Abstract
The synthesis, structure−activity relationship (SAR), and pharmacological evaluation of analogs of the acid-sensing ion channel (ASIC) inhibitor A-317567 are reported. It was found that the compound with an acetylenic linkage was the most potent ASIC-3 channel blocker. This compound reversed mechanical hypersensitivity in the rat iodoacetate model of osteoarthritis pain, although sedation was noted. Sedation was also observed in ASIC-3 knockout mice, questioning whether sedation and antinociception are mediated via a non-ASIC-3 specific mechanism.
Keywords: Pain, acid-sensing, ion channel, ASIC-3, degenerin
For millions of people, disease or injury results in chronic pain due to damage or persistent inflammation of neuronal or somatic tissue. Consequently, a significant medical need remains for new analgesics (1) to treat those who suffer from chronic pain and the comorbidities associated with it.
Under conditions of tissue damage, acidosis often occurs and contributes to pain (2). Highly sensitive proton-activated ion channels are present in sensory neurons and are activated by tissue acidification (3). The TRPV1 receptor (vanilloid receptor subtype-1) represents such a proton-activated ion channel and is widely viewed as a major detector of multiple pain-causing stimuli (4). A number of highly potent TRPV1 antagonists are undergoing clinical evaluation (5).
Acid-sensing ion channels (ASICs) represent a proton-activated subgroup of the degenerin/epithelial Na+ channel family (6). Evidence indicates that ASICs serve as proton sensors and play an important role in conveying the pain from tissue acidosis (7,8). While TRPV1 requires extreme acidification to a pH less than 6.0 for activation (9), the ASICs are more sensitive and, depending on the exact composition of the subunits, can detect an extracellular pH decrease from 7.4 to 7.0 (10). A number of inflammatory mediators enhance ASIC activity and expression and further implicate neuronal ASICs as key players in pain from tissue acidosis (11).
Several ASIC subunits (ASIC-1a/b, ASIC-1b2, ASIC-2a/b, ASIC-3, and ASIC-4) have been identified that are encoded by four genes (12). Channels containing the ASIC-3 subunit have received particular interest as a target for blocking chronic inflammatory pain because it is highly abundant in nociceptors where it may act as a sensor for pain from tissue acidosis (13,14). Mice lacking the ASIC-3 channel do not develop chronic muscle pain from repeated administration of acid, while ASIC-1 knockout mice were similar to wild-type (15).
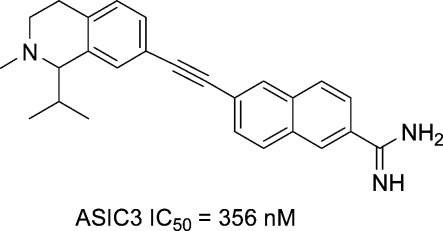
The dearth of potent and selective small-molecule inhibitors of ASIC channels has hampered elucidation of the physiological role of ASIC-3. Amiloride 1 is a potassium-sparing diuretic agent that is also a weak, nonselective inhibitor of ASIC channels (16). Amiloride exhibits modest efficacy in rat pain models at high concentrations (17). Abbott has reported that the amidine A-317567 (2) was a more potent blocker of ASIC-3 than amiloride (18). In addition, A-317567 was effective in the rat complete Freund’s adjuvant (CFA) model of inflammatory pain and in the skin incision model of postoperative pain (Figure 1).
Figure 1.
Known ASIC channel blockers.
While ASIC-3 has generated significant interest in the pain therapeutic field, ASIC-1a activity is associated with anxiety, a symptom that can often accompany depression-like behaviors (19,20). Recently, Wemmie and co-workers disclosed that A-317567 (2) also blocks ASIC-1a and produced antidepressant effects in mice when dosed intracerebroventricularly (21). In light of this work, we now report our results on the pharmacological characterization of an analog of A-317567 and question whether the observed antinociceptive effects attributed to ASIC-3 may be due to off-target effects, potentially including, but not limited to, other ASIC channel subtypes.
Results and Discussion
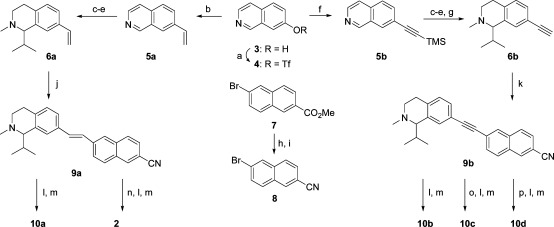
The synthesis of 2 and related analogs 10a−d is shown in Scheme 1(22). Commercially available 7-hydroxy-isoquinoline 3 was converted to the triflate 4, which was then transformed via a Suzuki cross-coupling with vinyl potassium fluoroborate to olefin 5a. Conversely, Sonogashira cross-coupling of 4 with trimethylsilylacetylene produced alkyne 5b. Compounds 5a and 5b may be converted through a three-step sequence (methylation, isopropyl Grignard addition, NaBH4 reduction) to tetrahydroisoquinolines 6a and 6b, the latter requiring an additional tetra-n-butylammonium fluoride (TBAF)-mediated deprotection of the trimethylsilyl group.
Scheme 1.
(a) Tf2O, CH2Cl2, pyridine, 0 °C; (b) vinyl potassium fluoroborate, Pd(dppf)Cl2, Et3N, DMF, 80 °C; (c) MeI, CH3CN, 60 °C; (d) iPrMgBr, THF, 0 °C; (e) NaBH4, MeOH, rt; (f) trimethylsilylacetylene, Pd(Ph3P)4, CuI, Et3N, DMF; (g) TBAF, THF, rt; (h) NH3−MeOH, 80 °C; (i) TFAA, TEA, CH2Cl2, 0 °C−rt; (j) 8, Pd(OAc)2, P(o-tolyl)3, Et3N, DMF, 80 °C; (k) 8, Pd(Ph3P)4, CuI, Et3N, DMF; (l) HCl(g), EtOH, 80 °C; (m) NH3−EtOH, rt−45 °C; (n) ZnEt2, CH2CI2, toluene, 80 °C; (o) Pd/CaCO3, H2, quinoline, MeOH; (p) Pd/C, H2, MeOH.
Naphthalene 8 is produced in two steps (ammonolysis, dehydration) from commercially available ester 7. Heck olefination of 8 with 6a affords E-olefin 9a. The nitrile in 9a was directly converted to the corresponding amidine 10a via generation of the O-ethyl imidate under acidic conditions followed by reaction with ammonia. A-317567, 2, was prepared via cyclopropanation of 9a and subsequent amidine formation. The alkyne nitrile 9b was prepared via Sonogashira coupling of 8 and 6b followed by amidine construction to afford 10b. Nitrile 9b was also fully (Pd/C, H2) or partially (Pd/CaCO3, quinoline, H2) hydrogenated to the corresponding alkane and Z-alkene variants 10c and 10d after the aforementioned amidine generation.
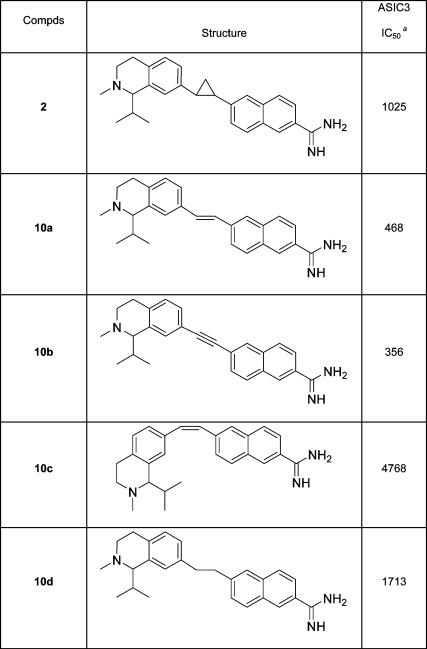
The initial structure−activity relationship (SAR) exploration for compounds 2 and 10a−d is shown in Table 1. These compounds were evaluated on HEK293 cells expressing the human ASIC-3 channel using an automated patch clamp assay. Compounds lacking the amidine functionality, such as nitriles 9a,b and their corresponding amides, all exhibited <50% channel inhibition at 20 μM (data not shown). Cyclopropane A-317567, 2, gave an ASIC-3 IC50 = 1025 nM, while the related E-olefin variant 10a provided ∼2-fold greater inhibition. The isomeric Z-olefin 10c was ∼10-fold less potent than the E-olefin 10a, and reduction of the double bond to alkane 10d also led to a marked decrease in ASIC-3 inhibition. The alkyne analog 10b proved to be the most potent among the series with an ASIC-3 IC50 = 356 nM and was selected for additional in vivo characterization.
Table 1. ASIC-3 Titration of Select Amiloride Derivatives.
 |
Electrophysiology recording; inhibition (nM) of current was expressed as percent inhibition of peak current vs baseline peak current. N = 3.
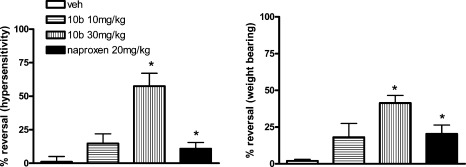
Compound 10b was initially evaluated in the rat iodoacetate model of osteoarthritis pain, which involves intraarticular injection of iodoacetate into the knee joint. In this study (Figure 2), 10b showed a robust reversal of both mechanical hypersensitivity in the hind paw and decreased weight bearing 30 min postdosing in male Sprague−Dawley rats. The magnitude of reversal at the 10 mg/kg dose of 10b was similar to that from the NSAID naproxen (dosed at 20 mg/kg p.o.) and that at the 30 mg/kg dose of 10b was markedly superior. The average plasma concentration (23) at 30 min was 0.75 μM at 10 mg/kg and 3.1 μM at 30 mg/kg with very low levels in brain (∼0.072 μM), implying that the primary site of action of this compound was likely peripheral and not within the central nervous system (CNS) (24).
Figure 2.
Evaluation of 10b and naproxen on mechanical hypersensitivity (left panel) and weight bearing (right panel) in the rat iodoaceate model of osteoarthritis 30 min postdosing: ∗ denotes p < 0.05 (ANOVA).
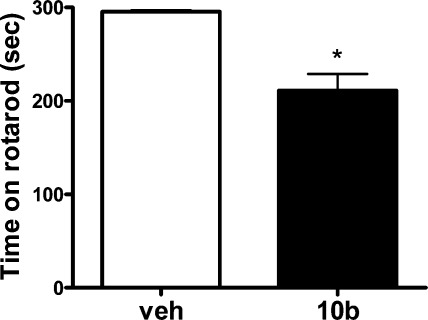
The rats treated with 10b appeared sedated following compound administration but were easily aroused and normal when handled. As a result, there was some concern that the mechanism mediating sedation could contribute to the antinociceptive behavior. Accordingly, a rotarod study in rat was conducted to assess motor coordination and balance, and as can be seen in Figure 3, performance on the rotarod was significantly impaired by treatment with 30 mg/kg 10b.
Figure 3.
Evaluation of 10b (30 mg/kg) on rat rotarod performance 60 min postdosing.
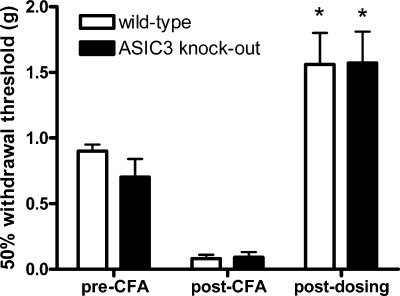
In order to evaluate whether the antinociceptive and sedative effects of 10b were ASIC-3-mediated, wild-type and ASIC-3 knockout mice were evaluated in the mouse complete Freund’s adjuvant (CFA) model of inflammatory pain (Figure 4) (25). Compound 10b (30 mg/kg) reversed CFA-induced mechanical hypersensitivity to a similar extent in both wild type and ASIC-3 knockout mice suggesting that the observed antinociceptive effects of 10b are not solely mediated by ASIC-3. Additionally, 10b administration reduced sensitivity to mechanical stimulation of the hind paw in both groups of mice compared with pre-CFA injection thresholds (i.e., >100% reversal of hypersensitivity), indicative of sedation in both groups and possible impaired motor coordination.
Figure 4.
Evaluation of 10b (30 mg/kg) on mechanical hypersensitivity in wild-type and ASIC-3 knockout mice in the complete Freund’s adjuvant (CFA) model of inflammatory pain 60 min postdosing.
Sensory neurons express both ASIC-1 and ASIC-3 subtypes and both subtypes have been implicated in nociception (26). Relative to their wild-type littermates, mice lacking ASIC-3 do not develop mechanical hyperalgesia to intramuscular acid injection (15). In the same model, ASIC-1 knockout mice develop hyperalgesia similar to wild-type. These results suggest a role for ASIC-3 and not ASIC-1 in acid-evoked muscle pain. Wemmie and colleagues have shown that ASIC-1a knockout mice exhibit deficits in fear responses suggesting an anxiety-related mechanism for ASIC-1 action via the CNS (27). A-317567, 2, blocks currents mediated by ASIC-1a-containing channels and produces antidepressant-like effects in a forced swim test (21). These findings support a role for ASIC-1 in CNS-related behaviors. As a close structural relative to A-317567, 2, 10b was evaluated in ASIC-1a expressing cells and found to be nonselective for ASIC-3 with an ASIC-1a IC50 = 450 nM. This raises the possibility that the antinociceptive effects seen with 10b in our rodent models of pain and inflammation may be due to anxiolytic-related effects of ASIC-1a inhibition and not blockade of ASIC-3.
We know that ASIC-3 inhibition has an analgesic effect locally (via treatment with peptide antagonist APETx2) (28) and ASIC-3 inhibitor 2 has an anxiolytic (nonsedating) effect centrally that has been attributed to ASIC-1a inhibition. However, a couple of points should be noted. First, compound 10b was evaluated against a number of receptors and enzymes (MDS Pharma Service, Taipei, Taiwan) and found to be highly promiscuous. This could indicate that ASIC-1a is not responsible for the perceived effects. For example, 10b had binding affinities with IC50’s < 10 μM against 39 targets that could contribute to the behavioral effects. These include muscarinic, adrenergic, dopamine, norepinephrine, and serotonin receptors. Second, 10b is poorly brain-penetrant, raising a question as to whether its effects are mediated peripherally or centrally, if indeed being driven by ASIC. Wemmie et al. concludes that ASIC-1a mediates anxiety behaviors because compound 2 was administered intracerebroventricularly to bypass the blood−brain barrier (20). Recently, an ASIC-1a selective antagonist was reported to reduce thermal and mechanical hyperalgesia in a human inflammatory UVB pain model (26,29). Nonselective inhibition of both ASIC-1 and ASIC-3 by compound 10b diminishes its usefulness as a tool to probe ASIC-3 as a pain target. The analgesic efficacy of nonselective ASIC-3 antagonists as represented by these structurally related compounds is tainted by their CNS-related effects through ASIC-1 or by another mechanism altogether. Previous structurally distinct nonselective ASIC-3 series that we have investigated showed similar sedative behaviors (30,31). What needs to be understood is whether the apparent peripheral sedating effect is mediated by another ASIC subtype or by some other off-target effect common to the range of inhibitors. Accordingly, an ASIC-3 subtype-selective inhibitor lacking other off-target effects would be an ideal research tool to investigate the contribution of ASIC-3 in CNS related behaviors.
In summary, we report that compound 10b, a close analog of A-317567, 2, is a more potent ASIC-3 channel blocker that is highly efficacious in the rat iodoacetate model of OA. However, 10b also caused sedative or lethargic effects in wild-type and ASIC-3 knockout mice. This calls into question whether the analgesic effects of 10b are indeed ASIC-3 mediated or are a result of off-target inhibition. Compound 10b was an equipotent ASIC-1a inhibitor, which could account for the observed sedation and may have influenced pain-related behaviors in our behavioral models. Efforts to identify structurally distinct non-amidine ASIC-3 inhibitors are underway.
Methods
Electrophysiology Studies
Acid-evoked (pH 5.5 for 3 s) current was recorded at −60 mV using an automated patch clamp instrument (PatchXpress, MDS, Inc.). The intracellular solution contained (mM): 119 K-gluconate, 15 KCl, 3.2 MgCl2, 5 ethylene glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 5 N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 5 K2ATP, pH 7.3. The extracellular solution contained (mM): 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 12 dextrose, and 10 HEPES (pH 7.4) or 10 2-(N-morpholino)ethanesulfonic acid (MES) (pH5.5). Compounds were applied 120 s prior to acid application. Peak current following compound incubation was expressed as a fraction of the control (vehicle) peak current. IC50 values were determined by fitting data to the Hill equation.
Iodoacetate Model of Osteoarthtritis in Rats
Baseline hind paw withdrawal thresholds to punctate pressure is determined in rats using the von Frey filament test. Additionally, hind paw weight bearing is measured using an incapacitance instrument. Following determination of baseline pain-related behaviors, rats are briefly anesthetized using isoflurane (1−5% to effect, inhalation) and receive an intraarticular injection of monosodium iodoacetate (2 mg/25 μL, vehicle is pH 7.4 saline) into the left hind limb knee joint. Rats are continuously monitored until full recovery from the anesthetic (<5 min) and are subsequently returned to their cages where they are maintained on soft bedding. Rats are tested for decreased mechanical withdrawal thresholds and weight bearing at 6 weeks post-iodoacetate injection to determine the baseline.
von Frey Filament Test
The von Frey filament test is used to measure sensitivity to a non-noxious punctate pressure stimulus. Rats are placed on an elevated mesh galvanized steel platform in individual chambers with a hinged lid and allowed to acclimate for 60 min. Mechanical sensitivity is determined by applying a series of calibrated von Frey filaments (0.25−15 g) to the plantar aspect of the ipsilateral hind paw using the “up−down” method to determine median withdrawal threshold (32). The calibrated filament is applied perpendicular to the hind paw for 6 s. A positive response is indicated by a brisk withdrawal of the hind paw, and in this case, the next lower force filament is applied. If no positive response is observed, the next higher force filament is applied. Once the crossover threshold is determined the responses to the next four filaments are recorded to determine the median withdrawal threshold.
Weight Bearing Test
Rats are tested for hind paw weight bearing by placing the animal in a Plexiglas box such that the posterior half of the animal is loosely restrained. This box is placed on an incapacitance analgesia meter (Stoelting Co.) such that the rats hind paws are positioned on two mechano-transducers that measure weight bearing (g) on each paw. Rats will remain in this box for a period of ∼2 min during which average weight bearing on each hind paw will be measured and displayed via LCD readout.
CFA Model of Inflammatory Pain
Male and female ASIC-3 knockout mice (9−10 weeks) were obtained from Taconic Farms (Germantown, NY). Naïve male C57BL/6 mice (10 weeks, Taconic Farms, Germantown, NY) were used as controls. All animals were maintained in a climate-controlled room on a 12 h/12 h light/dark cycle with free access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Merck and Co., Inc., West Point, PA. Baseline tactile thresholds to von Frey filaments were measured by applying a series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) to the plantar aspect of the left hind paw and determining the median withdrawal threshold (grams) using the Dixon up−down method (32). Inflammation was induced by injection of 30 uL of 50% complete Freund’s adjuvant (CFA) (1:1 solution of complete and incomplete Freund’s adjuvant, Sigma, St. Louis, MO) subcutaneously into the plantar surface of the hind paw. Twenty-four hours following CFA injection, mice were tested for tactile hypersensitivity using von Frey filaments. Mice were then administered either drug (3, 10, or 30 mg/kg, ip) or vehicle (normal saline, 10 mL/kg, ip), and paw withdrawal thresholds were recorded at 60 min postadministration. Following testing at 60 min, brain and plasma samples were collected for analysis of drug content. Dose−response curves were compared by two-way repeated measures ANOVA (time vs dose) with posthoc Tukey’s test (SigmaStat). Significance was defined as p < 0.05.
Rotarod Test of Motor Coordination
Male Sprague−Dawley rats (150−250 g) were obtained from Taconic Farms (Germantown, NY). To determine the effects of test compound on motor coordination, male Sprague−Dawley rats were first trained to remain on a rotarod (IITC Model 755) (33) revolving at 12 rpm for 120 s. Rats that failed to meet this criteria were excluded from the study and immediately euthanized. Rats that successfully completed the training session were administered vehicle (normal saline, 3 mL/kg, ip) or test compound (30 mg/kg, ip) and subsequently tested on the rotarod apparatus at 60 min postdosing. For the postdosing evaluation, rats were placed on an accelerating rotarod (increasing from 4 to 33 rpm during a 5 min period), and the time the rats remain on the rotarod was recorded up to a 5 min cutoff period. Following testing at 60 min, brain and plasma samples were collected for analysis of drug content. Statistical significance was defined as p < 0.05 using Student’s t test (vehicle vs drug) (SigmaStat).
References
- Childers W. E.; Gilbert A. M.; Kennedy J. D.; Whiteside G. T. (2008) Advances in the development of novel analgesics. Expert Opin. Ther. Pat. 18, 1027–1067. [Google Scholar]
- Krishtal O. (2003) The ASICs: Signaling molecules? Modulators?. Trends Neurosci. 26, 477–483. [DOI] [PubMed] [Google Scholar]
- Reeh P. W.; Kress M. (2001) Molecular physiology of proton transduction in nociceptors. Curr. Opin. Pharmacol. 1, 45–51. [DOI] [PubMed] [Google Scholar]
- Caterina M. J.; Julius D. (2001) The vanilloid receptor: A molecular gateway to the pain pathway. Annu. Rev. Neurosci. 24, 487–517. [DOI] [PubMed] [Google Scholar]
- Gunthorpe M. J.; Chizh B. A. (2009) Clinical development of TRPV1 antagonists: Targeting a pivotal point in the pain pathway. Drug Discovery Today 14, 56–67. [DOI] [PubMed] [Google Scholar]
- Wemmie J. A.; Price M. P.; Welsh M. J. (2006) Acid-sensing ion channels: Advances, questions and therapeutic opportunities. Trends Neurosci. 29, 578–587. [DOI] [PubMed] [Google Scholar]
- Lingueglia E. (2007) Acid-sensing ion channels in sensory perception. J. Biol. Chem. 282, 17325–17329. [DOI] [PubMed] [Google Scholar]
- Jones N. G.; Slater R.; Cadiou H.; McNaughton P.; McMahon S. B. (2004) Acid-induced pain and its modulation in humans. J. Neurosci. 4, 10974–10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortright D. N.; Crandall M.; Sanchez J. F.; Zou T.; Krause J. E.; White G. (2001) The tissue distribution and functional characterization of human VR1. Biophys. Res. Commun. 281, 1183–1189. [DOI] [PubMed] [Google Scholar]
- Waldmann R. (2001) Proton-gated cation channels—neuronal acid sensors in the central and periphera. Adv. Exp. Med. Biol. 502, 293–304. [DOI] [PubMed] [Google Scholar]
- Mamet J.; Baron A.; Lazdunski M.; Voilley N. (2002) Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J. Neurosci. 22, 10662–10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S.; Schild L. (2002) Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiol. Rev. 82, 735–767. [DOI] [PubMed] [Google Scholar]
- Babinski K.; Le K. T.; Seguela P. (1999) Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J. Neurochem. 72, 51–57. [DOI] [PubMed] [Google Scholar]
- Yiangou Y.; Facer P.; Smith J. A.; Sangameswaran L.; Eglen R.; Knowles C.; Williams N.; Anand P. (2001) Increased acid-sensing ion channel ASIC-3 in inflamed human intestine. Eur. J. Gastroenerol. Hepatol. 13, 891–896. [DOI] [PubMed] [Google Scholar]
- Sluka K. A.; Price M. P.; Breese N. M.; Stucky C. L.; Wemmie J. A.; Welsh M. J. (2003) Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC-3, but not ASIC-1. Pain 106, 229–239. [DOI] [PubMed] [Google Scholar]
- Ugawa S.; Ueda T.; Ishida Y.; Nishigaki M.; Shibata Y.; Shimada S. (2002) Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J. Clin. Invest. 110, 1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J.; Santos A. R. S.; Calixto J. B. (1999) Antinociception produced by systemic, spinal and supraspinal administration of amiloride in mice. Life Sci. 65, 1059–1066. [DOI] [PubMed] [Google Scholar]
- Dubé G. R.; Lehto S. G.; Breese N. M.; Baker S. J.; Wang X.; Matulenko M. A.; Honore P.; Stewart A. O.; Moreland R. B.; Brioni J. D. (2005) Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain 117, 88–96. [DOI] [PubMed] [Google Scholar]
- Coryell M. W.; Ziemann A. E.; Westmoreland P. J.; Haenfler J. M.; Kurjakovic Z.; Zha X. M.; Price M.; Schnizler M. K.; Wemmie J. A. (2007) Targeting ASIC-1a reduces innate fear and alters neuronal activity in the fear circuit. Biol. Psychiatry 62, 1140–1148. [DOI] [PubMed] [Google Scholar]
- Coryell M. W.; Wunsch A. M.; Haenfler J. M.; Allen J. E.; McBride J. L.; Davidson B. L.; Wemmie J. A. (2008) Restoring acid-sensing ion channel-1a in the amygdala of knock-out mice rescues fear memory but not unconditioned fear responses. J. Neurosci. 28, 13738–13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell M. W.; Wunsch A. M.; Haenfler J. M.; Allen J. E.; Schnizler M. K.; McBride J. L.; Ziemann A. E.; Cook M. N.; Dunning J. P.; Price M. P.; Rainier J. D.; Liu A.; Light A. R.; Langbehn D. R.; Wemmie J. A. (2009) Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J. Neurosci. 29, 5381–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compounds were prepared in a manner similar to that described in ref (18), with modifications noted in Scheme 1.
- Compound 9g was found to be 92% bound to rat plasma proteins.
- These plasma levels were consistent with those observed by Dube and co-workers in ref (18) in the rat CFA model utilizing compound 2.
- Stein C.; Millan M. J.; Herz A. (1988) Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: Alterations in behavior and nociceptive thresholds. Pharmacol., Biochem. Behav. 31, 445–451. [DOI] [PubMed] [Google Scholar]
- Dubé G. R.; Elagoz A.; Mangat H. (2009) Acid sensing ion channels and acid nociception. Curr. Pharm. Des. 15, 1750–1766 as well as references and note added in proof cited within. [DOI] [PubMed] [Google Scholar]
- Askwith C. C.; Wemmie J. A.; Price M. P.; Rokhline T.; Welsh M. J. (2004) Membrane transport, structure, function, and biogenesis. J. Biol. Chem. 279, 18296–18305. [DOI] [PubMed] [Google Scholar]
- Deval E.; Noel J.; Lay N.; Alloui A.; Dichot S.; Friend V.; Jodar M.; Lazdunski M.; Lingueglia E. (2008) ASIC-3, a sensor of acidic and primary inflammatory pain. EMBO J. 27, 3047–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangat M; Schmudermaier M; Dubé G. R.; Schmudermaier B; Gervais F; Gustorff B. Presented at the 11th International Conference on the Mechanisms and Treatment of Neuropathic Pain, Bermuda, March 2008.
- Kuduk S. D.; Di Marco C. N.; Chang R. K.; DiPardo R. M.; Cook S. P.; Cato M. J.; Jovanaskova A.; Urban M. O.; Leitl M.; Spencer R. H.; Kane S. A.; Bilodeau M. T.; Hartman G. D.; Bock M. G. (2009) Amiloride derived inhibitors of acid-sensing ion channel-3 (ASIC-3). Bioorg. Med. Chem. Lett. 19, 2514–2518. [DOI] [PubMed] [Google Scholar]
- Kuduk S. D.; Chang R. K.; Wai J.; Cofre V.; Di Marco C. N.; DiPardo R. M.; Cook S. P.; Cato M. J.; Jovanaskova A.; Urban M. O.; Leitl M.; Spencer R. H.; Kane S. A.; Hartman G. D.; Bilodeau M. T. (2009) Amidine derived inhibitors of acid-sensing ion channel-3 (ASIC-3). Bioorg. Med. Chem. Lett. 19, 4059–4063. [DOI] [PubMed] [Google Scholar]
- Chaplan S. R.; Bach F. W.; Pogrel J. W.; Chung J. M.; Yaksh T. L. (1994) Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63. [DOI] [PubMed] [Google Scholar]
- Boyce S.; Wyatt A.; Webb J. K.; O’Donnell R.; Mason G.; Rigby M.; Sirinathsinghji D.; Hill R. G.; Rupniak N. M. J. (1999) Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: Correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology 38, 611–623. [DOI] [PubMed] [Google Scholar]