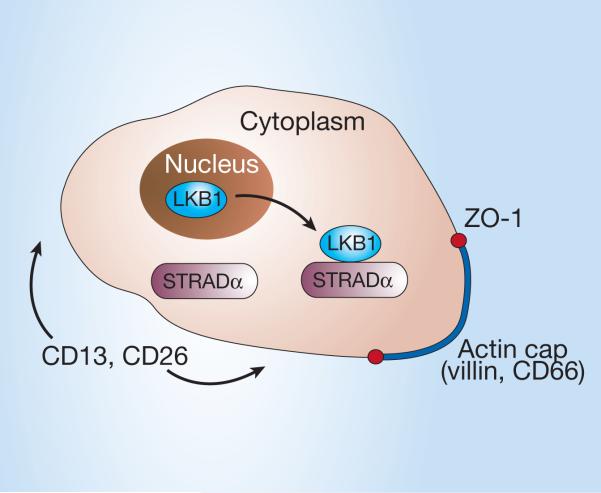
Proteins that are similar to LKB1 are involved in tissue organization and polarization in a variety of lower animals6,7, but Baas and colleagues1 are the first to study what happens within the cells. This group previously identified STRADα (STE20-related adaptor protein) as a protein that binds to LKB1 in the cytoplasm8. In their present study,using cells in culture that do not usually polarize, Baas et al. increased the production of STRADα so that LKB1 accumulated in the cytoplasm (Fig. 2; LKB can be found in the nucleus and the cytoplasm2). Before LKB1 accumulated, actin filaments of the cellular skeleton criss-crossed the cell and several other proteins had random distributions. Afterwards, actin and another protein, villin, re-localized into a ‘cap’ on the apical cell surface that contained long projections of membrane (Fig. 2). These actin-rich structures looked superficially like the intestinal brush border, so-called because of the abundance of highly structured microvilli (Fig. 1).
Figure 2.
LKB1 and cell polarization. Some aspects of cell-surface organization that are evident in polarized intestinal epithelial cells can be induced by expressing LKB1 in non-polarized cells. LKB1 binds to STRADα in the cytoplasm, and this is followed by re-localization of villin and the apical marker CD66 to an actin ‘cap’ with membrane projections. Around these projections is a ring of the protein ZO-1. CD13 and CD26 take up residence on the cell surface outside the actin cap.
The presence of a brush border is also a hallmark of epithelial polarity. Another protein, ZO-1, redistributed to a diffuse circle around the brush border when LKB1 was overexpressed. The distributions of several other proteins also changed when LKB1 accumulated in the previously unpolarized cells and resembled topologically those of polarized intestinal epithelial cells (Fig. 2). So several key characteristics of polarized cells were observed when LKB1 was introduced into cells that aren't normally polarized.
Unexpectedly, this apparent ability of LKB1 to induce polarity occurred in single cells and in the absence of a solid support1. Previous findings suggested that cells could not polarize until they had formed cell–cell junctions9 and that the surface10 to which they attached was also somehow involved.
Baas et al.1 also examined the effects of depleting LKB1 in cells that form a fully polarized epithelium in culture. Given the dramatic effects of overexpressing LKB1 on protein distributions in the unpolarized cells, it might have been expected that LKB1 depletion would have a negative effect on cell polarity. But although the locations of some apical markers, such as actin and villin, were altered, they were not completely disrupted — perhaps these cells have additional, LKB1-independent mechanisms to maintain cell polarity.
Formation of the villin-containing actin cap is striking and may be central to LKB1-induced cell polarization.Perhaps activation of LKB1 by STRADα (ref.8) or PAR1 (ref.7), another protein involved in cell polarity6, affects the behaviour of other proteins that induce the assembly of actin filaments and formation of membrane projections; villin would be in this pathway as its overexpression induces actin-containing membrane projections11. These membrane projections may act as a platform for the assembly of other components necessary to generate a functional apical surface.
How are protein distributions altered when LKB1 accumulates? We don't know, but such redistribution might result from the directed delivery of proteins to different membrane areas; their retention in specific regions — for example,due to their ability to bind actin; or their exclusion from specific areas, such as the actin cap, and localization by default to the rest of the membrane.This is particularly pertinent in light of the fact that Baas et al.1 never found LKB1 to have a polarized distribution.
It remains unclear how LKB1-induced changes in cell organization relate to its roles in apoptosis and inhibiting cell growth. Baas et al. point out that changes in cell organization occurred before the effects on cell growth. So does LKB1 have several functions depending on whether it is localized in the nucleus or cytoplasm, or does binding to different proteins (for example, STRADα or p53) or activation by proteins (such as PAR1 or protein kinase A12) direct LKB1 into different pathways?
References
- 1.Baas AF, et al. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 2.Boudeau J, Sapkota G, Alessi DR. FEBS Lett. 2003;546:159–165. doi: 10.1016/s0014-5793(03)00642-2. [DOI] [PubMed] [Google Scholar]
- 3.Jenne DE, et al. Nature Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 4.Bardeesy N, et al. Nature. 2002;419:162–167. doi: 10.1038/nature01045. [DOI] [PubMed] [Google Scholar]
- 5.Karuman P, et al. Mol. Cell. 2001;7:1307–1319. doi: 10.1016/s1097-2765(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 6.Watts JL, Morton DG, Bestman J, Kemphues KJ. Development. 2000;127:1467–1475. doi: 10.1242/dev.127.7.1467. [DOI] [PubMed] [Google Scholar]
- 7.Martin SG, St Johnston D. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- 8.Baas AF, et al. EMBO J. 2003;22:3062–3072. doi: 10.1093/emboj/cdg292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vega-Salas DE, Salas PJ, Rodriguez-Boulan EJ. Cell Biol. 1988;107:1717–1728. doi: 10.1083/jcb.107.5.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Streuli CH, Bailey N, Bissell MJ. J. Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friederich E, Huet C, Arpin M, Louvard D. Cell. 1989;59:461–475. doi: 10.1016/0092-8674(89)90030-5. [DOI] [PubMed] [Google Scholar]
- 12.Sapkota GP, et al. J. Biol. Chem. 2001;276:19469–19482. doi: 10.1074/jbc.M009953200. [DOI] [PubMed] [Google Scholar]