SUMMARY
An essential part of sexual reproduction typically involves the identification of an appropriate mating partner. Males of many moth species utilize the scent of sex pheromones to track and locate conspecific females. However, before males engage in flight, warm-up by shivering of the major flight muscles is necessary to reach a thoracic temperature suitable to sustain flight. Here we show that Helicoverpa zea males exposed to an attractive pheromone blend (and in some instances to the primary pheromone component alone) started shivering earlier and took off at a lower thoracic temperature than moths subjected to other incomplete or unattractive blends. This resulted in less time spent shivering and faster heating rates. Two interesting results emerge from these experiments. First, the rate of heat generation can be modulated by different olfactory cues. Second, males detecting the pheromone blend take off at lower thoracic temperatures than males exposed to other stimuli. The take-off temperature of these males was below that for optimal power production in the flight muscles, thus generating a trade-off between rapid departure and suboptimal flight performance. Our results shed light on thermoregulatory behaviour of unrestrained moths associated with the scramble competition for access to females and suggest ecological trade-offs between rapid flight initiation and sub-optimal flight performance.
KEY WORDS: thermoregulation, shivering, flight, sex pheromone, Noctuidae, insect
INTRODUCTION
Successful reproduction involves the identification and location of a mating partner. In many species, olfactory stimuli comprise the crucial sensory input shaping this behavior. A well-established model for the role of chemicals in long-distance sexual communication is the release of pheromones by females and the pheromone-mediated upwind flight of many male moth species (Mafra-Neto and Cardé, 1995; Vetter and Baker, 1983; Vetter and Baker, 1984; Vickers, 2002; Vickers et al., 1991; Willis and Arbas, 1991; Willis et al., 1991). Male moths have enhanced sensitivity to sex pheromone [e.g. seen in the great feathery antennae of males (Kaissling, 2009)] and are adapted to track and fly within complex odor plumes (Vickers et al., 2001; Wyatt, 2003). Males bear the major costs associated with mate finding, such as energy expenditure and risk (Greenfield, 1981; Thornhill and Alcock, 1983), and are therefore considered to be in a scramble competition in which the strongest flyers as well as the most effective searchers and trackers arrive at a calling female first and presumably mate with her (Lloyd, 1979; Wyatt, 2003).
In noctuid moths, as in several other insects, female receptivity and sex pheromone production decrease subsequent to mating (e.g. Barth, 1968; Gillot and Friedel, 1977; Raina et al., 1986; Webster and Cardé, 1984). After mating, female sexual receptiveness and calling behaviors are suppressed for at least 24 h (Kingan et al., 1993; Mbata and Ramaswamy, 1990; Raina, 1989; Raina, 1993; Raina et al., 1986; Raina and Stadelbacher, 1990) such that they do not mate twice or even call again on the same night (Raina, 1989; Raina et al., 1986; Raina and Stadelbacher, 1990). However, males have been reported to copulate up to three times on the same night (Callahan, 1958). Additionally, sperm precedence in twice-mated females is biased towards the second mate (e.g. LaMunyon, 2001) and females usually begin oviposition immediately after mating (Raina and Stadelbacher, 1990). Thus, if a male copulates with a female on a given night, there is a significant chance that the female's eggs are fertilized with his sperm. This means that strong selective pressure must exist for male moths to arrive as fast as possible at the location of the female. In short, males that have a low pheromone detection threshold, warm-up in a timely manner and effectively track the pheromone plume will outcompete other males.
Flight performance is therefore a major component of successfully locating a calling female. However, because muscle performance is strongly temperature dependent, flight muscles of most moths need to be heated endothermically before a moth can engage in upwind locomotion (Dorsett, 1962; Krogh and Zeuthen, 1941; Heinrich and Mommsen, 1985). Muscle temperature at the end of pre-flight warm-up dictates power production during flight (Heinrich, 2007), with concomitant effects on flight speed and maneuverability. The relationship between vertical force production during tethered flight and thoracic temperature has been shown to have an inverted U shape, demonstrating that there is a species-specific optimal thoracic temperature range within which insects produce maximal vertical force (e.g. Coelho, 1991). In addition, other variables such as the ratio of flight muscle mass to body mass (Buchwald and Dudley, 2010; Dillon and Dudley, 2004), ambient temperature and thoracic insulation (Heinrich, 1971; Heinrich, 1977) affect flight performance.
Little is known about how pre-flight warm-up behavior is modulated by internal physiological or external variables (Kammer, 1981). Upwind flight will be affected by the ambient temperature and wind conditions, which will influence heat loss during flight. Additionally, presence of sensory stimuli could affect the motivation for rapid pre-flight warm-up. Calling in female moths and the ensuing scramble competition between males to gain access to mates should constitute a strong stimulus for modulation of pre-flight warm-up. Two main questions are of interest in regard to the effects of the presence of female pheromone: (1) does it alter the onset and rate of thoracic heating in males and (2) does it change the thoracic temperature at which male moths take off?
In an effort to understand the pre-flight behavior of these insects in the context of sexual selection, we measured the thermoregulatory shivering activities of male Helicoverpa zea (Lepidoptera: Noctuidae) exposed to different sex-related olfactory cues. Interestingly, males heat up more quickly in the presence of female pheromone and take-off at lower thoracic temperature than males exposed to other scents. They therefore engage in a trade-off between sub-optimal flight performance and rapid onset of directed flight.
MATERIALS AND METHODS
Insects
Helicoverpa zea (Boddie 1850) were maintained at the University of Utah and reared on a modified pinto-bean diet (Shorey and Hale, 1965). Male pupae were separated and placed in environmental chambers (Percival Scientific, Boone, IA, USA) at 25°C and 60% relative humidity on a reversed light cycle (14 h:10 h light:dark photoperiod) until adult emergence. Moths were aged daily and separated in plastic containers with access to a 9% sucrose solution ad libitum. Virgin males between the ages of 2 and 6 days were utilized for both wind-tunnel experiments and force measurements, and each experiment was conducted between the fourth and eighth hour of scotophase (Vetter and Baker, 1983; Vetter and Baker, 1984). Because experiments were performed during a 4 h interval and we tested six treatments in the wind tunnel, only three randomly selected treatments per day were tested. At least 24 h before the beginning of the experiment, males were randomly segregated into three plastic containers. On the day of experimentation, males were cooled down to 8–10°C to avoid disturbing them while they were handled and then they were carefully introduced into a cylindrical 3×3 cm diameter wire-screen cage in the wind tunnel. After males acclimatized to the experimental room conditions, experiments were started. For the force measurement test, males were not segregated into different containers 24 h before the experiments, and they were cooled or heated according to the experiment protocol (see Force production assays).
Wind tunnel
A scaled-down version of the larger wind tunnel at the University of Utah (e.g. Vickers, 2002) was constructed with a working section area of 28×14×14 cm (length×height×width). The conditions in the wind tunnel were as follows (means ± s.d.): 21.9±0.8°C, 29.7±9.5% relative humidity and a wind speed of 44.6±8.2 cm s–1. Illumination was provided by a single red incandescent light bulb above the wind tunnel and the odor plume was vented out of the building at the downwind end of the wind tunnel via an exhaust duct. An infrared (IR) video camera (thermaCAM® S65HS, FLIR Systems, Wilsonville, OR, USA) above the wind tunnel was used to record temperature changes in freely behaving animals.
Pheromone test blends
Concentrated solutions of cis-11-hexadecenal (Z11-16:Ald), cis-9-hexadecenal (Z9-16:Ald) and cis-11-hexadecenyl acetate (Z11-16:OAc; Sigma-Aldrich, St Louis, MO, USA; maintained at –20°C) were used to make dilutions in hexane of 100 ng μl–1 for Z11-16:Ald and 10 ng μl–1 for both Z9-16:Ald and Z11-16:OAc (Vickers et al., 1991). All dilutions were checked with capillary gas chromatography (Shimadzu GC 17A, Shimadzu Scientific Instruments, Columbia, MD, USA). Odors were prepared with respect to the main pheromone component of H. zea (Z11-16:Ald), which was always 1000 ng (denoted 1), and sequentially loaded onto a circular filter paper disk (Whatman No. 4, 1 cm diameter). Ratios of compounds were as follows: 1:0.05:0.1 Z11-16:Ald to Z9-16:Ald to Z11-16:OAc. The six treatments utilized were: (1) attractive, Z11-16:Ald + Z9-16:Ald; (2) primary component, Z11-16:Ald; (3) non-attractive, Z11-16:Ald + Z9-16:Ald + Z11-16:OAc; (4) secondary component, Z9-16:Ald; (5) primary component + acetate, Z11-16:Ald + Z11-16:OAc; and (6) blank, hexane. Once the compounds were admixed on the disk, hexane was allowed to evaporate in the fume hood. The odor source was kept in place by a small alligator clip mounted on a plastic post at the upwind end of the wind tunnel (6 cm above the wind tunnel floor), and the distance between the take-off cage and the odor source was 12 cm.
Behavioral assays
The cooled-down moths were positioned singly in a wire-screen cage downwind of the position where the pheromone source was later placed. Once it was established that the moth had not been disturbed (defined as no apparent wing movement for at least 1 min), the IR video camera began recording and the odor source was introduced into the wind tunnel (replaced every 60 min to ensure that the odor stimulus was not depleted; N.J.V., personal observation). This was considered to be the beginning of the experiment. The duration of each trial was from this point until either the moth took off or 20 min elapsed. Those males that did not respond were checked for their ability to fly, and moths that appeared incapable of flight were discarded in these experiments. Moths that did not warm up as seen with the IR camera but were capable of flight were scored as ‘non-responders’.
Force production assays
Moths were restrained inside a disposable pipette tip with the end tip cut so that the male's head could pass through (the head was immobilized by dental wax). Additionally, a small window was cut on the pipette tip to allow exposure of the dorsal part of the moth's thorax. A minimum number of the dorsal thoracic scales were removed in order to wax (Ted Pella, Redding, CA, USA) an entomological pin (size 2) and a 0.003 inch copper-constantan thermocouple (Omega Engineering, Stamford, CT, USA) to the dorsal surface of the thorax. The thermocouple was connected to a thermocouple thermometer (Bat-12; Physitemp Instruments, Inc., Clifton, NJ, USA) to monitor the thoracic temperature (Tth) and the temperature recorded with an MP100 system and AcqKnowledge 9.2 software (Biopac Systems, Goleta, CA, USA). Moths were fixed to a force transducer (force displacement transducer FT03, Grass Technologies, West Warwick, RI, USA) and Tth was manipulated by exposure to cold air or with a 250 W IR bulb connected to a rheostat. Force measurements were recorded at a Tth equivalent to 20°C and then at increments of 2°C until the moth could no longer beat its wings (note: at extreme temperatures in some cases the moth became detached from the force transducer). To induce moths to fly, every force measurement (i.e. for every 2°C) was performed under both a 15 W black light tube (BioQuip Products, Inc., Compton, CA, USA) and a 40 W white light bulb positioned above the insect. At the end of each experiment, the moth was immediately frozen and flight muscle mass was measured (modified from Marden, 1987) to calculate mass-specific force production.
Temperature calibration
Because of different methodological approaches, we measured the temperature at the surface of the thoracic scales in the wind tunnel assays and at the cuticular surface in the force production assays. This necessitated a transformation of both surface temperature measurements to a core Tth (see below). For the wind tunnel experiments, a thermocouple was inserted into the thorax (along the midline of the dorsal surface of the thorax, 1.5 mm behind the anterior edge of the thorax and 1.5 mm below its surface) to measure the core Tth of live males while simultaneously recording the temperature at the surface of the thoracic scales with the IR camera. All other conditions were held constant. For the force production experiments, a second thermocouple was implanted as described above while all other conditions were held constant. A calibration curve was constructed for the temperature range 5–50°C for 2°C increments.
Data analyses
Recorded behavioral responses were analyzed using ThermaCAM Researcher Professional 2.8 SR-1 (software accompanying the IR video camera, FLIR Systems). Individual take-off angles were measured in 5 deg intervals starting from the position of the source (0 deg). The IR images generated a thermal imprint of the moving moth, which was used to visualize and calculate the take-off angles. Mean take-off direction was calculated for each treatment and analyzed by the Rayleigh test. Differences in warm-up behavior (i.e. time to start shivering, thoracic temperature at take-off, duration of shivering and thoracic heating rate during shivering) were subjected to a Kruskal–Wallis test and then to a multiple comparison test (see Siegel and Castellan, 1988) to identify significant differences between treatments. For the force measurements, at least four full wing-beat cycles (measured from peak to peak) were selected and the area under the curve was integrated to obtain the mean vertical force production at a particular Tth (Marden, 1995). Means of the maximal force production at the different Tth were then used to trace the upper limit of vertical force production (Marden, 1995). All statistical analyses were performed using the R statistical package (R Development Core Team, 2008).
RESULTS
Sex pheromone odor affects take-off orientation
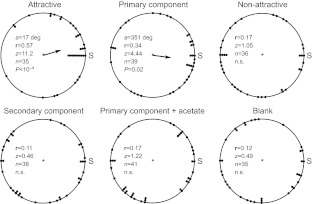
Because male moths fly upwind to a pheromone source, it was expected that they would also orient upwind at take-off. Fig. 1 shows that the take-off direction of males exposed to the attractive blend (Rayleigh test, P<10–4), as well as to the primary component alone (Rayleigh test, P=0.02), was significantly oriented toward the source (i.e. upwind). During exposure to the other treatments (in which the pheromone blend was altered or completely absent), males took off in random directions (Rayleigh test, P>0.1). These behavioral data not only show that males are attracted to the pheromone blend but also that the experimental approach utilized in this study (see Materials and methods) is appropriate for evaluating the pre-flight behavior of male moths. Furthermore, at least 80% of males tested in all treatments took off (data not shown), and no differences were found between treatments (chi-square test, P>0.08).
Fig. 1.
Helicoverpa zea male orientation at take-off when exposed to different female pheromone compounds. Each point represents a single individual and arrows indicate the preferred direction (Rayleigh test). S indicates the position of the odor source. Treatments are as follows: attractive, Z11-16:Ald + Z9-16:Ald; primary component, Z11-16:Ald; non-attractive, Z11-16:Ald + Z9-16:Ald + Z11-16:OAc; secondary component, Z9-16:Ald; primary component + acetate, Z11-16:Ald + Z11-16:OAc; and blank, hexane. The averaged preferred take-off angles (a), Rayleigh vector lengths (r), z-statistics (z), sample sizes (n) and P-values are shown for each treatment.
Sex pheromone odor affects warming-up behavior
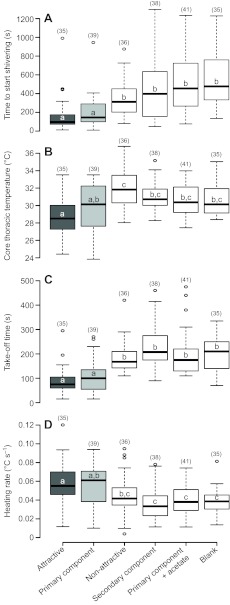
To characterize warm-up behavior in response to different sexual pheromone odors, the following variables were measured: (1) time to start shivering (measured from the start of the experiment; Fig. 2A), (2) core Tth with which moths took off (see Materials and methods for definition; Fig. 2B), (3) time spent shivering (Fig. 2C) and (4) the rate of heating calculated during shivering (Fig. 2D). For all measured variables, males exposed to the attractive blend showed a significant difference when compared with those of the last four treatments (i.e. non-attractive, secondary component, primary component + acetate, and blank; Kruskal–Wallis multiple comparison test, P<0.05). In other words, males that detected the attractive blend started shivering earlier, took off at a lower thoracic temperature, spent less time shivering and heated up at a faster rate than those exposed to other olfactory stimuli (excluding the primary component treatment; Fig. 3). Moreover, treatments other than the attractive blend and major component alone were not significantly different from each other (Kruskal–Wallis multiple comparison test, P>0.05).
Fig. 2.
Helicoverpa zea male warming-up differences when exposed to different female pheromone compounds. (A) Time elapsed since the start of the experiment until the start of shivering. (B) Core thoracic temperature at take-off. (C) Time elapsed during shivering (take-off time). (D) Thoracic heating rate during shivering. Treatments are as follows: attractive, Z11-16:Ald + Z9-16:Ald; primary component, Z11-16:Ald; non-attractive, Z11-16:Ald + Z9-16:Ald + Z11-16:OAc; secondary component, Z9-16:Ald; primary component + acetate, Z11-16:Ald + Z11-16:OAc; and blank, hexane. Sample sizes are indicated in brackets. Different letters indicate statistical differences (Kruskal–Wallis multiple comparison test, P<0.05). Boxplot shows the first quartile (lower limit of box), median and third quartile (upper limit of the box). Outliers are data points exceeding 1.5 times the interquartile range.
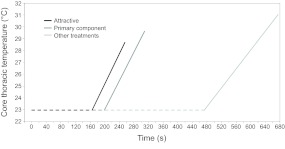
Fig. 3.
Differences in thoracic temperature over time between Helicoverpa zea males exposed to different chemical stimuli from first exposure to the stimulus until take-off. Faster heating rate and lower thoracic temperature at take-off result in more rapid take-off in males stimulated with sex pheromone. Treatments are as follows: attractive, Z11-16:Ald + Z9-16:Ald; primary component, Z11-16:Ald; and other treatments, grand mean of the last four treatments (i.e. non-attractive, Z11-16:Ald + Z9-16:Ald + Z11-16:OAc; secondary component, Z9-16:Ald; primary component + acetate, Z11-16:Ald + Z11-16:OAc; and blank, hexane). Dashed line represents time elapsed since the start of the experiment until the start of shivering.
Interestingly, when only the primary sex pheromone component was present, some males appeared to behave like those exposed to the attractive blend (Fig. 3). However, when both core Tth at take-off and the heating rate were analyzed, a mixed response owing to large inter-individual variability was observed (Fig. 2B,D). Previously, between 88 and 100% of H. zea males exposed to the primary pheromone component alone were found to take flight in a wind tunnel (Vickers et al., 1991).
Maximal vertical force is produced at a certain Tth
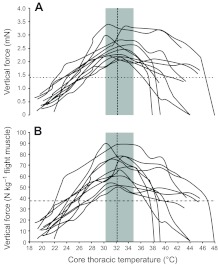
To understand whether the lower Tth found in males that were exposed to the attractive blend (Fig. 2B) manifested itself as a change in flight performance; we measured how maximal vertical force varied with Tth. Distributions of maximal vertical force production as a function of core Tth showed that the mean (±s.d.) optimal Tth (i.e. the temperature at which the peak force occurred) was 32.1±1.4°C (N=11; Fig. 4). Using the mass of tested individuals (145.0±26.3 mg), the projected Tth for sufficient force production to stay airborne was approximately 26°C. On average, males produced a maximal vertical force of 1.42±0.26 mN, but muscle response to temperature varied substantially (Fig. 4A). A similar distribution was observed when maximal vertical force was calculated based on thoracic muscle mass (Fig. 4B). Finally, the mean maximal vertical force found in this study was 67.0±16.2 N kg–1 flight muscle, which lies within the values found by Marden (Marden, 1987) for a wide variety of insects and other animals.
Fig. 4.
Upper limit traces of maximal vertical force production as a function of core thoracic temperature for each of the 11 Helicoverpa zea males tested. (A) Vertical force versus core thoracic temperature. (B) Flight muscle mass-specific vertical force versus core thoracic temperature. The horizontal dashed line depicts the mean vertical force needed to counteract the mean body mass of males tested. The vertical dashed line shows the mean core thoracic temperature at which mean maximal vertical force is exerted. The gray area indicates the range of temperatures at which peak maximal force production was observed for different males. Incomplete traces show missing data due to detachment from the force transducer during the experiment.
DISCUSSION
Our results demonstrate that sex pheromone cues modulate shivering behavior and influence the decision at which muscle temperature take-off is initiated in H. zea males. Moths stimulated with an attractive scent adjusted their warm-up behavior and took flight sooner in the direction of the odor source. These results also indicate that males initiated flight with thoracic temperatures that generated vertical force below the maximum. However, when a non-attractive scent was presented, take-off temperature overlapped with the range of temperature where maximal force was measured. Thus, the safety factor for flight was considerably reduced in moths exposed to the attractive blend, generating a trade-off between rapid take-off at a suboptimal temperature and delayed departure with greater flight performance.
Even though much research has been aimed at understanding the pheromone-mediated upwind flight of moths, quantitative measures of their take-off orientation have not been reported [however, see reports on other insects (e.g. Minoli and Lazzari, 2006)]. Our data show that male moths exposed to either the attractive pheromone blend or the major component alone take off in the direction of the source whereas those exposed to other odors exhibit a non-oriented response. The fact that the principal sex pheromone component is enough to elicit take-off in almost all tested insects (Vickers et al., 1991) (present study) and that they orient towards the source (present study) suggests that this chemical component has an arousal effect. Furthermore, Vickers et al. (Vickers et al., 1991) reported that 14–22% of these males flew upwind in the plume and 0–7% eventually located the source and landed on it. These results indicate that the primary pheromone component is enough, at least in some males, to elicit a similar thermoregulatory and even tracking behavior as the attractive blend. In fact, some aspects of the warming-up behavior of males exposed to the primary component alone do not significantly differ from those of males exposed to the attractive blend (Fig. 2), further supporting this statement.
Shivering before take-off allows moths to increase the temperature of their flight muscles until the power output of these muscles is sufficient to lift the insect's body mass (Heinrich and Bartholomew, 1971). Depending on external (e.g. ambient temperature) and internal (e.g. nutritional state) conditions, one would expect that shivering insects would spend a variable period of time warming up. For example, low ambient temperatures force a moth to shiver longer because it takes longer for the flight muscles to attain the minimal temperature required to produce enough power to fly (Heinrich, 1970). The increase in thoracic temperature during warm-up is usually linear (Heinrich and Kammer, 1973; Casey and Hegel-Little, 1987) (present study), suggesting that the rate of heat production must increase rapidly to both account for the heat storage that produces the rise in thoracic temperature and counteract the increasing heat loss as the temperature differential with the surroundings increases (Kammer, 1981). Once the core thoracic temperature starts rising, it is expected that muscle metabolism as well as central nervous system activity increase, generating a positive feedback on the thoracic heat production (Heinrich and Kammer, 1973; McCrea and Heath, 1971). In other words, because these insects possess neurogenic flight muscles, at higher core temperatures the frequency of action potentials should increase and so should flight muscle contractions, causing the thoracic temperature to rise (Mafra-Neto and Cardé, 1994).
Interestingly, Kammer (Kammer, 1981), despite citing data supporting variable warm-up rates, presented an energetic argument that pre-flight warm-up should not be modulated. The observed variation was attributed to individual unknown factors resulting from laboratory (e.g. restraining due to thermocouple leads) or physiological (e.g. age or ‘central excitatory state’) conditions. In this study, our methodology allowed us to test unrestrained moths in a similar physiological state (age, treatment before the experiment) and show that the rate of heat production during warm-up can vary when different olfactory stimuli are present. The observed differences in heating rates during warm-up must be reflected in different metabolic rates (Casey and Hegel-Little, 1987; Goller and Esch, 1991). Thus, under particular circumstances, the rate of heat production during warm-up can be increased, as in the case of male moths that sense the sex pheromone and are activated by it.
Potential mechanisms for increasing the heating rate include additional recruitment of motor units, increased activation of individual motor units, changing phase relationships of the antagonistic flight muscles and possible modulation of heat generation within a muscle. This latter possibility addresses more generally the potential for modulating power production. George et al. (George et al., 2012) showed that a temperature gradient exists in the different subunits of the dorsolongitudinal flight muscles of Manduca sexta, and this gradient influences force and work production. Furthermore, the nervous system might control the phase of activation of different muscular subunits so that locomotion typically occurs at sub-maximal levels of power production, leaving a safety margin for extreme behaviors (e.g. escape maneuvers). Such a neural control mechanism for modulating power production during flight may also be used during shivering. During pre-flight warm-up, a female pheromone, signaling the opportunity to mate, might present such a sensory cue that engages males in extreme activation for heat generation and also subsequent tracking behaviors.
In addition to heating faster, males exposed to the attractive blend take off at a lower thoracic temperature than those exposed to other scents. This difference in core thoracic temperature at take-off must have an effect on flight performance. Maximum lift production during take-off has been previously measured in a wide range of flying animals and revealed to be very similar between taxonomic groups (Marden, 1987). In the present study, we found not only that the mean of the maximal vertical force produced during tethered flight agrees with that of previous studies, but also that males stimulated with the attractive blend take off at a core thoracic temperature that may compromise their flight endeavors. Such a trade-off between becoming airborne as soon as possible to arrive first at the female and producing enough force to efficiently navigate through the environment has important evolutionary and ecological implications. Given that males are adapted to efficiently search through the pheromone plume (e.g. Mafra-Neto and Cardé, 1994), one might expect the moth's maneuverability (e.g. airspeed, course angles, turning frequency, etc.) during tracking to be affected.
It is unclear to what extent the lower take-off temperature may affect flight performance. Upon take-off, thoracic temperature typically decreases as a result of reduced heat production (more energy converted into mechanical energy and movement) and increased heat loss (forced convection from wing movement and air speed) (Heath and Heath, 1982; Kammer, 1981). The persistence of differences in thoracic temperature during flight will depend on environmental conditions such as ambient temperature and wind as well as flight speed and heating rate. However, the data from the present study show that moths stimulated with conspecific sex pheromone start flight with less safety margin in power production than those exposed to other olfactory cues.
Although our experimental design does not capture the full range and complexity of natural environmental conditions that male moths experience, it is likely to simulate one natural situation. Fitt (Fitt, 1989) reported that adult activity begins at dusk and lasts for a period of 1–2 h, during which time heliothine moths were observed to feed. This activity is followed by a period of inactivity before behaviors related to mating are initiated. If ambient temperature is sufficiently low, it is likely that inactive males allow their thoracic temperature to drop because sustained shivering would be energetically too costly. These males therefore will encounter pheromone with low thoracic temperatures, as simulated in our experiment, and upwind flight will require pre-flight warm-up. The present study provides evidence that sedentary males exposed to the sex pheromone modulate the onset and rate of thoracic heating and also the thoracic temperature at which they take off. These behavioral changes indicate a trade-off between sub-optimal flight performance and rapid onset of directed flight. This trade-off may be even more complex in the natural environment, where additional variables such as wind speed affect the thermal physiology of flight.
ACKNOWLEDGEMENTS
We thank K. F. Schramm for help with the insect rearing.
FOOTNOTES
FUNDING
This project was partially supported by the National Science Foundation [grants IOS-0416861 to N.J.V. and IOS-1110836 to J.G.C.] and the National Institutes of Health [grant DC 06876 to F.G.]. Deposited in PMC for immediate release.
REFERENCES
- Barth R. H. J. (1968). The comparative physiology of reproductive processes in cockroaches. Part I. Mating behavior and its endocrine control. Adv. Reprod. Physiol. 3, 167-207 [Google Scholar]
- Buchwald R., Dudley R. (2010). Limits to vertical force and power production in bumblebees (Hymenoptera: Bombus impatiens). J. Exp. Biol. 213, 426-432 [DOI] [PubMed] [Google Scholar]
- Callahan P. S. (1958). Behavior of the imago of the corn earworm, Heliothis zea (Boddie), with special reference to emergence and reproduction. Ann. Entomol. Soc. Am. 51, 271-283 [Google Scholar]
- Casey T. M., Hegel-Little J. R. (1987). Instantaneous oxygen consumption and muscle stroke work in Malacosoma americanum during pre-flight warm-up. J. Exp. Biol. 127, 389-400 [Google Scholar]
- Coelho J. R. (1991). The effect of thorax temperature on force production during tethered flight in honeybee (Apis mellifera) drones, workers, and queens. Physiol. Zool. 64, 823-835 [Google Scholar]
- Dillon M. E., Dudley R. (2004). Allometry of maximum vertical force production during hovering flight of neotropical orchid bees (Apidae: Euglossini). J. Exp. Biol. 207, 417-425 [DOI] [PubMed] [Google Scholar]
- Dorsett D. A. (1962). Preparation for flight by hawk-moths. J. Exp. Biol. 39, 579-588 [Google Scholar]
- Fitt G. P. (1989). The ecology of Heliothis species in relation to agroecosystems. Ann. Rev. Entomol. 34, 17-52 [Google Scholar]
- George N. T., Sponberg S., Daniel T. L. (2012). Temperature gradients drive mechanical energy gradients in the flight muscle of Manduca sexta. J. Exp. Biol. 215, 471-479 [DOI] [PubMed] [Google Scholar]
- Gillot C., Friedel T. (1977). Fecundity-enhancing and receptivity-inhibiting substances produced by male insects: a review. In Advances in Invertebrate Reproduction No. 1 (ed. Adiyodi K. G., Adiyodi R. G.), pp. 199-218 Karivellur: Peralam-Kenoth; [Google Scholar]
- Goller F., Esch H. E. (1991). Oxygen consumption and flight muscle activity during heating in workers and drones of Apis mellifera. J. Comp. Physiol. B 161, 61-67 [Google Scholar]
- Greenfield M. D. (1981). Moth sex pheromone: an evolutionary perspective. Fla. Entomol. 64, 4-17 [Google Scholar]
- Heath J. E., Heath M. S. (1982). Energetics of locomotion in endothermic insects. Ann. Rev. Physiol. 44, 133-143 [DOI] [PubMed] [Google Scholar]
- Heinrich B. (1970). Thoracic temperature stabilization in a free-flying moth. Science 168, 580-582 [DOI] [PubMed] [Google Scholar]
- Heinrich B. (1971). Temperature regulation of the sphinx moth Manduca sexta: I. Flight energetic and body temperature during free and tethered flight. J. Exp. Biol. 54, 141-152 [DOI] [PubMed] [Google Scholar]
- Heinrich B. (1977). Why have some animals evolved to regulate a high body temperature? Am. Nat. 111, 623-640 [Google Scholar]
- Heinrich B. (2007). The origin of insect thermoregulatory studies. J. Exp. Biol. 210, 177-179 [DOI] [PubMed] [Google Scholar]
- Heinrich B., Bartholomew G. A. (1971). An analysis of pre-flight warm-up in the sphinx moth, Manduca sexta. J. Exp. Biol. 55, 223-239 [Google Scholar]
- Heinrich B., Kammer A. E. (1973). Activation of the fibrillar muscles in the bumblebee during warm-up, stabilization of thoracic temperature and flight. J. Exp. Biol. 58, 677-688 [Google Scholar]
- Heinrich B., Mommsen T. P. (1985). Flight of winter moths near 0°C. Science 228, 177-179 [DOI] [PubMed] [Google Scholar]
- Kaissling K.-E. (2009). The sensitivity of the insect nose: the example of Bombyx mori. In Biologically Inspired Signal Processing for Chemical Sensing, Studies in Computational Intelligence No. 188 (ed. Gutiérrez A., Marco S.), pp. 45-52 Berlin: Springer-Verlag; [Google Scholar]
- Kammer A. E. (1981). Physiological mechanisms of thermoregulation. In Insect Thermoregulation (ed. Heinrich B.), pp. 115-158 New York: Wiley-Interscience; [Google Scholar]
- Kingan T. G., Thomas-Laemont P. A., Raina A. K. (1993). Male accessory gland factors elicit change from “Virgin to mated” Behavior in the female corn earworm moth Helicoverpa zea. J. Exp. Biol. 183, 61-76 [Google Scholar]
- Krogh A., Zeuthen E. (1941). The mechanism of flight preparation in some insects. J. Exp. Biol. 18, 1-10 [Google Scholar]
- LaMunyon C. W. (2001). Determinants of sperm precedence in a noctuid moth Heliothis virescens: a role for male age. Ecol. Entomol. 26, 388-394 [Google Scholar]
- Lloyd J. E. (1979). Sexual selection in luminescent beetles. In Sexual Selection and Reproductive Competition in Insects (ed. Blum M. S., Blum N. A.), pp. 293-342 New York: Academic Press; [Google Scholar]
- Mafra-Neto A., Cardé R. T. (1994). Fine-scale structure of pheromone plumes modulates upwind orientation of flying moths. Nature 369, 142-144 [Google Scholar]
- Mafra-Neto A., Cardé R. T. (1995). Effect of the fine-scale structure of pheromone plumes: pulse frequency modulates activation and upwind flight of almond moth males. Physiol. Entomol. 20, 229-242 [Google Scholar]
- Marden J. H. (1987). Maximum lift production during takeoff in flying animals. J. Exp. Biol. 130, 235-258 [Google Scholar]
- Marden J. H. (1995). Large-scale changes in thermal sensitivity of flight performance during adult maturation in a dragonfly. J. Exp. Biol. 198, 2095-2102 [DOI] [PubMed] [Google Scholar]
- Mbata G. N., Ramaswamy S. B. (1990). Rhythmicity of sex pheromone content in female Heliothis virescens: impact of mating. Physiol. Entomol. 15, 423-432 [Google Scholar]
- McCrea M. J., Heath J. E. (1971). Dependence of flight on temperature regulation in the moth, Manduca sexta. J. Exp. Biol. 54, 415-435 [DOI] [PubMed] [Google Scholar]
- Minoli S. A., Lazzari C. R. (2006). Take-off activity and orientation of triatomines (Heteroptera: Reduviidae) in relation to the presence of artificial lights. Acta Trop. 97, 324-330 [DOI] [PubMed] [Google Scholar]
- Raina A. K. (1989). Male-induced termination of sex pheromone production and receptivity in mated females of Heliothis zea. J. Insect. Physiol. 35, 821-826 [Google Scholar]
- Raina A. K. (1993). Neuroendocrine control of sex pheromone biosynthesis in Lepidoptera. Annu. Rev. Entomol. 38, 329-349 [DOI] [PubMed] [Google Scholar]
- Raina A. K., Klun J. A., Stadelbacher E. A. (1986). Diel periodicity and effect of age and mating on female sex pheromone titer in Heliothis zea (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 79, 128-131 [Google Scholar]
- Raina A. K., Stadelbacher E. A. (1990). Pheromone titer and calling in Heliothis virescens (Lepidoptera: Noctuidae): effect of mating with normal and sterile backcross males. Ann. Entomol. Soc. Am. 83, 987-990 [Google Scholar]
- R Development Core Team (2008). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; [Google Scholar]
- Shorey H. H., Hale R. L. (1965). Mass-rearing of the larvae of nine noctuid species on a simple artificial medium. J. Econ. Entomol. 58, 522-524 [Google Scholar]
- Siegel S., Castellan N. J., Jr (1988). The case of k independent samples. In Nonparametric Statistics for the Behavioral Sciences, pp. 213-214 New York: McGraw Hill; [Google Scholar]
- Thornhill R., Alcock J. (1983). The Evolution of Insect Mating Systems. Cambridge: Harvard University Press; [Google Scholar]
- Vetter R. S., Baker T. C. (1983). Behavioral responses of male Heliothis virescens in a sustained-flight tunnel to combinations of seven compounds identified from female sex pheromone glands. J. Chem. Ecol. 9, 747-759 [DOI] [PubMed] [Google Scholar]
- Vetter R. S., Baker T. C. (1984). Behavioral responses of male Heliothis zea moths in sustained-flight tunnel to combinations of 4 compounds identified from female sex pheromone gland. J. Chem. Ecol. 10, 193-202 [DOI] [PubMed] [Google Scholar]
- Vickers N. J. (2002). Defining a synthetic pheromone blend attractive to male Heliothis subflexa under wind tunnel conditions. J. Chem. Ecol. 28, 1255-1267 [DOI] [PubMed] [Google Scholar]
- Vickers N. J., Christensen T. A., Mustaparta H., Baker T. C. (1991). Chemical communication in heliothine moths III: Flight behavior of male Helicoverpa zea and Heliothis virescens in response to varying ratios of intra- and interspecific sex pheromone components. J. Comp. Physiol. A 169, 275-280 [Google Scholar]
- Vickers N. J., Christensen T. A., Baker T. C., Hildebrand J. G. (2001). Odour-plume dynamics influence the brain’s olfactory code. Nature 410, 466-470 [DOI] [PubMed] [Google Scholar]
- Webster R. P., Cardé R. T. (1984). The effects of mating, exogenous juvenile hormone and a juvenile hormone analogue of pheromone titre, calling and oviposition in the omnivorous leafroller moth (Platynota stultana). J. Insect Physiol. 30, 113-118 [Google Scholar]
- Willis M. A., Arbas E. A. (1991). Odour-modulated upwind flight of the sphinx moth, Manduca sexta L. J. Comp. Physiol. A 169, 427-440 [DOI] [PubMed] [Google Scholar]
- Willis M. A., Murlis J., Cradé R. T. (1991). Pheromone-mediated upwind flight of male gypsy moths, Lymantria dispar, in a forest. Physiol. Entomol. 16, 507-521 [Google Scholar]
- Wyatt T. D. (2003). Pheromones and Animal Behavior: Communication by Smell and Taste. New York: Cambridge University Press; [Google Scholar]