Abstract
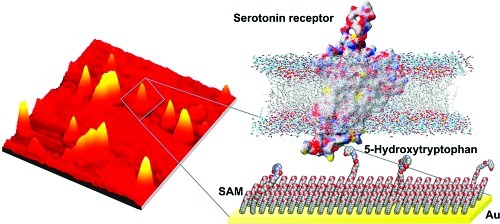
Recognition of small diffusible molecules by large biomolecules is ubiquitous in biology. To investigate these interactions, it is important to be able to immobilize small ligands on substrates; however, preserving recognition by biomolecule-binding partners under these circumstances is challenging. We have developed methods to modify substrates with serotonin, a small-molecule neurotransmitter important in brain function and psychiatric disorders. To mimic soluble serotonin, we attached its amino acid precursor, 5-hydroxytryptophan, via the ancillary carboxyl group to oligo(ethylene glycol)-terminated alkanethiols self-assembled on gold. Anti-5-hydroxytryptophan antibodies recognize these substrates, demonstrating bioavailability. Interestingly, 5-hydroxytryptophan-functionalized surfaces capture membrane-associated serotonin receptors enantiospecifically. By contrast, surfaces functionalized with serotonin itself fail to bind serotonin receptors. We infer that recognition by biomolecules evolved to distinguish small-molecule ligands in solution requires tethering of the latter via ectopic moieties. Membrane proteins, which are notoriously difficult to isolate, or other binding partners can be captured for identification, mapping, expression, and other purposes using this generalizable approach.
Keywords: 5-Hydroxytryptamine, membrane-associated receptors, receptor binding, functionalized surfaces, self-assembled monolayers, chemical patterning
Molecular interactions are often investigated using immobilization strategies (1−20). Tethered ligands, referred to as probes, are used to capture, to identify, and to determine affinities for binding partners, known as targets. Planar substrates, particles, quantum dots, and cells have been employed as scaffolds to present ligands to binding partners (9,21−24). Self-assembled monolayers (SAMs) are particularly useful in this regard because lithographic and other methods can be employed to impart precise control over surface chemistries (2,25−32). Carbohydrates (4), antibodies (6−9), peptides (10,11,33), nucleic acids (9,12), lipids (13,34), and membrane-associated receptors (35,36) have been investigated as probes. Materials functionalized with these ligands have been shown to capture small molecules, complementary nucleic acids (9,12), protein binding partners (10,13,14), receptors (15,16,33,37), and living cells (17−20) in the context of biosensing and biomedical applications.
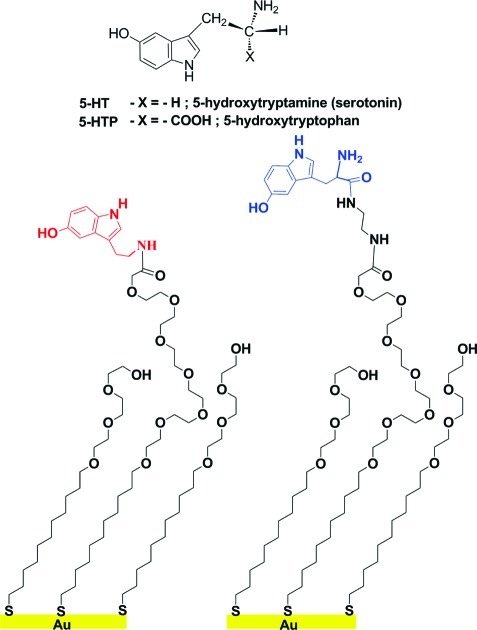
In addition to intermolecular interactions between macromolecules, binding of small-molecule ligands such as neurotransmitters, hormones, intracellular signaling molecules, or cofactors to cognate receptors and other large biomolecules is of central importance to many biological processes including cell signaling and gene regulation. Understanding interactions between small molecules and macromolecules, discovering new receptors for small molecules, and investigating groups of binding partners related by their affinity for small-molecule ligands are key to identifying novel targets for drug development and advancing our understanding of biological systems (38−40). Despite this, immobilization of small molecules (ca. 200 Da) has not been widely accomplished, due in part to limitations associated with maintaining molecular recognition (41,42). There have been a few reports of small-molecule enzyme substrates or inhibitors tethered to SAMs (14,43) or estradiol to dendrimers (44) with evidence of functional recognition. Research has also been carried out on γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system (24,45−47). We recently reported an approach to tethering the small-molecule neurotransmitter serotonin (5-hydroxytryptamine; 5-HT; Figure 1) to SAMs (5). We used insertion-directed self-assembly (26,29,30,48−50) to place carboxyl-terminated hexa(ethylene glycol) alkanethiols (HEG) into defects at step edges and domain boundaries in pre-existing SAMs consisting of hydroxyl-terminated tri(ethylene glycol) alkanethiols (TEG). This method enables dilution and appropriate spacing of the tether molecules to facilitate recognition of small-molecule probes by much larger binding partners. Functionalization was carried out by amide bond formation between the primary amine moiety of 5-HT and the terminal carboxyl group of the isolated HEGs (Figure 1). Surfaces prepared by this method show selective recognition of monoclonal and polyclonal anti-serotonin antibodies with low nonspecific binding (5).
Figure 1.
Self-assembled monolayers on gold are functionalized with serotonin (left) or its amino acid precursor, 5-hydroxytryptophan (right). Tethering of 5-hydroxytryptophan (5-HTP) via its carboxyl group and a diethylamine linker retains the primary amine for recognition, mimicking free serotonin (5-hydroxytryptamine, 5-HT). Tethering occurs via dilute carboxyl-terminated oligo(ethylene glycol) alkanethiols. The oligo(ethylene glycol) matrix resists nonspecific binding.
To date, our research on immobilized serotonin and the work of others on GABA has relied on antibodies to demonstrate biospecific recognition. However, small-molecule neurotransmitters are of insufficient size to elicit immune responses; therefore, producing antibodies against these haptens requires that they be coupled to large presenting macromolecules such as bovine serum albumin (51). Unlike endogenous receptors, which have evolved to recognize small signaling molecules that diffuse freely through the extracellular space and/or intracellular compartments, antibodies against small molecules necessarily recognize tethered small molecules. For example, anti-5-HT antibodies show excellent molecular recognition of serotonin conjugated by its primary amine to brain proteins via paraformaldehyde fixation (52,53) or to acylated serotonin (54), which approximates the immunizing hapten-conjugate. The limitation of this approach is evident as anti-5-HT antibodies have poor affinity for serotonin in solution (55).
Our ultimate goals are to devise surfaces functionalized with small-molecule probes for two purposes. First, these surfaces will be used as tools for functionally directed proteomics, as described below. Second, they will be employed to identify molecular recognition elements for small molecules selected from combinatorial libraries. Molecular recognition elements will be coupled to nanowires (3,56,57) or other nanostructures (58) for high-resolution in vivo sensing. Neurotransmitters act at the scale of nanometers and in milliseconds. Currently, in vivo sensors for serotonin function at the micrometer scale and suffer from low time resolution (min) (52,59−61) or poor chemical selectivity for measuring endogenous serotonin (62,63). In vivo detection of many other neurotransmitters is comparable, although dopamine (64,65) and, more recently, a few nonelectroactive transmitters (66,67), have been detected relatively selectively and rapidly by in vivo voltammetry. Advances that enhance chemical selectivity and spatial and temporal resolution, and that enable multiplexing for in vivo sensing applications will be critical to deciphering information encoded in chemical signaling in the brain and the periphery.
Here, we describe the next generation of capture surfaces designed to mimic small molecules in solution. We have functionalized surfaces with the biological amino acid precursor of serotonin, 5-hydroxytryptophan (5-HTP; Figure 1). We hypothesized that tethering via the carboxyl group on 5-HTP would leave all functional groups associated with the serotonin core structure accessible for recognition by native membrane-associated receptors that bind to free serotonin in the extracellular space (68). We find that 5-HTP-functionalized surfaces but not serotonin-functionalized surfaces selectively capture native serotonin membrane-associated receptors. Thus, tethering small-molecule ligands via ectopic functional groups represents a fundamental synthetic strategy for producing materials capable of capturing native (or nonnative) biomolecules with ultimate specificity for the associated free small-molecule ligands.
Results
5-Hydroxytryptophan-Functionalized Substrates Recognize 5-HTP Antibodies
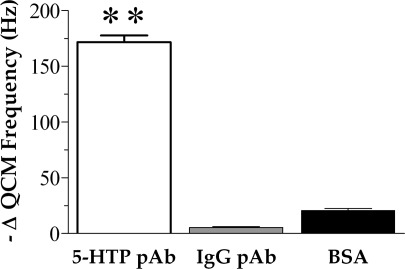
To assess bioavailability, 5-HTP-functionalized surfaces were incubated with polyclonal antibodies raised against 5-HTP, and binding was evaluated using quartz crystal microgravimetry (QCM). The specificity of these surfaces was investigated by challenging separate samples with either rabbit anti-IgG polyclonal antibodies or BSA. The IgG antibody was chosen as a representative nonspecific antibody. Additionally, BSA was used to investigate nonspecific adsorption of proteins onto 5-HTP-functionalized surfaces. As illustrated in Figure 2, 5-HTP-functionalized surfaces bind 5-HTP antibodies as indicated by a large decrease in mean QCM resonance frequency. By contrast, changes in QCM frequencies in response to anti-IgG antibodies or BSA were minimal and were significantly lower than those observed in response to anti-5-HTP antibodies (P < 0.01 compared with 5-HTP antibody binding).
Figure 2.
Changes in quartz crystal microbalance resonance frequencies in response to antibody binding. 5-Hydroxytryptophan-functionalized quartz crystals were exposed to polyclonal (pAb) antibodies raised against 5-hydroxytryptophan (5-HTP), anti-rabbit polyclonal antibodies against immunoglobulin-G (IgG), or bovine serum albumin (BSA). The large decrease in mean QCM frequency after 5-HTP pAb exposure suggests selective binding of this antibody relative to IgG pAb or BSA. Error bars represent SEMs for N = 3 samples [F(2,6) = 610; P < 0.001]; ∗∗ indicates P < 0.01 vs IgG pAb and BSA.
Substrates Bearing 5-HTP Capture Serotonin Receptors with Enantioselectivity
In previous experiments, we used carbodiimide coupling chemistry to form amide bonds between carboxyl-terminated HEG alkanethiols and the primary amine of serotonin to form 5-HT-functionalized surfaces (5). These materials showed selective molecular recognition of serotonin antibodies vs antibodies directed against other neurotransmitters or neuronal enzymes. However, in this strategy, the serotonin primary amine is used in the linking chemistry and is not freely available for recognition by native binding partners such as endogenously expressed receptor proteins. To test the importance of the primary amine moiety, we developed 5-HTP-functionalized surfaces and investigated biospecific recognition and capture of membrane-associated serotonin receptors on 5-HTP- vs 5-HT-functionalized surfaces (Figure 3a). 5-Hydroxytryptophan is the amino acid precursor of serotonin, having a structure similar to serotonin but with an α-carboxyl group (Figure 1).
Figure 3.
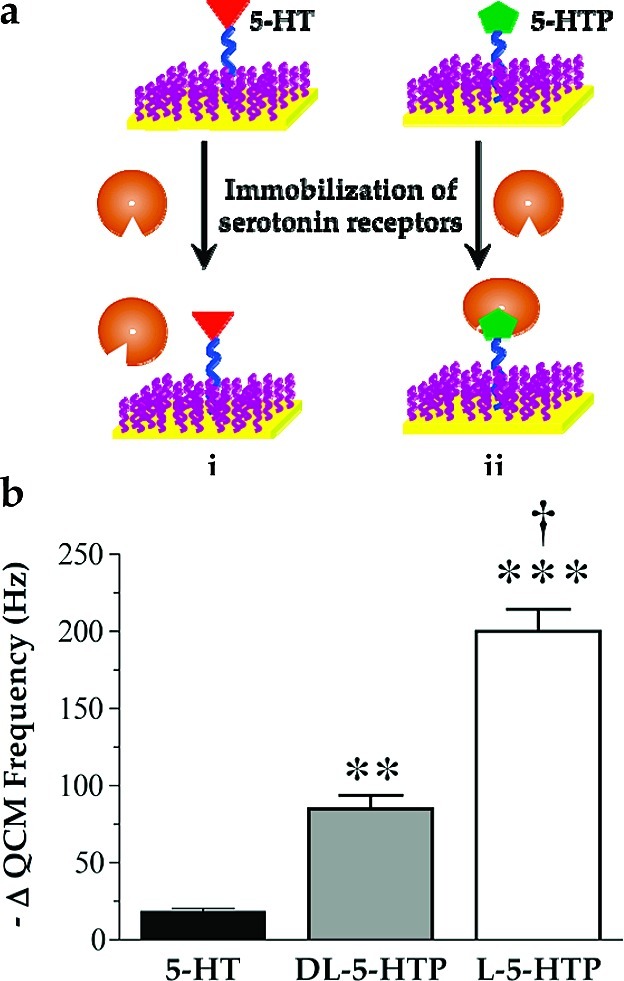
5-HT7 receptor binding to functionalized surfaces: (a) schematic illustration of selective capture of serotonin receptors by (ii) 5-HTP- but not (i) 5-HT-functionalized surfaces; (b) QCM responses of l-5-HTP-functionalized surfaces are twice those for dl-5-HTP-functionalized surfaces for binding to recombinant 5-HT7 receptors. In contrast, 5-HT-functionalized surfaces show very little binding of 5-HT7 receptors. Error bars represent SEMs for N = 3 samples [F(2,6) = 88; P < 0.001]; ∗∗ indicates P < 0.01; ∗∗∗ indicates P < 0.001 vs 5-HT-functionalized surfaces; † indicates P < 0.001 vs dl-5-HTP-functionalized surfaces.
Self-assembled monolayers formed on QCM crystals were functionalized with dilute 5-HTP or 5-HT and incubated with solutions of recombinant membrane-associated 5-HT7 receptor proteins. The data in Figure 3b show that serotonin-functionalized surfaces bind relatively small amounts of 5-HT7 receptors, as indicated by the negligible change in mean QCM resonance frequency. By contrast, surfaces functionalized with dl-5-HTP show significantly larger changes in mean QCM frequency, suggesting that receptor capture occurs (P < 0.01 vs 5-HT-functionalized surfaces).
We further tested the importance of probe selection by functionalizing SAMs with the biologically active stereoisomer l-5-HTP. As seen in Figure 3b, compared with dl-5-HTP-functionalized surfaces, decreases in QCM frequencies doubled when l-5-HTP-functionalized surfaces were exposed to the same concentration of 5-HT7 receptors (P < 0.001 vs 5-HT-functionalized surfaces and dl-5-HTP-functionalized surfaces). Thus, 5-HTP-functionalized surfaces appear to recognize and to bind recombinant 5-HT7 receptors in a stereospecific manner. These data also suggest that binding is limited by the low surface coverage of the small-molecule probe.
Serotonin-Receptor-Containing Nanovesicles Bind to Substrates
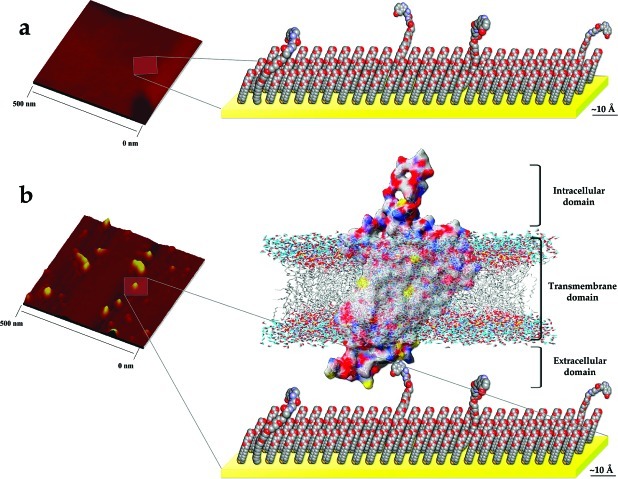
As described in the Supporting Information, membrane-associated receptor preparations containing 5-HT7 receptors were analyzed by dynamic light scattering (DLS) to determine the size distributions of the membrane vesicles. In addition to QCM, we analyzed 5-HT7 receptor binding on l-5-HTP-functionalized surfaces by atomic force microscopy (AFM). Figure 4a shows a representative topographic image of an l-5-HTP-functionalized substrate prior to receptor vesicle immobilization. Here, we observed large, flat domains on the surface. These surfaces were then incubated with 5-HT7 receptor-containing vesicles, which resulted in the appearance of new features (Figure 4b). In order to determine the sizes of the vesicles bound to 5-HTP-functionalized surfaces, we analyzed the volumes of a representative number of captured vesicles and compared these with vesicle volumes determined from DLS measurements of vesicles in solution. In both cases, vesicle shapes were assumed to be spherical for determination of volumes. A Gaussian fit to the volume histogram of the captured vesicles showed an average volume of 4000 nm3. Measurements made by DLS indicate that smaller vesicles have a volume of approximately 3000 nm3. Together, the AFM and DLS data suggest that small vesicles containing 5-HT7 receptors with diameters on the order of ∼20 nm dominate binding on 5-HTP-functionalized surfaces.
Figure 4.
Representative AFM images and schematics for dilute l-5-HTP-functionalized surfaces. Substrates (a) before and (b) after 5-HT7 receptor vesicle immobilization. The sizes of features observed are consistent with vesicles sized prior to capture (see text and Supporting Information). Discussions of the schematics generated from molecular dynamics simulations and the captured vesicle volumes are given in the Supporting Information.
5-HTP-Functionalized Surfaces Demonstrate Selective Receptor Capture
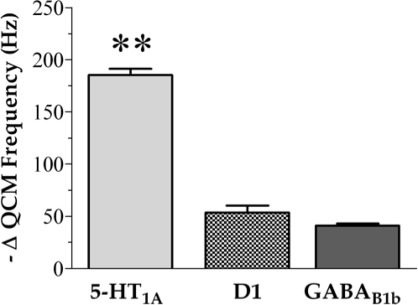
Surfaces functionalized with l-5-HTP were exposed to additional recombinant membrane receptors to study binding interactions. Here, 5-HT1A receptors were used as the native binding partners for serotonin, whereas G-protein-coupled receptors for the neurotransmitters GABA or dopamine were used to test selectivity. As shown in Figure 5, 5-HTP-functionalized surfaces capture 5-HT1A receptors, as indicated by a large decrease in mean QCM resonance frequency. We observed a similar trend for 5-HT1A receptors, whereby changes in QCM frequencies for binding to l-5-HTP surfaces were approximately twice those found using dl-5-HTP-functionalized surfaces. By comparison, receptors for the neurotransmitters dopamine (D1) or GABA (GABAB1b) elicited substantially smaller frequency responses (P < 0.01 vs 5-HT1A receptor binding), indicating nonspecific adsorption at approximately 20% of the specific binding of the same concentration of the serotonin receptors on these surfaces. Surfaces functionalized with dl-5-HTP similarly showed only small responses when exposed to D1 receptors (37.5 ± 2.5 Hz). Together, these data indicate that 5-HTP-functionalized surfaces capture membrane-associated serotonin receptors selectively compared with receptors for closely related small-molecule neurotransmitters.
Figure 5.
Selective binding of membrane receptors to l-5-HTP-functionalized surfaces. Serotonin 1A receptors show 4−5-fold higher binding to 5-HTP-functionalized surfaces compared with G-protein-coupled receptors for the neurotransmitters dopamine (D1) or GABA (GABAB1b) assessed by quartz crystal microgravimetry. Error bars represent SEMs for N = 3 samples [F(2,6) = 234; P < 0.001]; ∗∗ indicates P < 0.01 for 5-HT1A vs D1 or GABAB1b.
Discussion
Computer simulations and molecular biological and biophysical approaches have been used to investigate specific interactions between serotonin and its receptors (69). Modeling data suggest that the primary amine, indole nitrogen, and 5-hydroxyl groups of serotonin are all involved in recognition by 5-HT1A receptors (70). In studies on 5-HT2A71 and 5-HT2B72 receptors, modeling and mutagenesis experiments indicate that a critical aspartate residue in the third transmembrane domain associates with the protonated primary amine during high affinity binding of serotonin. This appears to be a common motif across the superfamily of G-protein coupled receptors, which includes the serotonin receptors (69). The current findings further support this model because the primary ethylamine moiety of serotonin is only available to interact with serotonin receptors in 5-HTP- but not 5-HT-functionalized materials. Steric hindrance associated with direct coupling of 5-HT via the primary amine might also impair recognition by serotonin receptors. Interestingly, N,N-dimethyltryptamine (DMT) analogs, which are characterized by steric hindrance at the ethylamine moiety, are selective ligands for 5-HT2A/5-HT2C73 and 5-HT6 receptors (74). Future experiments aimed at comparing recognition of these receptor subtypes by 5-HT- vs 5-HTP-fuctionalized surfaces, in addition to DMT-functionalized surfaces, are anticipated to yield further insight into the structural requirements of tethering small molecules so as to impart selective molecular recognition by native receptors.
Serotonin receptors appear to recognize immobilized 5-HTP in a stereoselective manner, which was unexpected at first given that serotonin itself is not chiral. However, receptor binding pockets are asymmetrical; that is, they have specific three-dimensional conformations (70). Thus, l-5-HTP tethered to HEG appears to present the 5-HT “pharmacophore” in an orientation that allows it to fit into the serotonin-binding cleft as opposed to HEG-tethered d-5-HTP. This concept will be explored further by comparing the ability of oligo(ethylene glycol)-tethered 5-HTP enantiomers to stimulate serotonin receptor activity as a test of functional recognition. Along similar lines, a biotinylated form of the GABA receptor agonist, muscimol (5-aminomethyl-3-hydroxyisoxazole), has been conjugated via avidin to silanized silicon substrates (75). Vu and co-workers showed that this free “mucimol−biotin” complex retains biological activity in electrophysiological studies using Xenopus oocytes (76). Furthermore, muscimol-conjugated quantum dots were recently shown to bind to GABAC receptors (24), although physiological activity has yet to be demonstrated.
Recently, an amine-terminated hexa(ethylene glycol) alkanethiol became commercially available (ProChimia, Sopot, Poland). We have used this alkanethiol for direct conjugation of the carboxyl moiety of 5-HTP, obviating the need for the diethylamine linker used here. As the number of alkanethiols or other tethers available for self-assembly increases, direct attachment of different classes of small molecules on surfaces will become possible. Additionally, solution-phase syntheses of tethered small molecules for direct self-assembly presents an alternate route well suited for producing multiplexed capture surfaces. For example, Nelson et al. used solution-phase synthesis to link biotin to oligo(ethylene glycol) followed by attachment to an alkanethiol to produce a biotin-terminated oligo(ethylene glycol) alkanethiol (77). This “pre-functionalized” tether was used to form SAMs for streptavidin immobilization. These strategies, as well as the use of different coupling chemistries including those that circumvent the need to utilize protecting group chemistries (78), could be used to couple native precursors of biologically important small molecules or synthetic analogs containing appropriate ectopic groups to tethers in a generalizable approach for producing substrates functionalized with small-molecule solution mimics.
Conclusions and Prospects
We have developed methods for tethering the serotonin analog 5-hydroxytryptophan via its ancillary carboxyl moiety to substrates. This immobilization strategy allows all functional groups associated with the serotonin structure to be available for molecular recognition. We demonstrate bioavailability of surface-immobilized 5-HTP via binding of anti-5-HTP antibodies. Moreover, 5-HTP-functionalized surfaces show enantiospecific recognition of native membrane-associated serotonin receptors. In contrast, surfaces functionalized with serotonin itself show poor serotonin receptor recognition.
Small-molecule-functionalized surfaces can be used as proteomics tools, in combination with mass spectrometry (28) and other techniques, to identify novel interacting proteins or to study relative changes in the expression levels of groups of proteins related functionally by their affinities for small biomolecules. We anticipate that small-molecule-functionalized surfaces of the type synthesized here can be used to investigate interactions between small molecules and DNA, to identify endogenous siRNAs or microRNAs, or to screen for nucleic acid aptamers for use in biosensors designed to detect free small molecules. When combined with lithographic techniques for patterning SAMs (2,25−32), small-molecule microarrays will be able to be fabricated for a wide range of applications in biology and medicine.
Methods
Preparation and Functionalization of SAMs
Self-assembled monolayers on Au functionalized with serotonin were prepared essentially as previously described (5), with the exception that carboxyl-terminated hexa(ethylene glycol) alkanethiol tethers were co-deposited from solution at a low molar ratio with hydroxyl-terminated tri(ethylene glycol) alkanethiols, instead of using insertion-directed self-assembly (25,30). The tethering chemistry for attaching 5-HTP to SAMs is described in detail in the online Supporting Information.
Characterization of Receptor Protein Preparations
Recombinant receptor preparations were composed of membrane fractions containing heterologously expressed human receptors from transfected Chinese hamster ovary (CHO) cells. Dynamic light scattering was used to determine the sizes of vesicles (10 μg/mL solutions in Tris-HCl buffer) and was performed with a Viscotek 802DLS system (Viscotek, Houston, TX).
Quartz Crystal Microbalance Measurements
Quartz crystal microgravimetry was used to quantify antibody and receptor protein binding on functionalized surfaces. Measurements were taken using a 10 MHz lever oscillator (International Crystal Manufacturing, Oklahoma City, OK) and an Agilent digital multimeter and frequency counter (Agilent, Palo Alto, CA). Biomolecule binding was carried out on gold-coated QCM crystals (International Crystal Manufacturing, Oklahoma City, OK) prepared with SAMs and functionalized with 5-HT, dl-5-HTP, or l-5-HTP. Crystals had a base resonance frequency of 10 MHz and electrode areas of 0.2 cm2. Functionalized QCM crystals were placed into an acrylic liquid flow cell (International Crystal Manufacturing, Oklahoma City, OK) with a 70-μL volume. The flow cell was sealed with an O-ring, and each QCM crystal was allowed to reach a stable baseline frequency. After stabilization, 10 cell volumes of Tris-HCl buffer (50 mM, pH 7.4) were flowed through the cell, and the baseline resonance frequency was recorded.
To test for biomolecule capture on l-5-HTP-functionalized surfaces, antibodies were diluted 1:400 in phosphate buffer (PB) (11 mM NaH2PO4, 39 mM NaH2PO4, pH 7.4) and injected into the flow cell and allowed to equilibrate for 10 min. Subsequently, 10 cell volumes of PB buffer were injected, and the resonance frequencies were recorded. The QCM resonance frequency changes were calculated as the differences between the frequencies obtained before and after injection of antibodies. Similarly, to assess nonspecific binding on 5-HTP-functionalized surfaces, BSA at 300 nM in PB buffer was incubated, and binding was measured as described above. For receptor binding studies, solutions containing 10 μg/mL of each receptor preparation in Tris-HCl buffer were injected into the flow cell and allowed to equilibrate with functionalized QCM crystals for 10 min. Functionalized QCM crystals were only challenged with one protein per crystal, and crystals were not reused.
Atomic Force Microscopy
Imaging was performed with a Thermo Microscopes Autoprobe CP Research atomic force microscope (Veeco, Santa Barbara, CA) in noncontact mode under ambient conditions. Silicon-nitride-coated plank-style AFM tips (Mikromasch, Portland, OR) with a spring constant of 40 N/m and a resonance frequency of 170 kHz were used to produce all images. Imaging was performed on commercially available Au{111} on mica (Molecular Imaging, Tempe, AZ) prepared with SAMs and dilute l-5-HTP functionalization. Images were collected at a scan rate of 1.0 Hz and a scan size of 0.5 μm. For receptor binding and volume analysis, 10 μg/mL of 5-HT7 receptor preparations in Tris-HCl buffer were incubated on 5-HTP-functionalized surfaces for 15 min, followed by rinsing with deionized H2O and drying under a stream of nitrogen. For comparison, 5-HTP-functionalized surfaces prior to incubation with receptor proteins were scanned under identical conditions. Images were analyzed using WSxM software (Nanotec Electronica, Madrid, Spain) to calculate the volumes of captured vesicles containing receptor proteins (79). Threshold filtering was performed using the WSxM flooding routine to calculate vesicle volumes. This method was previously used by Kad et al. to calculate the volume of proteins on mica surfaces (80). Images were first flattened, and analysis of each vesicle was performed. Individual vesicles were highlighted and the WSxM flooding routine was used to calculate vesicle volumes.
Statistical Analysis
Analyses were performed using Graphpad Prism (GraphPad Software, La Jolla, CA). Two group comparisons were analyzed by unpaired two-tailed t-tests. Multiple group comparisons were evaluated for overall significant differences by one-way analysis of variance (ANOVA) followed by either a priorit-tests or Tukey’s post hoc comparisons to evaluate differences between individual group means. Data are reported as means ± standard errors of the means (SEMs) with P < 0.05 considered statistically significant.
Acknowledgments
The authors acknowledge the Pennsylvania State University node of the National Nanotechnology Infrastructure Network and Dr. Tad Daniel for assistance with X-ray photoelectron spectroscopy. Drs. Shelley Claridge and Dongbo Li are gratefully acknowledged for assistance with the AFM experiments.
Supporting Information Available
Details of SAM functionalization, surface characterization, vesicle characterization, the description of the molecular dynamics simulation from which Figure 4 was assembled, and details of the chemicals used are available online. This material is available free of charge via the Internet at http://pubs.acs.org.
A.M.A. and A.V. designed the experiments with contributions from P.S.W. A.V. performed the experiments with contributions from M.J.S. and S.C. A.V. and A.M.A. analyzed the data with contributions from P.S.W. Y.S.S. provided structure design and interpretation. A.V., P.S.W., and A.M.A. wrote the manuscript.
The authors declare no conflict of interest.
The authors thank the Pennsylvania State University Center for Nanoscale Science, a National Science Foundation Materials Research and Science Engineering Center (Grant DMR-0820404), the LAM Research Corporation, and the Kavli Foundation for support of this work.
Supplementary Material
References
- Gillmor S. D.; Rugheimer P. P.; Lagally M. G. (2002) Computation with DNA on surfaces. Surf. Sci. 500, 699–721. [Google Scholar]
- Rosi N. L.; Mirkin C. A. (2005) Nanostructures in biodiagnostics. Chem. Rev. 105, 1547–1562. [DOI] [PubMed] [Google Scholar]
- Patolsky F.; Zheng G.; Lieber C. M. (2006) Nanowire sensors for medicine and the life sciences. Nanomedicine 1, 51–65. [DOI] [PubMed] [Google Scholar]
- Murrey H. E.; Hsieh-Wilson L. C. (2008) The chemical neurobiology of carbohydrates. Chem. Rev. 108, 1708–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster M. J.; Vaish A.; Szapacs M. E.; Anderson M. E.; Weiss P. S.; Andrews A. M. (2008) Biospecific recognition of tethered small molecules diluted in self-assembled monolayers. Adv. Mater. 20, 164–167. [Google Scholar]
- Garno J. C.; Amro N. A.; Wadu-Mesthrige K.; Liu G. Y. (2002) Production of periodic arrays of protein nanostructures using particle lithography. Langmuir 18, 8186–8192. [Google Scholar]
- Wadu-Mesthrige K.; Amro N. A.; Garno J. C.; Xu S.; Liu G. (2001) Fabrication of nanometer-sized protein patterns using atomic force microscopy and selective immobilization. Biophys. J. 80, 1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. B.; Park S. J.; Mirkin C. A.; Smith J. C.; Mrksich M. (2002) Protein nanoarrays generated by dip-pen nanolithography. Science 295, 1702–1705. [DOI] [PubMed] [Google Scholar]
- Brunker S. E.; Cederquist K. B.; Keating C. D. (2007) Metallic barcodes for multiplexed bioassays. Nanomedicine 2, 695–710. [DOI] [PubMed] [Google Scholar]
- Wegner G. J.; Lee H. J.; Corn R. M. (2002) Characterization and optimization of peptide arrays for the study of epitope-antibody interactions using surface plasmon resonance imaging. Anal. Chem. 74, 5161–5168. [DOI] [PubMed] [Google Scholar]
- Houseman B. T.; Huh J. H.; Kron S. J.; Mrksich M. (2002) Peptide chips for the quantitative evaluation of protein kinase activity. Nat. Biotechnol. 20, 270–274. [DOI] [PubMed] [Google Scholar]
- Thaxton C. S.; Georganopoulou D. G.; Mirkin C. A. (2006) Gold nanoparticle probes for the detection of nucleic acid targets. Clin. Chim. Acta 363, 120–126. [DOI] [PubMed] [Google Scholar]
- Stine R.; Pishko M. V.; Schengrund C. L. (2005) Comparison of glycosphingolipids and antibodies as receptor molecules for ricin detection. Anal. Chem. 77, 2882–2888. [DOI] [PubMed] [Google Scholar]
- Hodneland C. D.; Lee Y. S.; Min D. H.; Mrksich M. (2002) Selective immobilization of proteins to self-assembled monolayers presenting active site-directed capture ligands. Proc. Natl. Acad. Sci. U.S.A. 99, 5048–5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minic J.; Grosclaude J.; Aioun J.; Persuy M. A.; Gorojankina T.; Salesse R.; Pajot-Augy E.; Hou Y.; Helali S.; Jaffrezic-Renault N.; Bessueille F.; Errachid A.; Gomila G.; Ruiz O.; Samitier J. (2005) Immobilization of native membrane-bound rhodopsin on biosensor surfaces. Biochim. Biophys. Acta 1724, 324–332. [DOI] [PubMed] [Google Scholar]
- Vidic J.; Pla-Roca M.; Grosclaude J.; Persuy M. A.; Monnerie R.; Caballero D.; Errachid A.; Hou Y. X.; Jaffrezic-Renault N.; Salesse R.; Pajot-Augy E.; Samitier J. (2007) Gold surface functionalization and patterning for specific immobilization of olfactory receptors carried by nanosomes. Anal. Chem. 79, 3280–3290. [DOI] [PubMed] [Google Scholar]
- Slaughter G. E.; Bieberich E.; Wnek G. E.; Wynne K. J.; Guiseppi-Elie A. (2004) Improving neuron-to-electrode surface attachment via alkanethiol self-assembly: an alternating current impedance study. Langmuir 20, 7189–7200. [DOI] [PubMed] [Google Scholar]
- Hsiao S. C.; Crow A. K.; Lam W. A.; Bertozzi C. R.; Fletcher D. A.; Francis M. B. (2008) DNA-coated AFM cantilevers for the investigation of cell adhesion and the patterning of live cells. Angew. Chem., Int. Ed. 47, 8473–8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo Z.; Avci R.; Yang X.; Pascual D. W. (2008) Efficient immobilization and patterning of live bacterial cells. Langmuir 24, 4161–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrksich M.; Dike L. E.; Tien J.; Ingber D. E.; Whitesides G. M. (1997) Using microcontact printing to pattern the attachment of mammalian cells to self-assembled monolayers of alkanethiolates on transparent films of gold and silver. Exp. Cell Res. 235, 305–313. [DOI] [PubMed] [Google Scholar]
- Aparicio O., Geisberg J. V., Struhl K. (2004) Chromatin immunoprecipitation for determining the association of proteins with specific genomic sequences in vivo, In Current Protocols in Cell Biology (Bonifacino Juan S.,. et al. , Ed.), p 17.17, John Wiley & Sons, Inc., New York. [DOI] [PubMed] [Google Scholar]
- Cheng M. M.; Cuda G.; Bunimovich Y. L.; Gaspari M.; Heath J. R.; Hill H. D.; Mirkin C. A.; Nijdam A. J.; Terracciano R.; Thundat T.; Ferrari M. (2006) Nanotechnologies for biomolecular detection and medical diagnostics. Curr. Opin. Chem. Biol. 10, 11–19. [DOI] [PubMed] [Google Scholar]
- Rosenthal S. J.; Tomlinson I.; Adkins E. M.; Schroeter S.; Adams S.; Swafford L.; McBride J.; Wang Y.; DeFelice L. J.; Blakely R. D. (2002) Targeting cell surface receptors with ligand-conjugated nanocrystals. J. Am. Chem. Soc. 124, 4586–4594. [DOI] [PubMed] [Google Scholar]
- Gussin H. A.; Tomlinson I. D.; Little D. M.; Warnement M. R.; Qian H.; Rosenthal S. J.; Pepperberg D. R. (2006) Binding of muscimol-conjugated quantum dots to GABAC receptors. J. Am. Chem. Soc. 128, 15701–15713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen T. J.; Andrews A. M.; Weiss P. S. (2007) Self- and directed assembly strategies for nanopatterning. Aldrichimica Acta 40, 21–31. [Google Scholar]
- Smith R. K.; Lewis P. A.; Weiss P. S. (2004) Patterning self-assembled monolayers. Prog. Surf. Sci. 75, 1–68. [Google Scholar]
- Love J. C.; Estroff L. A.; Kriebel J. K.; Nuzzo R. G.; Whitesides G. M. (2005) Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 105, 1103–1169. [DOI] [PubMed] [Google Scholar]
- Mrksich M. (2008) Mass spectrometry of self-assembled monolayers: A new tool for molecular surface science. ACS Nano 2, 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan C.; Mullen T. J.; Hohman J. N.; Anderson M. E.; Dameron A. A.; Andrews A. M.; Dickey E. C.; Horn M. W.; Weiss P. S. (2007) Scanning electron microscopy of nanoscale chemical patterns. ACS Nano 1, 191–201. [DOI] [PubMed] [Google Scholar]
- Mullen T. J.; Srinivasan C.; Hohman J. N.; Gillmor S. D.; Shuster M. J.; Horn M. W.; Andrews A. M.; Weiss P. S. (2007) Microcontact insertion printing. Appl. Phys. Lett. 90, 063114. [Google Scholar]
- Anderson M. E.; Srinivasan C.; Hohman J. N.; Carter E. M.; Horn M. W.; Weiss P. S. (2006) Combining conventional lithography with molecular self-assembly for chemical patterning. Adv. Mater. 18, 3258–3260. [Google Scholar]
- Wen K.; Maoz R.; Cohen H.; Sagiv J.; Gibaud A.; Desert A.; Ocko B. M. (2008) Postassembly chemical modification of a highly ordered organosilane multilayer: New insights into the structure, bonding, and dynamics of self-assembling silane monolayers. ACS Nano 2, 579–599. [DOI] [PubMed] [Google Scholar]
- Kroger D.; Hucho F.; Vogel H. (1999) Ligand binding to nicotinic acetylcholine receptor investigated by surface plasmon resonance. Anal. Chem. 71, 3157–3165. [DOI] [PubMed] [Google Scholar]
- Goluch E. D.; Shaw A. W.; Sligar S. G.; Liu C. (2008) Microfluidic patterning of nanodisc lipid bilayers and multiplexed analysis of protein interaction. Lab Chip 8, 1723–1728. [DOI] [PubMed] [Google Scholar]
- Fang Y.; Frutos A. G.; Lahiri J. (2002) Membrane protein microarrays. J. Am. Chem. Soc. 124, 2394–2395. [DOI] [PubMed] [Google Scholar]
- Hong Y.; Webb B. L.; Su H.; Mozdy E. J.; Fang Y.; Wu Q.; Liu L.; Beck J.; Ferrie A. M.; Raghavan S.; Mauro J.; Carre A.; Mueller D.; Lai F.; Rasnow B.; Johnson M.; Min H.; Salon J.; Lahiri J. (2005) Functional GPCR microarrays. J. Am. Chem. Soc. 127, 15350–15351. [DOI] [PubMed] [Google Scholar]
- Das A.; Zhao J.; Schatz G. C.; Sligar S. G.; Van Duyne R. P. (2009) Screening of type I and II drug binding to human cytochrome P450−3A4 in nanodiscs by localized surface plasmon resonance spectroscopy. Anal. Chem. 81, 3754–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich R. L.; Hoth L. R.; Geoghegan K. F.; Brown T. A.; LeMotte P. K.; Simons S. P.; Hensley P.; Myszka D. G. (2002) Kinetic analysis of estrogen receptor/ligand interactions. Proc. Natl. Acad. Sci. U.S.A. 99, 8562–8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. A. (2004) Advances in membrane receptor screening and analysis. J. Mol. Recognit. 17, 286–315. [DOI] [PubMed] [Google Scholar]
- Huber W.; Mueller F. (2006) Biomolecular interaction analysis in drug discovery using surface plasmon resonance technology. Curr. Pharm. Des. 12, 3999–4021. [DOI] [PubMed] [Google Scholar]
- Bachas L. G.; Meyerhoff M. E. (1986) Theoretical models for predicting the effect of bridging group recognition and conjugate substitution on hapten enzyme immunoassay dose-response curves. Anal. Biochem. 156, 223–238. [DOI] [PubMed] [Google Scholar]
- Ishikawa E.; Hashida S.; Kohno T. (1991) Development of ultrasensitive enzyme immunoassay reviewed with emphasis on factors which limit the sensitivity. Mol. Cell Probes 5, 81–95. [DOI] [PubMed] [Google Scholar]
- Mrksich M.; Grunwell J. R.; Whitesides G. M. (1995) Biospecific adsorption of carbonic-anhydrase to self-assembled monolayers of alkanethiolates that present benzenesulfonamide groups on gold. J. Am. Chem. Soc. 117, 12009–12010. [Google Scholar]
- Harrington W. R.; Kim S. H.; Funk C. C.; Madak-Erdogan Z.; Schiff R.; Katzenellenbogen J. A.; Katzenellenbogen B. S. (2006) Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol. Endocrinol. 20, 491–502. [DOI] [PubMed] [Google Scholar]
- Saifuddin U.; Vu T. Q.; Rezac M.; Qian H.; Pepperberg D. R.; Desai T. A. (2003) Assembly and characterization of biofunctional neurotransmitter-immobilized surfaces for interaction with postsynaptic membrane receptors. J. Biomed. Mater. Res. A 66, 184–191. [DOI] [PubMed] [Google Scholar]
- Wang T.; Ehteshami G.; Massia S.; Muthuswamy J. (2006) Immobilization and characterization of gamma-aminobutyric acid on gold surface. J. Biomed. Mater. Res. A 79, 201–209. [DOI] [PubMed] [Google Scholar]
- Wang T.; Muthuswamy J. (2008) Immunosensor for detection of inhibitory neurotransmitter gamma-aminobutyric acid using quartz crystal microbalance. Anal. Chem. 80, 8576–8582. [DOI] [PubMed] [Google Scholar]
- Bumm L. A.; Arnold J. J.; Cygan M. T.; Dunbar T. D.; Burgin T. P.; Jones L.; Allara D. L.; Tour J. M.; Weiss P. S. (1996) Are single molecular wires conducting?. Science 271, 1705–1707. [Google Scholar]
- Cygan M. T.; Dunbar T. D.; Arnold J. J.; Bumm L. A.; Shedlock N. F.; Burgin T. P.; Jones L.; Allara D. L.; Tour J. M.; Weiss P. S. (1998) Insertion, conductivity, and structures of conjugated organic oligomers in self-assembled alkanethiol monolayers on Au{111}. J. Am. Chem. Soc. 120, 2721–2732. [Google Scholar]
- Donhauser Z. J.; Mantooth B. A.; Kelly K. F.; Bumm L. A.; Monnell J. D.; Stapleton J. J.; Price D. W. Jr.; Rawlett A. M.; Allara D. L.; Tour J. M.; Weiss P. S. (2001) Conductance switching in single molecules through conformational changes. Science 292, 2303–2307. [DOI] [PubMed] [Google Scholar]
- Singh K. V.; Kaur J.; Varshney G. C.; Raje M.; Suri C. R. (2004) Synthesis and characterization of hapten-protein conjugates for antibody production against small molecules. Bioconjugate Chem. 15, 168–173. [DOI] [PubMed] [Google Scholar]
- Luellen B. A.; Bianco L. E.; Schneider L. M.; Andrews A. M. (2007) Reduced brain-derived neurotrophic factor is associated with a loss of serotonergic innervation in the hippocampus of aging mice. Genes Brain Behav. 6, 482–490. [DOI] [PubMed] [Google Scholar]
- Luellen B. A.; Szapacs M. E.; Materese C. K.; Andrews A. M. (2006) The neurotoxin 2′-NH2-MPTP degenerates serotonin axons and evokes increases in hippocampal BDNF. Neuropharmacology 50, 297–308. [DOI] [PubMed] [Google Scholar]
- Bethea C. L.; Lu N. Z.; Reddy A.; Shlaes T.; Streicher J. M.; Whittemore S. R. (2003) Characterization of reproductive steroid receptors and response to estrogen in a rat serotonergic cell line. J. Neurosci. Methods 127, 31–41. [DOI] [PubMed] [Google Scholar]
- Gow I. F.; Corrie J. E.; Williams B. C.; Edwards C. R. (1987) Development and validation of an improved radioimmunoassay for serotonin in platelet-rich plasma. Clin. Chim. Acta 162, 175–188. [DOI] [PubMed] [Google Scholar]
- Wang W. U.; Chen C.; Lin K. H.; Fang Y.; Lieber C. M. (2005) Label-free detection of small-molecule-protein interactions by using nanowire nanosensors. Proc. Natl. Acad. Sci. U.S.A. 102, 3208–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow T. J.; Li M.; Kim J.; Mayer T. S.; Keating C. D. (2009) Programmed assembly of DNA-coated nanowire devices. Science 323, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehashi K.; Katsura T.; Kerman K.; Takamura Y.; Matsumoto K.; Tamiya E. (2007) Label-free protein biosensor based on aptamer-modified carbon nanotube field-effect transistors. Anal. Chem. 79, 782–787. [DOI] [PubMed] [Google Scholar]
- Mathews T. A.; Fedele D. E.; Coppelli F. M.; Avila A. M.; Murphy D. L.; Andrews A. M. (2004) Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J. Neurosci. Methods 140, 169–181. [DOI] [PubMed] [Google Scholar]
- Szapacs M. E.; Mathews T. A.; Tessarollo L.; Ernest Lyons W.; Mamounas L. A.; Andrews A. M. (2004) Exploring the relationship between serotonin and brain-derived neurotrophic factor: Analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. J. Neurosci. Methods 140, 81–92. [DOI] [PubMed] [Google Scholar]
- Guiard B. P.; David D. J.; Deltheil T.; Chenu F.; Le Maitre E.; Renoir T.; Leroux-Nicollet I.; Sokoloff P.; Lanfumey L.; Hamon M.; Andrews A. M.; Hen R.; Gardier A. M. (2008) Brain-derived neurotrophic factor-deficient mice exhibit a hippocampal hyperserotonergic phenotype. Int. J. Neuropsychopharmacol. 11, 79–92. [DOI] [PubMed] [Google Scholar]
- Baganz N. L.; Horton R. E.; Calderon A. S.; Owens W. A.; Munn J. L.; Watts L. T.; Koldzic-Zivanovic N.; Jeske N. A.; Koek W.; Toney G. M.; Daws L. C. (2008) Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 105, 18976–18981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws L. C. (2009) Unfaithful neurotransmitter transporters: Focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol. Ther. 121, 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M. C.; Shi G.; Borland L.; Michael A. C.; Weber S. G. (2006) Simultaneous determination of biogenic monoamines in rat brain dialysates using capillary high-performance liquid chromatography with photoluminescence following electron transfer. Anal. Chem. 78, 1755–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P. E.; Stuber G. D.; Heien M. L.; Wightman R. M.; Carelli R. M. (2003) Subsecond dopamine release promotes cocaine seeking. Nature 422, 614–618. [DOI] [PubMed] [Google Scholar]
- Quintero J. E.; Day B. K.; Zhang Z.; Grondin R.; Stephens M. L.; Huettl P.; Pomerleau F.; Gash D. M.; Gerhardt G. A. (2007) Amperometric measures of age-related changes in glutamate regulation in the cortex of rhesus monkeys. Exp. Neurol. 208, 238–246. [DOI] [PubMed] [Google Scholar]
- Burmeister J. J.; Coates T. D.; Gerhardt G. A. (2004) Multisite microelectrode arrays for measurements of multiple neurochemicals. Conf. Proc. IEEE Eng. Med. Biol. Soc. 7, 5348–5351. [DOI] [PubMed] [Google Scholar]
- Roth B. L. (2006) The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics, Humana Press, Totowa, NJ. [Google Scholar]
- Kristiansen K. (2004) Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: Molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol. Ther. 103, 21–80. [DOI] [PubMed] [Google Scholar]
- Seeber M.; De Benedetti P. G.; Fanelli F. (2003) Molecular dynamics simulations of the ligand-induced chemical information transfer in the 5-HT(1A) receptor. J. Chem. Inf. Comput. Sci. 43, 1520–1531. [DOI] [PubMed] [Google Scholar]
- Kristiansen K.; Kroeze W. K.; Willins D. L.; Gelber E. I.; Savage J. E.; Glennon R. A.; Roth B. L. (2000) A highly conserved aspartic acid (Asp-155) anchors the terminal amine moiety of tryptamines and is involved in membrane targeting of the 5-HT(2A) serotonin receptor but does not participate in activation via a “salt-bridge disruption” mechanism. J. Pharmacol. Exp. Ther. 293, 735–746. [PubMed] [Google Scholar]
- Manivet P.; Schneider B.; Smith J. C.; Choi D. S.; Maroteaux L.; Kellermann O.; Launay J. M. (2002) The serotonin binding site of human and murine 5-HT2B receptors: Molecular modeling and site-directed mutagenesis. J. Biol. Chem. 277, 17170–17178. [DOI] [PubMed] [Google Scholar]
- Berg K. A.; Maayani S.; Goldfarb J.; Scaramellini C.; Leff P.; Clarke W. P. (1998) Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: Evidence for agonist-directed trafficking of receptor stimulus. Mol. Pharmacol. 54, 94–104. [PubMed] [Google Scholar]
- Glennon R. A.; Lee M.; Rangisetty J. B.; Dukat M.; Roth B. L.; Savage J. E.; McBride A.; Rauser L.; Hufeisen S.; Lee D. K. (2000) 2-Substituted tryptamines: Agents with selectivity for 5-HT(6) serotonin receptors. J. Med. Chem. 43, 1011–1018. [DOI] [PubMed] [Google Scholar]
- Nehilla B. J.; Popat K. C.; Vu T. Q.; Chowdhury S.; Standaert R. F.; Pepperberg D. R.; Desai T. A. (2004) Neurotransmitter analog tethered to a silicon platform for neuro-BioMEMS applications. Biotechnol. Bioeng. 87, 669–674. [DOI] [PubMed] [Google Scholar]
- Vu T. Q.; Chowdhury S.; Muni N. J.; Qian H.; Standaert R. F.; Pepperberg D. R. (2005) Activation of membrane receptors by a neurotransmitter conjugate designed for surface attachment. Biomaterials 26, 1895–1903. [DOI] [PubMed] [Google Scholar]
- Nelson K. E.; Gamble L.; Jung L. S.; Boeckl M. S.; Naeemi E.; Golledge S. L.; Sasaki T.; Castner D. G.; Campbell C. T.; Stayton P. S. (2001) Surface characterization of mixed self-assembled monolayers designed for streptavidin immobilization. Langmuir 17, 2807–2816. [Google Scholar]
- Young I. S.; Baran P. S. (2009) Protecting-group-free synthesis as an opportunity for invention. Nat. Chem. 1, 193–205. [DOI] [PubMed] [Google Scholar]
- Ratcliff G. C.; Erie D. A. (2001) A novel single-molecule study to determine protein−protein association constants. J. Am. Chem. Soc. 123, 5632–5635. [DOI] [PubMed] [Google Scholar]
- Kad N. M.; Myers S. L.; Smith D. P.; Smith D. A.; Radford S. E.; Thomson N. H. (2003) Hierarchical assembly of beta2-microglobulin amyloid in vitro revealed by atomic force microscopy. J. Mol. Biol. 330, 785–797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.