Abstract
The molecular modes of action of antipsychotic drugs are poorly understood beyond their effects at the dopamine D2 receptor. Previous studies have placed Akt signaling downstream of D2 dopamine receptors, and recent data have suggested an association between psychotic illnesses and defective Akt signaling. To characterize the effect of antipsychotic drugs on the Akt pathway, we used the model organism C. elegans, a simple system where the Akt/forkhead box O transcription factor (FOXO) pathway has been well characterized. All major classes of antipsychotic drugs increased signaling through the insulin/Akt/FOXO pathway, whereas four other drugs that are known to affect the central nervous system did not. The antipsychotic drugs inhibited dauer formation, dauer recovery, and shortened lifespan, three biological processes affected by Akt signaling. Genetic analysis showed that AKT-1 and the insulin and insulin-like growth factor receptor, DAF-2, were required for the antipsychotic drugs to increase signaling. Serotonin synthesis was partially involved, whereas the mitogen activated protein kinase (MAPK), SEK-1 is a MAP kinase kinase (MAPKK), and calcineurin were not involved. This is the first example of a common but specific molecular effect produced by all presently known antipsychotic drugs in any biological system. Because untreated schizophrenics have been reported to have low levels of Akt signaling, increased Akt signaling might contribute to the therapeutic actions of antipsychotic drugs.
Keywords: Antipsychotic drugs, C. elegans, schizophrenia, insulin signaling, Akt, FOXO
Several lines of evidence implicate the insulin-signaling (Akt) pathway in both schizophrenia and bipolar disorder, the two most common psychotic illnesses. Genetic risk studies have linked schizophrenia to components of the Akt pathway such as phosphatidyl inositol 3-kinase (PI3K), Akt, FOXO and 14-3-3 proteins (1). Furthermore, drug naïve schizophrenics have reduced signaling through the insulin-signaling pathway, low levels of IGF-1, insulin resistance (2), and a higher incidence of diabetes mellitus (3). Akt and its downstream effectors FOXO and glycogen synthase kinase-3 regulate neuronal function and behavior in normal and genetically modified mice (4,5), and we previously showed that the antipsychotic drug olanzapine stimulates phosphorylation of Akt in mammalian neuronal cells in culture (6,7). An AKT1 (protein kinase B) haplotype is associated with increased risk for schizophrenia, and protein levels of Akt1 are reduced in post-mortem schizophrenic brains and in lymphocyte-derived cell lines from schizophrenic patients (8). Interestingly, an early treatment for schizophrenia involved repeated injections with high doses of insulin (9), which activates Akt signaling.
Akt can also be linked to the pathogenesis of schizophrenia through disrupted in schizophrenia 1 (DISC1), a susceptibility gene for schizophrenia and other major mental illnesses. DISC1 acts as a scaffolding protein, and many of its binding partners are codependent or independent genetic risk factors for schizophrenia (reviewed by Chubb et al. (10)). Critical functions of DISC1 are mediated by Girdin, which regulates Akt (11). Kim et al. (11) demonstrated that physiological interaction of DISC1 with Girdin results in increased phosphorylation of Akt and its downstream effector S6. They also mimicked DISC1 suppression phenotypes by expressing constitutively active Akt, suppressing PTEN (a suppressor of Akt), and overexpressing Girdin. The effects of DISC1 suppression could be compensated for by treatment with rapamycin, an inhibitor of the Akt effector mTOR.
The molecular mechanism by which antipsychotic drugs activate Akt is not known, although G proteins appear to be involved (12). Akt signaling can be activated in response to cross-talk between insulin/IGF receptors and G proteins downstream of G protein coupled receptors (GPCR) (13,14). Direct interactions between insulin/IGF-1 receptors and G proteins have been reported (15,16), which provide a mechanism for cross-talk involving receptor tyrosine kinases and GPCRs. Perhaps the antipsychotic drugs stimulate coactivation of receptor tyrosine kinases via their effects on G proteins.
Over the past decade, simple model organisms such as C. elegans have proven to be effective tools for drug discovery and drug target identification (reviewed by Artal-Sanz et al. (17)). In addition to powerful genetic approaches, they are often superior to cells in culture because they provide preliminary information on bioavailability and whole animal toxicity. Potential new targets for many drugs, including nicotine, ethanol, and volatile anesthetics have been identified in C. elegans(18−20). In a blind drug screen that used C. elegans, prednisone was identified as an active molecule against dystrophin deficiency (21). Prednisone is already used to treat muscular dystrophy, which highlights the conservation of molecular mechanisms between C. elegans and humans. C. elegans was used to show that the antidepressants fluoxetine and imipramine bind to 5-HT transporters in vivo and synergistically activate pathways within the serotonergic network independently of 5-HT receptors (22,23). Previous studies of antipsychotic drugs in C. elegans have revealed potential novel targets of these drugs, including transient receptor potential vanilloid (TRPV) proteins, calmodulin dependent kinase II (24), and ligand-gated chloride channels (25). Work in this system has also led to the discovery that antipsychotic drugs adversely affect neuronal development (26) and cause an adaptive response in serotonergic neurons (27).
Additional functional properties of the antipsychotic drugs may have heretofore been obscured by the complexity of their effects on the human nervous system. Therefore, to determine whether earlier studies may have missed novel targets of the antipsychotics related to Akt signaling, we chose to investigate the effects of these drugs in C. elegans, a system in which the Akt pathway has been well characterized. These studies reveal that all major classes of antipsychotic drugs increased signaling through the Akt pathway, which required the insulin-like growth factor receptor DAF-2 and AKT-1.
Results and Discussion
Antipsychotic Drugs Clozapine, Olanzapine, and Trifluoperazine Increase Akt Signaling to FOXO
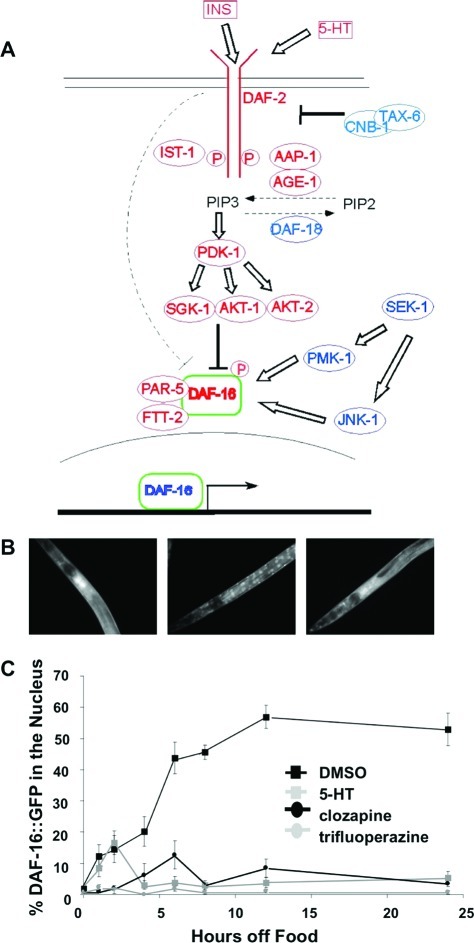
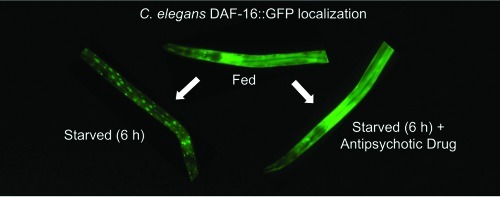
The Akt signaling pathway in C. elegans (Figure 1A) is both activated and repressed by insulin-like molecules that bind to the DAF-2 receptor. DAF-2 is homologous to both the human insulin and IGF-1 receptors (28) and is the only insulin receptor-like protein in C. elegans. Signaling through the DAF-2 receptor regulates metabolism, behavior, lifespan, and induction of dauer, an alternative larval stage adopted during development under harsh conditions such as starvation (29). Starvation reduces signaling through the insulin/Akt pathway leading to DAF-16 (FOXO) translocation from the cytoplasm to the nucleus (30,31). As in mammals, the insulin signaling network in C. elegans is regulated by phosphorylation. The phosphorylation of DAF-16 by an AKT-1/AKT-2/SGK-1 complex maintains DAF-16 in the cytoplasm (32) and a decrease in the phosphorylation of DAF-16 by PP2A allows translocation from the cytoplasm to the nucleus (33). To study the effects of the antipsychotic drugs on this pathway, we monitored redistribution of the fluorescence of a DAF-16::GFP fusion protein (34) as a function of drug treatment. For these experiments, well-fed transgenic daf-16::gfp larvae (L4 stage) were placed on NGM plates at 20 °C with food (fed), without food (starved), or without food in the presence of antipsychotic drugs. The normal response to starvation is reduced signaling through the Akt pathway, which results in accumulation of DAF-16::GFP in the nuclei and the appearance of discrete fluorescent foci throughout the animal (Figure1B, center). In animals exposed to the antipsychotic drug clozapine, DAF-16::GFP remained in the cytoplasm (Figure 1B, right). Importantly, this is the opposite of the effect expected if the drugs induced a stress response.
Figure 1.
Clozapine and trifluoperazine increase insulin signaling to DAF-16. (A) The C. elegans Akt pathway (adapted with permission from ref (59). Copyright 2006 Society for Endocrinology). DAF-2 signals through the PI3K orthologue AGE-1, and the phosphoinositide-dependent kinase orthologue PDK-1 to a complex of kinases that includes serum glucocorticoid-inducible kinase (SGK-1), AKT-1, and AKT-2. These kinases phosphorylate the FOXO transcription factor DAF-16, which results in its retention in the cytoplasm (30−32,60,61) by association with two 14−3−3 proteins, PAR-5 and FTT-2 (62,63). Activating mutations in akt-1 and pdk-1 suppress dauer arrest in age-1 null mutants (31), but do not suppress dauer formation in daf-2(e1370) mutants indicating that DAF-2 signals to DAF-16 through both the classical Akt signaling pathway and an unknown pathway (dashed line). (B) DAF-16::GFP localization in C. elegans that were well fed (left), starved (center), and starved while exposed to 160 μM clozapine (right). (C) Time course of DAF-16::GFP nuclear accumulation during starvation. Serotonin, clozapine, and trifluoperazine suppressed nuclear accumulation. (For panel C, n = 30 for each condition; each data point represents the mean ± SEM.)
Figure 1C shows the kinetics of DAF-16::GFP nuclear accumulation following the removal of animals from food in the presence and absence of antipsychotic drugs. In animals starved in the absence of drug, nuclear accumulation reached a maximum after approximately 12 h. However, in the presence of either the first-generation (typical) antipsychotic drug trifluoperazine or the second-generation (atypical) antipsychotic drug clozapine, nuclear accumulation of DAF-16::GFP was inhibited. This effect was seen uniformly in all the animals on the plate and in various cell types throughout the body. Serotonin, which is known to increase signaling through the Akt pathway by acting at or upstream of DAF-2 (35), served as a positive control.
To further characterize the effect of antipsychotic drugs on the Akt pathway, we developed a quantitative assay for DAF-16::GFP nuclear localization as described in the Methods. With this assay, we determined the effective concentration range where the antipsychotic drugs inhibit DAF-16::GFP nuclear localization in starved animals.
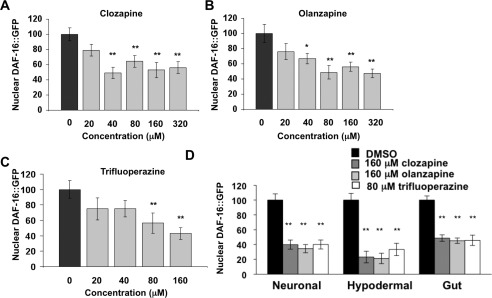
The atypical antipsychotic drugs olanzapine and clozapine and the typical antipsychotic drug trifluoperazine were selected as representative drugs for evaluation in dose−response studies (Figure 2A−C). All three drugs inhibited nuclear localization of DAF-16::GFP in starved animals in a dose-dependent manner. The concentrations used in these experiments describe the concentration of drug on NGM plates. C. elegans cuticle is an effective barrier against various drugs and exogenous agents. Consequently, the final concentration of drug in the animals is likely to be much lower than the concentrations present on the NGM plates. Nonetheless, all three of these drugs inhibited nuclear localization of DAF-16::GFP at concentrations equivalent to those required to antagonize dopamine receptors in C. elegans under identical conditions (36) and similar to those reached with accumulation in tissues including the human brain (37,38). Thus, stimulation of Akt signaling and antagonism of dopamine receptors occur in the same concentration range in this species. Additionally, these drugs had roughly the same effect on neurons, hypodermal cells, and gut cells (Figures 1B and 2D), despite the fact that the latter two cell types do not express dopamine or serotonin receptors.
Figure 2.
Clozapine, olanzapine, and trifluoperazine inhibit DAF-16::GFP nuclear localization within similar concentration ranges. (A−C) Dose−response for clozapine, olanzapine, and trifluoperazine suppression of DAF-16::GFP nuclear accumulation. In this and later figures, Nuclear DAF-16::GFP represents the ratio of cytoplasmic to nuclear fluorescence as a percent of the cytoplasmic/nuclear fluorescence of the no-drug controls as described in Methods (for each condition, n = 20; two tailed Student’s t-test, *P < 0.05, **P < 0.01 mean ± SEM). (D) Clozapine (160 μM), olanzapine (160 μM), and trifluoperazine (80 μM) have roughly the same effect on neurons, hypodermal cells, and gut cells in vivo. Neurons, hypodermal cells, and gut cells were measured for each condition as described in Methods (n = 200, two tailed Student’s t-test, *P < 0.05, **P < 0.01 mean ± SEM).
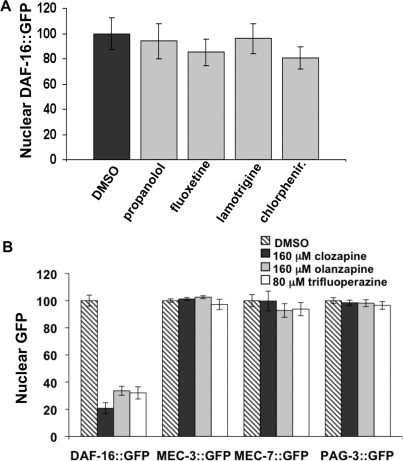
Four drugs that affect the CNS but lack antipsychotic properties were tested as controls. The β-adrenergic receptor antagonist propranolol, the selective serotonin reuptake inhibitor fluoxetine, the antihistamine chlorpheniramine, and the anticonvulsant lamotrigine had little if any effect on DAF-16::GFP nuclear localization when tested at a concentration of 160 μM under conditions identical to those used to test the antipsychotic drugs (Figure 3A). None of these drugs appreciably inhibited DAF-16::GFP nuclear accumulation. Weinshenker et al. (39) demonstrated that fluoxetine is taken up by C. elegans, and all of these control drugs can cross the blood−brain barrier in mammals.
Figure 3.
Negative Controls. (A) Four drugs that act on the nervous system but do not have antipsychotic properties including propanolol, fluoxetine, lamotrigine, and chlorpheniramine (chlorphenir) failed to suppress DAF-16::GFP nuclear localization. (B) Clozapine, olanzapine, and trifluoperazine do not affect general nuclear transport. Nuclear localization of proteins known to shuttle in and out of the nucleus was measured in response to treatment with clozapine, olanzapine, and trifluoperazine. GFP-tagged proteins tested include the transcription factors MEC-3 and PAG-3 and a β-tubulin, MEC-7, with an added nuclear localization sequence.
To be sure that the antipsychotic drugs were not blocking general transport of proteins into the nucleus, we tested the effect of clozapine, olanzapine, and trifluoperazine on the nuclear localization of three proteins known to localize to the nucleus. Nuclear localization of GFP-tagged transcription factors MEC-3 and PAG-3 and a β-tubulin (MEC-7) with an added nuclear localization sequence was not altered by treatment of the animals with antipsychotic drugs (Figure 3B).
Broad Range of Antipsychotic Drugs Increases Signaling to DAF-16::GFP
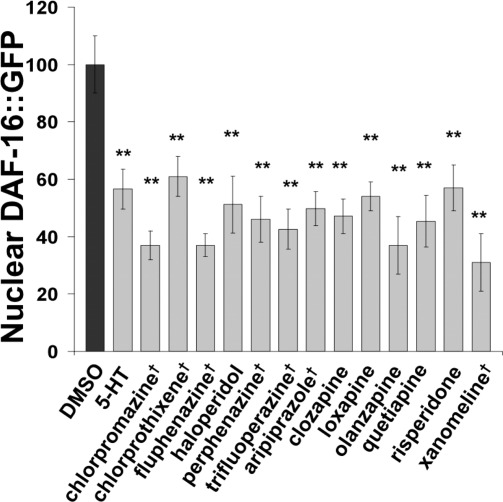
Next, we evaluated a broad range of drugs for their ability to inhibit the nuclear localization of DAF-16::GFP in starved animals. The drugs tested include all of the major categories of typical antipsychotic drugs, butyrophenones, phenothiazines, and thioxanthenes, and all of the commonly prescribed atypical antipsychotic drugs (Figure 4). Serotonin again served as a positive control. All of the antipsychotic drugs suppressed nuclear localization of DAF-16::GFP except ziprasidone (not shown), which formed an insoluble film on the plates and was therefore discounted.
Figure 4.
Broad range of antipsychotic drugs increase signaling to DAF-16. Inhibition of DAF-16::GFP nuclear accumulation by a broad range of antipsychotic drugs that include all of the major classes of typical antipsychotics, all of the commonly prescribed atypical antipsychotics, and the muscarinic receptor agonist xanomeline, which represents a new class of antipsychotic drug. The concentrations chosen were in each case based on previous experiments. (Drugs marked by † were tested at 80 μM, all other drugs were tested at 160 μM.) Serotonin (5-HT, 2.5 mM) provided a positive control (for each condition n = 20; two tailed Student’s t-test, *P < 0.05, **P < 0.01 mean ± SEM).
Xanomeline, a selective muscarinic receptor agonist, was recently shown to have antipsychotic efficacy while having no activity at the D2 dopamine receptor (40). Xanomeline, therefore, provided a useful drug for testing the hypothesis that all antipsychotic drugs will increase signaling through the Akt pathway. Like the other antipsychotic drugs tested, xanomeline significantly increased signaling to DAF-16::GFP (Figure 4). Thus, all antipsychotic drugs tested so far increased signaling through the Akt pathway.
Increased Akt Signaling by the Antipsychotic Drugs is Biologically Relevant
To assess whether stimulation of Akt signaling by antipsychotic drugs is sufficient to produce biologically relevant effects, we examined three processes regulated by this pathway: dauer formation, dauer recovery, and aging.
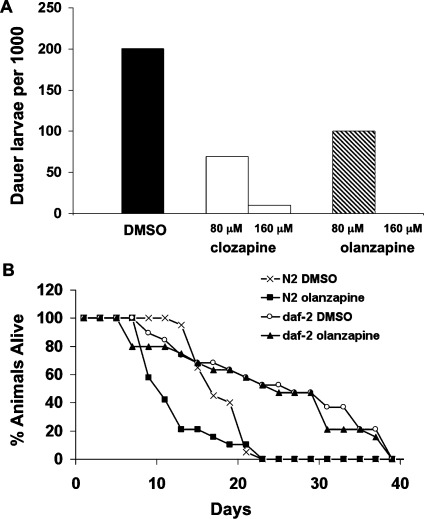
High levels of signaling through the Akt pathway inhibit dauer formation and stimulate dauer recovery (41). Therefore, the antipsychotic drugs should both stimulate dauer recovery and inhibit dauer formation. Seven antipsychotic drugs including aripiprazole, chlorprothixene, clozapine, haloperidol, olanzapine, quetiapine, and trifluoperazine were tested and found to stimulate dauer recovery in a daf-2-dependent manner (Table 1). Clozapine and olanzapine were tested in a dauer formation assay. Clozapine inhibited dauer formation in starved animals, and olanzapine at 160 μM completely eliminated dauer formation under these conditions (Figure 5A).
Table 1. Antipsychotic Drugs Stimulate Recovery from Dauera.
| concentration (μM) |
|||||||
|---|---|---|---|---|---|---|---|
| drug | 160 | 80 | 40 | 20 | 10 | 5 | 2.5 |
| chlorprothixene | − | R | R | R | R | R | R |
| clozapine | R | R | R | R | R | (±) | − |
| haloperidol | R | (±) | D | D | D | D | − |
| olanzapine | R | R | R | (±) | D | D | − |
| quetiapine | R | R | (±) | D | D | D | − |
| trifluoperazine | − | R | R | R | (±) | D | D |
| xanomeline | − | (±) | D | D | D | D | D |
| DMSO | D | D | D | D | D | D | − |
| % | |||||||
| 1 | 0.25 | 0.0625 | 0.0156 | 0.00391 | 0.0009 | − | |
| yeast extract | R | R | R | (±) | D | D | − |
Antipsychotic drugs stimulate recovery from dauer. Yeast extract served as a positive control. DMSO was used as the solvent for each of the drugs; therefore, DMSO alone at the concentrations used in each well was tested as a negative control. Haloperidol formed crystals at 160 μM so that concentration is not accurate. When this experiment was repeated with the daf-2(e1370) mutant, no animals recovered from the dauer state.
Figure 5.
Antipsychotic drugs inhibit dauer formation and decrease lifespan. (A) Clozapine (80 and 160 μM) and olanzapine (80 and 160 μM) inhibited dauer formation under dauer-inducing conditions. The number of dauer larvae per 1000 L3-adult stage animals is shown for each condition. (B) Olanzapine decreased the lifespan of wild type animals, but not in a daf-2 background. The percent of animals surviving at 25 °C is plotted as a function of time. Drug concentrations are provided in Methods.
Low levels of signaling through the Akt pathway extend lifespan, whereas high levels shorten lifespan (42). Therefore, chronic exposure to antipsychotic drugs should shorten lifespan. Data presented below show that the DAF-2 receptor is required for the drugs to increase Akt signaling; therefore, their effect on aging should be daf-2-dependent. We performed a lifespan study on animals exposed to DMSO (vehicle) alone or olanzapine in both wild type and daf-2 mutant backgrounds. Olanzapine significantly decreased lifespan relative to the vehicle control in a manner that was almost fully daf-2 dependent (Figure 5B). Clozapine and trifluoperazine also decreased lifespan in a manner that was partially but not fully daf-2 dependent suggesting that these drugs decrease lifespan through daf-2-dependent and daf-2-independent mechanisms.
There is also evidence that the antipsychotic drugs are affecting gene expression through DAF-16. Estevez et al. (43) showed that DAF-16 regulates the transcription of the tryptophan hydroxylase gene, tph-1, and we previously showed that the same antipsychotic drugs increase the expression of tph-1(24).
Clozapine, Olanzapine, and Trifluoperazine Require the DAF-2 Receptor and Signal to DAF-16 through AKT-1
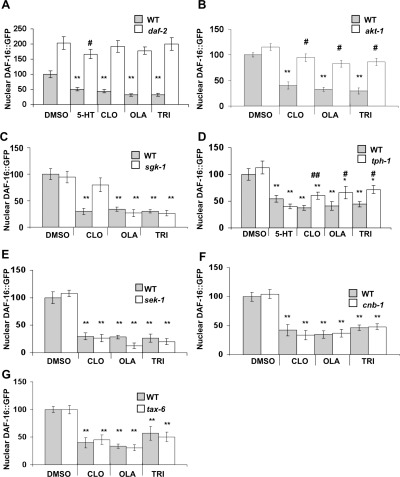
A strong point for the use of C. elegans in this study is that it provides the powerful tool of epistasis analysis, which in a well-characterized pathway allows one to identify where in the pathway the drugs act. To determine where in the insulin-signaling network (Figure 1A) the drugs act, we tested their effects on DAF-16::GFP localization in animals with mutations in various genes encoding network components. Central components of the insulin-signaling network, DAF-2 and AKT-1, are required for the drugs to inhibit nuclear localization of DAF-16::GFP in response to starvation (Figure 6A and B). This provides evidence that the drugs act at or upstream of DAF-2 and stimulate signaling through the classical Akt pathway. AKT-1 and SGK-1 are both downstream of PI3K (AGE-1) and are thought to act in parallel on DAF-16. Interestingly, clozapine, but not olanzapine or trifluoperazine, required both AKT-1 and SGK-1 to affect DAF-16::GFP localization (Figure 6B and C). Clozapine was recently reported to activate PI3K in C. elegans(44), which is consistent with our results. We previously showed that antipsychotic drugs increase serotonin production in C. elegans(24), and drug-induced cytoplasmic retention of DAF-16::GFP was modestly reduced in a serotonin-absent tph-1 mutant (Figure 6D). This suggests a role for serotonin in the response. The MAPK pathway positively regulates FOXO nuclear localization. However, C. elegans MAPK kinase SEK-1 did not contribute to this response (Figure 6E). We previously showed that antipsychotic drugs inhibit calcineurin activity in C. elegans(27). Calcineurin regulates FOXO activity through DAF-2, but neither the C. elegans calcineurin regulatory subunit CNB-1 nor the catalytic subunit TAX-6 affected localization of DAF-16::GFP in response to the antipsychotic drugs (Figure 6F and G).
Figure 6.
Genetic analysis of the effect of antipsychotic drugs on the Akt network. (A) DAF-2 is required for clozapine (CLO), olanzapine (OLA), and trifluoperazine (TRI) to block the nuclear accumulation of DAF-16::GFP. Serotonin (5-HT) served as a positive control. (B) AKT-1 is required for clozapine, olanzapine, and trifluoperazine to block the nuclear accumulation of DAF-16::GFP. (C) In an sgk-1(ok538) null background, clozapine did not block nuclear accumulation of DAF-16::GFP; however, olanzapine and trifluoperazine did. (D) In a tph-1(mg280) null background, antipsychotic drugs are slightly less effective at blocking the nuclear accumulation of DAF-16::GFP. (E) In a sek-1(km4) null background, clozapine, olanzapine, and trifluoperazine blocked the nuclear accumulation of DAF-16::GFP indicating that these drugs do not require SEK-1. (F and G) In the tax-6(p675) strong loss of function mutant or cnb-1(jh103) null backgrounds, clozapine, olanzapine, and trifluoperazine blocked the nuclear accumulation of DAF-16::GFP. tax-6 encodes the calcineurin catalytic domain, and cnb-1 encodes the calcineurin regulatory domain (for each condition, n = 20; two tailed Student’s t-test, *P < 0.05, **P < 0.01 mean ± SEM compared to those of DMSO-treated WT animals; #P < 0.05, ##P < 0.01 mean ± SEM compared to those of DMSO-treated mutants).
We do not know how the antipsychotic drugs increase signaling through Akt/FOXO. Because the drugs increase Akt signaling in both mammalian cells in culture (6,7) and C. elegans cells in culture (Weeks et al., unpublished data), they are likely to either directly activate the DAF-2 receptor or produce coactivation via DAF-2 through cell autonomous intermediaries. Candidate targets at or upstream of DAF-2 include G proteins and adaptor molecules such as Grb2 and β-arrestin. Consistent with this hypothesis, in preliminary experiments, the trimeric G protein β-subunit GPB-1 was required for the antipsychotic drugs to stimulate Akt signaling (Weeks, K., Dwyer, D., and Aamodt, E., unpublished observation). The drugs have roughly the same effect on neurons, hypodermal cells, and gut cells in vivo (Figures 1B and 2D); therefore, these diverse cell types must all express the drug target. Therefore, it seems unlikely that the drugs activate Akt by blocking their main targets, dopamine or serotonin neurotransmitter receptors, because these receptors are not expressed in all of the affected cell types. Moreover, the receptor agonist serotonin, which served as a positive control in these experiments, also increased Akt signaling through DAF-2 in all of these cells. Thus, the antipsychotic drugs may affect novel molecular targets that are broadly expressed and are also affected by high concentrations (2.5 mM) of serotonin. It is remarkable and we suspect significant that all of the antipsychotics share the ability to activate Akt in C. elegans, despite differences in the molecular details (e.g., clozapine’s actions require SGK-1, whereas xanomeline works in a noncell autonomous fashion (Weeks, K., Dwyer, D., and Aamodt, E., unpublished observation)).
Previous research has demonstrated that antipsychotic drugs increase Akt activity. Olanzapine has been shown to increase Akt phosphorylation in mammalian neuronal cells in culture (6,7) and haloperidol has been shown to increase Akt phosphorylation in the brains of mice (8). Acute treatment with risperidone, olanzapine, clozapine, haloperidol, quetiapine, or ziprasidone increased phosphorylation of GSK-3beta, a downstream effector of Akt, in the murine brain (45,46). Clozapine enhances Akt/GSK-3 phosphorylation in SH-SY5Y human neuroblastoma cells (47).
Whereas the present studies implicate Akt in some of the actions of antipsychotic drugs, their major therapeutic target is generally considered to be the D2 dopamine receptor. The importance of this target is reflected in the dopamine hypothesis of schizophrenia, which grew out of the observation that typical antipsychotics have high affinities for D2 dopamine receptors. According to the hypothesis, schizophrenia arises from excessive dopamine signaling in the brain. Pharmacological support for this idea as well as contrary evidence has been reviewed by Miyamoto et al. (48) who pointed out that the kinetics of the clinical response does not match the kinetics of D2 receptor blockade, and despite the effective antagonism of D2 receptors, many symptoms of schizophrenia persist. In addition to disturbances in dopaminergic activity, there are functional and structural anomalies in the brains of schizophrenic patients, such as changes in gray matter density in the frontal and temporal lobes, which provide support for a neurodevelopmental basis for this disorder (49). Perhaps in schizophrenia, defects in receptor signaling or cross-talk during brain development decrease Akt function, thereby interfering with the normal proliferation and differentiation of neurons and other brain cells.
Presently, it is unclear whether increased Akt signaling ameliorates psychotic symptoms and/or contributes to the metabolic side effects of antipsychotic drugs. Akt activity increases dopamine reuptake transporter activity, which reduces the concentration of dopamine at the synapse (50). In addition, FOXO regulates the expression of both tyrosine hydroxylase (51) and tryptophan hydroxylase (43), which are rate-limiting enzymes in the synthesis of dopamine and serotonin, respectively. Thus, the effect of the drugs on Akt and downstream gene expression may offer an alternative mechanism for restoring the balance between dopamine and serotonin in schizophrenia. Akt activity also inhibits GSK3, which alters the level of N-methyl-d-aspartic acid (NMDA) receptors and their subunits and reduces the level of apoptosis (52,53). Therefore, treatment with drugs that increase Akt activity may be beneficial in helping to normalize dopamine and NMDA neurotransmission and in protecting against gray matter loss (54). However, by increasing Akt activity, antipsychotic drugs may eventually induce resistance to insulin, which is a common side effect of many of the antipsychotic drugs currently used (reviewed by Chabroux et al. (55)). In any case, a wide array of antipsychotic drugs increases Akt signaling in multiple cell types including neurons in C. elegans.
Conclusions
A major finding from these studies is that antipsychotic drugs from all major categories increase signaling through the Akt pathway in C. elegans. By contrast, four other drugs known to affect the CNS did not affect Akt signaling. This result confirms findings that olanzapine, quetiapine, and clozapine activate Akt in neuronal cells (12) and complement observations that untreated schizophrenics have low levels of Akt signaling (8). This suggests that increased Akt signaling may have therapeutic benefits. This is the first example of a specific molecular effect produced by all antipsychotic drugs, including xanomeline, in any biological system.
A second observation is also highly significant, namely, that insulin/IGF-1 receptor signaling is required for the activation of Akt. This finding suggests that receptor cross-talk may play an unsuspected role in the actions of the antipsychotic drugs. Furthermore, these studies have begun to identify key components involved in the coactivation of DAF-2 receptors. The effect of antipsychotic drugs on this pathway is evolutionarily conserved, and we are struck by the fact that so many components of the Akt network map to genetic risk loci associated with schizophrenia (1).
Methods
Drugs
Eli Lilly and Co. provided olanzapine and xanomeline; Bristol-Myers Squibb, AstraZeneca, and Pfizer provided aripiprazole, quetiapine, and ziprasidone, respectively. We purchased loxapine and risperidone from Research Biomedicals International and MP Biomedicals Inc., respectively. All other drugs were from Sigma-Aldrich. Antipsychotic drugs were prepared as previously described (36). Briefly, drugs were dissolved in DMSO (clozapine, chlorpromazine, chlorprothixene, fluphenazine, haloperidol, loxapine, olanzapine, perphenazine, quetiapine, risperidone, trifluoperazine, xanomeline, and ziprasidone), ethanol (aripiprazole), or M9 buffer (serotonin) to obtain a stock solution. The stock solution was further diluted in dilute acetic acid (1.7 mM) to a final volume of 200 μL. Once the drug was added, the plates were equilibrated overnight.
Strains
We acquired all strains [GR1310 akt-1(ok525), KJ300 cnb-1(jh103), CB1370 daf-2(e1370ts), KU4 sek-1(km4), VC345 sgk-1(ok538), PR675 tax-6(p675), GR1321 tph-1(mg280), and TJ356 daf-16::gfp(zls356)] from the Caenorhabditis Genetics Center. We generated the daf-16::gfp double mutant strains by standard crosses between each mutant and the TJ356 daf-16::gfp(zls356) strain and screened for the double mutants in the F2 generation. When the akt-1(ok525) strain is crossed into the daf-16::gfp(zls356) background, 100% of the animals arrest as dauer larvae; therefore, we grew akt-1; daf-16::gfp animals on daf-16 RNAi plates (56) at 15 °C until the L4 larval stage to prevent dauer formation. All other strains were grown on NGM plates under standard conditions (57). Assays with daf-2(e1370ts) were performed at the restrictive temperature of 25 °C. For all other strains, assays were performed at 20 °C.
DAF-16::GFP Nuclear Localization Assay
We starved L4 larvae expressing DAF-16::GFP for 6 h on peptone-free nematode growth medium (NGM) plates that contained the drug being tested or as a control, solvent (DMSO) alone. All of the results were first observed by eye while the animals were still on the plates by the use of a dissecting microscope equipped with fluorescence optics. The animals were washed off the plates with M9 buffer and immediately before being photographed were immobilized with 10 mM sodium azide. Control experiments showed that exposure to sodium azide did not affect DAF-16::GFP nuclear localization for over 10 min; therefore, all images were collected within 10 min of exposure to sodium azide. We photographed animals on a Nikon LE300 microscope at 200× magnification with an exposure time of 500 ms. To standardize the focal plane, we focused on the grinder in the pharynx of each animal. Images were analyzed with Image J software. We measured DAF-16::GFP nuclear localization on the basis of the ratio of fluorescence in the nuclei compared to the adjacent cytoplasm for 4 head neurons, 6 hypodermal cells, and 10 gut cells. We tried to use the same cells in each animal. We normalized the data to control animals that had been exposed to solvent alone.
Lifespan Assay
The lifespan assay was modified from the methods of Johnson and Wood (58). We placed 20 L4-stage wild type (N2 var. Bristol) or daf-2(e1370ts) mutant animals on NGM plates containing DMSO, 160 μM clozapine, 160 μM olanzapine, or 80 μM trifluoperazine and counted the number of live animals each day until all the animals had died. Animals were determined to be dead if they were motionless and did not respond when touched by a platinum wire. Animals were transferred to fresh plates every 2 days to separate original animals from their offspring and to maintain exposure to fresh drug.
Dauer Formation Assay
To induce dauers, 20 L4-stage N2 larvae were transferred to seeded NGM plates containing DMSO alone or clozapine (80 μM, or 160 μM), olanzapine (80 μM or 160 μM), or trifluoperazine (40 μM) and were grown at 25 °C. Once the food supply was depleted, the animals were starved for an additional 24 h. Animals were then washed off the plates, treated with 1% SDS for 30 min to kill nondauers, rinsed 3× with M9 buffer, transferred to seeded plates, and then incubated at 20 °C overnight. The number of surviving animals per thousand was determined.
Dauer Recovery Assay
To measure dauer recovery, we followed the methods of Golden and Riddle (41). M9 buffer, DMSO, or ethanol were used as negative controls, depending on the solvent used to dissolve the drugs, and 1% yeast extract served as a positive control. We incubated animals at 20 °C for 4 h and then scored them as recovered/nondauers (R) on the basis of movement, appearance of a clear cuticle, and development of a clear area behind the pharynx; partially recovered (±) by the presence of some dauers, some recovered animals, and some animals that presented one or more of the recovered phenotypes; and dauers (D) if they were not moving, rod-like, and dark throughout their gut.
Acknowledgments
We thank the Caenorhabditis Genetic Center for strains and J. McGhee for comments on an earlier version of this manuscript.
Abbreviations
5-HT, 5-hydroxytryptamine or serotonin; chlorphenir, chlorpheniramine; CLO, clozapine; DISC1, disrupted in schizophrenia 1; DMSO, dimethyl sulfoxide; FOXO, forkhead box O transcription factor; GFP, green fluorescent protein; GPCR, G protein coupled receptor; GSK-3, glycogen synthase kinase-3; IGF1, insulin-like growth factor 1; MAPK, mitogen activated protein kinase; mTOR, mammalian target of rapamycin; NGM, nematode growth medium; OLA, olanzapine; PDK1, phosphoinositide-dependent kinase 1; PI3K, phosphatidyl inositol 3-kinase; SDS, sodium dodecyl sulfate; SGK1, serum- and glucocorticoid-inducible kinase 1; TRI, trifluoperazine; TRPV, transient receptor potential vanilloid.
K.R.W. performed the experiments, analyzed the data, and generated the figures. E.J.A. and D.S.D. contributed equally by generating hypotheses and designing experiments. All authors contributed equally to writing the paper.
We declare no conflicts of interest related to the data in this manuscript.
This work was supported by a Bridging Award funded by the Edward P. Stiles Trust Fund (LSUHSC) and the Biomedical Research Foundation of Northwest Louisiana.
References
- Dwyer D. S.; Weeks K. R.; Aamodt E. J. (2008) Drug discovery based on genetic and metabolic findings in schizophrenia. Expert Rev. Clin. Pharmacol. 1, 773–789. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G.; Chittiprol S.; Neelakantachar N.; Naveen M.; Thirthall J.; Gangadhar B.; Shetty K. T. (2007) Insulin and insulin-like growth factor-1 abnormalities in antipsychotic-naive schizophrenia. Am. J. Psychiatry 164, 1557–1560. [DOI] [PubMed] [Google Scholar]
- van Nimwegen L. J.; Storosum J. G.; Blumer R. M.; Allick G.; Venema H. W.; de Haan L.; Becker H.; van Amelsvoort T.; Ackermans M. T.; Fliers E.; Serlie M. J.; Sauerwein H. P. (2008) Hepatic insulin resistrance in antpsychotic naive schizophrenia patients: stable isotope studies of glucose metabolism. J. Clin. Endocrinol. Metab. 93, 572–577. [DOI] [PubMed] [Google Scholar]
- Beaulieu J. M.; Sotnikova T. D.; Yao W.-D.; Kockeritz L.; Woodgett J. R.; Gainetdinov R. R.; Caron M. G. (2004) Lithium antagonizes dopamine-dependent behaviors mediated by an Akt/glycogen synthase kinase 3 signaling cascade. Proc. Natl. Acad. Sci. U.S.A. 101, 5099–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai W.-S.; Xu B.; Westphal K. G. C.; Paterlini M.; Olivier B.; Pavlidis P.; Karayiorgou M.; Gogos J. A. (2006) Akt1 deficiency affects neuronal morphology and predisposes to abnormalities in prefrontal cortex functioning. Proc. Natl. Acad. Sci. U.S.A. 103, 16906–16911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer D. S.; Lu X. H.; Freeman A. M. III (2003) Neuronal glucose metabolism and schizophrenia: therapeutic prospects?. Expert. Rev. Neurother. 3, 29–40. [DOI] [PubMed] [Google Scholar]
- Lu X. H.; Bradley R. J.; Dwyer D. S. (2004) Olanzapine produces trophic effects in vitro and stimulates phosphorylation of Akt/PKB, ERK1/2, and the mitogen-activated protein kinase p38. Brain Res. 1011, 58–68. [DOI] [PubMed] [Google Scholar]
- Emamian E.; Hall D.; Birnbaum M.; Karayiorgou M.; Gogos J. (2004) Convergent evidence for impaired AKT-GSK3beta signaling in schizophrenia. Nat. Genet. 36, 131–137. [DOI] [PubMed] [Google Scholar]
- Sakel M. (1938) The methodical use of hypoglycemia in the treatment of psychosis. Am. J. Psychiatry 111–129. [DOI] [PubMed] [Google Scholar]
- Chubb J. E.; Bradshaw N. J.; Soares D. C.; Porteous D. J.; Miller J. K. (2008) The DISC locus in psychiatric illness. Mol. Psychiatry 13, 36–64. [DOI] [PubMed] [Google Scholar]
- Kim J. Y.; Duan X.; Liu C.; Jang M. H.; Gou J. U.; Pow-anpongkul N.; Kang E.; Song H.; Ming G. L. (2009) DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron 63, 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X. H.; Dwyer D. S. (2005) Second-generation antipsychotic drugs, olanzapine, quetiapine and clozapine enhance neurite outgrowth in PC12 cells via PI3K/Akt, ERK, and pertussis toxin-sensitive pathways. J. Mol. Neurosci. 27, 43–64. [DOI] [PubMed] [Google Scholar]
- Imamura T.; Vollenweider P.; Egawa K.; Clodi M.; Ishibashi K.; Nakashima N.; Ugi S.; Adams J. W.; Brown J. H.; Olefsky J. M. (1999) G alpha-q/11 protein plays a key role in insulin-induced glucose transport in 3T3-L1 adipocytes. Mol. Cell. Biol. 19, 6765–6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell L. M.; van Biesen T.; Hawes B. E.; Koch W. J.; Touhara K.; Lefkowitz R. J. (1995) G beta gamma subunits mediate Mitogen-activated protein kinase activation by the tyrosine kinase insulin-like growth factor 1 receptor. J. Biol. Chem. 270, 16495–16498. [DOI] [PubMed] [Google Scholar]
- Hallak H.; Seiler A. E.; Green J. S.; Ross B. N.; Rubin R. (2000) Association of heterotrimeric Gi with the insulin-like growth factor-1 receptor. J. Biol. Chem. 275, 2255–2258. [DOI] [PubMed] [Google Scholar]
- Dalle S.; Ricketts W.; Imamura T.; Vollenweider P.; Olefsky J. M. (2001) Insulin and insulin like growth factor 1 receptors utilize different G protein signaling components. J. Biol. Chem. 276, 15688–15695. [DOI] [PubMed] [Google Scholar]
- Artal-Sanz M.; de Jong L.; Tavernarakis N. (2006) Caenorhabditis elegans: a versatile platform for drug discovery. Biotechnol. J. 1, 1405–1418. [DOI] [PubMed] [Google Scholar]
- Gottschalk A.; Almedom R. B.; Schedletzky T.; Anderson S. E.; Yates J. R. III; Schafer W. R. (2005) Identification and characterization of novel nicotinic receptor-associated proteins in Caenorhabditis elegans. EMBO J. 24, 2566–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. G.; Pierce-Shimomura J. T.; Kim H.; VanHoven M. K.; Thiele T. R.; Bonci A.; Bargmann C. I.; McIntire S. L. (2003) A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell 115, 655–666. [DOI] [PubMed] [Google Scholar]
- Hawasli A. H.; Saifee O.; Liu C.; Nonet M. L.; Crowder C. M. (2004) Resistance to volatile anesthetics by mutations enhancing excitatory neurotransmitter release in Caenorhabditis elegans. Genetics 168, 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaud A.; Simon J. M.; Witzel T.; Carre-Pierrat M.; Wermuth C. G.; Segalat L. (2004) Prednisone reduces muscle degeneration in dystrophin-deficient Caenorhabditis elegans. Neuromuscul. Disord. 14, 365–370. [DOI] [PubMed] [Google Scholar]
- Dempsey C. M.; Mackenzie S. M.; Gargus A.; Blanco G.; Sze J. Y. (2005) Serotonin (5-HT), fluoxetine, imipramine, and dopamine target distinct 5-HT receptor signaling to modulate Ceanohabiditis elegans egg-laying behavior. Genetics 169, 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletta T.; Hengartner M. O. (2006) Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug. Discovery 5, 387–398. [DOI] [PubMed] [Google Scholar]
- Donohoe D. R.; Phan T.; Weeks K.; Aamodt E.; Dwyer D. S. (2008) Antipsychotic drugs up-regulate tryptophan hydroxylase in ADF neurons of Caenorhabditis elegans: role of calcium-calmodulin-dependent protein kinase II and transient receptor potential vanilloid channel. J. Neurosci. Res. 86, 2553–2563. [DOI] [PubMed] [Google Scholar]
- Ringstad N.; Abe N.; Horvitz H. R. (2009) Ligand-gated chloride channels are receptors for biogenic amines in C. elegans. Science 325, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe D. R.; Weeks K.; Aamodt E. J.; Dwyer D. S. (2008) Antipsychotic drugs alter neuronal development including ALM neuroblast migration and PLM axonal outgrowth in Caenorhadbitis elegans. Int. J. Dev. Neurosci. 26, 371–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe D. R.; Jarvis R. A.; Weeks K.; Aamodt E. J.; Dwyer D. S. (2009) Behavioral adaptation in C. elegans produced by antipsychotic drugs requires serotonin and is associated with calcium signaling and calcineurin inhibition. Neurosci. Res. 64, 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K. D.; Tissenbaum H. A.; Liu Y.; Ruvkun G. (1997) daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942–946. [DOI] [PubMed] [Google Scholar]
- Riddle D. L.; Swanson M. M.; Albert P. S. (1981) Interacting genes in nematode dauer larva formation. Nature 290, 668–671. [DOI] [PubMed] [Google Scholar]
- Lee R.; Hench J.; Ruvkun G. (2001) Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr. Biol. 11, 1950–1957. [DOI] [PubMed] [Google Scholar]
- Paradis S.; Ailion M.; Toker A.; Thomas J. H.; Ruvkun G. (1999) A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 13, 1438–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertweck M.; Gobel C.; Baumeister R. (2004) C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev. Cell 6, 577–588. [DOI] [PubMed] [Google Scholar]
- Padmanabhan S.; Mukhopadhyay A.; Narasimhan S. D.; Tesz G.; Czech M. P.; Tissenbaum H. A. (2009) A PP2A regulatory subunit regulates C. elegans insulin/IGF-1 signaling by modulating AKT-1 phosphorylation. Cell 136, 816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S. T.; Johnson T. E. (2001) daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 11, 1975–1980. [DOI] [PubMed] [Google Scholar]
- Liang B.; Moussaif M.; Kuan C.; Gargus J. J.; Sze J. Y. (2006) Serotonin targets the DAF-16/FOXO signaling pathway to modulate stress responses. Cell Metab. 4, 429–440. [DOI] [PubMed] [Google Scholar]
- Donohoe D. R.; Aamodt E. J.; Osborn E.; Dwyer D. S. (2006) Antipsychotic drugs disrupt normal development in Caenorhabditis elegans via additional mechanisms besides dopamine and serotonin receptors. Pharmacol. Res. 5, 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldessarini R. J.; Centorrino F.; Flood J. G.; Volpicelli S. A.; Houston-Lyons D.; Cohen B. M. (1993) Tissue concentrations of clozapine and its metabolites in the rat. Neuropsychopharmacology 9, 117–124. [DOI] [PubMed] [Google Scholar]
- Kornhuber J.; Shultz A.; Wiltfang J.; Meineke I.; Gleiter C. H.; Zochling R.; Boissl K. W.; Leblhuber F. (1999) Persistence of haloperidol in human brain tissue. Am. J. Psychiatry 156, 885–890. [DOI] [PubMed] [Google Scholar]
- Weinshenker D.; Garriga G.; Thomas J. H. (1995) Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J. Neurosci. 15(10), 6975–6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A.; Potter W. Z.; Lightfoot J.; Lienemann J.; Dube S.; Mallinckrodt C.; Bymaster F. P.; McKinzie D. L.; Felder C. C. (2008) Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry 165, 931–936. [DOI] [PubMed] [Google Scholar]
- Golden J. W.; Riddle D. L. (1984) The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev. Biol. 102, 368–378. [DOI] [PubMed] [Google Scholar]
- Ogg S.; Paradis S.; Gottlieb S.; Patterson G. I.; Lee L.; Tissenbaum H.; Ruvkun G. (1997) The Fork Head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389, 994–999. [DOI] [PubMed] [Google Scholar]
- Estevez A. O.; Cowie R. H.; Gardner K. L.; Estevez M. (2006) Both insulin and calcium channel signaling are required for developmental regulation of serotonin synthesis in the chemosesnsory ADF neurons of Caenorhabditis elegans. Dev. Biol. 298, 32–44. [DOI] [PubMed] [Google Scholar]
- Karmacharya R.; Sliwoski G. R.; Lundy M. Y.; Suckow R. F.; Cohen B. M.; Buttner E. A. (2009) Clozapine interaction with phosphatidyl inositol 3-kinase (PI3K)/insulin signaling pathway in Caenorhabditis elegans. Neuropsychopharmacology 34, 1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Rosborough K. M.; Friedman A. B.; Zhu W.; Roth K. A. (2007) Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int. J. Neuropsychopharmacol. 10, 7–19. [DOI] [PubMed] [Google Scholar]
- Roh M. S.; Seo S. M.; Kim Y.; Kim S. H.; Jeon W. J.; Ahn Y. M.; Kang U. G.; Juhnn Y. S.; Kim Y. S. (2007) Haloperidol and clozapine differentially regulate signals upstream of glycogen synthase kinase 3 in the rat frontal cortex. Exper. Mol. Biol. 39, 353–360. [DOI] [PubMed] [Google Scholar]
- Kang U. G.; Seo M. S.; Roh M. S.; Kim Y.; Yoon S. C.; Kim Y. S. (2004) The effects of clozapine on the GSK-3-mediated signaling pathway. FEBS Lett. 27, 115–119. [DOI] [PubMed] [Google Scholar]
- Miyamoto S.; Duncan G. E.; Marx C. E.; Lieberman J. A. (2005) Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry 10, 79–104. [DOI] [PubMed] [Google Scholar]
- Lewis D. A.; Levitt P. (2002) Schizophrenia as a disorder of neurodevelopment. Annu. Rev. Neurosci. 25, 409–432. [DOI] [PubMed] [Google Scholar]
- Carvelli L.; Moron J. A.; Kahig K. M.; Ferrer J. V.; Sen N.; Lechleiter J. D.; Leeb-Lundberg L. M.; Merrill G.; Lafer E. M.; Ballou L. M.; Shippenberg T. S.; Javitch J. A.; Lin R. Z.; Galli A. (2002) PI 3-kinase regulation of dopamine uptake. J. Neurochem. 81, 859–869. [DOI] [PubMed] [Google Scholar]
- Ferri A. L.; Lin W.; Mavromatakis Y. E.; Sasaki H.; Whitsett J. A.; Ang S.-L. (2007) Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic development in a dosage-dependent manner. Development 134, 2761–2769. [DOI] [PubMed] [Google Scholar]
- Hetman M.; Cavanaugh J. E.; Kimelman D.; Xia Z. (2000) Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J. Neurosci. 20, 2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.; Gu Z.; Liu W.; Yan Z. (2007) Glycogen synthase kinase 3 regulates N-methyl-D-aspartate receptor channel trafficking and function in cortical neurons. Mol. Pharmacol. 72, 40–51. [DOI] [PubMed] [Google Scholar]
- Lieberman J. A.; Bymaster F. P.; Meltzer H. Y.; et al. (2008) Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol. Rev. 60, 358–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabroux S.; Haffen E.; Penfornis A. (2009) Diabetes and second-generation (atypical) antispychotics. Ann. Endocrinol. 70, 202–210. [DOI] [PubMed] [Google Scholar]
- Fraser A. G.; Kamath R. S.; Zipperlen P.; Martinez-Campos M.; Sohrmann M.; Ahringer J. (2000) Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408, 325–330. [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974) The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. E.; Wood W. B. (1982) Genetic analysis of lifespan in C. elegans. Proc. Natl. Acad. Sci. U.S.A. 79, 6603–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister R.; Schaffetzel E.; Hertweck M. (2006) Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J. Endocrinol. 190, 191–202. [DOI] [PubMed] [Google Scholar]
- Lin K.; Dorman J. B.; Rodan A.; Kenyon C. (1997) DAF-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278, 1319–1322. [DOI] [PubMed] [Google Scholar]
- Lin K.; Hsin H.; Libina N.; Kenyon C. (2001) Regulation of the Caenorhabditis elegans longevity protein DAF-16 insulin/IGF-1 and germline signaling. Nat. Genet. 28, 139–145. [DOI] [PubMed] [Google Scholar]
- Berdichevsky A.; Viswanathan M.; Horvitz H. R.; Guarente L. (2006) C. elegans SIR-2.1 interacts with 14−3-3 proteins to activate DAF-16 and extend life span. Cell 125, 1165–1177. [DOI] [PubMed] [Google Scholar]
- Li J.; Tewari M.; Vidal M.; Lee S. S. (2007) The 14−3-3 protein FTT-2 regulates DAF-16 in Caenorhabditis elegans. Dev. Biol. 301, 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]