Abstract

The present study was performed to investigate the possible role of protein kinase C (PKC) in morphine tolerance at spinal levels of rats. Intrathecal injection of 10 μg of morphine induced increases in the hindpaw withdrawal latency (HWL) to noxious thermal and mechanical stimulation in rats. After intrathecal injections of 10 μg of morphine (twice a day) lasted for 5 days, the antinociceptive effects induced by intrathecal injections of morphine decreased significantly in rats. Interestingly, we found that there were significant increases in the content of PKC in the dorsal horn of the spinal cord and the dorsal root ganglion, but not in the ventral horn of the spinal cord, in rats with morphine tolerance determined by Western blot, suggesting that PKC is involved in morphine tolerance at spinal levels of rats. Furthermore, our results demonstrated that chronic intrathecal injection of the PKC inhibitor significantly inhibited the development of morphine tolerance. Moreover, we found that the maintenance of morphine tolerance was blocked by intrathecal administration of a PKC inhibitor in rats, and the inhibitory effects of the PKC inhibitor on morphine tolerance lasted for more than two days. Taken together, the present study clearly showed that PKC is involved in morphine tolerance at the spinal level of rats and that intrathecal administration of a PKC inhibitor can block the development and maintenance of morphine tolerance.
Keywords: Development of morphine tolerance, dorsal root ganglion, hindpaw withdrawal latency, maintenance of morphine tolerance, PKC, spinal cord
Morphine still proves to be clinically indispensible in treating moderate to severe pain. However, the development of tolerance, antinociceptive effect produced by a given dose of morphine declined over time (1−3), largely limits its extensive application. Protein kinase C (PKC), a family of phospholipid-dependent serine/threonine kinases, has been demonstrated to play an important role in cellular signal transduction. PKC can be activated upon external stimulation of cells by various ligands including growth factors, hormones, and neurotransmitters (4−6).
With the method of [3H] phorbol-12,13-dibutyrate (PdBu) binding, Mayer et al. demonstrated that daily injections of morphine showed an increase of membrane-bound PKC, particularly in spinal laminae I and II (7). The results indicate a strong correlation between PKC and morphine tolerance, which was later confirmed by a wide range of other studies (8−11). The present study was performed to explore the role of PKC in morphine tolerance at the spinal level of rats, especial in the development and maintenance of morphine tolerance.
Results and Discussion
Influence of Morphine Tolerance on the Expression of PKC at the Spinal Level of Rats
To investigate the role of PKC in morphine tolerance at the spinal level, rats received intrathecal administration of 10 μg of morphine twice a day for 5 days to produce morphine tolerance. Another group of rats without any treatment was as the control group (n = 8).
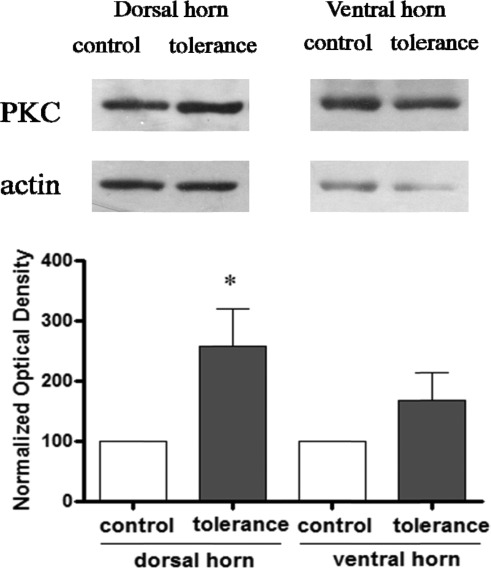
As shown in Figure 1, there were significant increases in the content of PKC (n = 5; F = 6.34; P < 0.05) in the dorsal horn of the spinal cord in rats with morphine tolerance compared with that of naïve rats determined by Western blot. However, there were no significant changes in the expression of PKC observed in the ventral horn of the spinal cord of rats with morphine tolerance (n = 4; F = 2.02; P = 0.21) compared with that in naïve rats tested by Western blot. The results indicate that morphine tolerance induces significant PKC expression in the dorsal horn, but not the ventral horn, of the spinal cord in rats.
Figure 1.
Changes in the expression of PKC in the spinal cord of rat after morphine tolerance. Data are presented as mean ± SEM, *P < 0.05 compared with the control group.
Our additional results demonstrated that there were also significant increases in the content of PKC (n = 3; F = 2207.21; P < 0.001) in the dorsal root ganglion in rats with morphine tolerance compared with that in naïve rats determined by Western blot, as shown in Figure 2.
Figure 2.
Changes in the expression of PKC in the DRG of rats after morphine tolerance. Data are presented as mean ± SEM, ***P < 0.001 compared with the control group. DRG, dorsal root ganglion.
The above results strongly suggest the involvement of PKC in morphine tolerance at the spinal level of rats.
Effects of the PKC Inhibitor on the Development of Morphine Tolerance
As the above results strongly suggest the involvements of PKC in morphine tolerance at the spinal level of rats, the experiments were performed to explore the influence of chronic intrathecal injection of the PKC inhibitor on chronic morphine-induced tolerance. One group of rats received intrathecal administration of 10 μg of morphine, followed 5 min later by intrathecal injections of 1 nmol of the PKC inhibitor chelerythrine, twice a day (n = 5). Another group of rats received intrathecal injections of 10 μg of morphine, followed 5 min later by 5 μL of 0.9% saline as a control (n = 6), twice a day. The above treatments lasted for five days.
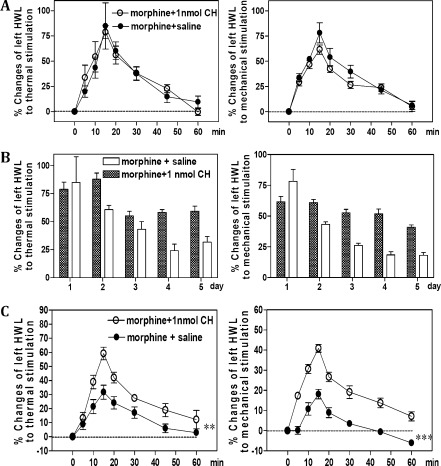
At the first day after morphine injection, the the hindpaw withdrawal latencies (HWLs) to thermal and mechanical stimulation in both groups of rats increased markedly and lasted for more than 50 min as shown in Figure 3A. There were no significant differences in the increased HWLs induced by morphine in the group of rats that received the intrathecal injection of morphine plus chelerythrine compared to those of rats that received morphine plus saline (hot-plate test, F = 0.04 and P = 0.86; Randall Selitto test, F = 1.47 and P = 0.26).
Figure 3.
Comparison of the antinociceptive effects induced by intrathecal administration of morphine in rats that received an intrathecal injection of morphine plus chelerythrine (CH) or morphine plus saline for 5 days. (A) Effects of intrathecal injection of morphine on the left HWL to thermal and mechanical stimulation in rats on day 1. Time = 0 min, intrathecal injection of 10 μg of morphine; time = 5 min, intrathecal injection of 1 nmol of chelerythrine or 5 μL of saline as a control. (B) The decrease of antinociceptive effects induced by intrathecal administration of morphine twice a day. The antinociceptive effects were assessed at 15 min after morphine injection each morning. (C) Effects of intrathecal injection of morphine on the left HWL to thermal and mechanical stimulation in rats on day 5. Time = 0 min, intrathecal injection of 10 μg of morphine; time = 5 min, intrathecal injection of 1 nmol of chelerythrine or 5 μL of saline as a control. Data are presented as mean ± SEM, **P < 0.01 and ***P < 0.001 compared to the control group. HWL, hindpaw withdrawal latency.
Figure 3B shows the influences of chronic intrathecal injection of PKC inhibitor on the chronic intrathecal injection of morphine-induced tolerance tested by the hot plate test and the Randall Selitto test. In the group of rats with 10 μg of morphine plus saline, the morphine-induced antinociception decreased significantly, while in the group of rats with 10 μg of morphine plus chelerythrine, the morphine-induced HWLs to thermal (first day, F = 0.07 and P = 0.81; second day, F = 17.90 and P < 0.01; third day, F = 1.95 and P = 0.20; fourth day, F = 25.64 and P < 0.01; fifth day, F = 14.74 and P < 0.01) and mechanical stimulation (first day, F = 2.01 and P = 0.19; second day, F = 30.30 and P < 0.001; third day, F = 70.42 and P < 0.001; fourth day, F = 52.32 and P < 0.001; fifth day, F = 53.56 and P < 0.001) remained at high levels compared to those of the control group.
Figure 3C shows the results tested on the fifth day after the injections. The HWLs to thermal and mechanical stimulation increased markedly in the two groups of rats after receiving intrathecal injections of morphine. Interestingly, the increased HWLs induced by the intrathecal injection of morphine were more pronounced in the group of rats receiving the intrathecal injection of morphine plus chelerythrine (hot-plate test, F = 21.76 and P < 0.01; Randall Selitto test, F = 65.47 and P < 0.001) than the HWLs in rats receiving the morphine plus saline. The results clearly showed that chronic intrathecal injection of the PKC inhibitor can inhibit the development of morphine tolerance.
Inhibition of the PKC Inhibitor on the Maintenance of Morphine Tolerance
We further explored the influence of the PKC inhibitor on the maintenance of morphine tolerance. Rats with chronic morphine-induced tolerance received intrathecal injections of 1 nmol of chelerythrine (n = 6) or 5 μL of 0.9% saline as a control (n = 5); 5 min later, all of the rats received intrathecal injections of 10 μg of morphine. The results are shown in Figure 4. In the control group (saline plus morphine), the HWLs to thermal and mechanical stimulation increased less than 25%, while in the group treated by intrathecal injections of chelerythrine plus morphine, the HWLs to both thermal and mechanical stimulation increased significantly after morphine injection (hot-plate test, F = 28.57 and P < 0.01; Randall Selitto test, F = 46.13 and P < 0.001) compared to those of the control group. The results clearly showed that morphine tolerance was reversed by intrathecal administration of the PKC inhibitor in rats.
Figure 4.
Effects of intrathecal injection of morphine on the left HWL to thermal and mechanical stimulation in rats that received an intrathecal injection of chelerythrine (CH) plus morphine or saline plus morphine. Five minutes before the morphine injection, intrathecal injection of 1 nmol of chelerythrine or 5 μL of saline as a control; time = 0 min, intrathecal injection of 10 μg of morphine. Data are presented as mean ± SEM. The statistical difference between groups was determined by two-way ANOVA. **P < 0.01 and ***P < 0.001 compared with the control group. HWL, hindpaw withdrawal latency.
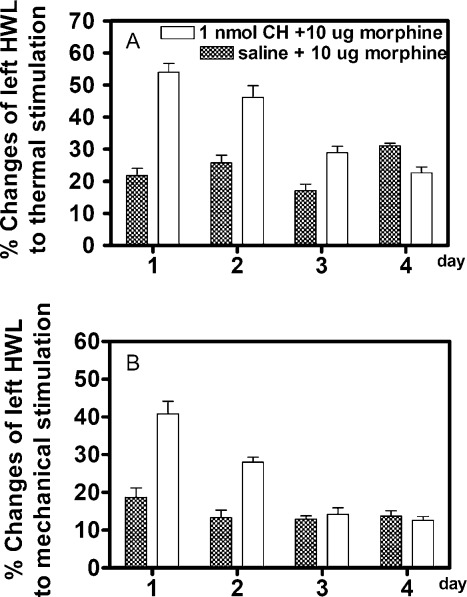
We further tested the lasting time of the inhibitory effects of the PKC inhibitor on morphine tolerance in rats. Two groups of rats with chronic morphine tolerance continued to receive intrathecal injections of 10 μg of morphine twice a day. Figure 5 shows the increased HWLs to thermal and mechanical stimulation tested at 15 min after morphine injection. Interestingly, the blockade effects of the PKC inhibitor on morphine tolerance lasted for three days in the hot-plate test (first day, F = 75.92 and P < 0.001; second day, F = 19.61 and P < 0.01; third day, F = 15.78 and P < 0.01) and lasted for 2 days in the Randall Selitto test (first day, F = 25.96 and P < 0.01; second day, F = 36.89 and P < 0.001; third day, F = 0.36 and P = 0.54), as shown in Figure 5. The results clearly showed that the PKC inhibitor-induced inhibition on the maintenance of morphine tolerance lasted for more than two days.
Figure 5.
Comparison of the antinociceptive effects induced by intrathecal administration of morphine on left HWLs in morphine-tolerant rats that received an intrathecal injection of chelerythrine (CH) or saline for 4 days. The antinociceptive effects were assessed at 15 min after the morphine injection each morning. Data are presented as mean ± SEM. HWL, hindpaw withdrawal latency.
Tolerance to morphine is believed to result from a neuronal adaptation produced by continuous drug administration. It has been reported that some signaling proteins and neuropeptides are involved in morphine tolerance (3,7,10−14). The role of PKC in morphine tolerance in the brain has been studied (15−18). Gabra et al. reported that there was a significant increase in the total phosphatase activity in the periaqueductal gray matter in morphine tolerant mice (15). They further demonstrated that intracerebroventricular (icv) administration of the PKC inhibitors bisindolylmaleimide I or Go6976 reversed the enhanced level of morphine tolerance induced by okadaic acid treatment (15).
It is known that μ-opioid receptor desensitization may play an important role in morphine tolerance. A recent study by Bailey et al. showed that μ-opioid receptor desensitization was observed in single rat brainstem locus coeruleus neurons following exposure to morphine in vitro and in vivo (16). Interestingly, their study found that μ-opioid receptor desensitization has a significant PKC-dependent component and that this desensitization underlies the maintenance of morphine tolerance (16).
There are also some studies for the roles of PKC in morphine tolerance at the spinal level (19,20). It has been demonstrated that spinal morphine tolerance results from PKC-mediated phosphorylation. Granados-Soto and his colleagues found that coinjection of morphine with chelerythrine, a PKC inhibitor, prevented tolerance to the probe morphine dose (19). They further demonstrated that bolus intrathecal injection of another PKC inhibitor GF109203X also blocked the development of morphine tolerance, indicating that morphine-induced tolerance is dependent upon an increase in local PKC phosphorylating activity (19). The present study found that morphine tolerance induced significant increases in PKC expression in the dorsal horn of the spinal cord and the dorsal root ganglion. Furthermore, chronic intrathecal injection of the PKC inhibitor significantly inhibited the development of morphine tolerance. Moreover, we found that morphine tolerance was blocked by intrathecal administration of PKC inhibitors in rats, and the effects of the PKC inhibitor lasted for more than two days. The results clearly showed that PKC is involved in morphine tolerance at the spinal level of rats, and intrathecal administration of the PKC inhibitor can block the development and maintenance of morphine tolerance. These results are supported by the previous findings that PKC inhibitors attenuated tolerance development (19,20).
Interestingly, significant up-regulation of PKC expression was found in morphine-tolerant rats in the dorsal horn of the spinal cord and the dorsal root ganglion but not in the ventral horn of the spinal cord compared with that in naïve rats. These results were consistent with the previous findings that superfluous PKC in the cell could desensitize the μ-opiate receptors on the plasma membrane (17,18,21), which finally facilitated the development of morphine tolerance. Meanwhile, the results also tallied with the notion that the ventral horn of the spinal cord does not participate in the modulation of pain.
Taken together, the present study found that PKC is involved in morphine tolerance at the spinal level of rats, and intrathecal administration of the PKC inhibitor can block the development and maintenance of morphine tolerance.
Materials and Methods
Animal Preparation
All experiments were carried out on freely moving male Sprague−Dawley rats weighing between 220 and 300 g (Experimental Animal Center, Academy of Military Medical Sciences, Beijing, China). The rats were housed in cages with free access to food and water, and maintained at a room temperature of 20 ± 4 °C with a 12 h light−dark cycle. All experiments were performed according to the guidelines of the International Association for the Study of Pain (22), and every effort was made to minimize both the suffering of animals and the number of animals used.
Chemicals
Solutions for intrathecal infusion were prepared with 0.9% sterilized saline, each containing a total volume of 5 μL of 1 nmol of chelerythrine chloride (Tocris Cookson, Bristol, UK) or 10 μg of morphine (morphine HCl; Shenyang First Pharmaceutical Factory, China). The following chemicals were used in immunoblotting: lysis buffer (Beyotime, China), BCA Protein Assay Kit (Pierce, Rockford, IL, USA), rabbit anti-PKC antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), HRP-conjugated goat antirabbit antibody (Zhongshan Goldenbridge Biotechnology Co., Ltd., China), and rabbit antiactin antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Nociceptive Tests
Rats were allowed to become accustomed to the test conditions for 5 days before the experiment to minimize the stress induced by handling and measurements. The HWLs during thermal and mechanical stimulation were measured as described previously (3,23). Briefly, the entire ventral surface of the rat hindpaw was placed manually on a hot plate, which was maintained at a temperature of 52 ± 2 °C. The time to hindpaw withdrawal was measured in seconds and referred to as the HWL to thermal stimulation. The Randall Selitto test (Ugo Basile, type 7200, Italy) was used to assess the HWL to mechanical stimulation. A wedge-shaped pusher at a loading rate of 30 g/s was applied to the dorsal surface of the hindpaw. The latency required to initiate the withdrawal response was assessed and expressed in seconds. Before intrathecal injections, the HWLs were tested three times and regarded as the basal HWLs. The HWLs recorded during subsequent experiments were expressed as percentage changes of the basal level for each rat (% changes of the HWL). Each rat was tested by both types of stimulation. Every measurement of the HWL to both thermal and mechanical stimulation was finished within 2 min. A cutoff limit of 15 s was set up to avoid tissue damage.
Intrathecal Injection
The method of intrathecal injection has been previously described by Yaksh and Rudy (24) and modified by Kong and Yu (25). A chronic polyethylene catheter (Intramedic PE 10) was implanted intrathecally with the inner tip at L3 to L5 in each rat under anaesthetization by intraperitoneal injection of trichloroacetaldehyde monohydrate (400 mg/kg; Xudong Chemical Factory, Beijing, China). Rats exhibiting postsurgical motor disorder (e.g., limb paralysis) were excluded from the experiment (26). On the experimentation day, the PE-10 tube was connected to a 50 μL syringe with a steel injection tip, and then 5 μL of solution was injected intrathecally followed by 15 μL of 0.9% saline to flush the catheter. After the injection, the rats usually recovered in 2 to 3 min.
Morphine Tolerance Model
The rat model of morphine tolerance has been described (1,3). Rats received intrathecal injections of 10 μg of morphine twice a day (at 10:30 a.m. and 22:30 p.m.), which lasted for 5 days. Tolerance was assessed by determining the antinociceptive effects of morphine at 15 min after the first injection of each day. Morphine-induced increases in HWLs to thermal and mechanical stimulation decreased because of after repeated morphine treatments, and morphine tolerance appeared on the fifth day.
Preparation of Protein Extracts
At the end of the experiments, the rats received an injection of a high dose of trichloroacetaldehyde monohydrate (800 mg/kg). The lumbar dorsal horn and the ventral horn of the spinal cord, and the lumbar dorsal root ganglions of the rats were then separately harvested. Tissues weighing 20 mg were homogenized in 100 μL of lysis buffer with 1% phenylmethanesulfonyl fluoride (PMSF). After centrifugation at 14,000 rpm for 10 min, the supernatant was collected and stored at −80 °C. The protein concentrations were determined by BCA Protein Assay Kit as described by the manufacturer.
Western Blot
Equal amount of proteins were separated on 10% sodium dodecyl sulfate−polyacrylamide gel electrophoresis (SDS−PAGE). Proteins were then transferred to Immobilon-PTM polyvynilidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% nonfat milk in Tris-buffered saline containing 0.05% Tween-20 (TBST) for 1 h at room temperature and then incubated overnight at 4 °C with the primary antibody. After washing 3 × 10 min in TBST, membranes were incubated with horseradish peroxidase (HRP)-conjugated goat antirabbit antibody for two hours at room temperature. After another three washes with TBST, protein was visualized with an ECL detection system (Applygen, China). The photographs were subjected to Transilluminator Gel-Pro 4400 (Media Cybernmetics. L.P., Silver Spring, MD, USA) for density measurements. The relative density was calculated by the total absolute density of (PKC/actin) × 100.
Statistical Analysis
Data from the experiment were expressed as the mean ± SEM. Statistical differences between groups were determined by either two-way analysis of variance (ANOVA) or one-way analysis of variance (ANOVA). *P < 0.05, **P < 0.01, and ***P < 0.001 were considered as significant differences.
Wu-Yang Jin, undergraduate student in the College of Life Sciences, Peking University, supported by a grant from National Undergraduate Innovative Test Program Research Endowment sponsored by the National Ministry of Education.
Long-Chuan Yu, professor and director, Neurobiology Laboratory, College of Life Sciences, Peking University, Beijing 100871, PR China, supported by grants from the National Natural Science Foundation of China (NSFC, 30870802, 30470542) and the National Program of Basic Research sponsored by the Ministry of Science and Technology of China (2009CB522002).
This study was supported by grants from the National Natural Science Foundation of China (NSFC, 30870802 and 30470542), National Undergraduate Innovative Test Program Research Endowment sponsored by the National Ministry of Education, and the National Program of Basic Research sponsored by the Ministry of Science and Technology of China (2009CB522002).
References
- Wu X.; Yu L. C. (2006) Plasticity of galanin in nociceptive modulation in the central nervous system of rats during morphine tolerance: a behavioral and immunohistochemical study. Brain Res. 1086, 85–91. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L.; Onofrio B. M. (1987) Retrospective consideration of the doses of morphine given intrathecally by chronic infusion in 163 patients by 19 physicians. Pain 31, 211–223. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Li J. J.; Yu L. C. (2003) Plastic changes of calcitonin gene-related peptide in morphine tolerance: behavioral and immunohistochemical study in rats. J. Neurosci. Res. 74, 622–629. [DOI] [PubMed] [Google Scholar]
- Murray N. R.; Weems C.; Chen L.; Leon J.; Yu W.; Davidson L. A.; Jamieson L.; Chapkin R. S.; Thompson E. A.; Fields A. (2002) Protein kinase C betaII and TGFbetaRII in omega-3 fatty acid-mediated inhibition of colon carcinogenesis. J. Cell Biol. 157, 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecha A. L.; Genaro A. M.; Gorelik G.; Arcos M. L. B.; Silberman D. M.; Schuman M.; Garcia S. I.; Pirola C.; Cremaschi G. A. (2006) Integrative study of hypothalamus−pituitary−thyroid−immune system interaction: thyroid hormone-mediated modulation of lymphocyte activity through the protein kinase C signaling pathway. J. Endocrinol. 189, 45–55. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Yu F. H.; Surmeier D. J.; Scheuer T.; Catterall W. A. (2006) Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase C. Neuron 49, 409–420. [DOI] [PubMed] [Google Scholar]
- Mayer D. J.; Mao J.; Price D. D. (1995) The development of morphine tolerance and dependence is associated with translocation of protein kinase C. Pain 61, 365–374. [DOI] [PubMed] [Google Scholar]
- Gabra B. H.; Bailey C. P.; Kelly E.; Smith F. L.; Henderson G.; Dewey W. L. (2008) Pre-treatment with a PKC or PKA inhibitor prevents the development of morphine tolerance but not physical dependence in mice. Brain Res. 1217, 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton P. M.; Kim J. A.; McGeehan A. J.; Paredes J. P.; Chu K.; Wallace M. J.; Roberts A. J.; Hodge C. W.; Messing R. O. (2007) Increased response to morphine in mice lacking protein kinase C epsilon. Genes Brain Behav. 6, 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla P. K.; Tang L.; Wang Z. J. (2006) Phosphorylation of neurogranin, protein kinase C, and Ca2+/calmodulin dependent protein kinase II in opioid tolerance and dependence. Neurosci. Lett. 404, 266–269. [DOI] [PubMed] [Google Scholar]
- Zeitz K. P.; Malmberg A. B.; Gilbert H.; Basbaum A. I. (2001) Reduced development of tolerance to the analgesic effects of morphine and clonidine in PKC gamma mutant mice. Pain 94, 245–253. [DOI] [PubMed] [Google Scholar]
- Law P. Y.; Loh H. H.; Wei L.-N. (2004) Insights into the receptor transcription and signaling: implications in opioid tolerance and dependence. Neuropharmacology 47, 300–311. [DOI] [PubMed] [Google Scholar]
- Ma W.; Zheng W. H.; Kar S.; Quirion R. (2000) Morphine treatment induced calcitonin gene-related peptide and substance P increases in cultured dorsal root ganglion neurons. Neuroscience 99, 529–539. [DOI] [PubMed] [Google Scholar]
- Ray S. B.; Gupta H.; Gupta Y. K. (2004) Up-regulation of mu-opioid receptors in the spinal cord of morphine-tolerant rats. J. Biosci. 29, 51–56. [DOI] [PubMed] [Google Scholar]
- Gabra B. H.; Bailey C. P.; Kelly E.; Sanders A. V.; Henderson G.; Smith F. L.; Dewey W. L. (2007) Evidence for an important role of protein phosphatases in the mechanism of morphine tolerance. Brain Res. 1159, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. P.; Llorente J.; Gabra B. H.; Smith F. L.; Dewey W. L.; Kelly E.; Henderson G. (2009) Role of protein kinase C and mu-opioid receptor (MOPr) desensitization in tolerance to morphine in rat locus coeruleus neurons. Eur. J. Neurosci. 29, 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. P.; Smith F. L.; Kelly E.; Dewey W. L.; Henderson G. (2006) How important is protein kinase C in μ-opioid receptor desensitization and morphine tolerance?. Trends Pharmacol. Sci. 27, 558–565. [DOI] [PubMed] [Google Scholar]
- He L.; Fong J.; Zastrow M. V.; Whistler J. L. (2002) Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell 108, 271–282. [DOI] [PubMed] [Google Scholar]
- Granados-Soto V.; Kalcheva I.; Hua X. Y.; Newton A.; Yaksh T. L. (2000) Spinal PKC activity and expression: role in tolerance produced by continuous spinal morphine infusion. Pain 85, 395–404. [DOI] [PubMed] [Google Scholar]
- Wu G. J.; Wen Z. H.; Chang Y. C.; Yang S. N.; Tao P. L.; Wong C. S. (2006) Protein kinase C inhibitor chelerythrine attenuates the morphine-induced excitatory amino acid release and reduction of the antinociceptive effect of morphine in rats injected intrathecally with pertussis toxin. Life Sci. 78, 1801–1807. [DOI] [PubMed] [Google Scholar]
- Ueda H.; Inoue M.; Matsumoto T. (2001) Protein kinase C-mediated inhibition of mu-opioid receptor internalization and its involvement in the development of acute tolerance to peripheral mu-agonist analgesia. J. Neurosci. 1, 2967–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16, 109–110. [DOI] [PubMed] [Google Scholar]
- Li J. J.; Zhou X.; Yu L. C. (2005) Involvement of neuropeptide Y and Y1 receptor in antinociception in the arcuate nucleus of hypothalamus, an immunohistochemical and pharmacological study in intact rats and rats with inflammation. Pain 118, 232–242. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L.; Rudy T. A. (1976) Analgesia mediated by a direct spinal action of narcotics. Science 192, 1357–1358. [DOI] [PubMed] [Google Scholar]
- Kong L. L.; Yu L. C. (2006) Involvement of mu- and delta-opioid receptors in the antinociceptive effects induced by AMPA receptor antagonist in the spinal cord of rats. Neurosci. Lett. 402, 180–183. [DOI] [PubMed] [Google Scholar]
- Mao J. (2002) Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain 100, 213–217. [DOI] [PubMed] [Google Scholar]